Abstract
Age-related diseases are often attributed to immunopathology, which results in self-damage caused by an inappropriate inflammatory response. Immunopathology associated with early-life inflammation also appears to cause faster ageing, although we lack direct experimental evidence for this association. To understand the interactions between ageing, inflammation and immunopathology, we used the mealworm beetle Tenebrio molitor as a study organism. We hypothesized that phenoloxidase, an important immune effector in insect defence, may impose substantial immunopathological costs by causing tissue damage to Malpighian tubules (MTs; functionally equivalent to the human kidney), in turn accelerating ageing. In support of this hypothesis, we found that RNAi knockdown of phenoloxidase (PO) transcripts in young adults possibly reduced inflammation-induced autoreactive tissue damage to MTs, and increased adult lifespan. Our work thus suggests a causative link between immunopathological costs of early-life inflammation and faster ageing. We also reasoned that if natural selection weakens with age, older individuals should display increased immunopathological costs associated with an immune response. Indeed, we found that while old infected individuals cleared infection faster than young individuals, possibly they also displayed exacerbated immunopathological costs (larger decline in MT function) and higher post-infection mortality. RNAi-mediated knockdown of PO response partially rescued MTs function in older beetles and resulted in increased lifespan after infection. Taken together, our data are consistent with a direct role of immunopathological consequences of immune response during ageing in insects. Our work is also the first report that highlights the pervasive role of tissue damage under diverse contexts of ageing and immune response.
Keywords: ageing, infection, inflammation, immunopathology, Malpighian tubules
1. Introduction
Immunopathology refers to self-damage caused by an over-reactive immune system in response to infection, and contributes to the pathology of several human diseases [1–3]. In insects, fast-acting but non-specific inflammatory responses are efficient against invading pathogens, but they also lead to immunopathology [4]. Cytotoxin-producing effector systems such as the phenoloxidase (PO) response pathway can also act against host cells [4–6]. However, the role of immunopathology in natural populations is still unclear, with little information on whether immunopathology is a measurable cost of immune response, with major impacts on fitness. It is important to address these gaps, because immunopathology owing to inflammatory responses can have long-term implications. For instance, it was proposed that reduced inflammatory exposure during childhood may have contributed significantly to increased lifespan in modern human industrialized societies [7].
Recently, the impact of an early-life immune response on faster ageing was studied experimentally in the model insect Tenebrio molitor [8]. The study suggested that accelerated ageing is caused by immunopathological damage to Malpighian tubules (MTs). MTs are fluid-secreting epithelia that are functionally analogous to the human kidney [9,10], playing a critical role in osmoregulation as well as detoxification of haemolymph [11]. They are exposed to haemolymph owing to functional necessities and extend throughout the body cavity of insects [12]. The lack of an impermeable protective membrane combined with the spatial disposition makes MTs particularly vulnerable to damage during an immune response [13]. However, we lack direct experimental evidence for the role of damage to MTs as a mediator of faster ageing owing to early immune response. The PO pathway, a fast-acting immune effector in insects [14], is a likely candidate to cause autoreactive tissue damage via cytotoxic intermediates in T. molitor [13]. Previous studies have already established a link between an overactive PO response and increased mortality in Drosophila melanogaster. For instance, flies that carried mutations in serpin-encoded spn27A failed to inhibit overexpression of the PO response, and died faster owing to excessive melanization [15]. Also, mutations that inactivate a signalling serine protease (CG3066) in fruit flies—required for activation of pro-PO—increased lifespan after Streptococcus pneumoniae infection [16]. Based on these results, we propose that the immunopathological damage to MTs caused by the PO cascade results in accelerated ageing after early immune response.
In most animals, ageing is associated with a progressive decline in immune function leading to increased mortality and morbidity [17–19]. Yet recent experiments seem to contradict this. For example, in D. melanogaster different immune genes show age-specific upregulation—older fruit flies increased the expression of antimicrobial peptides after infection [20] and harboured lower pathogen load [21]. Similar results were reported for the red flour beetle Tribolium castaneum, where ageing enhanced multiple components of immunity such as haemolymph antibacterial activity and PO response [22]. However, despite this increased immune response with age, older beetles are more likely to die from infection. This highlights a mismatch between immune function and the ability to survive after infection, and questions whether the observed age-related increase in immunity is beneficial. Instead, relaxed natural selection in aged individuals [23,24] may result in deregulation of the immune system, increasing immunopathological costs. We hypothesize that immunopathology associated with such a deregulated (over-reactive) immune response can directly contribute to greater post-infection mortality with age.
To begin to understand the complex interplay between ageing, immune response and immunopathology, we conducted experiments with the holometabolous insect mealworm beetle T. molitor. We first investigated whether PO-mediated immunopathological damage to MTs during early-life inflammation resulted in faster ageing. We then tested whether older individuals experience increased immunopathological risk and whether this is also mediated by the same mechanisms as in early-life inflammation.
2. Methods
All experiments were conducted with female T. molitor. The mealworm beetle is a storage pest of food grain, so it lives in relatively high-density populations in dark places [25]. In our experiments, we collected experimental beetles from an outbred stock population maintained at 30 ± 2°C and supplied with an ad libitum diet of wheat bran and rat chow, supplemented with apple every 3 days. Under natural conditions, Tenebrio is exposed regularly to a number of pathogens, including fungi [26] and microsporidia [27]. However, we have no evidence for virulent microbial infections in our stock population. To produce inflammatory responses without pathogenesis (see below for experiment 1), we used peptidoglycans (Sigma) derived from Staphylococcus aureus strain JLA513, a tetracycline resistant strain known to cause persistent infections in T. molitor [28]. For all live infections (see below for experiments 2 and 3), we used stationary-phase S. aureus culture (same strain as mentioned above). We collected early pupae and determined their sex by observing the terminal abdominal segments. Upon emergence, only females weighing between 120 and 150 mg were retained individually as virgins in grid box containers. We did not use males in our experiments owing to logistical reasons. All experimental females were thus controlled for age, mating status and size. Typically, female Tenebrio beetles attain sexual maturity and begin to oviposit within a week from adult emergence [29,30]. This applies to the beetle population used in this study, with more than 97% mating success on day 7 post-eclosion (I.K. and A. Prakash 2016, unpublished data). We further found that female beetles undergo reproductive senescence within 42 days post-eclosion (electronic supplementary material, figure S1), and the mean adult lifespan is 60 days under standard laboratory conditions. Based on these findings, we used 7- and 49-day-old individuals as ‘young’ and ‘old’ adults, respectively. We subjected both young and old females to experiments on the same day to enable a direct comparison between age classes.
(a). Experiment 1: impact of immunopathology associated with early immune activation on ageing
(i). RNAi
To investigate whether reduced immunopathology minimizes the survival costs of early inflammation, we experimentally manipulated the degree of immunopathology during early-life immune response using RNA interference (RNAi) of prophenoloxidase (proPO) expression and quantified survival. T. molitor has at least two proPO genes as documented in Johnston et al. [31] which likely encode two subunits of a heterodimer. We used RNAi to knock down the expression of both the proPO genes in 7-day-old virgin females: a previously sequenced Tenebrio proPO gene transcript (NCBI accession AB020738.1; henceforth PO1) [32], and an orthologue to T. castaneum proPO subunit 1 (henceforth, PO2). We amplified the internal region of the cDNA sequences encoding PO with T7-tailed primers (electronic supplementary material, table S1), and used them as templates to synthesize dsRNA using the T7 MEGAscript kit (Ambion) according to the manufacturer's instructions. We extracted the resulting RNA with phenol/chloroform, resuspended it in sterile nuclease-free Ringer solution (128 mM NaCl, 18 mM CaCl2, 1.3 mM KCl, 2.3 mM NaHCO3 [28]), and stored it at −80°C until further use. We annealed the complementary strands by heating at 65°C for 30 min and incubating at 22°C for an hour, before injecting 100 ng purified dsRNA into each beetle. As RNAi control (or early immune-challenged control), we injected individuals with an internal region of the cDNA sequence encoding a lysozyme (Gm-Lys) from Galleria mellonella (Swiss-Prot accession: P82174), available in our laboratory [33]. Two days after RNAi manipulation, we injected each beetle with 5 µl peptidoglycan (concentration: 100 ng in 1 ml of Ringer solution) to deplete the basal amount of PO in the haemolymph. Finally, after another 2 days, we challenged each beetle with a higher dose of peptidoglycan (5 µl; 5 µg in 1 ml of Ringer solution) to induce a strong immune response. Twenty hours later, we quantified RNAi efficacy by performing qPCR (see electronic supplementary material, supporting methods for replicate sizes, RNA isolation and qPCR protocol, and qPCR primers; electronic supplementary material, table S2). We used the comparative CT method [34] to estimate relative gene expression (see electronic supplementary material, figure S2 and table S3 for RNAi efficacy).
Following the immune challenge, a subset of beetles from all RNAi treatments (n = 30 beetles per treatment) was monitored for total lifespan. Four days after immune challenge, the remaining beetles were either assayed for MT activity (n = 20–28 beetles per treatment) or PO response (n = 16 beetles per treatment; see below for methods). A set of 30 beetles served as unhandled full controls (control) that remained in the grid box container throughout the experimental window. In addition, as a procedural control for the impact of early inflammation on adult lifespan, 30 beetles received ds-Lys injection (mock RNAi) followed by a mock immune challenge (with insect Ringer). Below, we briefly describe the methods for quantifying the immunopathology and PO responses.
(ii). Malpighian tubule activity
MT activity is of particular importance in this study owing to its vulnerability to damage during an immune activation [13]. A previous study used a modified ‘oil drop’ technique [35,36] to demonstrate a large reduction in MT function owing to immunopathology associated with immune induction [13]. The method provides an in vitro functional estimate of the ability of isolated tubules to transport saline across the active cell wall into the tubule lumen. We estimated the fluid transporting capacity of MTs harvested from experimental beetles 4 days after immune challenge, as a proxy for immunopathology owing to immune response. Each beetle has three pairs (dorsal, lateral and ventral) of large MTs of varying length but similar secretion rates [37]. Hence, we dissected one tubule from each cold-anaesthetized animal under cold sterile modified Tenebrio Ringer saline, prepared as described in Wiehart et al. [38]. We severed the tubule at the point where it connects to the gut, and removed another approximately 0.5 mm length from the open end (to control for the condition of the cut end). Following this, we transferred a single tubule per beetle to a 60 µl drop of sterile modified Ringer saline supplemented with 0.05% w/v phenol red to facilitate visualization, and 0.1 mM l−1 dibutyryl cyclic AMP to stimulate fluid secretion [13,36]. We covered the whole preparation with mineral oil (Sigma). Next, we pulled the open end of the tubule out of the saline drop and wrapped it around 0.1 mm pins (Fine Science Tools) in the mineral oil, where it secreted fluid. Six hours later, we measured the volume of the secreted droplet, as well as the length of tubule that remained within the saline drop using ImageJ software. The volume of the secreted droplet is negatively correlated with the degree of immunopathological harm to MTs.
(iii). Phenoloxidase response
We measured the PO activity of RNAi-treated beetles by measuring the rate of formation of dopachrome with a spectrophotometer [39]. We mixed 2 µl undiluted haemolymph (collected from a wound between the head and thorax) with 8 µl PBS, and centrifuged the sample at 6500 r.p.m. for 15 min at 4°C. We transferred 5 µl of the supernatant to a 96-well-microplate containing 20 µl PBS and 140 µl distilled water to measure activated PO enzyme (henceforth, PO activity). We then added 20 µl of l-DOPA substrate into each well, and transferred the plate immediately into a Microplate reader. We allowed the reaction to proceed at 30°C for 60 min, and then measured absorption at 490 nm once every minute. We quantified PO enzyme activity as the slope of the linear phase (between 15 and 45 min) of the reaction in each well (Vmax: change in absorbance per minute).
(b). Experiment 2: impact of ageing on immune function and post-infection survival
We grew a S. aureus culture overnight in liquid LB medium to an OD600 of 95%. We then centrifuged the culture, washed the pellet three times before re-suspending in insect Ringer solution. We injected 5 µl of this suspension directly into the haemocoel of each individual (approx. 4 × 106 colony-forming units (CFUs) per inoculum; see [28]). Control individuals were injected with 5 µl of Ringer solution. After infection, we redistributed beetles individually into grid boxes under standard conditions with access to food. For a subset of beetles (n = 14–15 beetles per age group per treatment), we monitored individual survival daily at 20.00 for 40 days. Another subset was tested for clearance of S. aureus infection from haemolymph after 6 h, 1 day, 7 days and 14 days. At each time point, we harvested bacterial cells from a group of nine to 11 individuals to estimate remaining CFUs per beetle using the perfusion bleeding method described by Haine et al. [28]. Briefly, perfused haemolymph was collected from each beetle and plated on LB agar containing 5 µg ml−1 tetracycline as a selective agent. The number of colonies observed after 48 h of incubation at 30°C should be negatively correlated with the ability to clear bacterial infection.
Next, we tested whether induced haemolymph antimicrobial activity against S. aureus cells differed across beetle age groups 1 or 7 days after infection (n = 9–13 beetles per infection treatment per age group), using an in vitro cell killing reaction as described by Haine et al. [28]. At each time point, we collected 2 µl of undiluted haemolymph sample as described above, diluted it with 48 µl PBS and 2 µl of an overnight culture of S. aureus (approx. 106 CFU), and incubated at 30°C with shaking at 150 rpm for 2 h. Following this, we diluted the mixture 800 times and plated out as described above. The number of CFUs that appeared was counted after 48 h incubation at 30°C. The induced anti-S. aureus activity of the haemolymph was therefore estimated as the number of S. aureus cells killed during 2 h of exposure to beetle haemolymph.
Finally, we measured PO response (n = 30 beetles per age group) and estimated the relative expression of the antimicrobial peptide-coding genes attacin 2 and tenecin 1 (see electronic supplementary material for qPCR primer sequences and replicate sizes) as a function of age in naive beetles as described earlier. Because these assays were performed with uninfected beetles, they served as an estimate of age-associated changes in baseline constitutive innate immune function in the absence of immune induction.
(c). Experiment 3: impact of ageing and infection on immunopathology
Beetles from both age groups (7 versus 49 days old) were first infected (or sham-infected) as described earlier (n = 11–14 beetles per age group per infection status). Four days later, we estimated MT function as a proxy for immunopathology (see experiment 1 for methods). We also manipulated the impact of bacterial infection on MT activity and tested whether reducing damage to MTs can rescue the low post-infection survival of older beetles. To this end, we injected 47-day-old beetles with 5 µl of dsRNA (100 ng µl−1) of PO1 or Lys (n = 27–34 beetles per RNAi treatment) as described in experiment 1. Two days later, we infected beetles as described above. We monitored a subset of individuals for their post-infection survival daily at 20.00 for 25 days (n = 15–18 beetles per RNAi treatment). The remaining individuals were tested for MT activity as described above to quantify immunopathology (n = 12–16 beetles per RNAi treatment).
(d). Data analysis
Residuals of bacterial clearance data were not normally distributed (tested with Shapiro–Wilks test). Hence, we log-transformed the data, and confirmed that the transformed residuals were normally distributed. Following this, we used a two-way or one-way ANOVA to test the following effects: (i) bacterial clearance as a function of age and assay time (ii) PO response of early immune-challenged beetles as a function of RNAi treatments. We tested for pairwise differences between treatments after correcting for multiple comparisons, using Tukey's HSD. Non-normally distributed data that could not be transformed to a normal distribution were analysed using non-parametric Wilcoxon rank sum tests: (i) MT activity after early-immune response as a function of RNAi treatments; (ii) antimicrobial activity as a function of age (analysed separately for control and infected beetles); (iii) PO response as a function of age; (iv) MT activity as a function of age and infection; (v) MT activity of older beetles as a function of RNAi treatments. Here, we used a Steel–Dwass test to estimate pairwise differences.
We used Cox proportional hazard survival analysis to test the following effects: (i) beetle survival as a function of age and infection (ii) post-infection survival of old beetles as a function of RNAi treatments. We did not have any censored values while analysing the data, as all beetles died within the experimental window. We calculated the impact of treatment (e.g. bacterial infection or RNAi) as the estimated hazard ratio of the experimental versus the control group (hazard ratio = rate of deaths occurring in the experimental group per rate of deaths occurring in the control group). A hazard ratio significantly greater than one indicates an increased risk of mortality in the experimental group compared with control individuals.
We analysed median and maximum lifespans to measure the change in ageing rate following early-life immune activation. We used accelerated failure time (AFT) models [40] to examine the median lifespan in R using the ‘survival’ package, with each model separately analysing the difference in median lifespan between a treatment and unhandled control group. We also compared knockout (PO1 and PO2) versus early immune-challenged control (EI) beetles to analyse whether PO knockdown increased lifespan after an early immune response. We found that the Weibull distribution minimized the Akaike's information criterion (AIC) value of AFT models, and was thus the most appropriate method to use for each comparison. For each model, we estimated the c-parameter (exponential (estimated coefficient associated with lifespan)) representing the difference in median lifespan between the two groups, as suggested by Swindell [40]. A c-parameter value significantly less than 1 indicates reduction in lifespan and vice versa. For a given comparison, the value 100(c − 1) represents the per cent change in median lifespan of the experimental versus the control group [41].
Maximum lifespan has been suggested as an important indicator of the ageing process [42]; hence, we used it to test the impact of early immune response on ageing. We first estimated the 90th percentile lifespan when all treatments were combined, and then calculated the percentage of beetles in each treatment group living until this time. We then performed exact unconditional tests using a contingency table approach to compare percentage survival of each treatment group with unhandled control beetles (www.stat.ncsu.edu/exact). We also compared knockout beetles versus immune-challenged control beetles (i.e. PO1 versus EI or PO2 versus EI) to test whether PO knockdown increased maximum lifespan significantly. We used a binomial, two-way model to generate a pooled z-score test p-value for each comparison. To obtain the treatment effect for each comparison, we divided the percentage survival of the respective control groups by that of the experimental group.
3. Results
(a). Phenoloxidase response after early inflammation increases mortality and organ damage
We found that an early immune challenge in young adults caused an increase in PO response (figure 1a and electronic supplementary material, table S4a), with a concomitant reduction in MT activity (figure 1b and electronic supplementary material, table S4b). We further observed that RNAi knockdown of the PO response reduced MT damage in immune-challenged beetles, resulting in a limited reduction of fluid secretion rate (compare figure 1a,b and electronic supplementary material, table S4a,b). We note that if difficulty in MT excretion alters haemolymph pH in immune-challenged beetles, PO enzyme kinetics could also change without actual changes in proPO expression. This explanation can be ruled out, however, as the RNAi manipulation reduced PO transcripts which strongly correlates with the decreased PO enzymatic activity determined by the standard spectrophotometric method (compare electronic supplementary material, figure S2 and figure 1a).
Figure 1.
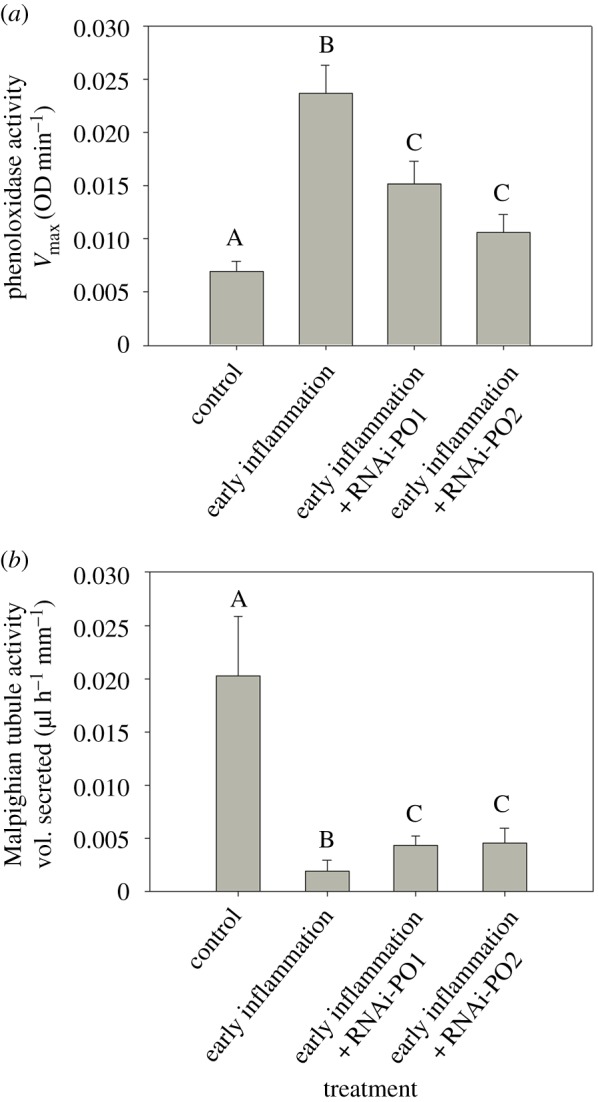
Impact of RNAi-mediated knockdown of prophenoloxidase transcripts on (a) phenoloxidase (PO) response (n = 16 beetles per treatment) and (b) Malpighian tubule (MT) activity (n = 20–28 beetles per treatment), serving as a proxy for immunopathological damage, measured at day 4 post-immune challenge (i.e. day 15 post-emergence). PO activity was measured as Vmax of the enzymatic reaction with l-DOPA substrate. MT activity was measured as the rate of fluid secretion. Significantly different groups are indicated by distinct alphabets (based on Tukey's HSD/Steel–Dwass test). Control = unhandled control beetles; early inflammation = early immune challenged control beetles; early inflammation + RNAi-PO1 = RNAi of PO1 transcript followed by an early inflammation; early inflammation + RNAi-PO2 = RNAi of PO2 transcript followed by an early inflammation.
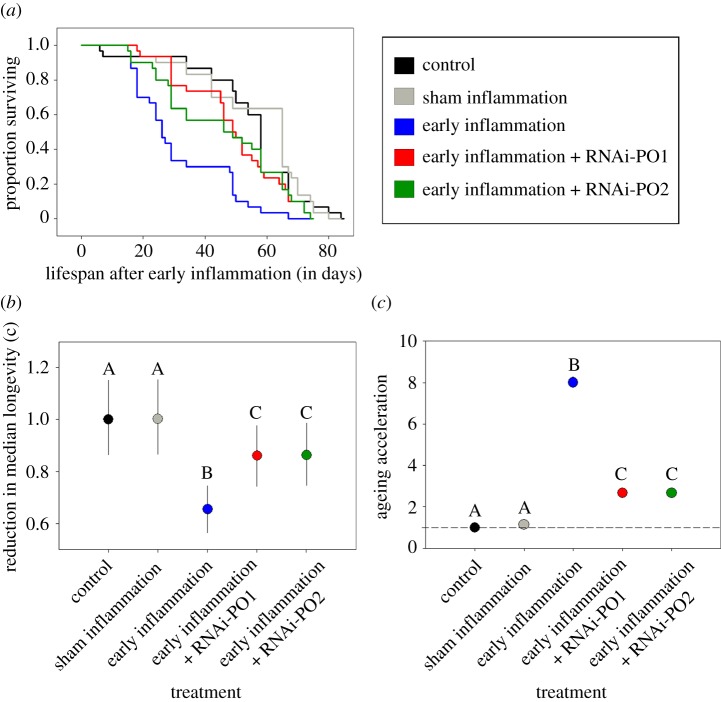
An AFT model showed that early immune-challenged beetles also had shorter lifespans compared with full control and procedural control beetles (figure 2a,b and electronic supplementary material, table S5a). We did not detect mortality until 16 days following the immune challenge, suggesting that early immune challenge did not have an immediate impact on survival (figure 2a). The c-parameter was lowest in immune-challenged control (EI) beetles, suggesting a reduction in median lifespan (per cent decline with respect to full control, 100(c − 1): approx. 35%) following an early-life immune response (figure 2b and electronic supplementary material, table S5a). The negative impact of an early immune challenge on beetle lifespan could also be reversed by RNA interference of proPO transcripts (e.g. PO1 and PO2) in immune-challenged beetles (figure 2a,b and electronic supplementary material, table S5a). RNAi of both the transcripts extended the lifespan by approximately 31–32% compared with immune-challenged control (EI) beetles (figure 2b and electronic supplementary material, table S5a).
Figure 2.
(a) Survival curves of adults after an early immune challenge that induced inflammation (n = 30 beetles per treatment). (b) Impact of phenoloxidase (PO) knockdown on median lifespan. Survival of each experimental group was compared with the unhandled control group using an accelerated failure time model. The c-parameter denotes the effect of the immune challenge treatment on survival, averaged over total survival time. Error bars represent 95% confidence intervals. (c) Impact of PO knockdown on maximum lifespan. ‘Ageing acceleration’ on the y-axis denotes the reduction in maximum lifespan caused by immune challenge treatments (the percentage of survivors to the 90th percentile survival time in the full control group/the percentage of survivors to the 90th percentile survival time in the experimental group). Control = unhandled control beetles; sham inflammation = procedural control for early life immune challenge; early inflammation = early life immune challenged control beetles; early inflammation + RNAi-PO1 = RNAi of PO1 transcript followed by an early inflammation; early inflammation + RNAi-PO2 = RNAi of PO2 transcript followed by an early inflammation.
Analysis of maximum lifespan produced similar results as that of median lifespan. The 90th percentile of overall survival time was 74 days (pooling all individuals across treatments); the percentage of immune-challenged individuals surviving to this time was significantly lower than control groups (EI = 3%, PO1 = 10%, PO2 = 10%, PC = 23%, control = 27%; also see figure 2c and electronic supplementary material, table S5b for treatment effects). These data suggest acceleration in ageing owing to early-life immune response (compare 90th percentile survival for each experimental group: FC = 79.5; PC = 83.1, EI = 57.6; PO1 = 73.8, PO2 = 73.8). Finally, we found that RNAi knockdown of the PO response significantly increased maximum lifespan compared with immune-challenged EI beetles with normal PO levels, suggesting delayed ageing in knockout groups (compare ageing acceleration in figure 2c and electronic supplementary material, table S5b).
(b). Immune responses increase with age
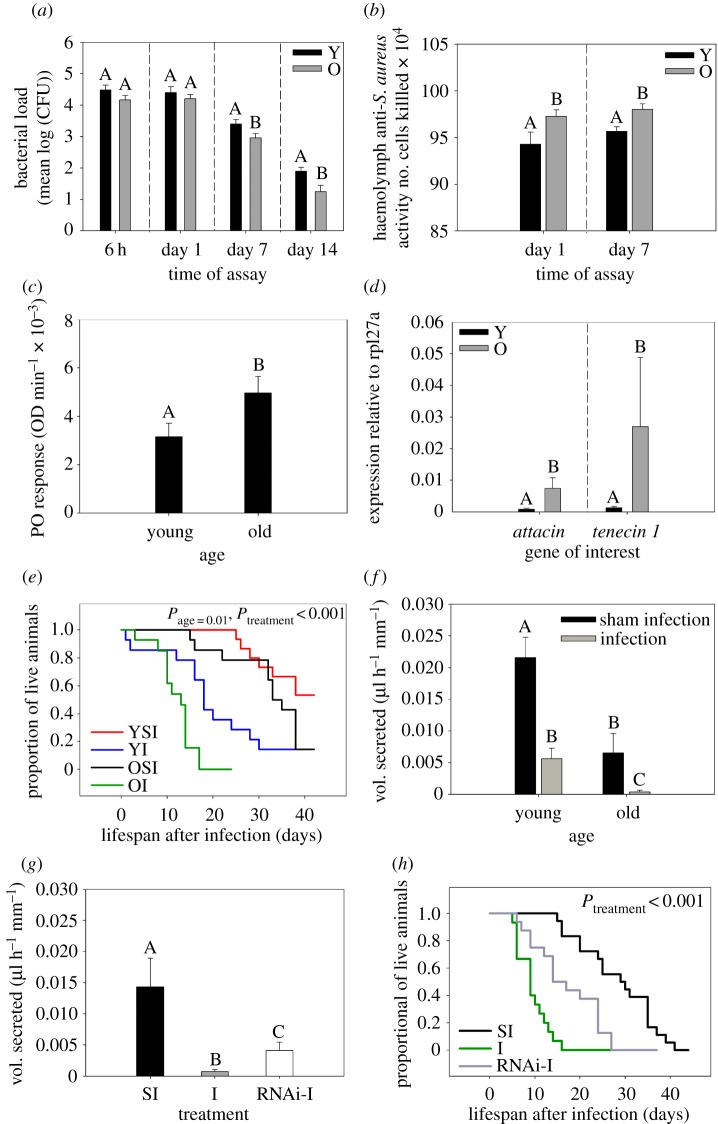
We found a rapid clearance of bacterial cells in both young and old beetles: more than 98% cells were removed within 6 h after infection. However, older individuals showed consistently lower bacterial loads across different time points (i.e. 6 h, 24 h, 7 days and 14 days) after infection (figure 3a and electronic supplementary material, table S6a). Next, we tested the ability of cell-free haemolymph to kill S. aureus cells 1 or 7 days after infection with live bacteria (or sham infection). We found that haemolymph from older infected beetles showed a significantly higher antibacterial response compared with younger beetles (figure 3b and electronic supplementary material, table S6b). In contrast, age did not have a strong influence on the antibacterial activity of cell-free haemolymph harvested from sham-infected beetles (electronic supplementary material, figure S3 and table S6b). Older beetles also showed an enhanced PO response (figure 3c and electronic supplementary material, table S6c) and higher expression of the antimicrobial peptides attacin 2 and tenecin 1 (figure 3d and electronic supplementary material, table S3), even in the absence of a previous bacterial infection.
Figure 3.
(a–f) Impact of age on (a) bacterial clearance at different time points after infection (n = 9–11 beetles per age group per treatment per time point); (b) haemolymph anti-S. aureus activity of infected beetles at different time points (n = 9–13 beetles per age group per treatment per time point); (c) phenoloxidase (PO) response of naive beetles (n = 30 beetles per age group); (d) expression of antimicrobial peptide genes attacin and tenecin-1 in naive beetles relative to an internal control (rpl27a) (n = 5–6 pairs per age group per gene); (e) post-infection survival (n = 14–15 beetles per age group per treatment) and (f) Malpighian tubule (MT) activity after bacterial infection (n = 11–14 beetles per age-group per infection status); (g,h) Effect of RNA interference of PO1 transcript on (g) immunopathological damage to MTs (n = 12–16 beetles per RNAi treatment) and (h) post-infection survival in older beetles (n = 15–18 beetles per RNAi treatment). Bacterial clearance was measured as log10 of colony-forming units (CFUs) recovered from perfused haemolymph after injection of S. aureus cells into each beetle. Haemolymph antibacterial activity was measured as the number of S. aureus cells killed during 2 h of exposure to beetle haemolymph. PO response and immunopathology were measured as described in figure 1. Significantly different groups are indicated by distinct alphabets (based on Tukey's HSD/Steel–Dwass test). For panels (a,b,d), alphabet assignments are meaningful only within each time point (or gene) (partitioned by dashed vertical lines), and are not comparable across time points (or genes). Y = young beetles; O = old beetles; YSI = young sham-infected beetles; OSI = old sham-infected beetles; YI = young infected beetles; OI = old infected beetles; PC = sham infection with insect ringer; I = S. aureus infection; RNAi + I = RNAi followed by S. aureus infection.
(c). Older beetles die faster after bacterial infection
To test if an age-related increase in immunity also confers greater survival benefits, we tested the impact of S. aureus infection on the survival of young versus old beetles. Compared with young beetles, we found that old beetles died much faster after an infection (hazard ratio: sham-infection versus infection in old beetles = 12.59, p < 0.001; sham-infection versus infection in young beetles = 5.18, p < 0.001; figure 3e and electronic supplementary material, table S6d). In contrast, beetle age did not alter the survival of sham-infected beetles significantly (figure 3e and electronic supplementary material, table S6d).
(d). Increased immune response impairs Malpighian tubule activity via immunopathology
We found that ageing itself reduced the baseline MT activity in sham-infected beetles. Bacterial infection impaired the fluid transport ability of MTs in both age groups, but the relative impact of infection was much larger in infected older beetles (approx. 95% reduction, compared with an approx. 73% reduction in young beetles; figure 3f and electronic supplementary material, table S7a). As observed for early immune-challenged beetles, we found that RNAi-mediated knockdown of pro-PO1 partially rescued the MT activity of infected older beetles (figure 3g and electronic supplementary material, table S7b), and proPO1 knockdown beetles survived longer after infection than wild-type beetles (hazard ratio: knockout versus infected wild-type beetles = 0.396; p = 0.01; figure 3h and electronic supplementary material, table S7c). We thus suggest that bacterial infection induced immune upregulation in older beetles, but also incurred greater immunopathological damage to MTs, resulting in greater mortality despite higher immune function.
4. Discussion
In the work presented here, we provide evidence that is consistent with an important role of immunopathological costs in the diverse contexts of ageing and immune response in the model insect T. molitor. We first show that early-life inflammation leads to faster ageing. Early-life immune activation, without the direct cost of a live and replicating pathogen, was sufficient to reduce the activity of MTs, a vital insect organ that is functionally equivalent to the vertebrate kidney. Subsequently, we demonstrated that experimental suppression of the PO response partially rescued MT activity and increased survival. These results highlight that greater collateral damage to vital organs by PO is possibly one of the mechanisms that cause accelerated death of older beetles after early-life inflammation. Although our work focuses on an insect system, it may provide the first empirical support for the proposed link between childhood inflammation, immunopathology and reduced human lifespan [7]. Next, we describe the immunopathological risk associated with an ageing immune system and its impact on the lifespan of older individuals. While ageing increased several aspects of beetle immunity such as PO response, antimicrobial peptide gene expression, induction of haemolymph antibacterial activity and the ability to clear S. aureus infection, it also severely impaired MTs and compromised the ability to survive after bacterial infection. We found that RNAi knockdown of the PO response once again minimized damage to MTs of old beetles, partially rescued fluid transport ability and extended adult lifespan after infection. Thus, one of the most interesting implications of our work is the likely role of immunopathological damage to MTs that underlies the effects of early immune activation as well as the later impacts of infection. Because we used fluid secretion rate as a proxy for immunopathological damage and given that fluid transportation can be energy-intensive, a possible alternative is that the reduced fluid transport after immune challenge indicates a trade-off between immune induction and MT excretion [9]. Yet another possibility is that immune activation may alter the phosphorylation state of dFOXO of the tubules, which may in turn change its fluid transport ability without direct immunopathological consequences [9]. In both cases, a general immune activation should lead to altered MT fluid secretion. However, previous work in Tenebrio shows that MT function decreased with local rather than with general immune activation [13], suggesting that immunopathology is indeed the most probable explanation for our observed results.
In a prior study, Ayres & Schneider [16] had predicted a direct role for the PO response pathway in immunopathological damage and increased post-infection mortality of fruit flies. Our data not only support this prediction, but also indicate impaired MT activity as an important mechanism underlying the immunopathological consequences of the PO response and associated reduction in lifespan. We note that other components of the immune system can also be involved in immunopathology, and the PO pathway may not be the sole determinant of immunopathology. For instance, the tumour necrosis factor-like protein encoded by the gene eiger is known to cause immunopathology in fruit flies [43]. NF-kB signalling, which is involved in chronic inflammation in vertebrates [44], may also cause immunopathology in insects, though it is poorly studied. We thus suggest the need for further studies to test the fitness impacts of immunopathology caused by other immune effectors.
Another important finding of our study is a mechanism to explain the discordance between immune response and the host fitness post-infection, reported in this study as well as in flour beetles [22] and fruitflies [45,46]. Such a mismatch suggests that the inherent ability to mount an immune response is not a reliable predictor of host fitness after infection. Our data suggest that these contradictory results may be mediated via a large reduction in MT activity in infected older individuals. We find that bacterial infection rendered MTs of older beetles almost dysfunctional (approx. 95% reduction). This implies that older infected beetles should have difficulty in maintaining the water balance and accumulate toxic waste products inside the haemocoel [47]—a condition analogous to potential uraemic poisoning. We thus propose that older beetles succumb to infection faster than younger beetles owing to the inability of damaged MTs to effectively maintain physiological homeostasis. This may also suggest that older beetles pay greater immunopathological costs of increased immune activation compared with young beetles. In fact, several studies in vertebrates show that ageing leads to chronic inflammatory responses via maladaptive impacts of the innate immune system, contributing to the pathology of several age-related illnesses [19,48]. An exaggerated inflammatory response with age can induce lethal immunopathology in mice: old individuals die faster owing to hepatocyte necrosis caused by an elevated level of interleukin-17 and neutrophil activation [44]. Another study found increased post-infection mortality in older mice with suppressed anti-inflammatory cytokine interleukin-10 expression, suggesting a link between overactive inflammatory responses and increased risk of mortality [49]. We thus conclude that the observed increase in beetle immunity with age actually represents immune activation at an unnecessarily high level without any adaptive value. Instead, ageing leads to a dramatic increase in the negative impacts of inflammation in beetles, and the net impact of the immune response compromises old individuals' fitness post-infection. Moreover, ageing may also reduce tolerance to infection, the ability to limit negative impacts of pathogens, or self-damage (discussed in reference [50]). Consequently, older individuals may increasingly rely on direct immune activation to limit pathogen burden, which could rapidly escalate the immunopathological risk. However, we stress that currently there is no evidence for this hypothesis in T. molitor.
Our work also highlights the intrinsic aspects of physiological ageing in insects. We find that multiple components of immunity (e.g. PO and antimicrobial peptides) increase as a function of age, even in the absence of pathogenic infection (in naive beetles). A plausible explanation is that the observed increase in baseline constitutive innate immune responses is a by-product of general relaxation of gene regulation associated with ageing (see reference [23]). We further speculate that the strength of selection on old individuals may be too weak [23] to effectively prevent such misregulation of the immune system, resulting in increased risk of immunopathology. Indeed, in support of the above prediction, our data reveal a baseline reduction in MT functioning with age in naive beetles. This perhaps indicates an age-dependent trade-off between investment in immune function and prevention of its immunopathological costs. It is possible that such increases in immune responses and immunopathological damage to vital organs (even without infection) could be a key feature of senescence. However, we lack direct empirical evidence for this hypothesis. Further manipulative experiments are thus necessary to test whether experimental suppression of inflammatory pathways (e.g. RNAi of PO response in insects) results in reduced organ damage in older uninfected individuals, in turn, improving their lifespan.
We note an alternative possibility where the observed age-specific differences in survival and MT function could be artefacts of using only virgin beetles. In some insects, virgin females also develop ovaries on par with their mated counterparts but refrain from laying eggs [51]. If storing unfertilized eggs bears additional costs, then it is possible that the physiological burden of carrying eggs increased with age, resulting in observed physiological defects. However, there is currently no evidence for such costs of egg retention in T. molitor or any related coleopterans (but see blood-feeding insects [52]). Furthermore, in previous work with T. castaneum flour beetles, we found that older individuals increased immunity and yet, die faster after infection, regardless of their mating status [22]. Hence, we do not expect that the physiological burden of carrying unfertilized eggs should impact our experimental results.
Overall, our work has important implications for the evolution of maladaptive immune pathology in natural populations. Natural selection might be almost blind to the self-damage associated with the immune response, because the costs are usually paid at a later stage in life [23]. From a public health perspective, such relaxed selection can pose a serious problem to an ageing human population, potentially exacerbating the autoimmune disease crisis worldwide [1,53,54]. We suggest that intimate connections between immune action and immunopathology may drive these hallmarks of ageing. We hope that our observations encourage further empirical work for a deeper understanding of life-history trade-offs and fitness impacts associated with immune function and its immunopathological outcome. These are necessary to understand how and why apparently maladaptive immunopathological features of an immune system have evolved.
Supplementary Material
Acknowledgements
We are grateful to Paul Johnston, Dino McMahon, Caroline Zanchi, Saurabh Mahajan and Vrinda Ravi Kumar for feedback on the manuscript. We thank Jayjit Das, Caroline Zanchi, Arun Prakash and Dipendra Nath Basu for their help during experiments and data analysis.
Data accessibility
Data available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.dk034 [55].
Authors' contributions
I.K. and J.R. conceived of and designed experiments; I.K. carried out experiments and analysed data; I.K. and J.R. wrote the manuscript. D.A. helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
We acknowledge funding and support from the Centre for International Collaboration, Free University of Berlin; DAAD and SERB-DST Young Investigator Grant supplements to I.K.; the National Centre for Biological Sciences, India; a DST Inspire Faculty fellowship to D.A.; and a European Research Council (EVORESIN) grant supplement to J.R.
References
- 1.Bach JF. 2002. The Effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347, 911–920. ( 10.1056/NEJMra020100) [DOI] [PubMed] [Google Scholar]
- 2.Dye C, Scheele S, Dolin P, Pathania V, Raviglione M. 1999. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA 282, 677–686. ( 10.1001/jama.282.7.677) [DOI] [PubMed] [Google Scholar]
- 3.Bekker LG, Moreira AL, Bergtold A, Freeman S, Ryffel B, Kaplan G. 2000. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect. Immun. 68, 6954–6961. ( 10.1128/IAI.68.12.6954-6961.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urabe K, Aroca P, Tsukamoto K, Mascagna D, Palumbo A, Prota G, Hearing VJ. 1994. The inherent cytotoxicity of melanin precursors: a revision. Biochim. Biophys. Acta - Mol. Cell Res. 1221, 272–278. ( 10.1016/0167-4889(94)90250-X) [DOI] [PubMed] [Google Scholar]
- 5.Nappi AJ, Vass E, Frey F, Carton Y. 1995. Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. Eur. J. Cell Biol. 68, 450–456. [PubMed] [Google Scholar]
- 6.Zhao P, Lu Z, Strand M, Jiang H. 2008. Antiviral, antiparasitic, and cytotoxic effects of 5,6- dihydroxyindole (DHI), a reactive compound generated by phenoloxidase during insect immune response. Insect Biochem. Mol. Biol. 141, 520–529. ( 10.1016/j.ibmb.2011.04.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finch CE, Crimmins EM. 2004. Inflammatory exposure and historical changes in human life-spans. Science 305, 1736–1739. ( 10.1126/science.1092556) [DOI] [PubMed] [Google Scholar]
- 8.Pursall ER, Rolff J. 2011. Immune responses accelerate ageing: proof-of-principle in an insect model. PLoS ONE 6, e19972 ( 10.1371/journal.pone.0019972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies SA, Overend G, Sebastian S, Cundall M, Cabrero P, Dow JAT, Terhzaz S. 2012. Immune and stress response ‘cross-talk’ in the Drosophila Malpighian tubule. J. Insect Physiol. 58, 488–497. ( 10.1016/j.jinsphys.2012.01.008) [DOI] [PubMed] [Google Scholar]
- 10.Pacheco CA, Ravazi A, de Azeredo Oliveria MTV, Alevi K. 2014. Review: malpighian tubule, an essential organ for insects. Entomol. Ornithol. Herpetol. Curr. Res. 3, 2–4. ( 10.4172/2161-0983.1000122) [DOI] [Google Scholar]
- 11.McGettigan J, et al. 2005. Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect Biochem. Mol. Biol. 35, 741–754. ( 10.1016/j.ibmb.2005.02.017) [DOI] [PubMed] [Google Scholar]
- 12.Chapman RF. 1998. The insects: structure and function. Cambridge, UK: University of Cambridge Press. [Google Scholar]
- 13.Sadd BM, Siva-Jothy MT. 2006. Self-harm caused by an insect's innate immunity. Proc. R. Soc. B 273, 2571–2574. ( 10.1098/rspb.2006.3574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerenius L, Lee BL, Söderhäll K. 2008. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 29, 263–271. ( 10.1016/j.it.2008.02.009) [DOI] [PubMed] [Google Scholar]
- 15.De Gregorio E, et al. 2002. An immune-responsive Serpin regulates the melanization cascade in Drosophila. Dev. Cell 3, 581–592. ( 10.1016/S1534-5807(02)00267-8) [DOI] [PubMed] [Google Scholar]
- 16.Ayres JS, Schneider DS. 2008. A signalling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol. 6, e305 ( 10.1371/journal.pbio.0060305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deveale B, Brummel T, Seroude L. 2004. Immunity and aging: the enemy within? Aging Cell 3, 195–208. ( 10.1111/j.1474-9728.2004.00106.x) [DOI] [PubMed] [Google Scholar]
- 18.Shanley DP, Aw D, Manley NR, Palmer DB. 2009. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 30, 374–381. ( 10.1016/j.it.2009.05.001) [DOI] [PubMed] [Google Scholar]
- 19.Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, Caruso C. 2005. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun. Ageing 2, 8 ( 10.1186/1742-4933-2-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zerofsky M, Harel E, Silverman N, Tatar M. 2005. Aging of the innate immune response in Drosophila melanogaster. Aging Cell 4, 103–108. ( 10.1111/j.1474-9728.2005.00147.x) [DOI] [PubMed] [Google Scholar]
- 21.Khan I, Prasad NG. 2013. The aging of the immune response in Drosophila melanogaster. J. Gerontol. A. Biol. Sci. Med. Sci. 68, 129–135. ( 10.1093/gerona/gls144) [DOI] [PubMed] [Google Scholar]
- 22.Khan I, Prakash A, Agashe D. 2015. Immunosenescence and the ability to survive bacterial infection in the red flour beetle Tribolium castaneum. J. Anim. Ecol. 85, 291–301. ( 10.1111/1365-2656.12433) [DOI] [PubMed] [Google Scholar]
- 23.Hamilton WD. 1966. The moulding of senescence by natural selection. J. Theor. Biol. 12, 12–45. ( 10.1016/0022-5193(66)90184-6) [DOI] [PubMed] [Google Scholar]
- 24.Mueller LD, Rose MR. 1996. Evolutionary theory predicts late-life mortality plateaus. Proc. Natl Acad. Sci. USA 93, 15 249–15 253. ( 10.1073/pnas.93.26.15249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos-Elorduy J, González EA, Hernández AR, Pino JM. 2002. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 95, 214–220. ( 10.1603/0022-0493-95.1.214) [DOI] [PubMed] [Google Scholar]
- 26.Meyling NV, Eilenberg J. 2007. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: potential for conservation biological control. Biol. Control 43, 145–155. ( 10.1016/j.biocontrol.2007.07.007) [DOI] [Google Scholar]
- 27.Armitage SAO, Siva-Jothy MT. 2005. Immune function responds to selection for cuticular colour in Tenebrio molitor. Heredity 94, 650–656. ( 10.1038/sj.hdy.6800675) [DOI] [PubMed] [Google Scholar]
- 28.Haine E, Moret Y, Siva-jothy M, Rolff J. 2008. Antimicrobial defence and persistent infection in insects. Science 322, 1257–1259. ( 10.1126/science.1165265) [DOI] [PubMed] [Google Scholar]
- 29.Tschinkel W, Willson C, Bern HA. 1967. Sex pheromone of the mealworm beetle. (Tenebrio molitor). J. Exp. Zool. 164, 81–85. ( 10.1002/jez.1401640108) [DOI] [PubMed] [Google Scholar]
- 30.Morales-Ramos JA, Rojas MG, Kay S, Shapiro-Lian DI, Tedders WL. 2012. Impact of adult weight, density, and age on reproduction of Tenebrio molitor (coleoptera: Tenebrionidae). J. Entomol. Sci. 47, 208–220. ( 10.18474/0749-8004-47.3.208) [DOI] [Google Scholar]
- 31.Johnston PR, Makarova O, Rolff J. 2014. Inducible defenses stay up late: temporal patterns of immune gene expression in Tenebrio molitor. G3 4, 947–955. ( 10.1534/g3.113.008516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobson AJ, Johnston PR, Vilcinskas A, Rolff J. 2012. Identification of immunological expressed sequence tags in the mealworm beetle Tenebrio molitor. J. Insect Physiol. 58, 1556–1561. ( 10.1016/j.jinsphys.2012.09.009) [DOI] [PubMed] [Google Scholar]
- 33.Johnston PR, Rolff J. 2015. Host and symbiont jointly control gut microbiota during complete metamorphosis. PLoS Pathog. 11, 1–11. ( 10.1371/journal.ppat.1005246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. ( 10.1038/nprot.2008.73) [DOI] [PubMed] [Google Scholar]
- 35.Maddrell SH, Overton JA. 1990. Transport in insect Malpighian tubules. Methods Enzymol. 192, 617–632. ( 10.1016/0076-6879(90)92099-Y) [DOI] [PubMed] [Google Scholar]
- 36.Neufeld DS, Leader JP. 1998. Cold inhibition of cell volume regulation during the freezing of insect Malpighian tubules. J. Exp. Biol. 201, 2195–2204. [DOI] [PubMed] [Google Scholar]
- 37.Nicolson S. 1992. Excretory function in Tenebrio molitor: fast tubular secretion in a vapour-absorbing insect. J. Insect Physiol. 38, 139–146. ( 10.1016/0022-1910(92)90043-D) [DOI] [Google Scholar]
- 38.Wiehart UIM, Nicolson SW, Eigenheer RA, Schooley DA. 2002. Antagonistic control of fluid secretion by the Malpighian tubules of Tenebrio molitor: effects of diuretic and antidiuretic peptides and their second messengers. J. Exp. Biol. 205, 493–501. [DOI] [PubMed] [Google Scholar]
- 39.Zanchi C, Troussard J, Martinaud G. 2011. Differential expression and costs between maternally and paternally derived immune priming for offspring in an insect. J. Anim. Ecol. 80, 1174–1183. ( 10.1111/j.1365-2656.2011.01872.x) [DOI] [PubMed] [Google Scholar]
- 40.Swindell WR. 2010. Accelerated failure time models provide a useful statistical framework for ageing research. Exp. Gerontol. 44, 190–200. ( 10.1016/j.exger.2008.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel K, Kay R, Rowell L. 2006. Comparing proportional hazards and accelerated failure time models: an application in influenza. Pharm. Stat. 5, 213–224. ( 10.1002/pst.213) [DOI] [PubMed] [Google Scholar]
- 42.Wang C, Li Q, Redden DT, Weindruch R, Allison DB. 2004. Statistical methods for testing effects on ‘maximum lifespan’. Mech. Ageing Dev. 125, 629–632. ( 10.1016/j.mad.2004.07.003) [DOI] [PubMed] [Google Scholar]
- 43.Brandt SM, Dionne MS, Khush RS, Pham LN, Vigdal TJ, Schneider DS. 2004. Secreted bacterial effectors and host-produced eiger/TNF drive death in a Salmonella-infected fruit fly. PLoS Biol. 2, e418 ( 10.1371/journal.pbio.0020418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stout-Delgado HW, Du W, Shirali AC, Booth CJ, Goldstein DR. 2009. Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe 6, 446–456. ( 10.1016/j.chom.2009.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corby-Harris V, Habel KE, Ali FG, Promislow DEL. 2007. Alternative measures of response to Pseudomonas aeruginosa infection in Drosophila melanogaster. J. Evol. Biol. 20, 526–533. ( 10.1111/j.1420-9101.2006.01267.x) [DOI] [PubMed] [Google Scholar]
- 46.Ramsden S, Cheung YY, Seroude L. 2008. Functional analysis of the Drosophila immune response during aging. Aging Cell 7, 225–236. ( 10.1111/j.1474-9726.2008.00370.x) [DOI] [PubMed] [Google Scholar]
- 47.Maddrell SH, Overton JA. 1988. Stimulation of sodium transport and fluid secretion by ouabain in an insect Malpighian tubule. J. Exp. Biol. 137, 265–276. [DOI] [PubMed] [Google Scholar]
- 48.Vasto S, et al. 2007. Inflammatory networks in ageing, age-related diseases and longevity. Mech. Ageing Dev. 128, 83–91. ( 10.1016/j.mad.2006.11.015) [DOI] [PubMed] [Google Scholar]
- 49.Belloni V, Faivre B, Guerreiro R, Arnoux E, Bellenger J, Sorci G. 2010. Suppressing an anti-inflammatory cytokine reveals a strong age-dependent survival cost in mice. PLoS ONE 5, e12940 ( 10.1371/journal.pone.0012940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medzhitov R, Schneider DS, Soares MP. 2012. Disease tolerance as a defense strategy. Science 335, 936–941. ( 10.1126/science.1214935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shukla S, Shilpa MC, Gadagkar R. 2013. Virgin wasps develop ovaries on par with mated females, but lay fewer eggs. Insect. Soc. 60, 345–350. ( 10.1007/s00040-013-0299-1) [DOI] [Google Scholar]
- 52.Xue R, Ali A, Barnard DR. 2005. Effects of forced egg-retention in Aedes albopictus on adult survival and reproduction following application of DEET as an oviposition deterrent. J. Vector Ecol. 30, 45–48. [PubMed] [Google Scholar]
- 53.Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A. 2006. Parasitic worms and inflammatory diseases. Parasite Immunol. 28, 515–523. ( 10.1111/j.1365-3024.2006.00879.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finch CE. 2010. Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc. Natl Acad. Sci. USA 107, 1718–1724. ( 10.1073/pnas.0909606106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan I, Agashe D, Rolff J. 2016. Data from: Early inflammation, immunopathology and aging. Dryad Digital Repository. ( 10.5061/dryad.dk034) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Khan I, Agashe D, Rolff J. 2016. Data from: Early inflammation, immunopathology and aging. Dryad Digital Repository. ( 10.5061/dryad.dk034) [DOI]
Supplementary Materials
Data Availability Statement
Data available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.dk034 [55].