Abstract
Although nanoparticles (NPs) have made incredible progress in the field of nanotechnology and biomedical research and their applications are demanded throughout industrial world particularly over the past decades, little is known about the fate of nanoparticles in ecosystem. Concerning the biosafety of nanotechnology, nanotoxicity is going to be the second most priority of nanotechnology that needs to be properly addressed. This review covers the chemical as well as the biological concerns about nanoparticles particularly titanium dioxide (TiO2) NPs and emphasizes the toxicological profile of TiO2 at the molecular level in both in vitro and in vivo systems. In addition, the challenges and future prospects of nanotoxicology are discussed that may provide better understanding and new insights into ongoing and future research in this field.
1. Introduction
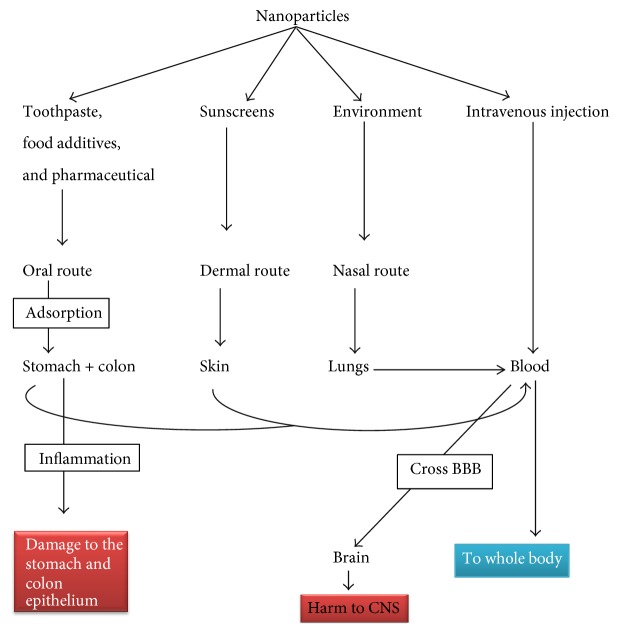
In the last decade, nanoscience has flourished a lot with rapidly advancing nanotechnology and its wider applications [1]. Nanomaterials (NMs) are being and have been exclusively developed and extensively used in a wide variety of products, including medicine, industry, personal care products [2, 3], cosmetics [4], sunscreens [5], toothpastes [6], paints, optics and electronics [7, 8], photocatalysts, antiultraviolet light agents [9], food packaging, medical devices, bandages, clothing, dental restoration material and water treatment facilities [10, 11], antibacterial agents [12], drug delivery systems, artificial organ, and tissue adhesives [13], and for cancer cells apoptosis under UV irradiations (Figure 1) [14]. Moreover, the nanoparticles (NPs) are eminent candidates to overcome drug resistance posed by microorganisms, a major challenge to scientific community [15]. Currently, more than 1000 products or product lines in market contain NPs [16, 17], and it has been estimated that the engineered NMs had reached 2.5 trillion US$ annual profit by 2015 [17]. Nevertheless, the consequently increasing interactions of NPs with biological, chemical, and ecosystems have raised concerns regarding their general and occupational health and safety profiles. The NPs enter the environment and affect both biotic and abiotic components of the ecosystem [18], including human beings [19]. The aquatic ecosystem has also been contaminated with NPs and their negative impacts suppress the immune system of fish and invertebrates [10].
Figure 1.
Nanoparticles containing products and their entrance ways into the biological system.
Among the NPs, titanium dioxide NPs (TiO2 NPs) are one of the most highly manufactured and widely used in the world [20]. TiO2 is a well-known semiconductor and a versatile compound that exists in three crystalline forms, anatase, rutile, and brookite [14, 21], which can only be activated with UV light due to its high band gap energy (3.0 eV for rutile phase and 3.2 eV for anatase phase). The anatase and rutile forms have natural and industrial importance, while the brookite is rarely used. Generally, anatase is more toxic than rutile and, unfortunately, being used abundantly [21, 22]. Many researchers have contributed to the use of TiO2 NPs in in vitro and in vivo systems. However, there is a lack of an overall evaluation of their toxicological effects in terms of harmful interactions with the biological and chemical systems and the environment. This review, therefore, specifically intends to provide a brief insight into the toxicological profile of TiO2 NPs with respect to biological and ecosystems.
2. Confliction about the Toxicological Impacts of TiO2 NPs
TiO2 is known for long time as “the environmental white knight” due to its limited toxicity [23], inertness, and biocompatibility [8, 24]. The lethal dose at 50% concentration (LD50) of TiO2 is greater than 10 g/kg [25], and it has been approved as a food additive since 1996 by the Food and Drug Administration (FDA). The FDA and Environmental Protection Agency (EPA) have specified 50 μg/kg body weight/day of nano-TiO2 (nTiO2) as safe dose for humans (Title 21, volume 1, revised as of April 1, 2014). Moreover, the European Commission's Scientific Committee on Food (SCF), the Joint Expert Committee on Food Additives of the Food and Agriculture Organization/World Health Organization (JECFA), and the European Food Safety Authority (EFSA)'s Scientific Panel on Food Additives, Flavorings, Processing Aids and Materials in Contact with Food have also approved the daily intake of nano-TiO2 in general food stuff. Looking from the perspective of potential adverse health effects, several experimental and epidemiological data have demonstrated that TiO2 is biologically inactive and physiologically inert, exhibiting relatively low toxicity, thus posing low risk to humans [26]. For example, in a study of chronic toxicity and carcinogenicity, a total of Fischer 344 rats and B6C3F1 mice at concentration of 0, 25000, and 50000 mg TiO2/kg diet for 103 weeks (2 years) showed no significant toxicity. In the same study, TiO2 coated mica at 0, 1, 2, and 5% in Fischer 344 rats for 130 weeks (2 and half years) had no toxicological or carcinogenic effects [27]. Furthermore, the intraperitoneal injections (IP) of TiO2 NPs (5 mg/kg) for 14 days caused no significant adverse effects on mouse kidney [28]. Similarly, both the JECFA and EFSA evaluations of TiO2 showed that there is no absorption or tissue storage of TiO2, as well as no health hazard effects for occupational workers and public health by Material Safety Data Sheets (MSDS) [8]. In addition, the World Health Organization (WHO)'s Environmental Health states that “titanium compounds are poorly absorbed from the gastrointestinal tract, which is the main route of exposure for the general population” (WHO 1982), and they pose low hazard potential in mammals or aquatic species (Daphnia magna, Oncorhynchus mykiss) [29]. Keeping in view the above-mentioned data, it is obvious to accept that the TiO2 NPs are health friendly and nontoxic to biological environment.
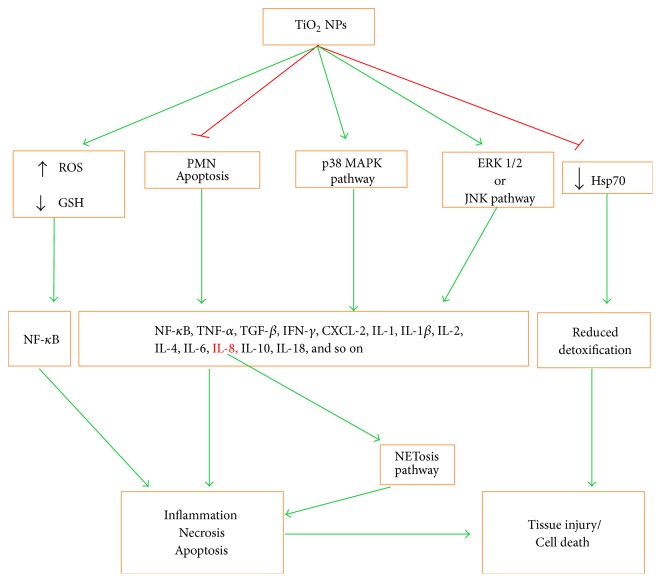
Contrarily, the Scientific Committee on Consumer Safety (SCCS) has described the genotoxic, carcinogenic, and photosensitization behavior of TiO2 NPs (SCCS/1516/13), and several in vitro and in vivo studies have shown the adverse effects of TiO2 NPs in biological systems [30, 31]. Recently, Yin et al. [8] have shown that all the molecular sizes and crystal forms (anatase and rutile) of nTiO2 may cause phototoxicity [mainly caused by reactive oxygen species (ROS)] under UV irradiations [8] and exert acute toxicity in mice at different dosages of 0, 324, 648, 972, 1296, 1944, or 2592 mg/kg body weight [32]. ROS may further upregulate the inflammatory cytokines and apoptosis-related genes [24, 33, 34], inhibit the heat shock proteins (HSP) [24, 35], and cause neuroinflammation (Figure 4) [36]. The small size (10–20 nm) TiO2 NPs may induce oxidative DNA damage, lipid peroxidation, and increased hydrogen peroxide (H2O2) and nitric oxide production in BEAS-2B cells (human bronchial epithelial cell line) without photoactivation [35, 37]. Collectively, on the basis of above-described data, it seems that there is no clear-cut evidence regarding the safe dose of TiO2 NPs and great attention is needed while dealing with these nanomaterials.
Figure 4.
Nano-TiO2-induced tissue injury and inflammation. These NPs cause elevation of ROS, decline of GSH levels, inhibition of PMN apoptosis, and tyrosine phosphorylation of p38MAPK and ERK1/2 or JNK. All these induce the production of different inflammatory cytokines that in turn lead to inflammation and consequent necrosis or apoptosis mechanism of cell death. Decreased detoxification due to CYP1A and HSP70 decline also leads to tissue injury or cell death.
3. Biological Perspective
NPs, being the advent of nanotechnology, have great impact on the environment. Their production and consumption are increasing day by day, which ultimately has increased the contact chances of NPs with the environment. How do these NPs enter the biological system? What mechanism do they follow? And what are the consequences to the cell viability? To answer these questions, one needs to look very carefully while dealing with NPs in in vivo or in vitro studies as discussed in detail in the next sections.
3.1. Biological Uptake of TiO2 NPs and Their Entry into the Human Cells
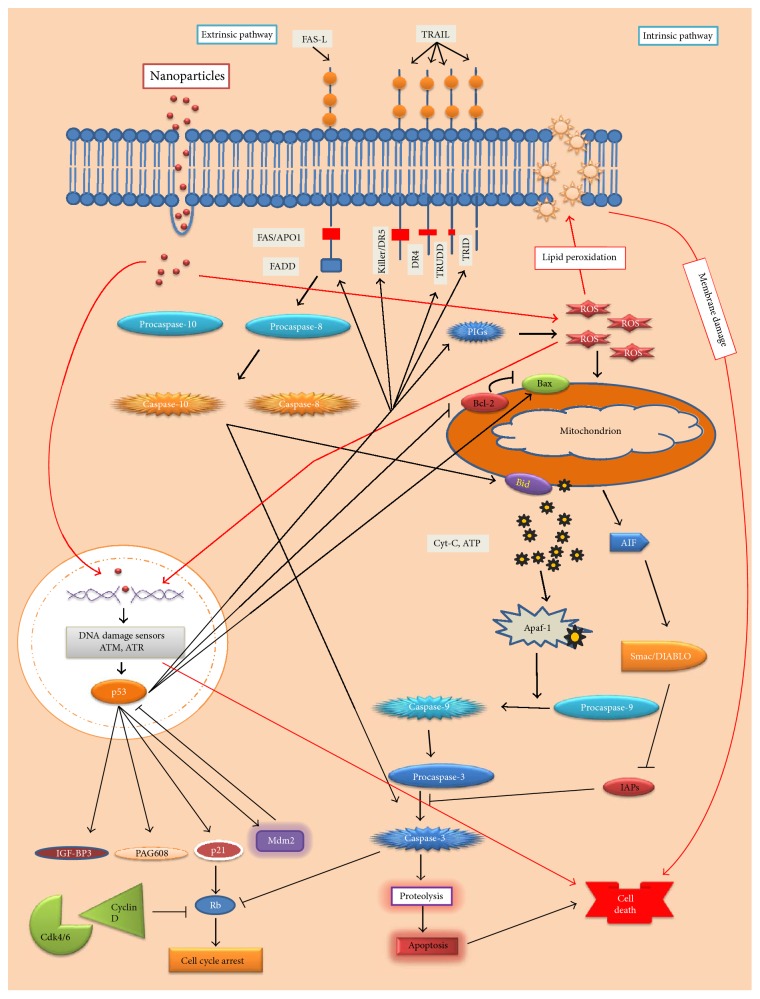
The cellular responses toward NPs depend not only on the properties of NPs, but also on the genetic, transcriptomic, and proteomic landscape of the target cells, imparting different cytotoxic and genotoxic outcomes in various cell types [38]. TiO2 NPs enter the human body through several ways, including inhalation, ingestion (food stuffs and daily use materials), skin uptake (through skin lesions), and medical injections [39], and may be distributed to different body organs through circulatory system (Figure 1). After internalization, the TiO2 NPs interact with cytoplasmic proteome and bring posttranslational modifications, such as acetylation (A549 cells), by oxidative stress and other mechanisms (Figure 3) [39, 40]. They reach the periregion of nucleus, impede the function of endoplasmic reticulum, and block the nuclear pore or enter the nucleus. Inside the nucleus, they interact with DNA [35] and cause the upregulation of cytokines-, oxidative stress-, and apoptosis-related genes [23, 24, 37]. Meanwhile, the defense system of the cell responds in such a way that the first-line defense is provided by superoxide dismutase (SOD) and catalase (CAT) against oxygen toxicity (ROS), and neutrophils participate against foreign particles (discussed in NETosis pathway in Section 3.2). The transformation of oxy-radicals occurs, such that superoxide radical •O2− is dismutated to O2 and H2O2 by catalytic activity of SOD enzyme and, then, CAT converts the H2O2 into water and oxygen. Oxidative stress (ROS) pathway is one of the mechanisms through which TiO2 and Ag NPs exert their toxic effects and disturb the life cycle of Drosophila via enhanced ROS generation and DNA damage that lead to related adverse consequences (Figure 3) [7, 41]. In sertoli cells (testicular), the exposure of TiO2 NPs (2.5, 5, or 10 mg/kg body weight) may cause severe testicular oxidative damage, apoptosis, ROS generation, and lipid peroxidation. TiO2 NPs may also cause suppression of SOD, CAT, glutathione peroxidase (GPx), glutathione S epoxide transferase (GST), glutathione reductase (GR), Cytochrome P450, Family 1, Subfamily B, Polypeptide 1 (Cyp1b1), carbonic anhydrase III (Car3), Bcl-2, acetyl-coenzyme A acyltransferase 2 (Acaa2), and Axin upregulated 1 (Axud1) in mouse testis, while enhancing the expression of apoptotic genes in mouse testis [42]. Moreover, the reverse correlation between ROS generation and reduction of glutathione (GSH) in human hepatocellular carcinoma cell line (SMMC-7721), rat hepatocarcinoma cell line (CBRH-7919), human liver cell line (HL-7702), and rat liver cell line (BRL-3A) has shown the toxicity of TiO2 NPs [13].
Figure 3.
The apoptosis induced by TiO2 NPs. The TiO2 NPs-induced apoptosis mostly follows the intrinsic pathway. TiO2 NPs enter the cell, induce ROS generation, and then enter the nucleus causing DNA damage. The DNA damage is sensed by sensor proteins (ATM/ATR) as a consequence of which p53 is upregulated, which further activates Bax (promoter of apoptosis) and inhibits Bcl2 (inhibitor of Bax).
3.2. NETosis Pathway
Neutrophils, the first line of immune defense, have the ability to extrude their DNA (either mitochondrial or nuclear) along with bactericidal, fungal, and protozoal pathogen molecules, thus creating neutrophils extracellular traps (NETs) and releasing them to the extracellular environment. The NETosis pathway is elicited by respiratory burst and ROS generation, causing release of NETs due to formation of superoxide ions. The H2O2 in phagosome consequently leads to NETs release and NETosis via triggering of the downstream signaling pathways (Figure 2). Exposure of neutrophils to nTiO2 may lead to an increased oxidative burst that coincides with NETs release [43]. The NETosis is often accompanied by cell death in order to control and limit extracellular infections, which may otherwise cause complicated human diseases, including sepsis and autoimmune disorders [44, 45].
Figure 2.
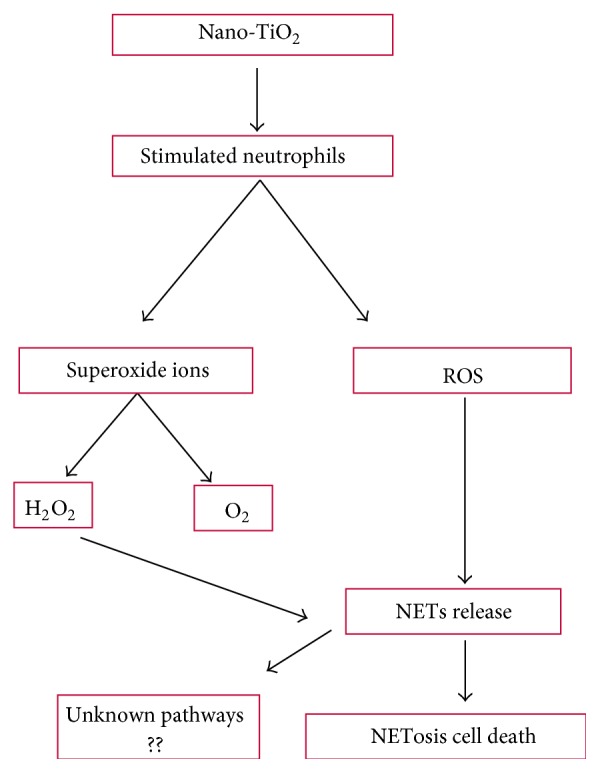
Nano-TiO2-induced NETosis cell death pathway.
3.3. Apoptosis Mediated by TiO2 NPs
Generally, cells remain under constant threats from the cytotoxic and mutagenic effects of DNA damaging agents comprising endogenous (e.g., ROS) and exogenous (such as UV light, ionizing radiations, and other agents like chemicals in foodstuffs, water, or air) or both. Upon DNA damage, the cells undergo either DNA repair or cell cycle arrest leading to apoptosis [46]. Apoptosis is the best described mechanism through which NPs may exert their toxic effects inducing (a) an intrinsic pathway, mediated by mitochondria, or (b) an extrinsic pathway, mediated by death receptors.
TiO2 NPs have been shown to induce apoptosis via intrinsic pathway in human bronchial epithelial cell line (BEAS-2B), independent of caspase 8/t-Bid (involved in extrinsic pathway), by enhancing ROS level and proinflammatory responses [28, 33]. During this pathway mitochondrial membrane permeability is enhanced because of caspase-3 release and subsequent PARP cleavage and release of cytochrome C, followed by induction of caspase-9 and caspase-3 (effector caspases) of apoptosis-inducing factor. The genotoxic effect of TiO2 NPs upregulates p53 gene that promotes the expression of Bax genes by suppressing Bcl-2 family regulator proteins, thus making an ease for opening the mitochondrial channels and release of cytochrome C (Figure 3) [7, 37, 47]. The accumulation of TiO2 NPs in mouse neurons manifests the apoptotic markers such as nuclear shrinkage and chromatin condensation [47]. Furthermore, TiO2 NPs may cause the upregulation of oxidative-stress-related genes, including heme oxygenase-1, thioredoxin reductase, glutathione-S-transferase, and cytokines such as interleukin- (IL-) 1, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-18, and IL-1β, transforming growth factor- (TGF-) β, tumor necrosis factor- (TNF-) α, and interferon- (IFN-) γ (Figure 4), which may cause inhibition of HSP70. The IL-8 gene expression is induced via p38 mitogen-activated protein kinase (MAPK) pathway and/or extracellular signal (ERK) pathway [24, 35]. Similarly, the intragastric exposure of TiO2 (2.5, 5, and 10 mg/kg) in mouse may lead to their accumulation in kidneys, inducing necrosis and inflammatory responses (Figure 3) [24].
3.4. Phototoxicity and Genotoxicity
TiO2 NPs induce phototoxicity upon UV irradiations. They have been shown to induce apoptosis by activating apoptosis-inducing factor (AIF) in human keratinocyte cells [48], as well as in retinal pigment epithelial cells (Figure 3) [2]. Moreover, TiO2 NPs have been demonstrated to cause pericardial oedema and premature hatch of Japanese medaka (Oryzias latipes) embryos when treated with aqueous suspensions at 0 and 14 μg/mL [49].
The genotoxicity of TiO2 NPs attributes to ROS generation and oxidative stress in human epidermal cells, which elicits signal transduction pathways leading to apoptosis or cellular death [33]. They have been shown to induce DNA double-strand breakage in bone marrow and human amnion epithelial (WISH) cells, as well as in mice in a dose-dependent manner, leading to cell cycle arrest [50–52]. The induction of ROS may reduce NADH levels, impairing mitochondrial membrane potential (ΔΨm) and causing mitochondrial dysfunction [53]. The exposure of TiO2 and Al2O3 NPs may also cause genotoxic effects in Chinese hamster ovary after 24 h treatment [54]. The genotoxicity, apoptosis, and mitotic arrest are caused by both nano- and microparticles of TiO2 in various tissues of mice [4], as mentioned by SCCS in 2013 (SCCS/1516/13) (discussed in Section 2).
In human lymphocytes, TiO2 NPs have been found genotoxic at a dose of 0.25 mM probably by the lipid peroxidation mechanism and at 4 mM to Allium cepa [55]. The viability of human epidermal cells was significantly decreased due to DNA damage, micronucleus formation, and reduction in glutathione [14]. They were readily uptaken by A549 cells (carcinomic human alveolar basal epithelial cells) in vitro. However, such rapid uptake was in contrast with a very low oral absorption in a differentiated Caco-2 monolayer system (human epithelial colorectal adenocarcinoma cells) and after oral gavage administration to rats [56]. The calculation of uptake, dispersion, and biological effects of ingested NMs is complicated in vivo due to interindividual differences in the composition, pH, thickness of the mucus layer, gastrointestinal flora, and gastrointestinal passage time [57]. The in vitro studies of NPs interactions are unrealistic and may not indicate the actual fate of NPs, while in in vivo studies, the biological molecules are absorbed on the surface of NPs, changing their biokinetics and the consequent fate of the biomolecules in natural environment [58].
3.5. Neurotoxicity
Brain tissues are more susceptible to oxidative stress-induced damage because of high metabolic rate, cellular content of lipids, proteins, and extensive axonal and dendritic networks, and low levels of endogenous scavengers. After exposure of TiO2 NPs, the integrity of blood brain barrier (BBB) is badly affected due to persistence of NPs in endothelial cells or via infiltration of immune cells, resulting in breakdown of BBB. In an experimental study, the TiO2 NPs have been demonstrated to cause injury to neurons via JNK/p53-mediated-apoptosis and ROS generation, which activated downstream p53/p21 pathway, causing G2/M arrest in in vitro model of dopaminergic neurons (PC12 cell) (Figure 3) [21, 59]. In another study, the TiO2 NPs were found to enter the brain via olfactory bulb and reside in the hippocampus region, damaging mitochondria and inducing oxidative stress in rat and human glial cell lines [60]. The anatase nano-TiO2 are more toxic to neuronal cells than rutile [21]. Whether these findings have definite neurotoxic implications needs further investigations.
3.6. Respiratory Toxicity
The exposures of NMs via inhalation (occupational and/or environmental) may affect the respiratory tract, resulting in an increased risk of lung cancer, fibrosis, blockage of interalveolar areas, and presence of inflammatory cells [17, 61]. The natural and engineered NPs penetrate the lungs through inhalation, reach different body organs via the blood circulatory system [51], and upregulate the inflammatory proteins (MIP and MCP) and genes of MHC class I via Th2-mediated pathway [62]. The IFN-γ is preferably released from Th1 cells and induces NPs-triggered cellular immune response along with ROS production in macrophages. They also elicit the expression of GTP-cyclohydrolase I (GCH-I) enzymes, which lead to formation of neopterin, and of indoleamine 2,3-dioxygenase (IDO) (first step is catalyzing enzyme in tryptophan breakdown by kynurenine pathway). The intratracheal exposure of TiO2 NPs in mouse may result in their substantial accumulation in lungs, causing bleeding and inflammation [34, 63]. The mutagenic potential of TiO2 NPs has been revealed by the treatment of pUC19/lacZ− plasmid with different concentrations of TiO2 NPs (average size 30.6 nm) and subsequent transfection of CaCl2-induced competent DH5α cells, which showed loss of transfection efficacy of the plasmid in comparison to untreated ones [64].
In a cytoplasmic proteome study involving human monocyte-derived macrophages, the abundancies of chloride intracellular channel protein 1, cathepsin D, and lysine acetylation were observed after exposure to nTiO2 [40]. Recently, Sheng et al. [65] have demonstrated the significant alterations in the expressions of 1041 genes involved in different types of processes, including immune/inflammatory responses, apoptosis, oxidative stress, stress responses, metabolic processes, ion transport, signal transduction, and cell proliferation/division and translation, in mice spleen [65].
TiO2 also caused lung cancer in rats after oral administration of 160 and 33 nm particles at doses of 40, 200, and 1000 mg/kg body weight [4]. The ultrafine TiO2 (UF-TiO2), less than 100 nm in diameter, induced pulmonary fibrosis, lung tumor, and genotoxicity in rats [66, 67]. Similarly, the NMs may also cause damage to liver cells during cleansing of toxins and pollutants in body. Furthermore, the TiO2 NPs may cause hepatotoxicity in human hepatocellular carcinoma cell line (SMMC-7721), human liver cell line (HL-7702), rat hepatocarcinoma cell line (CBRH-7919), and rat liver cell line (BRL-3A), which may be associated with changes in cell morphology, increased intercellular ROS production, and decreased GSH levels at 0.1–100 μg/mL [13].
3.7. Aquatic Nanotoxicity
The in vitro studies have raised concerns about the toxicity of TiO2 NPs in mammalian, but there are limited data on ecotoxicity to aquatic organisms. The heaping of NPs to sewage increases due to their excessive use in industry and commerce [68]. The engineered nanoparticles (ENPs) intermingle with various toxins, including metals in sediments and water phase, making agglomerates and resides [69], and causing damage to aquatic organisms [55]. The exposure of adult zebra fish to 1.0 mg/L TiO2 (both NP and bulk) for 21 days has been shown to lower the number of viable embryos [70] and inhibit the growth of goldfish (Carassius auratus) [71]. Similarly, the exposure of nTiO2 suspensions (100 and 200 mg/L) to carp (Cyprinus carpio) may cause a decrease in SOD, CAT, and POD, while inducing a significant increase in LPO levels in the liver [72]. The combined exposure of anatase and rutile NPs to freshwater microalgae, Chlorella sp., at 0.25, 0.5, and 1 mg/L under UV irradiations has been demonstrated to reduce the cell viability and chlorophyll content [22]. TiO2 has also adverse impacts on the survival, growth, and reproduction of D. magna. It has been determined that exposure of anatase (21 nm) particles is more toxic to D. magna as compared to anatase (250 nm) and rutile (500 nm) particles [73]. Therefore, the study of adverse effects of various NMs on aquatic species is necessary to assess their potential environmental hazardous effects.
4. Interactions of NPs in Ecosystem
The clean air is not only of scientific, environmental, and physiological importance but a basic need for living a healthy life. The chemicals and biological attacks may pose risk to human health and environment [74]. In this regard, the dangers of NPs to human health and environment have increased due to the prompt growth in nanotechnology. The adsorption of noxious pollutants on NPs has been extensively studied. In environment, the NPs always amalgamate with other pollutants. The interactions between conventional pollutants with NPs and their impact on environmental components are little considered. The heaping of NPs to sewage increases due to their excessive use in industry and commerce [68]. The chance of association of organic materials, including toxicants, increases with the aggregation of NPs in water. Hence, the bioavailability of these materials is altered. Thus, extra toxicological concerns are needed in presence of NPs [75].
The workers involved in the production of TiO2 NPs may have significant risk on cytotoxicity response at relatively high airborne concentrations of anatase TiO2 NPs [76]. Widespread use of nTiO2 may intensify the threat of combined exposure of nTiO2 with other environmental pollutants. The mixing of different compounds may bring astonishing toxic effects, even if the toxicities of the individual compounds are well known. For example, when bisphenol A (BPA) combines with nTiO2, it facilitates the movement of nTiO2 into exposed cells, causing synergistic toxicity by oxidative stress, inducing DNA double-strand breaks and micronuclei formation [77]. The growth inhibition of fresh water algae (Pseudokirchneriella subcapitata) was increased by the interaction of Cd(II) species with TiO2 [78]. Similarly, the adsorption of Cd(II) onto nTiO2 was enhanced by coating humic acid (HA) on nTiO2 [79]. The anatase NPs are superior sorbents than activated carbon and other metal oxide NPs [80]. The TiO2 and Al2O3 NPs enter the Chinese hamster ovary (CHO-K1) cells through endocytosis and attack on lysosomal and mitochondrial activities, thus causing cytotoxicity and genotoxicity as well as a decrease in cell viability [54].
The hydroxylated fullerenes/C60 (OH) 24 exert synergistic stimulative effect on genes related to circadian rhythm, vesicular transport, kinases, and immune responses in zebrafish embryos [81], while the presence of nitrite with TiO2 enhances the induction of apoptosis-related genes via NO signaling pathway [48].
5. Chemical Perspective
From chemical perspective, TiO2 NPs show phototoxic effects upon UVA irradiations. Upon photon energy absorption, the electrons of the NPs jump from valence band to the conduction band, leaving the valence band holes. Hydroxyl radicals (•OH) are produced when valence band holes take electrons from water or hydroxyl ions and other ROS such as singlet oxygen (1O2) and superoxide (•O2−) are also produced by different mechanisms. Free radicals (•OH and carbon centered free radicals) are also generated in dark. The generated ROS may be genotoxic or cytotoxic, affecting cell viability (Figure 3). Hence, TiO2 NPs are toxic to living system both in the presence and absence of light via generation of free radicals [2].
6. Effect of Exposure Time and Dose on Toxicity of TiO2 NPs
The primary particle size (the size of particle at the time of injection) of TiO2 NPs is not as important as that of secondary particle size (the size of particle after agglomeration) for in vivo toxicity. Likewise, the physicochemical characteristics and time of exposure of NPs before the toxicological study are important [82]. The dietary exposure of nTiO2 for 3 or 14 days may cause hazards to the terrestrial invertebrates [83]. Intratracheal instillation to rats with 0.5, 5, or 50 mg/kg of 5, 21, and 50 nm TiO2 primary particles, respectively, has been demonstrated to exhibit dose-dependent toxic responses. In the same way, intraperitoneal injection of TiO2 NPs (5 mg/kg) for 14 days did not have considerable effect on mouse kidney and the nephric dysfunction; however at doses of 50, 100, and 150 mg/kg body weight, it significantly induced inflammatory response and abnormal functions of kidney in mice.
Short-term exposure of TiO2 NPs may have low-to-medium ecological hazards on zebrafish [23]. Nano-TiO2 exposure for 3 h causes highest production of ROS in cytoplasm while at 24 h exposure ROS is only produced in perinuclear region due to aggregation [35].
Nano-TiO2 accumulation occurs around the nucleus for up to 25 days in retinal pigment epithelial cells after a single low-level long-term exposure [2]. The cytotoxicity in normal liver and carcinomatous liver cells of either rat or human increases as the time of exposure increases, even a low concentration of nTiO2 may induce higher toxicity with increase in time of exposure [13]. In long-term exposure, TiO2 NPs may cause pronounced adverse effect (growth inhibition and loss in liver weight) on zebrafish in time- and dose-dependent manner in vivo. It has also been shown that TiO2 NPs exposure for 6 months to zebrafish may elicit pronounced toxic consequences like organ injury, behavior alterations, mortality, and organ distribution at higher concentration [23]. Furthermore, at long-term exposure, TiO2 accumulates in the cell and causes toxic effects which are not evident at short-term exposure [84]. Several cell lines exposed to higher concentrations (100 μg/mL of TiO2) may exhibit morphological changes such as cell shrinkage or nuclear condensation [35]. The exposure of differentiated murine J774.2 macrophages to 1 μg/mL concentration may have no considerable effects on cell proliferation, while at concentration 10 μg/mL it may exhibit significant cytotoxic effects [12]. Unnithan and colleagues have shown that fine nano-TiO2 (~20 nm) at 40 mg/kg cause biochemical perturbations in Wistar rats [85]. Conclusively, the NPs even at their noncytotoxic doses may have pathophysiological concerns [28].
7. Effect of Size and Shape on Toxicity of TiO2 NPs
The major physicochemical properties to evaluate the toxicity are size, shape, surface area, phase, composition, coating, nature of surface, and agglomeration of NPs [16, 86]. The size and surface area of NPs may be responsible for their toxicity, but most of the studies do not reveal the relationship between physiochemical characteristics of NPs and their toxicity [82]. For example, 25 nm anatase and 31 nm anatase/rutile show greater phototoxicity than 142 nm anatase and 214 nm rutile NPs [2]. All the sizes and crystal forms (anatase and rutile) of TiO2 NPs exert toxic (phototoxic) effects on human skin keratinocytes under UVA irradiations in a dose-dependent way. The smaller size nTiO2 may cause greater cytotoxicity than larger size NPs, and anatase form may show more phototoxicity than rutile [8, 84]. Furthermore, the NPs (rod and sphere) of smaller size show higher toxicity than larger particles. Moreover, the nanorods exhibit more toxicity than spherical particles having the same size and surface area, showing the contribution of shape toward cytotoxicity [16]. The Ag (20 and 200 nm) and TiO2 (21 nm) NPs are significantly taken up by human epithelial, hepatic, and undifferentiated monocyte cells, resulting in decline of metabolic activation and cell death enhancement [87].
8. Conclusion and Future Perspectives
Concerning the biosafety of nanotechnology, nanotoxicity is going to be the second most priority of nanotechnology. The different responses toward the NPs in ecosystem may be very complex and diverse, involving a variety of parameters, demonstrating their difficult environmental fate [88]. In addition, the environmental hazards of ENPs can be documented by knowing their behavior and fate in the natural aquatic system [89].
To date no product (medicinal or food stuff) is available with 100 percent purity and efficiency, but for the safer use of nanosized particles with no or minimal hazardous effects on environment, the detailed understanding about their sources, interactions with environment, biodegradability, and possible risk assessment are utmost requirement prior to use. In addition, the interactions of NPs with biological molecules and their adverse effects need to be fully understood prior to their approval in clinical trials.
The cellular responses and toxicity produced by TiO2 NPs depend on the surface/mass ratio, purity, crystallinity, surface reactivity, adsorbed groups, coatings, solubility, shape, size [7, 54], zeta potential, and dispersion or propensity to agglomerate or aggregate in different media [90]. These parameters need to be considered for the safer use of NMs. The undefined health and environmental features of TiO2 NPs due to its widespread use are necessary to be managed by a systematic, coherent, and tested foundation. Therefore, the regulatory health risk assessment of such particles may be mandatory for the safe use of NMs in consumer products and medicines, including the potential effects on reproduction and fertility.
Acknowledgments
The authors thank all the researchers who contributed to their current understanding of hazardous effects of TiO2 NPs.
Abbreviations
- NMs:
Nanomaterials
- NPs:
Nanoparticles
- TiO2 NPs:
Titanium dioxide NPs
- LD50:
Lethal dose at 50% concentration
- FDA:
Food and Drug Administration
- EPA:
Environmental Protection Agency
- nTiO2:
Nano-TiO2
- SCF:
European Commission's Scientific Committee on Food
- JECFA:
Joint Expert Committee on Food Additives of the Food and Agriculture Organization/World Health Organization
- EFSA:
European Food Safety Authority
- MSDS:
Material Safety Data Sheets
- WHO:
World Health Organization
- SCCS:
Scientific Committee on Consumer Safety
- ROS:
Reactive oxygen species
- HSP:
Heat shock proteins
- H2O2:
Hydrogen peroxide
- SOD:
Superoxide dismutase
- CAT:
Catalase
- GPx:
Glutathione peroxidase
- GST:
Glutathione S epoxide transferase
- GR:
Glutathione reductase
- NETs:
Neutrophils extracellular traps
- TGF-β:
Transforming growth factor-β
- TNF-α:
Tumor necrosis factor-α
- IFN-γ:
Interferon-γ
- MAPK:
Mitogen-activated protein kinase
- AIF:
Apoptosis-inducing factor
- BBB:
Blood brain barrier
- IDO:
Indoleamine 2,3-dioxygenase
- UF-TiO2:
Ultrafine TiO2
- ENPs:
Engineered nanoparticles
- BPA:
Bisphenol A
- HA:
Humic acid
- CHO-K1:
Chinese hamster ovary
- •OH:
Hydroxyl radicals
- 1O2:
Singlet oxygen
- •O2−:
Superoxide.
Competing Interests
The authors declare no competing financial interests.
References
- 1.Gonçalves D. M., Girard D. Titanium dioxide (TiO2) nanoparticles induce neutrophil influx and local production of several pro-inflammatory mediators in vivo. International Immunopharmacology. 2011;11(8):1109–1115. doi: 10.1016/j.intimp.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Sanders K., Degn L. L., Mundy W. R., et al. In vitro phototoxicity and hazard identification of nano-scale titanium dioxide. Toxicology and Applied Pharmacology. 2012;258(2):226–236. doi: 10.1016/j.taap.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Khosravi K., Hoque M. E., Dimock B., Hintelmann H., Metcalfe C. D. A novel approach for determining total titanium from titanium dioxide nanoparticles suspended in water and biosolids by digestion with ammonium persulfate. Analytica Chimica Acta. 2012;713:86–91. doi: 10.1016/j.aca.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Sycheva L. P., Zhurkov V. S., Iurchenko V. V., et al. Investigation of genotoxic and cytotoxic effects of micro- and nanosized titanium dioxide in six organs of mice in vivo. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2011;726(1):8–14. doi: 10.1016/j.mrgentox.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Gonçalves D. M., Chiasson S., Girard D. Activation of human neutrophils by titanium dioxide (TiO2) nanoparticles. Toxicology in Vitro. 2010;24(3):1002–1008. doi: 10.1016/j.tiv.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Li B., Hu R., Cheng Z., et al. Titanium dioxide nanoparticles relieve biochemical dysfunctions of fifth-instar larvae of silkworms following exposure to phoxim insecticide. Chemosphere. 2012;89(5):609–614. doi: 10.1016/j.chemosphere.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 7.Kang S. J., Kim B. M., Lee Y. J., Hong S. H., Chung H. W. Titanium dioxide nanoparticles induce apoptosis through the JNK/p38-caspase-8-Bid pathway in phytohemagglutinin-stimulated human lymphocytes. Biochemical and Biophysical Research Communications. 2009;386(4):682–687. doi: 10.1016/j.bbrc.2009.06.097. [DOI] [PubMed] [Google Scholar]
- 8.Yin J.-J., Liu J., Ehrenshaft M., et al. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes—generation of reactive oxygen species and cell damage. Toxicology and Applied Pharmacology. 2012;263(1):81–88. doi: 10.1016/j.taap.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang R., Niu Y., Li Y., et al. Acute toxicity study of the interaction between titanium dioxide nanoparticles and lead acetate in mice. Environmental Toxicology and Pharmacology. 2010;30(1):52–60. doi: 10.1016/j.etap.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Jovanović B., Palić D. Immunotoxicology of non-functionalized engineered nanoparticles in aquatic organisms with special emphasis on fish—review of current knowledge, gap identification, and call for further research. Aquatic Toxicology. 2012;118-119:141–151. doi: 10.1016/j.aquatox.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Philbrook N. A., Winn L. M., Afrooz A. R. M. N., Saleh N. B., Walker V. K. The effect of TiO2 and Ag nanoparticles on reproduction and development of Drosophila melanogaster and CD-1 mice. Toxicology and Applied Pharmacology. 2011;257(3):429–436. doi: 10.1016/j.taap.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Gutierrez F., Thi E. P., Silverman J. M., et al. Antibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticles. Nanomedicine: Nanotechnology, Biology, and Medicine. 2012;8(3):328–336. doi: 10.1016/j.nano.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Sha B., Gao W., Wang S., Xu F., Lu T. Cytotoxicity of titanium dioxide nanoparticles differs in four liver cells from human and rat. Composites Part B: Engineering. 2011;42(8):2136–2144. doi: 10.1016/j.compositesb.2011.05.009. [DOI] [Google Scholar]
- 14.Markowska-Szczupak A., Ulfig K., Morawski A. W. The application of titanium dioxide for deactivation of bioparticulates: an overview. Catalysis Today. 2011;169(1):249–257. doi: 10.1016/j.cattod.2010.11.055. [DOI] [Google Scholar]
- 15.Mohanty S., Mishra S., Jena P., Jacob B., Sarkar B., Sonawane A. An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch-stabilized silver nanoparticles. Nanomedicine: Nanotechnology, Biology, and Medicine. 2012;8(6):916–924. doi: 10.1016/j.nano.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao I.-L., Huang Y.-J. Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. Science of the Total Environment. 2011;409(7):1219–1228. doi: 10.1016/j.scitotenv.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 17.Oosthuizen M. A., Oberholzer H. M., Scriba M. R., van der Spuy W. J., Pretorius E. Evaluation of the morphological changes in the lungs of BALB/c mice after inhalation of spherical and rod-shaped titanium nanoparticles. Micron. 2012;43(8):863–869. doi: 10.1016/j.micron.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Bhatt I., Tripathi B. N. Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere. 2011;82(3):308–317. doi: 10.1016/j.chemosphere.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y., Gopee N. V., Howard P. C., Yu L.-R. Proteomic analysis of early response lymph node proteins in mice treated with titanium dioxide nanoparticles. Journal of Proteomics. 2011;74(12):2745–2759. doi: 10.1016/j.jprot.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jomini S., Clivot H., Bauda P., Pagnout C. Impact of manufactured TiO2 nanoparticles on planktonic and sessile bacterial communities. Environmental Pollution. 2015;202:196–204. doi: 10.1016/j.envpol.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Wu J., Sun J., Xue Y. Involvement of JNK and P53 activation in G2/M cell cycle arrest and apoptosis induced by titanium dioxide nanoparticles in neuron cells. Toxicology Letters. 2010;199(3):269–276. doi: 10.1016/j.toxlet.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Iswarya V., Bhuvaneshwari M., Alex S. A., et al. Combined toxicity of two crystalline phases (anatase and rutile) of Titania nanoparticles towards freshwater microalgae: Chlorella sp. Aquatic Toxicology. 2015;161:154–169. doi: 10.1016/j.aquatox.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Chen J., Dong X., Xin Y., Zhao M. Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (Danio rerio) after a long-term exposure. Aquatic Toxicology. 2011;101(3-4):493–499. doi: 10.1016/j.aquatox.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Gui S., Zhang Z., Zheng L., et al. Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. Journal of Hazardous Materials. 2011;195:365–370. doi: 10.1016/j.jhazmat.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Zhou G., Chen C., et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicology Letters. 2007;168(2):176–185. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Hong Y. C., Bang C. U., Shin D. H., Uhm H. S. Band gap narrowing of TiO2 by nitrogen doping in atmospheric microwave plasma. Chemical Physics Letters. 2005;413(4–6):454–457. doi: 10.1016/j.cplett.2005.08.027. [DOI] [Google Scholar]
- 27.Bernard B. K., Osheroff M. R., Hofmann A., Mennear J. H. Toxicology and carcinogenesis studies of dietary titanium dioxide-coated mica in male and female fischer 344 rats. Journal of Toxicology and Environmental Health. 1990;29(4):417–429. doi: 10.1080/15287399009531402. [DOI] [PubMed] [Google Scholar]
- 28.Hussain S., Boland S., Baeza-Squiban A., et al. Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: role of particle surface area and internalized amount. Toxicology. 2009;260(1–3):142–149. doi: 10.1016/j.tox.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Warheit D. B., Hoke R. A., Finlay C., Donner E. M., Reed K. L., Sayes C. M. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicology Letters. 2007;171(3):99–110. doi: 10.1016/j.toxlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Iavicoli I., Leso V., Fontana L., Bergamaschi A. Toxicological effects of titanium dioxide nanoparticles: a review of in vitro mammalian studies. European Review for Medical and Pharmacological Sciences. 2011;15(5):481–508. [PubMed] [Google Scholar]
- 31.Iavicoli I., Leso V., Bergamaschi A. Toxicological effects of titanium dioxide nanoparticles: a review of in vivo studies. Journal of Nanomaterials. 2012;2012:36. doi: 10.1155/2012/964381.964381 [DOI] [PubMed] [Google Scholar]
- 32.Chen J., Dong X., Zhao J., Tang G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. Journal of Applied Toxicology. 2009;29(4):330–337. doi: 10.1002/jat.1414. [DOI] [PubMed] [Google Scholar]
- 33.Shukla R. K., Sharma V., Pandey A. K., Singh S., Sultana S., Dhawan A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicology in Vitro. 2011;25(1):231–241. doi: 10.1016/j.tiv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Sun Q., Tan D., Ze Y., et al. Pulmotoxicological effects caused by long-term titanium dioxide nanoparticles exposure in mice. Journal of Hazardous Materials. 2012;235-236:47–53. doi: 10.1016/j.jhazmat.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 35.Park E.-J., Yi J., Chung K.-H., Ryu D.-Y., Choi J., Park K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicology Letters. 2008;180(3):222–229. doi: 10.1016/j.toxlet.2008.06.869. [DOI] [PubMed] [Google Scholar]
- 36.Ze Y., Sheng L., Zhao X., et al. TiO2 nanoparticles induced hippocampal neuroinflammation in mice. PloS One. 2014;9(3) doi: 10.1371/journal.pone.0092230.e92230 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Shi Y., Wang F., He J., Yadav S., Wang H. Titanium dioxide nanoparticles cause apoptosis in BEAS-2B cells through the caspase 8/t-Bid-independent mitochondrial pathway. Toxicology Letters. 2010;196(1):21–27. doi: 10.1016/j.toxlet.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Ng K. W., Khoo S. P. K., Heng B. C., et al. The role of the tumor suppressor p53 pathway in the cellular DNA damage response to zinc oxide nanoparticles. Biomaterials. 2011;32(32):8218–8225. doi: 10.1016/j.biomaterials.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 39.Migdal C., Rahal R., Rubod A., et al. Internalisation of hybrid titanium dioxide/para-amino benzoic acid nanoparticles in human dendritic cells did not induce toxicity and changes in their functions. Toxicology Letters. 2010;199(1):34–42. doi: 10.1016/j.toxlet.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Sund J., Palomäki J., Ahonen N., Savolainen K., Alenius H., Puustinen A. Phagocytosis of nano-sized titanium dioxide triggers changes in protein acetylation. Journal of Proteomics. 2014;108:469–483. doi: 10.1016/j.jprot.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Zhu A., Sun K., Petty H. R. Titanium doping reduces superoxide dismutase activity, but not oxidase activity, of catalytic CeO2 nanoparticles. Inorganic Chemistry Communications. 2012;15:235–237. doi: 10.1016/j.inoche.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao X., Sheng L., Wang L., et al. Mechanisms of nanosized titanium dioxide-induced testicular oxidative stress and apoptosis in male mice. Particle and Fibre Toxicology. 2014;11, article 47 doi: 10.1186/s12989-014-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Jovanović B., Anastasova L., Rowe E. W., Zhang Y., Clapp A. R., Palić D. Effects of nanosized titanium dioxide on innate immune system of fathead minnow (Pimephales promelas Rafinesque, 1820) Ecotoxicology and Environmental Safety. 2011;74(4):675–683. doi: 10.1016/j.ecoenv.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Remijsen Q., Kuijpers T. W., Wirawan E., Lippens S., Vandenabeele P., Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death and Differentiation. 2011;18(4):581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mesa M. A., Vasquez G. NETosis. Autoimmune Diseases. 2013;2013:7. doi: 10.1155/2013/651497.651497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ChemIDplus. ChemIDplus Advanced. U.S. National Library of Medicine, National Institutes of Health, Department of Health and Human Services; 2005. (Search Terms: Titanium Dioxide; CAS Reg. No. 13463-67-7). http://chem.sis.nlm.nih.gov/chemidplus/ [Google Scholar]
- 47.Hu R., Zheng L., Zhang T., et al. Molecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticles. Journal of Hazardous Materials. 2011;191(1-3):32–40. doi: 10.1016/j.jhazmat.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 48.Tu M., Huang Y., Li H.-L., Gao Z.-H. The stress caused by nitrite with titanium dioxide nanoparticles under UVA irradiation in human keratinocyte cell. Toxicology. 2012;299(1):60–68. doi: 10.1016/j.tox.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Paterson G., Ataria J. M., Hoque M. E., Burns D. C., Metcalfe C. D. The toxicity of titanium dioxide nanopowder to early life stages of the Japanese medaka (Oryzias latipes) Chemosphere. 2011;82(7):1002–1009. doi: 10.1016/j.chemosphere.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 50.Chen Z., Wang Y., Ba T., et al. Genotoxic evaluation of titanium dioxide nanoparticles in vivo and in vitro. Toxicology Letters. 2014;226(3):314–319. doi: 10.1016/j.toxlet.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 51.Saquib Q., Al-Khedhairy A. A., Siddiqui M. A., Abou-Tarboush F. M., Azam A., Musarrat J. Titanium dioxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in human amnion epithelial (WISH) cells. Toxicology in Vitro. 2012;26(2):351–361. doi: 10.1016/j.tiv.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Trouiller B., Reliene R., Westbrook A., Solaimani P., Schiestl R. H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Research. 2009;69(22):8784–8789. doi: 10.1158/0008-5472.CAN-09-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freyre-Fonseca V., Delgado-Buenrostro N. L., Gutiérrez-Cirlos E. B., et al. Titanium dioxide nanoparticles impair lung mitochondrial function. Toxicology Letters. 2011;202(2):111–119. doi: 10.1016/j.toxlet.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 54.Di Virgilio A. L., Reigosa M., Arnal P. M., Fernández Lorenzo de Mele M. Comparative study of the cytotoxic and genotoxic effects of titanium oxide and aluminium oxide nanoparticles in Chinese hamster ovary (CHO-K1) cells. Journal of Hazardous Materials. 2010;177(1-3):711–718. doi: 10.1016/j.jhazmat.2009.12.089. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh M., Bandyopadhyay M., Mukherjee A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: plant and human lymphocytes. Chemosphere. 2010;81(10):1253–1262. doi: 10.1016/j.chemosphere.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 56.Janer G., Mas del Molino E., Fernández-Rosas E., Fernández A., Vázquez-Campos S. Cell uptake and oral absorption of titanium dioxide nanoparticles. Toxicology Letters. 2014;228(2):103–110. doi: 10.1016/j.toxlet.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Fröhlich E., Roblegg E. Models for oral uptake of nanoparticles in consumer products. Toxicology. 2012;291(1–3):10–17. doi: 10.1016/j.tox.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valant J., Drobne D., Novak S. Effect of ingested titanium dioxide nanoparticles on the digestive gland cell membrane of terrestrial isopods. Chemosphere. 2012;87(1):19–25. doi: 10.1016/j.chemosphere.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 59.Brun E., Carrière M., Mabondzo A. In vitro evidence of dysregulation of blood-brain barrier function after acute and repeated/long-term exposure to TiO2 nanoparticles. Biomaterials. 2012;33(3):886–896. doi: 10.1016/j.biomaterials.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 60.Huerta-García E., Pérez-Arizti J. A., Márquez-Ramírez S. G., et al. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radical Biology and Medicine. 2014;73:84–94. doi: 10.1016/j.freeradbiomed.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 61.Naya M., Kobayashi N., Ema M., et al. In vivo genotoxicity study of titanium dioxide nanoparticles using comet assay following intratracheal instillation in rats. Regulatory Toxicology and Pharmacology. 2012;62(1):1–6. doi: 10.1016/j.yrtph.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Park E.-J., Yoon J., Choi K., Yi J., Park K. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology. 2009;260(1-3):37–46. doi: 10.1016/j.tox.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Makumire S., Chakravadhanula V. S. K., Köllisch G., Redel E., Shonhai A. Immunomodulatory activity of zinc peroxide (ZnO2) and titanium dioxide (TiO2) nanoparticles and their effects on DNA and protein integrity. Toxicology Letters. 2014;227(1):56–64. doi: 10.1016/j.toxlet.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 64.Ahmad J., Dwivedi S., Alarifi S., Al-Khedhairy A. A., Musarrat J. Use of β-galactosidase (lacZ) gene α-complementation as a novel approach for assessment of titanium oxide nanoparticles induced mutagenesis. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2012;747(2):246–252. doi: 10.1016/j.mrgentox.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Sheng L., Wang L., Sang X., et al. Nano-sized titanium dioxide-induced splenic toxicity: a biological pathway explored using microarray technology. Journal of Hazardous Materials. 2014;278:180–188. doi: 10.1016/j.jhazmat.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Wang J. J., Sanderson B. J. S., Wang H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2007;628(2):99–106. doi: 10.1016/j.mrgentox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Warheit D. B., Sayes C. M., Reed K. L., Swain K. A. Health effects related to nanoparticle exposures: environmental, health and safety considerations for assessing hazards and risks. Pharmacology and Therapeutics. 2008;120(1):35–42. doi: 10.1016/j.pharmthera.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y., Westerhoff P., Hristovski K. D. Fate and biological effects of silver, titanium dioxide, and C60 (fullerene) nanomaterials during simulated wastewater treatment processes. Journal of Hazardous Materials. 2012;201-202:16–22. doi: 10.1016/j.jhazmat.2011.10.086. [DOI] [PubMed] [Google Scholar]
- 69.Hartmann N. B., Legros S., Von der Kammer F., Hofmann T., Baun A. The potential of TiO2 nanoparticles as carriers for cadmium uptake in Lumbriculus variegatus and Daphnia magna. Aquatic Toxicology. 2012;118-119:1–8. doi: 10.1016/j.aquatox.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Ramsden C. S., Henry T. B., Handy R. D. Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquatic Toxicology. 2013;126:404–413. doi: 10.1016/j.aquatox.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 71.Ates M., Demir V., Adiguzel R., Arslan Z. Bioaccumulation, subacute toxicity, and tissue distribution of engineered titanium dioxide nanoparticles in goldfish (Carassius auratus) Journal of Nanomaterials. 2013;2013:6. doi: 10.1155/2013/460518.460518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hao L., Wang Z., Xing B. Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio) Journal of Environmental Sciences. 2009;21(10):1459–1466. doi: 10.1016/s1001-0742(08)62440-7. [DOI] [PubMed] [Google Scholar]
- 73.Bang S. H., Le T.-H., Lee S. K., Kim P., Kim J. S., Min J. Toxicity assessment of titanium (IV) oxide nanoparticles using Daphnia magna (Water Flea) Environmental Health and Toxicology. 2011;26 doi: 10.5620/eht.2011.26.e2011002.e2011002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srisitthiratkul C., Pongsorrarith V., Intasanta N. The potential use of nanosilver-decorated titanium dioxide nanofibers for toxin decomposition with antimicrobial and self-cleaning properties. Applied Surface Science. 2011;257(21):8850–8856. doi: 10.1016/j.apsusc.2011.04.083. [DOI] [Google Scholar]
- 75.Govindasamy R., Rahuman A. A. Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus) Journal of Environmental Sciences (China) 2012;24(6):1091–1098. doi: 10.1016/s1001-0742(11)60845-0. [DOI] [PubMed] [Google Scholar]
- 76.Liao C.-M., Chiang Y.-H., Chio C.-P. Assessing the airborne titanium dioxide nanoparticle-related exposure hazard at workplace. Journal of Hazardous Materials. 2009;162:57–65. doi: 10.1016/j.jhazmat.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 77.Zheng D., Wang N., Wang X., et al. Effects of the interaction of TiO2 nanoparticles with bisphenol A on their physicochemical properties and in vitro toxicity. Journal of Hazardous Materials. 2012;199-200:426–432. doi: 10.1016/j.jhazmat.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 78.Hartmann N. B., Von der Kammer F., Hofmann T., Baalousha M., Ottofuelling S., Baun A. Algal testing of titanium dioxide nanoparticles—testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology. 2010;269(2-3):190–197. doi: 10.1016/j.tox.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 79.Chen Q., Yin D., Zhu S., Hu X. Adsorption of cadmium(II) on humic acid coated titanium dioxide. Journal of Colloid and Interface Science. 2012;367(1):241–248. doi: 10.1016/j.jcis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 80.Hu J., Shipley H. J. Evaluation of desorption of Pb (II), Cu (II) and Zn (II) from titanium dioxide nanoparticles. Science of the Total Environment. 2012;431:209–220. doi: 10.1016/j.scitotenv.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 81.Jovanović B., Ji T., Palić D. Gene expression of zebrafish embryos exposed to titanium dioxide nanoparticles and hydroxylated fullerenes. Ecotoxicology and Environmental Safety. 2011;74(6):1518–1525. doi: 10.1016/j.ecoenv.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 82.Menard A., Drobne D., Jemec A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environmental Pollution. 2011;159(3):677–684. doi: 10.1016/j.envpol.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 83.Drobne D., Jemec A., Pipan Tkalec Ž. In vivo screening to determine hazards of nanoparticles: nanosized TiO2. Environmental Pollution. 2009;157(4):1157–1164. doi: 10.1016/j.envpol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 84.Federici G., Shaw B. J., Handy R. D. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquatic Toxicology. 2007;84(4):415–430. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 85.Unnithan J., Rehman M. U., Ahmad F. J., Samim M. Concentration dependent toxicity of ∼20 nm anatase titanium dioxide nanoparticles—an in vivo study on wistar rats. Journal of Biomedical Nanotechnology. 2011;7(1):207–208. doi: 10.1166/jbn.2011.1271. [DOI] [PubMed] [Google Scholar]
- 86.Posgai R., Cipolla-McCulloch C. B., Murphy K. R., Hussain S. M., Rowe J. J., Nielsen M. G. Differential toxicity of silver and titanium dioxide nanoparticles on Drosophila melanogaster development, reproductive effort, and viability: size, coatings and antioxidants matter. Chemosphere. 2011;85(1):34–42. doi: 10.1016/j.chemosphere.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 87.Lankoff A., Sandberg W. J., Wegierek-Ciuk A., et al. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicology Letters. 2012;208(3):197–213. doi: 10.1016/j.toxlet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 88.García A., Espinosa R., Delgado L., et al. Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests. Desalination. 2011;269(1–3):136–141. doi: 10.1016/j.desal.2010.10.052. [DOI] [Google Scholar]
- 89.von der Kammer F., Ottofuelling S., Hofmann T. Assessment of the physico-chemical behavior of titanium dioxide nanoparticles in aquatic environments using multi-dimensional parameter testing. Environmental Pollution. 2010;158(12):3472–3481. doi: 10.1016/j.envpol.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 90.Asare N., Instanes C., Sandberg W. J., et al. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells. Toxicology. 2012;291(1-3):65–72. doi: 10.1016/j.tox.2011.10.022. [DOI] [PubMed] [Google Scholar]