Graphical abstract
Keywords: Xylose, Fermentation, Biofuels, Xylose isomerase, Xylose reductase, Xylitol dehydrogenase
Highlights
-
•
Publicly available xylose-fermenting Saccharomyces cerevisiae were compared.
-
•
Yeast using xylose reductase/xylitol dehydrogenase had higher ethanol production.
-
•
Yeast using xylose isomerase had better ethanol yields from xylose.
-
•
Yeast using xylose reductase/xylitol dehydrogenase finish fermentations faster.
-
•
Comparisons between the two strains provide benchmarks for future engineering.
Abstract
Economical biofuel production from plant biomass requires the conversion of both cellulose and hemicellulose in the plant cell wall. The best industrial fermentation organism, the yeast Saccharomyces cerevisiae, has been developed to utilize xylose by heterologously expressing either a xylose reductase/xylitol dehydrogenase (XR/XDH) pathway or a xylose isomerase (XI) pathway. Although it has been proposed that the optimal means for fermenting xylose into biofuels would use XI instead of the XR/XDH pathway, no clear comparison of the best publicly-available yeast strains engineered to use XR/XDH or XI has been published. We therefore compared two of the best-performing engineered yeast strains in the public domain—one using the XR/XDH pathway and another using XI—in anaerobic xylose fermentations. We find that, regardless of conditions, the strain using XR/XDH has substantially higher productivity compared to the XI strain. By contrast, the XI strain has better yields in nearly all conditions tested.
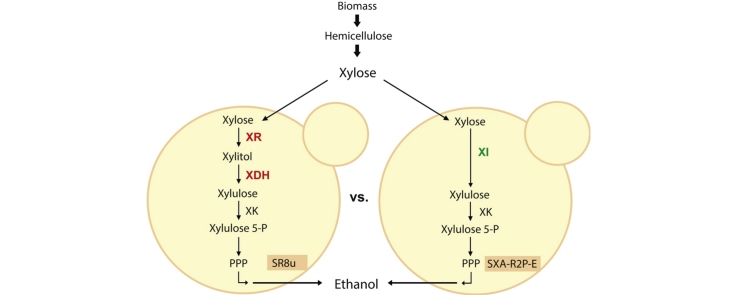
Xylose is the second most abundant sugar next to glucose in plant cell wall hydrolysates [10]. Therefore, efficient and rapid utilization of xylose along with glucose is essential for economic and sustainable production of fuels and chemicals from lignocellulosic biomass [16]. Although Saccharomyces cerevisiae has been exclusively used for producing bioethanol, this yeast cannot ferment xylose. In order to confer xylose-fermenting capabilities into S. cerevisiae, two metabolic pathways from other microorganisms have been introduced [4], [18]. An oxidoreductase pathway consisting of xylose reductase (XR) and xylitol dehydrogenase (XDH) [10], [17], produces reduced byproducts such as xylitol and glycerol during anaerobic xylose fermentation, likely due to the difference in cofactor specificities of XR and XDH [2], [10]. XR uses NADPH, whereas XDH uses NAD+. Therefore, xylose fermentation facilitated by the XR/XDH pathway under anaerobic conditions can lead to surplus NADH production [2], [17], eliciting the production of glycerol and xylitol. As a solution of the redox imbalance problem, the second pathway involving xylose isomerase (XI) pathway was proposed [19]. Several XIs from anaerobic fungi and bacteria have been identified that are functionally expressed in S. cerevisiae, allowing construction of xylose-fermenting S. cerevisiae using the XI pathway [1], [3], [14], [11].
In addition to the introduction and functional expression of two different metabolic pathways for xylose consumption (XR/XDH and XI), rational and evolutionary metabolic engineering strategies have been used to construct efficient xylose-fermenting S. cerevisiae strains [5], [7], [11], [12]. An outstanding question in the field is whether one pathway or the other would be preferred for large-scale fermentation [18]. However, there has not been a direct comparison of the best performing strains with each pathway available in the public domain. Such a comparison would benefit future efforts to develop bioethanol production from lignocellulosic biomass.
Combined rational and evolutionary engineering efforts have led to the generation of strains SR8 [7] and SXA-R2P-E [12], two of the best-performing strains reported to utilize XR/XDH and XI pathway, respectively. SR8 contains two or more chromosomal copies of Scheffersomyces stipitis XYL1 (XR), XYL2 (XDH) and XYL3 (xylulokinase, XK) each with optimized expression, along with deletion of acetaldehyde dehydrogenase, ALD6. It was evolved on xylose in the laboratory, which resulted in the loss of phosphatase Pho13 function. It was subsequently made auxotrophic for uracil (ura3-52) for additional engineering (SR8u) [7]. SXA-R2P-E, also evolved on xylose, has two copies of Piromyces sp. xylA3* (an improved XI mutant), S. stipitis TAL1 (transaldolase) integrated, native XKS1 (XK) overexpressed, and GRE3 (aldose reductase) and PHO13 deleted [12]. It has been recently reported that PHO13 deletion leads to up-regulation of the pentose phosphate genes including TAL1 [8].
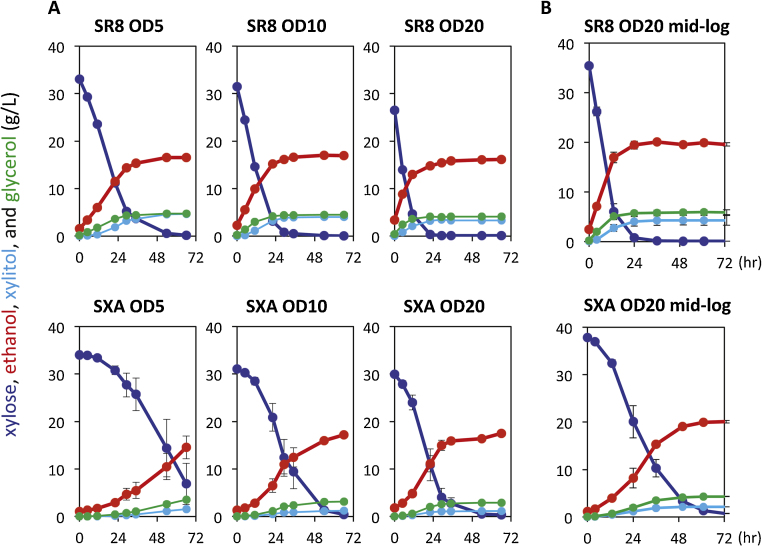
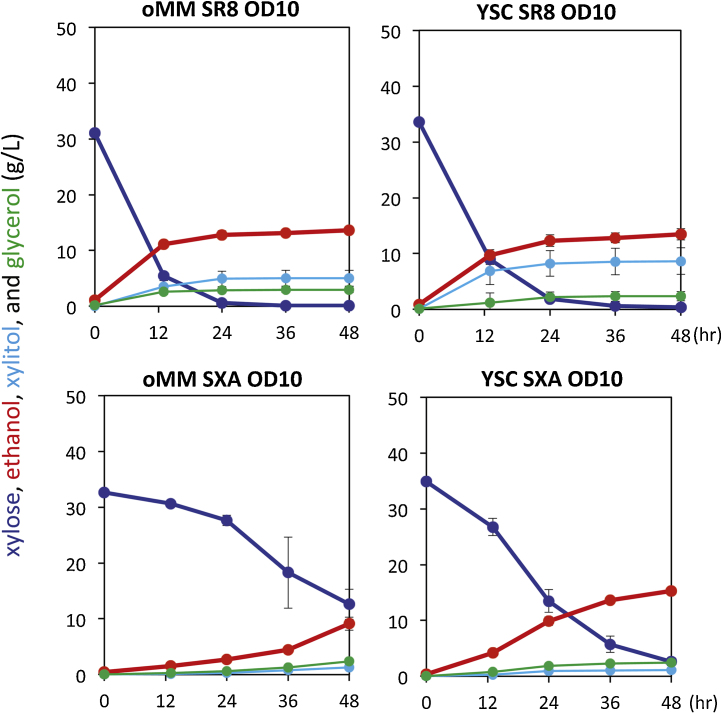
The xylose fermentation performance of the two strains, SR8u and SXA-R2P-E, was compared in various anaerobic batch culture conditions (Table 1). Over a range of cell loadings (5–20 Optical Density (OD) at 600 nm), strain SR8u expressing XR/XDH always displayed higher rates of ethanol production when compared to strain SXA-R2P-E expressing XI (Fig. 1A). This result was also true for seed cultures prepared from mid-log cultures (Fig. 1B) and for fermentation carried out in both optimized Minimal Medium (oMM) and YSC media, which was used to evolve strain SXA-R2P-E (Fig. 2). While xylose consumption and ethanol production rates were higher in strain SR8u expressing the XR/XDH pathway, strain SXA-R2P-E had slightly higher ethanol yields and produced less xylitol as a byproduct (Table 1). Overall, strain SR8u showed 16–104% higher productivities (g ethanol/OD·h) and 5–19% lower yields (g ethanol/g xylose) than strain SXA-R2P-E in the tested xylose fermentation conditions.
Table 1.
Fermentation performance of the two xylose-utilizing S. cerevisiae strains.
| Mediuma | Seed condition | Strainb | Xylose consumption ratec (g xylose OD−1 H−1) [OD]e |
Ethanol production ratec (g ethanol OD−1 H−1) [OD]e |
Ethanol yieldd (g ethanol·g xylose−1) |
|---|---|---|---|---|---|
| oMM | late-log phase, 5 OD | SR8u | 0.127 [5.9] | 0.063 [5.9] | 0.369 |
| oMM | late-log phase, 5 OD | SXA-R2P-E | 0.072 [8.3] | 0.038 [6.6] | 0.402 |
| oMM | late-log phase, 10 OD | SR8u | 0.112 [12.6] | 0.052 [12.6] | 0.378 |
| oMM | late-log phase, 10 OD | SXA-R2P-E | 0.085 [14.4] | 0.045 [14.8] | 0.419 |
| oMM | late-log phase, 20 OD | SR8u | 0.102 [24.4] | 0.044 [24.4] | 0.390 |
| oMM | late-log phase, 20 OD | SXA-R2P-E | 0.046 [26.0] | 0.022 [26.0] | 0.430 |
| oMM | mid-log phase, 20 OD | SR8u | 0.097 [23.7] | 0.049 [23.7] | 0.392 |
| oMM | mid-log phase, 20 OD | SXA-R2P-E | 0.052 [21.8] | 0.024 [24.6] | 0.412 |
| YSC | late-log phase, 10 OD | SR8u | 0.129 [14.7] | 0.046 [14.7] | 0.378 |
| YSC | late-log phase, 10 OD | SXA-R2P-E | 0.077 [15.7] | 0.033 [15.7] | 0.453 |
| oMM+ 0.1% Ac | late-log phase, 10 OD | SR8u | 0.087 [11.0] | 0.042 [11.0] | 0.374 |
| oMM+ 0.1% Ac | late-log phase, 10 OD | SXA-R2P-E | 0.062 [14.6] | 0.031 [14.6] | 0.403 |
Fermentations were carried out anaerobically in batch conditions using serum bottles and 40 g/L xylose.
Strain characteristics: (1) SR8u: XYL1, XYL2, XYL3, Δald6, ura3-52, evolved, (2) SXA-R2P-E: xylA3*, XKS1, TAL1, Δgre3, Δpho13, evolved.
Maximal values in g of xylose consumed or ethanol produced per OD per hour.
Grams of ethanol yield per g of xylose.
OD value at the maximum rate of xylose consumption or ethanol production.
Fig. 1.
Comparison of XI and XR/XDH pathway fermentation performance.
Xylose fermentations using strain SR8u (XR/XDH pathway) and strain SXA-R2P-E (XI pathway) with (A) different starting cell loadings (5-20 OD600), (B) mid-log phase cultures as fermentation seeds. The strain and starting OD600 values are indicated above each panel. The medium used in these fermentations was oMM. Error bars represent the standard deviation of biological triplicates. Xylose fermentation samples were resolved as previously described [13].
Fig. 2.
Xylose fermentation performance of strains SR8u (XR/XDH) and SXA-R2P-E (XI), using either oMM or YSC medium.
The media, strain, and starting OD600 values are given above each panel. Error bars represent the standard deviation of biological triplicates.
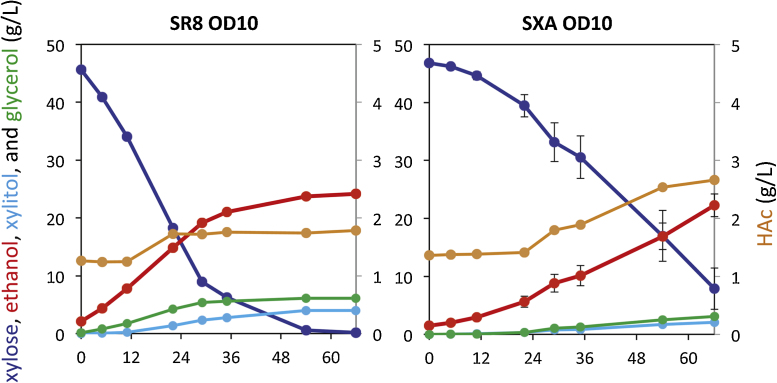
Acetic acid is present in cellulosic hydrolysates due to the acetylation of hemicellulose in the plant cell wall [15]. However, its toxicity has inhibitory effects on fermentation [9], [20]. We therefore compared xylose fermentation in the presence of 1 g/L of acetate, and found that strain SR8u consumed xylose and produced ethanol faster than strain SXA-R2P-E (Fig. 3). It is notable that no acetate reduction pathway was used in these experiments. This suggests that an acetate reduction pathway could be coupled with the XR/XDH pathway to improve ethanol productivity and yield even further by not only resolving the problematic redox cofactor imbalance but also using it to drive co-consumption of xylose and acetate [20].
Fig. 3.
Xylose fermentation performance of strains SR8u (XR/XDH) and SXA-R2P-E (XI) in the presence of acetate.
The strain and starting OD600 values are given above each panel. The medium used in these fermentations was oMM. Error bars represent the standard deviation of biological triplicates.
Here we found that in two high-performance engineered yeast strains, the XR/XDH xylose utilization pathway was capable of higher xylose consumption and ethanol production rates compared to the XI pathway, despite formation of more xylitol as a byproduct. It is worth noting that the XR/XDH pathway has been shown to produce much less xylitol or no xylitol when xylodextrins are used rather than xylose in fermentations [13], or when undetoxified lignocellulose hydrolysate is used [6]. Thus, xylodextrin consumption by means of the XR/XDH pathway could result in yeast strains with both high ethanol productivity and yield, without the drawback of xylitol byproduct formation. Here we used media conditions chosen previously in the development of these strains, to enable comparisons to previously published results. In the future, our results can serve as a baseline for optimizing industrially relevant media, and for in-depth metabolic engineering of industrial yeast strains. It will be important to continue benchmarking high-performance engineered yeast strains with these alternative xylose utilization pathways, to identify and remove remaining bottlenecks in yeast strains to be used in industrial applications.
1. Materials and methods
1.1. Anaerobic xylose fermentation
Yeast strains SR8u or SXA-R2P-E were pre-grown aerobically to mid-log or late-log phase in oMM or YSC medium containing 2% glucose, washed 3 times with water, and re-suspended in either oMM or YSC medium, depending on the batch fermentation. Media containing 4% w/v xylose (5% in Fig. 3) was inoculated with a range of starting OD values, as indicated in the figure panels, and then purged with N2. Anaerobic batch fermentations were carried out in 50 mL of oMM or YSC medium in 125 mL serum bottles, closed with butyl rubber stoppers, shaking at 220 rpm in a 30 °C shaker. At the indicated time points, 1 mL samples were removed with a syringe and pelleted. 5 μL supernatants were analyzed by ion-exclusion HPLC to determine xylose, xylitol, glycerol, and ethanol concentrations.
During the anaerobic fermentations, we collected OD values at every time point and used the average value of each time interval for the rate calculations. Xylose consumption rate (g xylose/OD·H) and ethanol production rate (g ethanol/OD·H) were calculated from the steepest slope of the curve using the average OD of that time interval. Ethanol yield (g ethanol/g xylose) was calculated using grams of xylose and ethanol directly measured at the initial and final time points.
To account for variations from the stated initial xylose concentration of 40 g/L (50 g/L in Fig. 3), rate and yield calculations in Table 1 and the text used values measured by HPLC, including values measured for the time = 0 h time point. This addressed some variation that occurred due to the time needed to set up fermentations in parallel. Experiments to directly compare SR8u and SXA-R2P-E in a given condition and with a given OD were prepared from the same batches of reagents.
In the experiment in which strains were pre-grown to mid-log phase (Fig. 1B), we observed ethanol production over the theoretical maximum. We believe this violation likely arose from cell loading of glucose, as the cells were pre-grown on media containing glucose.
Optimal minimal medium (oMM) contained 1.7 g/L YNB (Y1251, Sigma, Saint Louis, MO, USA), two-fold complete CSM, 10 g/L (NH4)2SO4, 1 g/L MgSO4·7H2O, 6 g/L KH2PO4, 100 mg/L adenine hemisulfate, 10 mg/L inositol, 100 mg/L glutamic acid, 20 mg/L lysine, 375 mg/L serine and 0.1 M 2-(N-morpholino) ethanesulfonic acid (MES) pH 6.0. Yeast synthetic complete (YSC) medium contained 6.7 g/L YNB and appropriate CSM dropout mixture.
Acknowledgements
We thank Prof. Hal Alper for sharing strain SXA-R2P-E. This work was funded by the Energy Biosciences Institute.
References
- 1.Brat D., Boles E., Wiedemann B. Functional expression of a bacterial xylose isomerase in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009;75:2304–2311. doi: 10.1128/AEM.02522-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruinenberg P.M., Debot P.H.M., Vandijken J.P., Scheffers W.A. The role of redox balances in the anaerobic fermentation of xylose by yeasts. Eur. J. Appl. Microbiol. 1983;18:287–292. [Google Scholar]
- 3.Ha S.J., Kim S.R., Choi J.-H., Park M., Jin Y.S. Xylitol does not inhibit xylose fermentation by engineered Saccharomyces cerevisiae expressing xylA as severely as it inhibits xylose isomerase reaction in vitro. Appl. Microbiol. Biotechnol. 2011;92:77–84. doi: 10.1007/s00253-011-3345-9. [DOI] [PubMed] [Google Scholar]
- 4.Hahn-Hägerdal B., Karhumaa K., Jeppsson M., Gorwa-Grauslund M.F. Metabolic engineering for pentose utilization in Saccharomyces cerevisiae. Adv. Biochem. Eng. Biotechnol. 2007;108:147–177. doi: 10.1007/10_2007_062. [DOI] [PubMed] [Google Scholar]
- 5.Jin Y.S., Alper H., Yang Y.T., Stephanopoulos G. Improvement of xylose uptake and ethanol production in recombinant Saccharomyces cerevisiae through an inverse metabolic engineering approach. Appl. Environ. Microbiol. 2005;71:8249–8256. doi: 10.1128/AEM.71.12.8249-8256.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karhumaa K., Sanchez R.G., Hahn-Hagerdal B., Gorwa-Grauslund M.F. Comparison of the xylose reductase-xylitol dehydrogenase and the xylose isomerase pathways for xylose fermentation by recombinant Saccharomyces cerevisiae. Microb. Cell Fact. 2007;6(5):1–10. doi: 10.1186/1475-2859-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S.R., Skerker J.M., Kang W., Lesmana A., Wei N., Arkin A.P., Jin Y.S. Rational and evolutionary engineering approaches uncover a small set of genetic changes efficient for rapid xylose fermentation in Saccharomyces cerevisiae. PLoS One. 2013:8. doi: 10.1371/journal.pone.0057048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S.R., Xu H., Lesmana A., Kuzmanovic U., Au M., Florencia C., Oh E.J., Zhang G., Kim K.H., Jin Y.S. Deletion of PHO13 encoding haloacid dehalogenase type IIA phosphatase, results in upregulation of the pentose phosphate pathway in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2015;81:1601–1609. doi: 10.1128/AEM.03474-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klinke H.B., Thomsen A.B., Ahring B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004;66:10–26. doi: 10.1007/s00253-004-1642-2. [DOI] [PubMed] [Google Scholar]
- 10.Kotter P., Ciriacy M. Xylose fermentation by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1993;38:776–783. [Google Scholar]
- 11.Kuyper M., Hartog M.M.P., Toirkens M.J., Almering M.J.H., Winkler A.A., Van Dijken J.P., Pronk J.T. Metabolic engineering of a xylose-isomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Res. 2005;5:399–409. doi: 10.1016/j.femsyr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Lee S.-M., Jellison T., Alper H. Systematic and evolutionary engineering of a xylose isomerase-based pathway in Saccharomyces cerevisiae for efficient conversion yields. Biotechnol. Biofuels. 2014;7:122. doi: 10.1186/s13068-014-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X., Yu V.Y., Lin Y., Chomvong K., Estrela R., Park A., Liang J.M., Znameroski E.A., Feehan J., Kim S.R., Jin Y.S., Glass N.L., Cate J.H.D. Expanding xylose metabolism in yeast for plant cell wall conversion to biofuels. eLife. 2015:4. doi: 10.7554/eLife.05896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhavan A., Tamalampudi S., Ushida K., Kanai D., Katahira S., Srivastava A., Fukuda H., Bisaria V.S., Kondo A. Xylose isomerase from polycentric fungus Orpinomyces: gene sequencing cloning, and expression in Saccharomyces cerevisiae for bioconversion of xylose to ethanol. Appl. Microbiol. Biotechnol. 2009;82:1067–1078. doi: 10.1007/s00253-008-1794-6. [DOI] [PubMed] [Google Scholar]
- 15.Palmqvist E., Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000;74:25–33. [Google Scholar]
- 16.Stephanopoulos G. Challenges in engineering microbes for biofuels production. Science. 2007;315:801–804. doi: 10.1126/science.1139612. [DOI] [PubMed] [Google Scholar]
- 17.Tantirungkij M., Nakashima N., Seki T., Yoshida T. Construction of xylose-assimilating Saccharomyces cerevisiae. J. Ferment. Bioeng. 1993;75:83–88. [Google Scholar]
- 18.van Maris A.J., Winkler A.A., Kuyper M., de Laat W.T., van Dijken J.P., Pronk J.T. Development of efficient xylose fermentation Saccharomyces cerevisiae: xylose isomerase as a key component. Adv. Biochem. Eng. Biotechnol. 2007;108:179–204. doi: 10.1007/10_2007_057. [DOI] [PubMed] [Google Scholar]
- 19.Walfridsson M., Bao X., Anderlund M., Lilius G., Bülow L., Hahn-Hägerdal B. Ethanolic fermentation of xylose with Saccharomyces cerevisiae harboring the Thermus thermophilus xylA gene, which expresses an active isomerase. Appl. Environ. Microbiol. 1996;62:4648–4651. doi: 10.1128/aem.62.12.4648-4651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei N., Quarterman J., Kim S.R., Cate J.H., Jin Y.S. Enhanced biofuel production through coupled acetic acid and xylose consumption by engineered yeast. Nat. Commun. 2013;4:2580. doi: 10.1038/ncomms3580. [DOI] [PubMed] [Google Scholar]