Highlights
-
•
Crude glycerol is a byproduct of biodiesel industry.
-
•
Crude glycerol is a valuable source for different valuable industrial products.
-
•
Bioconversion of glycerol is a sustainable approach.
-
•
This makes revenue to biodiesel industries and to overall economy of the biodiesel process.
Keywords: Biodiesel, Crude glycerol, Value-added products, Bio-conversion
Abstract
Crude glycerol that is produced as the by-product from biodiesel, has to be effectively utilized to contribute to the viability of biodiesel. Crude glycerol in large amounts can pose a threat to the environment. Therefore, there is a need to convert this crude glycerol into valued added products using biotechnological processes, which brings new revenue to biodiesel producers. Crude glycerol can serve as a feedstock for biopolymers, poly unsaturated fatty acids, ethanol, hydrogen and n-butanol production and as a raw material for different value added industrial products. Hence, in this review we have presented different bioconversion technologies of glycerol to value added industrial products.
1. Introduction
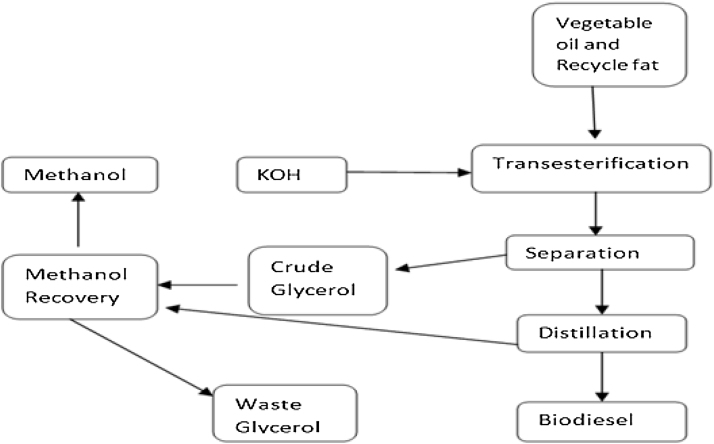
Nowadays, people are busy in inventing new machines and instruments which need a lot of energy for running their application, these energies that are supplied naturally, biologically, chemically, electrochemically or physically. One of these is petroleum and its products like petrol, diesel, gasoline, etc. These have certain drawbacks like they have created global ecological disturbance [1]. So this has resulted in the emergence of eco-friendly, alternative fuel biodiesel. There are many types of feedstock for its production of oils and fats [2]. Biodiesel can be defined as long chains of alkyl esters, which are formed by transesterification of triglycerides with alcohol that results in glycerol as a waste product [3]. It is estimated that the biodiesel market will reach to 37 billion gallons by 2016 with an annual growth of 42% which is indirectly producing 4 billion gallons of crude glycerol as a byproduct 10 kg of glycerol (crude) will be produced from for 100 kg of biodiesel [4]. The scheme of events for biodiesel production through transesetrification reaction is depicted in Fig. 1 [5].
Fig. 1.
Biodiesel production through transesterification reaction.
The up gradation of biofuel byproduct facilitates the added value of the economy of the process; moreover it falls under 4th generation biofuel strategy of minimum waste production in the process. Crude glycerol up gradation to value added products impinges a substantial effect on the economy of the biodiesel sector. Purification of crude glycerol is a cumbersome and hence utilization of crude glycerol as intact as source for any industrial product is a value added approach. The abundant surplus of glycerol from biodiesel production, makes the utilization of glucose more expensive while compared with crude glycerol. Furthermore, glucose competes directly with food and feed production, which is not the case for glycerol [6]. Glycerol has a greater degree of reduction than does sugars, and it is also cheaper and more readily available. In comparison with glucose fermentation, the almost exclusive synthesis of reduced products during glycerol fermentation reflects the highly reducible state of glycerol. Conversion of glycerol to phosphoenolpyruvate, or pyruvate, generates twice the amount of reducing equivalents than does producing pyruvate from glucose or xylose. As an example, glycerol fermentation produced ethanol and formic acid (or ethanol and hydrogen) with overall a yield of twice that of glucose fermentation since half of the glucose lost as carbon dioxide during bioconversion of glucose. Moreover, utilization of crude glycerol also alleviates the carbon catabolic repression/glucose effect present in case of glucose utilization. In case of carbon catabolite repression, the presence of a rapidly metabolizable carbon source such as glucose inhibits the expression of genes encoding proteins required for the utilization of alternative carbon sources such as glycerol and lactose etc. [7]. Glycerol has many uses in the different type of industries like pharmaceutical, soaps, food, paint, cosmetics, toothpaste [8]. There are many microbes that can metabolize glycerol aerobically and few microbes are able to metabolise it anaerobically, so none of them is used at industrial scale. Escherichia coli, Klebsiella, Enterobacter, Glucanobacter, Clostridium, Candida, Aspergillus can convert crude glycerol into value added products [9]. About 10 kg of biodiesel produces glycerol equivalent to 1 kg. The production cost of biodiesel increases by $0.021/L for every $0.22/kg reduction in glycerol selling price [10].
2. Bioconversion technologies of glycerol to value added products
The left unattended crude glycerol from the biodiesel industry is also a threat to environment. Hence conversion to other value added products through biological routes with the aid of microbes is a viable resource and which subsequently enhances the economy of the process. Different value added products from crude glycerol have been summarized as follows.
2.1. 1,3-Propanediol [CH2(CH2OH)2]
This three carbon diol is a colorless viscous liquid used to produce polymers such as polytrimethylene terephthalate (PTT). It is used widely to produce aliphatic polyesters, co-polyesters, adhesives, composites, coatings, moldings, laminates, wood paints, and antifreeze [11]. 1,3-PDO have been reported to be produced by a recombinant strain of E. coli which was constructed by transferring DhaB1 (B12-independent glycerol dehydratase) and its activating factor DhaB2 from the species Clostridium butyrium. The overall yield, concentration and overall productivity of 1,3-PDO was 1.09 mol/mol, 104.4 g/L and 2.61 g/L/h respectively [12]. Bacteria Citrobacter freundii (DSM 15979) is another potential candidate for 1,3-PDO production through fed-batch fermentation [13]. Klebsiella oxytoca converts biodiesel derived crude-glycerol into 1,3-PDO by creating a lactate deficient mutant (LDH3) under batch and fed-batch fermentation conditions, the yield and productivity increased to 0.53 g/mol from 0.41 and 0.83 g/L/h from 0.63 respectively. In fed-batch fermentation, ethanol is formed along with 1,3-PDO with an initial glycerol concentration of 126 g/L [14].
2.2. Hydrogen [H2]
Hydrogen, the only fuel to produce water as a by-product is seen as an ideal fuel for the future that can be produced in an eco-friendly manner. Crude glycerol serves as a raw material for hydrogen production through microbial fermentation. The broad spectrum of applicable substrates in fermentative hydrogen production facilitates the possibility of combing the energetic utilization of biomass to hydrogen with the simultaneous treatment of waste materials. Hydrogen produced through microbial fermentation is a suitable alternative as combustion of hydrogen generates only water as byproduct which drastically reducing CO2, NOx, particulate and other emissions, usually accompanied with the fossil fuels usage [15], [16].
Under batch fermentation, Clostridium species forms hydrogen. C. freundii H3 produces H2 to give a yield of 0.94 mol/mol by fermentation from chemical grade glycerol [17]. Under thermotolerant fermentation, Klebsiella pneumoniae TR17 forms 20 g/L of hydrogen by batch as well as continuous fermentation [18]. Pre-treated waste glycerol can be photo-fermented to hydrogen through Rhodopseudomonas palustris CGA009, a purple non-sulphur photosynthetic bacterium. It produces 6.1 mol/h/mol of glycerol [19]. A hydrogen yield of 0.80 mol/mol glycerol has been reported from Paenibacillus macerans strains through utilization of glycerol as a sole carbon source. The reported was very low as compared to the later research studies, where H2 has been produced by R. palustris and Enterobacter aerogenes [20].
Microbial electrolysis cells also used as a source for hydrogen production uses crude glycerol as a carbon source. The basic mechanism of microbial electrolysis cell for hydrogen production is—an electron is donated to the anode with the help of an external power supply during bioconversion of glycerol, then the free protons will be reduced on the cathode that subsequently produce H2 [21]. Different researchers reported the efficiency of glycerol as a substrate for hydrogen production through microbial electrolysis cells (MEC’s). Conversion of crude glycerol into H2 and electricity has been reported by Chookaew et al. [22] through a two-stage process of linking dark fermentation with a microbial fuel cell (MFC) or microbial electrolysis cell (MEC) and achieved a maximum H2 rate of 332 ml/L and a yield of 0.55 mol H2/mol glycerol. A hydrogen yield of 3.9 mol–H2/mol has been reported using glycerol as carbon source through MEC, which is higher than that possible by fermentation, at relatively high rates of 2.0 ± 0.4 m3/m3 d (Eap = 0.9 V) [23]. Sakai and Yagishita have reported the hydrogen production by E. aerogenes NBRC 12010 using crude glycerol as a feedstock in a bio-electrochemical two-compartment reactor with a cation-exchange membrane separating the compartments [24].
2.3. Propanoic acid and trehalose
Propanoic acid [CH3CH2COOH] is a universal preservative derived directly from a metabolic pathway similar to that of succinic acid. The numerous industrial applications of Propanoic acid account for increasing interest in the development of a biotechnological production process as it is used to produce solvents, pesticides, artificial flavours, thermoplastics and pharmaceutical products [25]. Propionibacterium acidipropionici, Propionibacterium acnes and Clostridium propionicum are the three major bacterial strains used to produce propionate from glycerol. Both in terms of yield and productivity, authors have concluded that for Propanoic acid production, glycerol is a promising substrate. Trehalose, a disaccharide of sugar is a reducing sugar with nutraceutical properties and sed as a stabilizer in therapeutic products (Herceptin®, Avastin®, Lucentis®, and Advate®). Propionibacterium freudenreichii subspecies shermanii produces Propanoic acid, lactic acid and trehalose from crude glycerol [26].
2.4. Single cell oil
Single cell oil or simply SCO has drawn interest in industrial applications as microbial lipids has the potential to replace the plant triacylglycerols. Fed-batch systems appear to enable an increase in lipid content and cell density. Batch experimentations that though have been predominantly used to investigate lipid production from glycerol were negatively impacted for species such as Cryptococcus curvatus by the impurities found in crude glycerol. Comparable cell densities were observed even in crude glycerol by switching to the fed-batch culture system. High lipid content above 70% was reported using the fungus Thamnidium elegans in batch cultures [27] while 60.7% lipid was accumulated in fed-batch system using the yeast Rhodotorula glutinis [28]. Novel fungi strains have to be fetched out that produce high cell densities that contain a copious amount of lipid in the form of SCO from crude glycerol.
2.5. n-Butanol
The production of biobutanol is of particular interest as it offers better physical properties in comparison to ethanol as an alternative fuel. Glycerol as a byproduct from biodiesel production has also been served as a potential substrate for n-butanol production. A maximum yield of 0.28 g/L × h n-butanol was obtained by the use of Clostridium pasteurianum, a gram positive anaerobe with the initial substrate concentration of 25 g/L at 37 °C [29]. Through in situ n-butanol removal by gas striping, Jensen et al. achieved a n-butanol productivity of as high as 1.3 g/L × h [30], [31]. C. pasteurianum DSMZ 525 have been reported as a promising strain for n-butanol production from mixed substrates of biomass hydrolysate and glycerol. The produced n-butanol has less downstream processing steps as the process is not producing acetone as a by-product. In this case, the formation of butanol was checked at different temperatures and also at different initial substrate’s concentration. Out of all the cases, the best temperature at which there was a maximum production of butanol was 37 °C [32]. Clostridium acetobutylicum KF158795 have been reported as a promising n-butanol producer using glycerol as a substrate and produced 13.57 g/L of butanol in 96 h under optimized conditions [33].
2.6. Glyceric acid
Glyceric acid is produced as a small by product of dihydroxyacetone production from glycerol by Glucanobacter sp. The microbe used in this was isolated from rotten apple they also optimized the amounts of glyceric acid by using various volumes of CaCl2, characterized trace elements present in the waste glycerol. Hence, they proposed that glyceric acid be potentially mass producible from waste glycerol. During fermentation it was observed that the initial pH of 6.0 favored the growth of microbes and as the conversion proceeded the pH dropped to 2.5 which tended to increase the production of glyceric acid. It was also observed that there was a maximum precipitation of glyceric acid calcium salt solution at various concentration ranges from 30 to 70%, 50% [34].
2.7. Citric acid
Citric acid is used as an emulsifying agent in ice creams, as a cleansing agent, in pharmaceutical industry, cosmetics, etc. It is usually produced by submerged microbial fermentation of molasses using Aspergillus niger, Yarrowia lipolytica [35]. The final concentration of citric acid that obtained was 77.4 g/L in the fermentation broth when raw glycerol as a substrate was used. However, by using Y. lipolytica N15, higher citric acid concentrations of 112 mg/g were obtained [36].
2.8. Ethanol
It is the highest produced alcohol during the fermentation. It is used in thermometers, as a solvent, as a fuel, etc. Pachysolen tannophilus (CBS4044) is used to produce ethanol by submerged batch fermentation from crude glycerol. The efficiency of this bacterium to use glycerol was found to be 50% v/v [37]. K. pneumoniae (GEM167) is used to produce ethanol in batch and fed batch fermentation conditions with a production rate of 0.93 g/L/h [38].
E. coli is also used to produce ethanol. To maximize the ethanol production, strain SY03 was constructed by inactivating fumarate reductase and phosphate acetyltransferase, which resulted in the production of 1 mol of ethanol and 1 mol of hydrogen gas per mole of glycerol consumed [39].
2.9. Polyunsaturated fatty acids
It was observed that T. elegans was able to produce 371 mg at an initial crude glycerol concentration of 90 g. In another study, Cunninghamella echinulata and Mortierella isabellina also produced GLA at concentrations of 190 and 116 mg, respectively from crude glycerol [40]. DHA (Docosahexanoic acid) and EPA (Eicosahexanoic acid) are both important omega-3 PUFA’s due to their essential role in the treatment of cancer, Alzheimer’s disease and cardiovascular disease. Most of the PUFA’s are fish extracted which are less preferred due to unpleasant odor and harmful pollutants that they might accumulate. EPA was produced from waste glycerol from the fungus Pythium irregular at a final concentration of 90 mg/L and a productivity of 14.9 mg/L/day [41]. DHA was produced using waste glycerol as substrate by the algal species Schizochytrium limacinum SR21 with DHA productivity of 0.51 g/L/day [42].
2.10. Biopolymers (PHA, PHB and acrylates)
It was observed that the biopolymers were produced by using the crude glycerol—a byproduct of biodiesel under the limited amount of nitrogen and phosphate. Several researchers reported the production of biopolymers through microbial fermentation using crude glycerol as a carbon source. Ammonium molybdate and ammonium sulphate were used in the medium that was nitrogen deficient instead of ammonium chloride. Hence, maximum biopolymer productions were observed in Bacillus sp. and Pseudomonas sp. That was equal to 13.3 g/100 ml and 11.2 g/100 ml respectively [43]. It has been seen that the Burkholderia cepacia ATCC 17759 produced poly-3-hydroxybutyrate (PHB) from different concentrations of glycerol ranging from 3% to 9% (v/v). It was observed that with increasing the concentration of glycerol, there was a gradual reduction of biomass, the yield of PHA, and molecular mass of PHB. In the end, the fermentation was successfully scaled up to 200 L for its production which resulted in 23.6 g/L yield of dry biomass and 7.4 g/L yield of PHB [44]. Polyhydroxyalkanoates (PHAs) represent naturally occurring biopolymers produced intracellularly through fermentation by several bacterial strains using glycerol as a carbon source. Halomonas sp. SA8, a soil bacteria from Finnish soils and sediments have been reported for the intracellular accumulation of PHA (56%) through fermentation by using crude glycerol as a carbon source using mineral medium. The produced PHA has been identified as a PHB homopolymer, which shows a great hindrance in the commercial production of PHA’s [45]. mcl-PHA produced through microbial fermentation by Pseudomonas spp. P. mediterranea 9.1 (CFBP 5447) using crude glycerol as a carbon source is a potential to use as a softener for biopolymeric blends of food packaging and medical devices material [46]. Bacillus thuringiensis EGU45 also reported for its efficiency in production of different PHA’s under optimized nitrogen conditions using crude glycerol as a carbon source. In presence of high nitrogen contents as media components, B. thuringiensis EGU45 produces a PHA in a concentrations around 1.5–3.5 g. B. thuringiensis EGU45 has been also reported for the production of co-polymer of P(3HB-co-3HV) under non-limiting N conditions (N containing media supplemented with propionic acid) using crude glycerol as a carbon source [47]. Several recombinant strains also have been reported for the production of biopolymers using crude glycerol as a carbon source. In a batch fermentation, P(3HB) has been reported to be produced by recombinant E. coli JM109 as glycerol as a carbon source [48]. An attempt of producing poly(3-hydroxypropionate), P(3HP) has been reported in the literature in a two stage process. The first stage involves conversion of glycerol to 3-hydroxypropionaldehyde (3HPA) by Lactobacillus reuteri followed by second stage of transformation of 3HPA to P(3HP) using recombinant E. coli strain with the co-expression of A-acylating propionaldehyde dehydrogenase (PduP) from L. reuteri and polyhydroxyalkanoate synthase (PhaCcs) from Chromobacterium sp. [49].
The several research attempts for production of value added products (with the yield information) and microbes used for the bioconversion of crude glycerol have been tabulated under Table 1 [50].
Table 1.
Microbial conversion of crude glycerol to value-added products.
| Sl. no. | Species | Strain | Product/yield (mol/mol glycerol) |
References |
|---|---|---|---|---|
| 1 | Clostridium |
C.pasteurianam (immobilized) C. butyricum AKR102a C. butyricum VPI 3266 Engineered C. acetobutylicum |
n-butanol/0.43 1,3-PDO/0.63 1,3-PDO/0.65 1,3-PDO/0.66 |
Wilkens et al. [51] Gonzales et al. [52] Gonzales et al. [53] Jensen et al. [54] |
| 2 | E. coli | Engineered E. coli SY03 E. coli AC521 Engineered E. coli |
Ethanol/1 Lactic acid/0.9 Succinate/0.8 |
Hong et al. [55] Zhang et al. [56] Metsoviti et al. [57] |
| 3 | Citrobacter |
C. freundii FMCC-B294 C. werkmanii DSM 17579 C. freundii H3 |
1,3-PDO/0.48 1,3-PDO/0.62 H2/0.94 |
Maervoet et al. [58] Maru et al. [59] Yang et al. [60] |
| 4 | Klebsiella | Engineered K. pneumonia K. pneumonia (Encapsulated) K. oxytoca (Lactate deficient) K. pneumonia (Inactivated ADH) |
Ethanol/0.89 1,3-PDO/0.65 1,3-PDO/0.41 1,3-PDO/0.70 |
Zhao et al. [61] Yang et al. [62] Zhang et al. [63] Zhang et al. [64] |
| 5 |
Propionibacterium Bacteria |
Engineered P. acidipropionici Strain P. freudenreichi subsp. Shermanii NCIM 5137 |
Propionic acid/0.66 Trehalose/391 mg/g biomass |
Ruhal et al. [65] Sabourin et al. [66] |
| 6 | Other bacteria and mixed culture |
R. palustris CGA009 P. macerans |
H2/6 H2/0.801 |
Gupta et al. [67] Andre et al. [68] |
| 7 | Fungi |
L. edodes strains A. niger strains Thamnidium elegans |
SCO/0.1 g/g biomass SCO/0.41 1 g/g biomass SCO and PUFAs |
Chatzifragku et al. [69] Imandi et al. [70] Rywinska et al. [71] |
| 8 | Yeast |
Y. lipolytica Wratislavia AWG7 Cryptococcus curvatus Rhodotorula glutinis Engineered S. cerevisiae |
Citric acid/0.33 SCO/52% lipid content SCO/36.5% lipid content Ethanol |
Liang et al. [72] Jung et al. [73] Chi et al. [74] Sánchez et al. [75] |
| 9 | Microalgae | S. limacinum SR21 | DHA | Pyle et al. [76] |
3. Future prospects
New technologies which involve glycerin-to-methanol production pathway and glycerin-to-ethanol production pathway can rapidly change the market scenario biofuels. Instead of seeing glycerin as a waste by biofuel producers, they have come to know that this by-product is a valuable resource for their own production processes. This could change the market situation for other industries which now fully rely on the glycerin production and supply from the biofuels industry. Compared to conventional catalysis, the biotransformation/bioconversion of crude glycerol are a less studied domain. As a sustainable approach, more focus has to be given for bioconversion of crude glycerol to poly urethane foams, biopolyols, polyglycerols and pigments. A more research has to done to enhance the yields of lactic acid, n-butanol and PUFA’s through crude glycerol based bioconversions. Scarce literature reports are available on crude glycerol pretreatment for better utilization in MEC for r hydrogen production. Hence, further investigation is still required like collective removal method for different types of impurities (like acetic acid and butyric acid) will fetch the process to industrial scale that may be helpful for crude glycerol to hydrogen production in near future. Further investigation of microbial hydrogen production on continuous mode and application of co-culture system is recommended. Further, genetically engineered microbial strains may enhance the hydrogen production potential of crude glycerol. However, many of the existing technologies offered still need further development to make them cost-effective and operationally feasible for incorporation into biorefineries.
4. Conclusion
Biodiesel, designated to be a future alternative fuel produces crude glycerol as a waste byproduct. The bioconversion of this crude glycerol to value added products is a sustainable approach compared to chemical conversion. With the advent of directed evolution and systems biology approaches, the constructed novel strains can convert to some more valuable products from the crude glycerol reservoir. There are many products which can be made from crude glycerol which can be used in cosmetics, pharmaceuticals, feed products etc. Bioconversion approaches seems to a viable source for economic development and also towards maintenance of nature.
Acknowledgement
The authors gratefully acknowledge JUIT, India, for providing library resource facilities to Uttara Shankar and Amrita Budhiraja.
References
- 1.Biodiesel 2020: Global Market Survey, Feedstock Trends and Forecasts. Emerging Markets Online, 2 2008. <http://www.healthtech.com/biodiesel2020/>, Multi-Client Study.
- 2.Anand P., Saxena R.K. A comparative study of solvent-assisted pretreatment of biodiesel derived crude glycerol on growth and 1,3-propanediol production from Citrobacter freundi. New Biotechnol. 2011:1–7. doi: 10.1016/j.nbt.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Milazzo M.F., Spina F., Vinci A., Espro C., Bart J.C.J. Brassica biodiesels: past, present and future. Renew. Sustain. Energy Rev. 2013;18C:350–389. [Google Scholar]
- 4.Kerr B.J., Dozier W.A., III, Bregendahl K. Nutritional value of crude glycerin for nonruminants. Proceedings of the 23rd Annual Carolina Swine Nutrition Conference; Raleigh, NC; 2007. pp. 6–18. [Google Scholar]
- 5.Yazdani S.S., Gonzalez R. Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007;18:213–219. doi: 10.1016/j.copbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura C.E., Whited G.M. Metabolic engineering for the microbial production of 1,3-propanediol. Curr. Opin. Biotechnol. 2003;14:454–459. doi: 10.1016/j.copbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 7.da silva G.P., Mack M., Contiero J. Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009;2:30–39. doi: 10.1016/j.biotechadv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Colin T., Bories A., Lavigne C., Moulin G. Effects of acetate and butyrate during glycerol fermentation by Clostridium butyricum. Curr. Microbiol. 2001;43(4):238–243. doi: 10.1007/s002840010294. [DOI] [PubMed] [Google Scholar]
- 9.Solomos B., Zeng A.P., Biebl H., Schlieker H., Posten C., Deckwer W.D. Comparison of the energetic efficiencies of hydrogen and oxochemicals formation in Klebsiella pneumonia and Clostridium butylicum during anaerobic growth on glycerol. J. Biotechnol. 1995;39:107–117. doi: 10.1016/0168-1656(94)00148-6. [DOI] [PubMed] [Google Scholar]
- 10.Da Silva G.P., Mack M., Contiero J. Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009;27:30–39. doi: 10.1016/j.biotechadv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Yazdani S.S., Gonzalez R. Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007;18:213–219. doi: 10.1016/j.copbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Tang X., Tan Y., Zhu H., Zhao K., Shen W. Microbial conversion of glycerol to 1,3-propanediol by an engineered strain of E. coli. Appl. Environ. Microbiol. 2009;75:1628–1634. doi: 10.1128/AEM.02376-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Y., Liu W., Zou H., Cheng T., Tian N., Xian M. Microbial production of short chain diols. Microb. Cell Fact. 2014;13:65. doi: 10.1186/s12934-014-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho S., Kim T., Woo H.M., Kim Y., Lee J., Um Y. High production of 2,3-butanediol from biodiesel-derived crude glycerol by metabolically engineered Klebsiella oxytoca M1. Biotechnol. Biofuels. 2015;8:146. doi: 10.1186/s13068-015-0336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priscilla A.S., Joe M.P., Wallis A.L., Bruce E.L. Enhanced hydrogen and 1,3-propanediol production from glycerol by fermentation using mixed cultures. Biotechnol. Bioeng. 2009;104:1098e106. doi: 10.1002/bit.22487. [DOI] [PubMed] [Google Scholar]
- 16.Sarma S.J., Brar S.K., Sydney E.B., Bihan Y.L., Buelna G., Soccol C.R. Microbial hydrogen production by bioconversion of crude glycerol: a review. Int. J. Hydrogen Energy. 2012;37:6473–6490. [Google Scholar]
- 17.Rahul M., Matti K., Ville S. Bioconversion of crude glycerol from biodiesel production to hydrogen. Int. J. Hydrogen Energy. 2012;37:12198–12204. [Google Scholar]
- 18.Yung C.L., Xue J.C., Chi Y.H., Ying J.Y., Jo S.C. Dark fermentative hydrogen production with crude glycerol from biodiesel industry using indigenous hydrogen-producing bacteria. Int. J. Hydrogen Energy. 2013;38(35):1–8. [Google Scholar]
- 19.Robert W.M.P., Christopher J.H., John S.D. Photofermentation of crude glycerol from biodiesel using Rhodopseudomonas palustris: comparison with organic acids and the identification of inhibitory compounds. Bioresour. Technol. 2013;130:725–730. doi: 10.1016/j.biortech.2012.11.126. [DOI] [PubMed] [Google Scholar]
- 20.Saurabh J.S., Gurpreet S.D., Satinder K.B., Yann L.B., Gerardo B., Mausam V. Investigation of the effect of different crude glycerol components on hydrogen production by Entero bacteraerogenes NRRL B-407. Renew. Energy. 2013;60:566–571. [Google Scholar]
- 21.Escapa A., Manuel M.F., Moran A., Gomez X., Guiot S.R., Tartakovsky B. Hydrogen production from glycerol in a membrane less microbial electrolysis cell. Energy Fuels. 2009;23:4612–4618. [Google Scholar]
- 22.Chookaew T., Prasertsan P., Ren Z.J. Two-stage conversion of crude glycerol to energy using dark fermentation linked with microbial fuel cell or microbial electrolysis cell. New Biotechnol. 2014;31(March (2)):179–184. doi: 10.1016/j.nbt.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Selembo P.A., Perez J.M., Lloyd W.A., Logan B.E. High hydrogen production from glycerol or glucose by electrohydrogenesis using microbial electrolysis cells. Int. J. Hydrogen Energy. 2009;34:5373e81. [Google Scholar]
- 24.Sakai S., Yagishita T. Microbial production of hydrogen and ethanol from glycerol-containing wastes discharged from a biodiesel fuel production plant in a bio-electrochemical reactor with thionine. Biotechnol. Bioeng. 2007:340e8. doi: 10.1002/bit.21427. [DOI] [PubMed] [Google Scholar]
- 25.Bertleff W., Roeper M., Sava X. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim: 2005. Carbonylation. [Google Scholar]
- 26.Ohtake S., Wang Y.J. Trehalose: current use and future applications. J. Pharm. Sci. 2011;100(6):2020–2053. doi: 10.1002/jps.22458. [DOI] [PubMed] [Google Scholar]
- 27.Chatzifragkou A., Makri A., Belka A., Bellou S., Mavrou M., Mastoridou M., Mystrioti P., Onjaro G., Aggelis G., Papanikolaou S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy. 2011;36:1097–1108. [Google Scholar]
- 28.Saenge C., Cheirsilp B., Suksaroge T.T., Bourtoom T. Potential use of the oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011;46:210–218. [Google Scholar]
- 29.Swati K., Arun G., Vijayanand S.M. Production of n-butanol from biodiesel derived crude glycerol using Clostridium pasteurianum immobilized on Amberlite. Fuel. 2013;112:557–561. [Google Scholar]
- 30.Jensen T.O., Kvist T., Mikkelsen M.J., Christensen P.V., Westermann P. Fermentation of crude glycerol from biodiesel production by Clostridium pasteurianum. J. Ind. Microbiol. Biotechnol. 2012;39:709–717. doi: 10.1007/s10295-011-1077-6. [DOI] [PubMed] [Google Scholar]
- 31.Jensen T.O., Kvist T., Mikkelsen M.J., Westermann P. Production of 1,3-PDO and butanol by a mutant strain of Clostridium pasteurianum with increased tolerance towards crude glycerol. AMB Express. 2012;2(1):44. doi: 10.1186/2191-0855-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabra W., Groeger C., Sharma P.N., Zeng A.P. Improved n-butanol production by a non-acetone producing Clostridium pasteurianum DSMZ 525 in mixed substrate fermentation. Appl. Microbiol. Biotechnol. 2014;98(9):4267–4276. doi: 10.1007/s00253-014-5588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yadav S., Rawat G., Tripathi P., Saxena R.K. A novel approach for biobutanol production by Clostridium acetobutylicum using glycerol: a low cost substrate. Renewable Energy. 2014;71(November):37–42. [Google Scholar]
- 34.De Ley J., Frateur J. The status of the generic name Gluconobacter. Int. J. Syst. Bacteriol. 1970;20:83–95. [Google Scholar]
- 35.Papanikolaou S., Fakas S., Fick M., Chevalot I., Galiotou-Panayotou M., Komaitis M., Marc I., Aggelis G. Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenergy. 2008;32:60–71. [Google Scholar]
- 36.Papanikolaou S., Aggelis G. Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytic. Lipid Technol. 2009;21:83–87. [Google Scholar]
- 37.Baek R.O., Jeong W.S., Sun Y.H., Won K.H., Lian H.L., Min J.h.., Don H.P., Chul H.K. Efficient production of ethanol from crude glycerol by a Klebsiella pneumonia mutant strain. Bioresour. Technol. 2011;102:3918–3922. doi: 10.1016/j.biortech.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Fakas S., Papanikolaou S., Batsos A., Galiotou-Panayotou M., Mallouchos A., Aggelis G. Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenergy. 2009;33:573–580. [Google Scholar]
- 39.Yazdani S.S., Gonzalez R. Engineering E. coli for the efficient conversion of glycerol to ethanol and co-products. Metab. Eng. 2008;10:340–351. doi: 10.1016/j.ymben.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Khanna S., Goyal A., Moholkar V.S. Production of n-butanol from biodiesel derived crude glycerol using Clostridium pasteurianum immobilized on amberlite. Fuel. 2011;112:557–561. [Google Scholar]
- 41.Athalye S.K., Garcia R.A., Wen Z. Use of biodiesel-derived crude glycerol for producing eicosapentaenoic acid (EPA) by the fungus Pythium irregulare. J. Agric. Food Chem. 2009;57:2739–2744. doi: 10.1021/jf803922w. [DOI] [PubMed] [Google Scholar]
- 42.Chi Z., Pyle D., Wen Z., Frear C., Chen S. A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem. 2007;42:1537–1545. [Google Scholar]
- 43.Sathianachiyara S., Devaraj A. Biopolymer production by bacterial species using glycerol, a byproduct of biodiesel. Int. J. Sci. Res. Publ. 2013;3 [Google Scholar]
- 44.Zhu C., Nomurg C.T., Perrotta J.A., Stipanovic A.J., Nakas J.P. Production and characterization of poly-3-hydroxybutyrate from biodiesel-glycerol by Burkholderia cepacia ATCC 17759. Biotechnol. Prog. 2009;26:424–430. doi: 10.1002/btpr.355. [DOI] [PubMed] [Google Scholar]
- 45.de Castro J.S., Nguyen L.D., Seppala J. Bioconversion of commercial and waste glycerol into value-added polyhydroxyalkanoates by bacterial strains. J. Microb. Biochem. Technol. 2014 [Google Scholar]
- 46.Pappalardo F., Fragalà M., Mineo P.G., Damigella A., Catara A.F., Palmeri R., Rescifina A. Production of filmable medium-chain-length polyhydroxyalkanoates produced from glycerol by Pseudomonas mediterranea. Int. J. Biol. Macromol. 2014;65(April):89–96. doi: 10.1016/j.ijbiomac.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Kumar P., Ray S., Patel S.K.S., Lee J.K., Kalia V.C. Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int. J. Biol. Macromol. 2015;78(July):9–16. doi: 10.1016/j.ijbiomac.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 48.Ganesh M., Senthamarai A., Shanmughapriya S., Natarajaseenivasan K. Effective production of low crystallinity poly(3-hydroxybutyrate) by recombinant E. coli strain JM109 using crude glycerol as sole carbon source. Bioresour. Technol. 2015;192(September):677–681. doi: 10.1016/j.biortech.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 49.Linares-Pastén J.A., Sabet-Azad R., Pessina L., Sardari R.R.R., Ibrahim M.H.A., Hatti-Kaul R. Efficient poly(3-hydroxypropionate) production from glycerol using Lactobacillus reuteri and recombinant E. coli harboring L. reuteri propionaldehyde dehydrogenase and Chromobacterium sp. PHA synthase genes. Bioresour. Technol. 2015;180:172–176. doi: 10.1016/j.biortech.2014.12.099. [DOI] [PubMed] [Google Scholar]
- 50.Li Cheng, Lesnik Keaton L., Liu Hong. Microbial conversion of waste glycerol from biodiesel production into value-added products. Energies. 2013;6:4739–4768. [Google Scholar]
- 51.Wilkens E., Ringel A.K., Hortig D., Willke T., Vorlop K.-D. High-level production of 1,3-propanediol from crude glycerol by Clostridium butyricum AKR102a. Appl. Microbiol. Biotechnol. 2012;93:1057–1063. doi: 10.1007/s00253-011-3595-6. [DOI] [PubMed] [Google Scholar]
- 52.González-Pajuelo M., Andrade J.C., Vasconcelos I. Production of 1,3-propanediol by Clostridium butyricum VPI 3266 in continuous cultures with high yield and productivity. J. Ind. Microbiol. Biotechnol. 2005;32:391–396. doi: 10.1007/s10295-005-0012-0. [DOI] [PubMed] [Google Scholar]
- 53.González-Pajuelo M., Meynial-Salles I., Mendes F., Andrade J.C., Vasconcelos I., Soucaille P. Metabolic engineering of Clostridium acetobutylicum for the industrial production of 1,3-propanediol from glycerol. Metab. Eng. 2005;7:329–336. doi: 10.1016/j.ymben.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Jensen T., Kvist T., Mikkelsen M.J., Christensen P.V., Westermann P. Fermentation of crude glycerol from biodiesel production by Clostridium pasteurianum. J. Ind. Microbiol. Biotechnol. 2012;39:709–717. doi: 10.1007/s10295-011-1077-6. [DOI] [PubMed] [Google Scholar]
- 55.Hong A.-A., Cheng K.-K., Peng F., Zhou S., Sun Y., Liu C.-M., Liu D.-H. Strain isolation and optimization of process parameters for bioconversion of glycerol to lactic acid. J. Chem. Technol. Biotechnol. 2009;84:1576–1581. [Google Scholar]
- 56.Zhang X., Shanmugam K.T., Ingram L.O. Fermentation of glycerol to succinate by metabolically engineered strains of E. coli. Appl. Environ. Microbiol. 2010;76:2397–2401. doi: 10.1128/AEM.02902-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Metsoviti M., Zeng A.-P., Koutinas A.A., Papanikolaou S. Enhanced 1,3-propanediol production by a newly isolated Citrobacter freundii strain cultivated on biodiesel-derived waste glycerol through sterile and non-sterile bioprocesses. J. Biotechnol. 2013;163:408–418. doi: 10.1016/j.jbiotec.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 58.Maervoet V.E.T., Beauprez J., de Maeseneire S.L., Soetaert W.K., de Mey M. Citrobacter werkmanii, a new candidate for the production of 1,3-propanediol: strain selection and carbon source optimization. Green Chem. 2012;14:2168–2178. [Google Scholar]
- 59.Maru B.T., Constanti M., Stchigel A.M., Medina F., Sueiras J.E. Biohydrogen production by dark fermentation of glycerol using Enterobacter and Citrobacter sp. Biotechnol. Prog. 2013;29:31–38. doi: 10.1002/btpr.1644. [DOI] [PubMed] [Google Scholar]
- 60.Yang C., Jiang P., Xiao S., Zhang C., Lou K., Xing X.-H. Fed-batch fermentation of recombinant Citrobacter freundii with expression of a violacein-synthesizing gene cluster for efficient violacein production from glycerol. Biochem. Eng. J. 2011;57:55–62. [Google Scholar]
- 61.Zhao Y.-N., Chen G., Yao S.-J. Microbial production of 1,3-propanediol from glycerol by encapsulated Klebsiella pneumoniae. Biochem. Eng. J. 2006;32:93–99. [Google Scholar]
- 62.Yang G., Tian J., Li J. Fermentation of 1,3-propanediol by a lactate deficient mutant of Klebsiella oxytoca under microaerobic conditions. Appl. Microbiol. Biotechnol. 2007;73:1017–1024. doi: 10.1007/s00253-006-0563-7. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y., Li Y., Du C., Liu M., Cao Z. Inactivation of aldehyde dehydrogenase: a key factor for engineering 1,3-propanediol production by Klebsiella pneumoniae. Metab. Eng. 2006;8:578–586. doi: 10.1016/j.ymben.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 64.Zhang A., Yang S.-T. Propionic acid production from glycerol by metabolically engineered Propionibacterium acidipropionici. Process Biochem. 2009;44:1346–1351. [Google Scholar]
- 65.Ruhal R., Choudhury B. Improved trehalose production from biodiesel waste using parent and osmotically sensitive mutant of Propionibacterium freudenreichii subsp. shermanii under aerobic conditions. J. Ind. Microbiol. Biotechnol. 2012;39:1153–1160. doi: 10.1007/s10295-012-1124-y. [DOI] [PubMed] [Google Scholar]
- 66.Sabourin-Provost G., Hallenbeck P.C. High yield conversion of a crude glycerol fraction from biodiesel production to hydrogen by photofermentation. Bioresour. Technol. 2009;100:3513–3517. doi: 10.1016/j.biortech.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 67.Gupta A., Murarka A., Campbell P., Gonzalez R. Anaerobic fermentation of glycerol in Paenibacillus macerans: metabolic pathways and environmental determinants. Appl. Environ. Microbiol. 2009;75:5871–5883. doi: 10.1128/AEM.01246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.André A., Diamantopoulou P., Philippoussis A., Sarris D., Komaitis M., Papanikolaou S. Biotechnological conversions of bio-diesel derived waste glycerol into added-value compounds by higher fungi: production of biomass, single cell oil and oxalic acid. Ind. Crops Prod. 2010;31:407–416. [Google Scholar]
- 69.Chatzifragkou A., Makri A., Belka A., Bellou S., Mavrou M., Mastoridou M., Mystrioti P., Onjaro G., Aggelis G., Papanikolaou S. Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy. 2011;36:1097–1108. [Google Scholar]
- 70.Imandi S.B., Bandaru V.R., Somalanka S.R., Garapati H.R. Optimization of medium constituents for the production of citric acid from byproduct glycerol using Doehlert experimental design. Enzyme Microb. Technol. 2007;40:1367–1372. [Google Scholar]
- 71.Rywińska A., Rymowicz W., Żarowska B., Wojtatowicz M. Biosynthesis of citric acid from glycerol by acetate mutants of Yarrowia lipolytica in fed-batch fermentation. Food Technol. Biotechnol. 2009;47:1–6. [Google Scholar]
- 72.Liang Y., Cui Y., Trushenski J., Blackburn J.W. Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010;101:7581–7586. doi: 10.1016/j.biortech.2010.04.061. [DOI] [PubMed] [Google Scholar]
- 73.Jung J.Y., Yun H.S., Lee J.W., Oh M.K. Production of 1,2-propanediol from glycerol in Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2011;21:846–853. doi: 10.4014/jmb.1103.03009. [DOI] [PubMed] [Google Scholar]
- 74.Chi Z., Pyle D., Wen Z., Frear C., Chen S. A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem. 2007;42:1537–1545. [Google Scholar]
- 75.Sánchez Ó.J., Cardona C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008;99:5270–5295. doi: 10.1016/j.biortech.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 76.Pyle D.J., Garcia R.A., Wen Z. Producing docosahexaenoic acid (DHA)-rich algae from biodiesel-derived crude glycerol: effects of impurities on DHA production and algal biomass composition. J. Agric. Chem. 2008;56:3933–3939. doi: 10.1021/jf800602s. [DOI] [PubMed] [Google Scholar]