Abstract
[Purpose] Although cardiac rehabilitation (CR) is recommended for patients with chronic heart failure (CHF), adequate exercise effect cannot be obtained in elderly patients. Administration of amino acids (AA) to CHF patients has been reported to improve exercise capacity, but the changes in AA composition in plasma before and after CR had not been reported. This study aimed to measure plasma levels of AA in CHF patients and compare with values of normal range. In addition the relationship between the change of exercise capacity and AA were examined. [Subjects and Methods] Twelve CHF patients (60% males, aged 68 ± 12 years) were studied. The correction between the rates of changes in exercise capacity parameters and in plasma AA levels was investigated. [Results] Anaerobic threshold (AT) and peak oxygen uptake (VO2) improved significantly after CR. The AA profile showed no specific pattern, and citrulline (Cit) was the amino acid showing a significant positive correlation with exercise capacity (∆Cit vs. ∆AT: r=0.602, ∆Cit vs. ∆AT-work rate (WR): r=0.681, ∆Cit vs. ∆VO2/WR: r=0.635). A tendency of positive correlation was observed between ∆Cit and ∆peak VO2 (r=0.456). [Conclusion] The AA profile showed no specific pattern, but a relationship between change in exercise capacity and Cit were found.
Key words: Chronic heart failure, Exercise capacity, Amino acid
INTRODUCTION
In patients with chronic heart failure (CHF), because of skeletal muscle atrophy and changes in muscle fibers, exercise capacity is decreased. Hence, exercise training is recommended for the purpose of improving exercise capacity in CHF patients1). However, study has shown that implementation of exercise intervention does not significantly improve exercise capacity in elderly patients with heart failure2). On the other hand, recent studies have reported that administration of essential amino acids improves exercise capacity in CHF patients3, 4).
Amino acids are an important component of the human body and the building blocks of structural proteins, functional proteins and neurotransmitters. Certain diseases are known to be associated with specific amino acid profile in blood. For example, liver failure is characterized by lowered branched chain amino acids (BCAA) and elevated aromatic amino acids (AAA) in blood, and BCAA is used as a treatment for end-stage liver failure5). However, there are few reports on the blood amino acid profile in CHF. In addition, the changes in amino acid levels in CHF patients before and after exercise training have not been reported. According to the report of Aquilani et al.4), amino acid supplementation in CHF patients can be expected to increase the effect of exercise training in improving exercise capacity.
This study aimed to measure plasma levels of amino acids in CHF patients and compare with values of normal range. In addition the relationship between the change of exercise capacity and change of plasma levels of amino acids were examined, before and after CR.
SUBJECTS AND METHODS
Among the patients who participated in the three-month outpatient phase II cardiac rehabilitation (CR) program at the Sakakibara Heart Institute between March 2011 and January 2013, 20 patients who gave informed consent were enrolled in the present study. The subjects comprised 14 males and 6 females, aged 68 ± 12 (mean ± standard deviation) years. The underlying diseases were dilated cardiomyopathy in 8 patients, ischemic cardiomyopathy in 4 patients, valvulopathy in 3 patients, hypertensive heart failure in 1 patient, amyloidosis in 1 patient, and others in 3 patients. Left ventricular ejection fraction (LVEF) was 30.0 ± 12.3%. Phase II CR was conducted according to the Guidelines for Rehabilitation in Patients with Cardiovascular Disease1). The exercise program was created based on the program by American Heart Association6). This program was provided on an outpatient basis, consisting of one to three sessions per week and 15 to 30 min of aerobic exercise per session. The exercise intensity was set at the anaerobic threshold (AT) calculated from the cardiopulmonary exercise test (CPX) or at Borg scale 11 to 13. And also at home, patients exercised aerobic training, such as walking, with the same exercise intensity.
Nutritional guidance was conducted by a certified dietitian at the beginning and at the end of the phase II CR program. The contents of guidance included salt intake less than 6 g, while the energy intake was determined by calculating the standard body weight and considering the intensity of daily activity of the patient. Around the same time of nutritional guidance, nurses also provided guidance on methods of self-care and diseases management.
Patient background variables such as age, gender, body weight, body mass index (BMI), underlying disease, LVEF, early diastolic mitral valve flow to tissue annular velocity ratio (E/E’), and tricuspid regurgitation pressure gradient (TRPG) were extracted from the medical records. The following data were obtained from laboratory test results: hemoglobin, total protein, albumin, aspartate aminotransferase, alanine aminotransferase, creatinine kinase, blood urea nitrogen, creatinine, uric acid, blood sugar, triglyceride, total cholesterol, high density lipoprotein cholesterol, and N terminal pro brain natriuretic peptide (NT-pro BNP).
For the evaluation of exercise capacity, AT and peak oxygen uptake (VO2), work rate (WR), ∆VO2/WR, and the minute ventilation to carbon dioxide output (VE vs. VCO2) slope were calculated from the CPX. Then WR were measured both at AT and peak VO2.
For the evaluation of motor functions, leg strength, hand grip, and short physical performance battery (SPPB) were measured.
Blood sampling for amino acid analysis was done in the early morning when fasting. Immediately after collection, the blood samples were cooled to 4 °C. After centrifugation, 0.5 ml of plasma was collected and stored at −80 °C until assay. Blood samples were sent to Ajinomoto Institute for Innovation for amino acid analysis using a high speed amino acid analyzer by high performance liquid chromatography. The normal ranges of plasma amino acid levels provided by the laboratory7) were used as the normal ranges in this study.
For the evaluation of amino acid profile, 19 proteinogenic amino acids and 3 non-proteinogenic amino acids in blood samples were measured: glycine (Gly), alanine (Ala), serine (Ser), threonine (Thr), valine (Val), isoleucine (Ile), leucine (Leu), lysine (Lys), arginine (Arg), histidine (His), tyrosine (Tyr), phenylalanine (Phe), tryptophan (Trp), methionine (Met), cysteine (Cys), proline (Pro), glutamine (Gln), glutamic acid (Glu), asparagine acid asparagine (Asn), citrulline (Cit), ornithine (Orn), and taurine (Tau). In this study, Aspartic acid (Asp) was not measured at Ajinomoto Institute of Innovation for amino acid analysis.
Blood samples were collected and motor function was assessed in all the subjects at the beginning of the phase II CR program (pre-CR) and at the completion of the three-month program (post-CR).
CPX was conducted using a bicycle ergometer (0 W Load Strength Ergo 8; Fukuda Denshi, Tokyo, Japan). After 4 min of warming up at 20 W, a ramp protocol was implemented at increments of 10 W/min until limited by symptoms8). A gas analyzer (AE 300S; Minato Medical Science Co. Ltd., Osaka, Japan) was used to measure oxygen uptake by the breath-by-breath method. AT was determined using the V-slope method.
Leg strength was measured using a hand-held dynamometer (μTas F-1; Anima Corp., Tokyo, Japan). Measurement was made with the subject sitting at the edge of a chair, with the hip and knee joints positioned at 90°. Leg muscle strength was measured when the subject exerted maximum effort in knee extension for approximately 5 s, while instructions were given to the subject to avoid the Valsalva effect. The left leg and right leg were each measured two times, and the results were averaged and expressed as muscle strength (kg) per body weight (kg) ×100% for analysis9).
Hand grip was measured using a standard adjustable-handle dynamometer (JAMAR; Sammons Preston Rolyan, Canada). Measurement was made with the subject sitting at the edge of a chair, while instructions were given to the subject to avoid the Valsalva effect. The left hand and right hand were each measured two times, and the greater values were used for analysis10).
The SPPB is a tool for evaluating physical performance using balance tests (side-by-side stand, semi-tandem stand, and tandem stand), 4-meter walk, and repeated chair stands. This test has also been used in the field of cardiac diseases11). The SPPB was performed by physiotherapists. Each item of the SPPB was scored on a scale of 0 to 4. The total score for the three items (0 to 12) was used for analysis as the SPPB score.
All the data are expressed as mean ± SD. Changes in exercise capacity and changes in amino acid levels were compared using Wilcoxon sign-rank test. The rates of changes (∆) in exercise capacity and amino acid levels were calculated as follows: (post-CR level − pre-CR level)/pre-CR level, and the relationship between the two were examined by Spearman’s correlation coefficient. A p level less than 0.05 was considered significant. All statistical analyses were performed using SPSS 19.0 J (SPSS, Chicago, IL, USA).
The present study was approved by the Ethical Committee of the Sakakibara Heart Institute and carried out in compliance with the Declaration of Helsinki. All subjects gave informed consent after receiving explanations from the investigators regarding the objective and contents of the study and the handling of test results. Plasma samples were delivered to Ajinomoto Institute of Innovation for amino acid analysis, and all the samples were masked so that individual identification was not possible.
RESULTS
All subjects completed the phase II CR program with no occurrence of vascular event. The implementation rate of CPX was 60%. The correlation examined in 12 cases who CPX was implemented. Table 1 shows the changes in clinical parameters and in SPPB, Leg strength, Hand grip, and Table 2 shows the results of CPX before and after the phase II CR program. Upon completion of phase II CR, body weight and BMI increased significantly (p<0.01), and AT and peak VO2 also improved significantly (p<0.05).
Table 1. Clinical characteristics of patients.
| pre-CR | post-CR | |
|---|---|---|
| BW (kg) | 59.1 ± 15.0 | 62.0 ± 16.3* |
| BMI (kg/m2) | 22.4 ± 4.7 | 23.4 ± 5.1* |
| LVEF (%) | 30.0 ± 12.3 | 31.0 ± 11.6 |
| E/E’ | 20.0 ± 18.0 | 20.0 ± 14.1 |
| TRPG (mmHg) | 23.0 ± 8.1 | 25.0 ± 18.5 |
| Hb (g/dl) | 13.0 ± 2.9 | 12.9 ± 1.8 |
| TP (g/dl) | 7.1 ± 0.5 | 7.1 ± 0.5 |
| Alb (g/dl) | 4.5 ± 0.6 | 4.4 ± 0.6 |
| AST (U/l) | 24.4 ± 5.5 | 24.1 ± 5.3 |
| ALT (U/l) | 18.4 ± 8.7 | 17.2 ± 5.0 |
| CK (U/l) | 108.3 ± 107.5 | 114.7 ± 68.7 |
| BUN (mg/dl) | 19.3 ± 9.2 | 19.2 ± 10.1 |
| Cre (mg/dl) | 1.0 ± 0.4 | 1.0 ± 0.4 |
| UA (mg/dl) | 7.2 ± 1.5 | 7.0 ± 1.0 |
| BS (mg/dl) | 109.2 ± 26.4 | 104.0 ± 24.9 |
| TG (mg/dl) | 123.0 ± 79.9 | 158.0 ± 130.5 |
| T-cho (mg/dl) | 180.2 ± 46.9 | 177.9 ± 41.4 |
| HDL-cho (mg/dl) | 50.0 ± 12.5 | 53.7 ± 17.3 |
| NT-pro BNP (pg/ml) | 2,294.7 ± 2,310.7 | 2,553.2 ± 3,428.2 |
| SPPB (points) | 11.2 ± 1.4 | 11.2 ± 1.3 |
| Leg strength (%BW/kg) | 29.8 ± 11.6 | 28.5 ± 12.1 |
| Hand grip (kg) | 23.9 ± 7.1 | 24.3 ± 7.3 |
pre-CR: before phase II cardiac rehabilitation program; post-CR: after phase II cardiac rehabilitation program; BW: body weight; BMI: body mass index; LVEF: left ventricular ejection fraction; E/E’: early diastolic mitral valve flow to tissue annular velocity ratio; TRPG: tricuspid regurgitation pressure gradient; Hb: hemoglobin; TP: total protein; Alb: albumin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; CK: creatinine kinase; BUN: blood urea nitrogen; CK: creatinine; BUN: blood urea nitrogen; BS: blood sugar; TG: triglyceride; T-cho: total cholesterol; HDL-cho: high density lipoprotein cholesterol; NT-pro BNP: N terminal pro-brain natriuretic peptide; SPPB: short physical performance battery. Pre-CR and post-CR data were compared using Wilcoxon signed-rank test. *p<0.05. Values are means ± SD, n=12
Table 2. Results of cardiopulmonary exercise test.
| pre-CR | post-CR | |
|---|---|---|
| AT-WR (W) | 36.1 ± 18.0 | 40.0 ± 19.9 |
| AT (ml/min/kg) | 10.2 ± 2.5 | 11.2 ± 3.1* |
| peak WR (W) | 68.8 ± 22.6 | 76.3 ± 31.9 |
| peak VO2 (ml/min/kg) | 15.2 ± 4.8 | 17.1 ± 6.0* |
| VE vs. VCO2 slope | 35.9 ± 12.0 | 34.2 ± 11.8 |
| VO2/WR | 8.6 ± 2.4 | 8.6 ± 3.6 |
pre-CR: before phase II cardiac rehabilitation program; post-CR: after phase II cardiac rehabilitation program; AT: anaerobic threshold; WR: work rate; VO2: oxygen uptake; VE: minute ventilation; VCO2: carbon dioxide output. Pre-CR and post-CR data were compared using Wilcoxon signed-rank test. *p<0.05. Values are means ± SD, n=12
Table 3 shows the results of 19 proteinogenic amino acids level and 3 non-proteinogenic amino acids level. Among the amino acids examined, plasma Cys and Cit levels were higher than the normal ranges while Orn level was lower than the normal range, both before and after the CR program. On the other hand, in all the amino acids examined, no significant changes in plasma level were observed after phase II CR compared to before the program.
Table 3. Plasma amino acid level profile.
| pre-CR | post-CR | normal range | |
|---|---|---|---|
| Gly (nmol/ml) | 255.8 ± 89.7 | 251.6 ± 74.3 | 151.0–351.0 |
| Ala (nmol/ml) | 409.4 ± 111.6 | 404.6 ± 111.7 | 208.7–522.7 |
| Ser (nmol/ml) | 107.1 ± 24.2 | 105.1 ± 28.5 | 72.4–164.5 |
| Thr (nmol/ml) | 101.7 ± 19.4 | 103.0 ± 23.2 | 66.5–188.9 |
| Val (nmol/ml) | 240.3 ± 37.1 | 251.6 ± 47.3 | 147.8–307.0 |
| Ile (nmol/ml) | 71.7 ± 15.3 | 77.1 ± 20.9 | 43.0–112.8 |
| Leu (nmol/ml) | 125.8 ± 22.7 | 131.8 ± 29.1 | 76.6–171.3 |
| Lys (nmol/ml) | 197.1 ± 38.6 | 200.7 ± 37.9 | 108.7–242.2 |
| Arg (nmol/ml) | 99.6 ± 20.6 | 98.1 ± 23.5 | 53.6–133.6 |
| His (nmol/ml) | 72.9 ± 10.6 | 76.7 ± 10.8 | 59.0–92.0 |
| Tyr (nmol/ml) | 71.9 ± 12.9 | 70.6 ± 17.2 | 40.4–90.3 |
| Phe (nmol/ml) | 67.0 ± 10.3 | 67.8 ± 13.8 | 42.6–75.7 |
| Trp (nmol/ml) | 54.2 ± 9.8 | 58.4 ± 9.5 | 37.0–74.9 |
| Met (nmol/ml) | 24.6 ± 5.9 | 27.0 ± 5.8 | 18.9–40.5 |
| Cys (nmol/ml) | 66.4 ± 18.6 | 70.4 ± 22.8 | 13.7–28.3 |
| Pro (nmol/ml) | 186.6 ± 53.5 | 183.7 ± 70.0 | 77.8–272.7 |
| Gln (nmol/ml) | 622.1 ± 65.1 | 604.4 ± 74.8 | 422.1–703.8 |
| Glu (nmol/ml) | 50.7 ± 31.3 | 48.7 ± 22.5 | 12.6–62.5 |
| Asn (nmol/ml) | 45.6 ± 17.3 | 47.4 ± 15.7 | 44.7–96.8 |
| Cit (nmol/ml) | 45.2 ± 17.3 | 47.4 ± 15.7 | 17.1–42.6 |
| Orn (nmol/ml) | 24.6 ± 5.9 | 27.0 ± 5.8 | 31.3–104.7 |
| Tau (nmol/ml) | 54.8 ± 15.4 | 55.0 ± 14.6 | 39.5–93.2 |
pre-CR: before phase II cardiac rehabilitation program; post-CR: after phase II cardiac rehabilitation program; Gly: glycine; Ala: alanine; Ser: serine; Thr: threonine; Val: valine; Ile: isoleucine; Leu: leucine; Lys: lysine; Arg: arginine; His: histidine; Tyr: tyrosine; Phe: phenylalanine; Trp: tryptophan; Met: methionine; Cys: cysteine; Pro: proline; Gln: glutamine; Glu: glutamic acid; Asn: asparagine; Cit: citrulline; Orn: ornithine; Tau: taurine. Between pre-CR and post-CR by Wilcoxon signed-rank test. Values are means ± SD, n=12
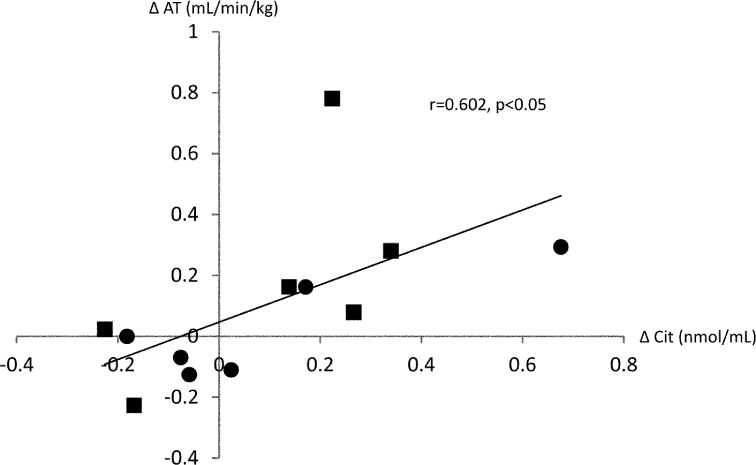
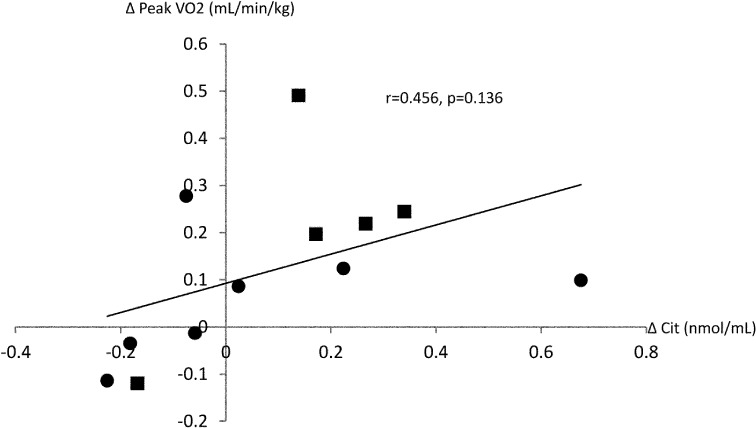
Table 4 shows the correlation between the rate of change in exercise capacity and rate of change in individual plasma amino acid level. The amino acid that showed a significant correlation with exercise capacity parameters were Cit, Ala and Pro. A significant positive correlation was observed between ∆Cit and ∆AT (r=0.602, p<0.05), between ∆Cit and ∆AT-WR (r=0.681, p<0.05), and between ∆Cit and ∆VO2/WR (r=0.635, p<0.05). In addition, a tendency of positive correlation was observed between ∆Cit and ∆peak VO2 (r=0.456, p=0.136). Figure 1 showed the correlation between ∆Cit and ∆AT. Data were showed divided into two groups in 9.4 which is the median value of AT at pre-CR. Figure 2 showed the correlation between ∆Cit and ∆peak VO2. As in the Fig.1, the data were showed divided into two groups in 14.
Table 4. Correlation between the rate of changes in plasma amino acid levels and changes in exercise capacity parameters after phase II cardiac rehabilitation.
| ∆ | peak VO2 | peak WR | AT | AT WR | VE vs. VCO2 slope | ΔVO2/WR |
|---|---|---|---|---|---|---|
| Gly | 0.171 | 0.338 | 0.117 | 0.094 | −0.061 | 0.149 |
| Ala | 0.228 | 0.068 | 0.591* | 0.552 | 0.452 | 0.438 |
| Ser | −0.117 | 0.267 | −0.644 | −0.581 | −0.321 | 0.144 |
| Thr | 0.063 | −0.155 | 0.434 | 0.308 | 0.46 | 0.617 |
| Val | 0.074 | −0.109 | −0.061 | −0.066 | 0.041 | 0.182 |
| Ile | −0.228 | −0.022 | −0.345 | −0.42 | −0.392 | −0.205 |
| Leu | −0.09 | 0.201 | −0.512 | −0.59 | −0.357 | 0.012 |
| Lys | 0.086 | 0.018 | −0.239 | −0.329 | 0.427 | 0.121 |
| Arg | 0.224 | 0.306 | −0.116 | −0.103 | 0.368 | 0.226 |
| His | −0.297 | −0.277 | 0.113 | −0.002 | −0.065 | −0.257 |
| Phe | −0.293 | 0.036 | −0.58 | −0.53 | −0.462 | −0.364 |
| Tyr | −0.067 | −0.206 | −0.077 | 0.074 | −0.007 | 0.171 |
| Trp | 0.139 | 0.397 | −0.557 | −0.531 | −0.144 | 0.175 |
| Met | −0.039 | 0.018 | −0.086 | −0.135 | −0.393 | −0.173 |
| Cys | 0.279 | 0.182 | −0.101 | −0.105 | −0.014 | 0.026 |
| Pro | 0.456 | 0.478 | 0.391 | 0.409 | 0.133 | 0.626* |
| Gln | −0.238 | −0.008 | −0.161 | −0.145 | 0.021 | 0.046 |
| Glu | 0.163 | 0.323 | 0.007 | 0.096 | −0.233 | 0.297 |
| Asn | 0.194 | 0.376 | −0.265 | −0.096 | 0.179 | 0.212 |
| Cit | 0.456 | 0.163 | 0.602* | 0.681* | 0.33 | 0.635* |
| Orn | −0.039 | 0.018 | −0.086 | −0.135 | −0.393 | −0.173 |
| Tau | 0.069 | −0.105 | −0.246 | −0.092 | 0.565 | −0.104 |
VO2: oxygen uptake; WR: work rate; AT: anaerobic threshold; VE: minute ventilation; VCO2: carbon dioxide output; Gly: glycine; Ala: alanine; Ser: serine; Thr: threonine; Val: valine; Ile: isoleucine; Leu: leucine; Lys: lysine; Arg: arginine; His: histidine; Tyr: tyrosine; Phe: phenylalanine; Trp: tryptophan; Met: methionine; Cys: cysteine; Pro: proline; Gln: glutamine; Glu: glutamic acid; Asn: asparagine acid; Cit: citrulline; Orn: ornithine; Tau: taurine. Correlation was evaluated using Spearman correlation coefficient. *p<0.05. Values are means ± SD, n=12
Fig. 1.
Correlation between the rate of change in citrulline level (∆Cit) and change in anaerobic threshold (∆AT) ■: less than median value (9.4), ●: 9.4 and more
Fig. 2.
Correlation between change in citrulline level (∆Cit) and change in peak oxygen uptake (∆peak VO2). ■: less than median value (14.1), ●: 14.1 and more
DISCUSSION
The subjects in the present study had baseline LVEF of 30% and NT-pro BNP of 2295 pg/mL, and CPX results showed AT of 2.9 metabolic equivalent of task (METs) and peak VO2 of 4.3 METs. These results indicate that the patients had moderate CHF equivalent to New York Heart Association (NYHA) class III12). Regarding motor functions, compared to the average leg strength of 31.2 kg reported for healthy persons of the same age group13), the average leg strength of 18.7 kg in this subjects was reduced. Especially, some elderly patients showed markedly reduced muscle strength and the implementation rate of CPX was 60%. Likewise, compared to the average hand grip reported for healthy persons of the same age group (35.4 kg in males, 22.8 kg in females)14), the hand grip of this subjects (mean 23.9 kg) was reduced. The above data indicate that due to the effects of old age and moderate or more severe heart failure, the subjects in the present study had markedly lowered exercise capacity and physical function.
When the effectiveness of phase II CR was assessed in these elderly patients with heart failure, AT increased significantly by 9.8% and peak VO2 by 12.5% (p<0.05). According to a systematic review, exercise training increased peak VO2 by 17% in patients with heart failure15). Although improvement of physical strength by exercise training was observed in the present study, the rate of increase in exercise capacity was lower than those in previous studies, probably due to the large number of patients with low physical strength. Witham et al.2) reported that exercise intervention did not improve exercise capacity in older patients with heart failure, and that in these patients, a comprehensive package combining exercise training with other behavioral interventions may be needed2).
Plasma amino acid analysis showed plasma levels higher than the normal ranges both before and after phase II CR in two amino acids only: Cys (pre-CR vs. post-CR: 66.4 ± 18.6 vs. 70.4 ± 22.8 nmol/ml; normal range 13.7–28.3) and Cit (45.2 ± 17.3 vs. 47.4 ± 15.7 nmol/ml; normal range 17.1–42.6). However, the differences were not significant, and no amino acid profile specific to CHF was observed. Cys and Cit have been reported to be elevated specifically in renal insufficiency16). The elevated Cys and Cit in the present study may be caused by the inclusion of patients with chronic renal diseases among the subjects. On the other hand, Orn was 24.6 ± 5.9 nmol/ml before and 27.0 ± 5.8 nmol/ml after the CR program, and were lower than the normal range (31.3–104.7). Previous study has reported that inducible NO synthase (iNOS) is induced in CHF17). Consequently Arg is consumed, which may lead to the relative decrease in Orn. However, the increase in NOS activates Arginase. These interaction may explain the mild decrease in Orn both before and after phase II CR, although the increases did not reach statistical significance.
Among previous studies that analyzed amino acids in heart diseases, Okamoto et al.18) studied patients who underwent cardiac surgery and reported decreases in BCAA and alanine and an increase in AAA as specific amino acid changes in patients who developed cardiac cachexia before cardiac surgery. In their study, cardiac cachexia was defined as NYHA class IV, concurrent tricuspid insufficiency with the mitral valve as main lesion, congested liver (four fingers’ width in right hypochondrium), and emaciation (80% or less of standard body weight), and many of their patients had severe CHF. The authors suggested that long-term catabolism caused by cachexia may cause breakdown of amino acid metabolism resulting in decreases in BCAA and alanine and increase in AAA. As mentioned above, the subjects in the present study had moderate CHF equivalent to NYHA class III and were capable of continuous aerobic exercise, which may explain why unlike the previous report, a specific amino acid profile in patients with CHF was not observed in this study.
In the present study, ∆Cit showed a positive correlation with ∆AT, ∆AT-WR, and ∆VO2/WR. Cit is one of the non-essential amino acids, and is known to be produced in the body when NOS catalyzes the enzymatic reaction of arginine via the L-arginine NO pathway to produce nitric oxide (NO) and citrulline19). NO acts on vascular endothelial cells to dilate peripheral blood vessels, and possesses pleiotropic functions such as improving skeletal muscle blood flow, lowering blood pressure, inhibiting platelet coagulation, and antioxidant effect. For this reason, the usefulness of Arg as a treatment for heart failure has been reported recently20).
Previous study in rats also showed that exercise training activated the L-arginine NO pathway, thereby increasing NO production and lowering blood pressure21). During this process, Cit is also increased in the body. In addition, it is a report that Cit, after a short exercise to target the healthy male, is mild upward trend22). Cit is present in abundance in watermelon, and is little affected by other foods and drugs. From these things, although not significantly different, the plasma Cit level in the present study showed an increase from 45.2 ± 17.3 nmol/ml before to 47.4 ± 15.7 nmol/ml after phase II CR, and was accompanied by improvement of exercise capacity. In addition, a significant positive correlation was observed between ∆Cit and ∆AT, as shown in Fig. 1. When data were divided into two groups by median of pre CR, the data have been shown with variation. It was suggested that there is a correlation between ΔAT and ΔCit regardless of exercise capacity before exercise. On the other hand, only a tendency of positive correlation was found between ∆Cit and ∆peak VO2, as shown in Fig. 2. The data were divided into two groups by median of pre CR as in Fig.1. This tendency of positive correlation between ∆Cit and ∆peak VO2 may be attributed to the low muscle strength that did not permit adequate implementation of CPX to symptom limitation, or beta error due to the small sample size. Nevertheless, the present findings suggest that Cit is associated with the change in exercise capacity by exercise training in CHF patients.
Because of the absence of a control group, it was impossible to study the effects of change in plasma Cit level on heart failure or whether higher Cit level synergistically improves exercise capacity achieved by exercise training. Hereafter, it is necessary to study comparative trial of the exercise training group vs no training group of CHF patients. Moreover, due to the small sample size, it was impossible to demonstrate the characteristic blood amino acid profile in patients with moderate CHF who are candidates for exercise training. In this study, because it was difficult to apply exercise load due to low physical strength by aging, there may not be enough to verify the effects of exercise.
Further studies are required to examine of evidence in the positive correlation between the ∆Cit and ∆AT.
A specific amino acid profile was not observed in this study, but a relationship between change in exercise capacity and change in plasma Cit level in CHF patients has been found, before and after phaseIICR.
Acknowledgments
We are grateful to Ajinomoto Pharmaceuticals Co., Ltd. for conducting amino acid analysis. We thank Mr. Masakazu Saitoh for cooperation in statistical analysis and drafting of the manuscript. This study was conducted jointly with Ajinomoto Pharmaceuticals Co., Ltd. All the blood samples were delivered to Ajinomoto Institute for Innovation for amino acid analysis. No financial supports including research grant were received from Ajinomoto Pharmaceuticals Co., Ltd.
REFERENCES
- 1.Guidelines for Rehabilitation in patients with cardiovascular disease (JCS 2012). http://www.j-circ.or.jp/guideline/pdf/JCS2012_nohara_h.pdf. [DOI] [PubMed]
- 2.Witham MD, Fulton RL, Greig CA, et al. : Efficacy and cost of an exercise program for functionally impaired older patients with heart failure: a randomized controlled trial. Circ Heart Fail, 2012, 5: 209–216. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi C, Carubelli V, Lazzarini V, et al. : Effects of oral amino acid supplements on functional capacity in patients with chronic heart failure. Clin Med Insights Cardiol, 2014, 8: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aquilani R, D’Antona G, Baiardi P, et al. : Essential amino acids and exercise tolerance in elderly muscle-depleted subjects with chronic diseases: a rehabilitation without rehabilitation? BioMed Res Int, 2014, 2014: 341603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muto Y, Sato S, Watanabe A, et al. Long-Term Survival Study Group: Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol, 2005, 3: 705–713. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher GF, Balady GJ, Amsterdam EA, et al. : Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation, 2001, 104: 1694–1740. [DOI] [PubMed] [Google Scholar]
- 7.http://www.srl.info/srlinfo/kensa_ref_CD/otherdata/57–0833.html.
- 8.Itoh H: Oxygen uptake: work rate relationship in patients with heart failure. In: Sato Y, et al (ed.), Integration of medical and sports sciences. Med Sport Sci. Basel: Karger, 1992, 37: pp 374–380. [Google Scholar]
- 9.Katoh M, Isozaki K, Sakanoue N, et al. : Reliability of isometric knee extension muscle strength measurement using a hand-held dynamometer with a belt: a study of test-retest reliability in healthy elderly subjects. J Phys Ther Sci, 2010, 22: 359–363. [Google Scholar]
- 10.Izawa KP, Watanabe S, Yokoyama H, et al. : Muscle strength in relation to disease severity in patients with congestive heart failure. Am J Phys Med Rehabil, 2007, 86: 893–900. [DOI] [PubMed] [Google Scholar]
- 11.Kawai K, Saitoh M, Ozawa T, et al. : Determinant factors of postoperative deterioration of lower extremity function in patients after elective cardiac surgery. JJCR, 2013, 18: 89–93(in Japanese). [Google Scholar]
- 12.The criteria committee of the New York Heart Association. Disease of the heart and vessels: nomenclature and criteria for diagnosis, 6th ed. Boston: Little Brown, 1964. [Google Scholar]
- 13.Katoh M, Isozaki K: Reliability of isometric knee extension muscle strength measurements of healthy elderly subjects made with a hand-held dynamometer and a belt. J Phys Ther Sci, 2014, 26: 1855–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seino S, Shinkai S, Fujiwara Y, et al. TMIG-LISA Research Group: Reference values and age and sex differences in physical performance measures for community-dwelling older Japanese: a pooled analysis of six cohort studies. PLoS One, 2014, 9: e99487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smart N, Marwick TH: Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med, 2004, 116: 693–706. [DOI] [PubMed] [Google Scholar]
- 16.Laidlaw SA, Berg RL, Kopple JD, et al. : Patterns of fasting plasma amino acid levels in chronic renal insufficiency: results from the feasibility phase of the Modification of Diet in Renal Disease Study. Am J Kidney Dis, 1994, 23: 504–513. [DOI] [PubMed] [Google Scholar]
- 17.Heusch P, Aker S, Boengler K, et al. : Increased inducible nitric oxide synthase and arginase II expression in heart failure: no net nitrite/nitrate production and protein S-nitrosylation. Am J Physiol Heart Circ Physiol, 2010, 299: H446–H453. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto K, Hashihira M, Ogino H, et al. : [The pathogenesis of cardiac cachexia–relationship between nutritional assessment and amino acid metabolism]. Nippon Kyobu Geka Gakkai Zasshi, 1987, 35: 810–817(in Japanese). [PubMed] [Google Scholar]
- 19.Tang WH, Shrestha K, Wang Z, et al. : Diminished global arginine bioavailability as a metabolic defect in chronic systolic heart failure. J Card Fail, 2013, 19: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilotra NA, Russell SD: Arginine vasopressin as a target in the treatment of acute heart failure. World J Cardiol, 2014, 6: 1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris MB, Mitchell BM, Sood SG, et al. : Increased nitric oxide synthase activity and Hsp90 association in skeletal muscle following chronic exercise. Eur J Appl Physiol, 2008, 104: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahlborg G, Felig P, Hagenfeldt L, et al. : Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest, 1974, 53: 1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]