Abstract
Conspecific aggression in outdoor-housed rhesus macaques (Macaca mulatta) at primate research facilities is a leading source of trauma and can potentially influence animal wellbeing and research quality. Although aggression between macaques is a normal part of daily social interactions, human presence might affect the frequency of various behaviors and instigate increases in conspecific aggression. We sought to determine how and which human management events affect conspecific aggression both immediately after an event and throughout the course of a day. From June 2008 through December 2009, we recorded agonistic encounters among macaques living in 7 social groups in large outdoor field cages. Behavioral data were then synchronized with specific management events (for example, feeding, enclosure cleaning, animal catching) that occurred within or near the enclosure. By using an Information Theoretical approach, 2 generalized linear mixed models were developed to estimate the effects of human management events on 1) aggression after individual management events and 2) daily levels of aggression. Univariate analysis revealed an increase in the rate of aggression after a management event occurred. The best predictor of aggression in a cage was the type of management event that occurred. Various factors including the number of daily management events, the total time of management events, the technicians involved, reproductive season, and their interactions also showed significant associations with daily aggression levels. Our findings demonstrate that human management events are associated with an increase in conspecific aggression between rhesus macaques and thus have implications regarding how humans manage primates in research facilities.
Abbreviation: AIC, Akaike Information Criterion
Trauma caused by conspecific aggression is a major source of morbidity at primate research facilities that house rhesus macaques in species-typical social groups.5,26,27,30 Although agonistic encounters in this species are a normal means of policing and maintaining social hierarchies,38 human presence is an important risk factor associated with increased aggression and subsequently can exacerbate trauma in NHP.10,16,19,22,29 For management purposes, primate research facilities require caretakers, research staff, and maintenance staff to enter primate enclosures; these events influence primate behavior, yet the consequences of these events on macaque agonistic behaviors are poorly understood.
Aggression in rhesus macaques (Macaca mulatta), which comprise the majority of NHP used in biomedical research, has long been studied and generally focused on group dynamics.7,12,36,37,44 Many factors affect the stability of and levels of aggression in primate groups, including age and sex composition, population density, and kinship structure.16,27,30 However, little research has been done on which human activities, interactions, or characteristics create the greatest risk for agonistic behavior. At the California National Primate Research Center, aggression-related trauma is often observed in animals living in large outdoor field cages, which each house 50 to 200 animals. Rhesus macaques develop complex matrilineal social hierarchies,33 the stability of which is maintained through social bonds, alliances, and conflict policing by high-ranking members.14 However, under captive conditions, conspecific aggression may occur frequently and can be prolonged by dominance displays, confinement, and the inability to escape attackers.25 The trauma caused by this aggression presents a management problem in facilities that house rhesus macaques in large social groups, with increased morbidity in less stable groups. 5,26,30
The primary objective of the current study was to evaluate the rate of agonistic behaviors relative to management events in the large outdoor social groups of macaques at our primate facility to determine 1) whether management events increased rates of conspecific aggression and 2) whether the frequency of management events affected overall aggression in these groups. We also determined which human-related factors were associated with increased frequency of aggression so that management techniques could be developed to minimize trauma and ultimately increase animal wellbeing at primate facilities.
Materials and Methods
Study site and animals.
The study subjects for this project were rhesus macaques (Macaca mulatta; age, 3 to 24 y [mean, 7 y]) housed in 7 social groups in large (0.2 ha; 60 m × 30 m) outdoor field cages at the California National Primate Research Center.4 Each cage contained 100 to 200 socially housed animals with the freedom to interact with each other for 24 h daily. Enclosure design across groups was similar, with chain-link walls and ceiling, a single cement feeding platform, multiple watering devices, A-frame shading structures, climbing structures, swings, perches, and gravel or grass substrate.
All groups were managed by a crew of animal care technicians that fed the macaques twice daily (0600 to 0700 and 1430 to 1530). All animals were fed a commercial monkey chow (Lab Diet 5047, PMI Nutrition, St Louis, MO) without restriction in feeding troughs at the feeding platform. Feed enrichment was provided almost every morning and consisted of a seed or fruit mixture scattered throughout the cage. The research was approved by the University of California–Davis IACUC (protocol no. 11843).
Study design.
Aggression data were collected on the macaques in each of the 7 social groups and compared with data on management events that were collected from those same groups over the same period of time. The design for this study has been published in 2 other related research projects studying factors influencing group stability.5,27 Briefly, data collection began in June 2008 and ended in December 2009. A total of 3 data collectors working in teams of 2 observed each social group for 6 h each day, 4 d each week, for 1 wk each month. Observations typically began at 0900 and ended at 1600, with a 1-h break at approximately 1200. The 2 observers used an event-sampling design to record all occurrences of agonistic interactions (interobserver agreement, 91% ± 3% [mean ± 1 SD; range, 86% to 94%], κ = 0.65, P = 0.0001 across 3 observers). Each observer was responsible for observing half of the group. For every agonistic event, observer initials, group ID, time, aggressor ID, recipient ID, and the severity of the agonistic encounter (rated on a scale of 1 to 8, with 1 representing simple threats, 4 representing a chase of less than 6 m, and 8 representing a bite for more than 5 s) were recorded. Further information regarding agonistic encounter severity is available.6
Management data were generated by the animal care staff, cage maintenance crew, and research staff when working in or around a cage. Staff members were asked to record the time they entered or approached the cage and the time they left the cage, as well as the procedure being performed and how many people were involved. Management events in this study fit 1 of 9 categories: morning health check, feeding, minor cleaning, major cleaning, animal catch, animal discharge, cage maintenance, enrichment, and approach cage (Figure 1). Events were linked to specific technicians, who initialed each event that they recorded. To provide anonymity, these initials were coded by number (for example, Technician 1) prior to analysis.
Figure 1.
Definitions of management events performed and recorded by technicians working in or around captive group housed rhesus macaques at the California National Primate Research Center from 2008 through 2009.
Analysis of individual management events.
Data were entered into a database (Access 2013, Microsoft, Redmond, WA). To study the effects of specific management activities on aggressive behavior, date and time stamps of behavioral observations were matched with the corresponding date and time stamps of management data. A management event was included in the analysis only when 30 min of observational data were available prior to and after the staff member entered or approached the cage. Agonistic encounters were counted during the 30-min intervals before and after the start of a management event. When available, agonistic encounters were also counted for the 30-min interval after an event ended (staff member exited the cage). However, when a management event lasted less than 5 min, agonistic encounters for the 30-min interval after the start of a management event were not counted, and only the 30-min intervals before and after the event were counted. This exception was made to buffer any differences in time keeping between technicians and observers and because 86.7% to 96.7% of the aggression counts during the 30-min interval after the management event began would actually be counts of aggression during the 30-min interval after the event ended. Time intervals for management events were noted as overlapping when they coincided with intervals of separate events that could affect behavior in the social group. For example, if 2 management events occurred within 45 min of each other, the 30-min interval after the first management event would overlap with the 30-min interval before the second management event, such that the 30-min interval before the second event potentially could be affected by the first event. However, in this example, the 30-min interval after the first management event would not be marked as overlap because it was unaffected by the second management event (that is, the interval occurred before a management event). Management events for which the reason was not noted by staff or for which the reason was inconsistent with a previously described management event were excluded from the data set. In addition, management events for which data were missing for one of the fixed effects used in analysis were excluded.
Data analysis was performed by using SPSS Statistics for Windows (version 22, IBM, Armonk, NY). To test for differences in rates of aggression related to individual management events, data were analyzed by using a generalized linear mixed repeated-measures model28 using Poisson distribution. The dependent variable was a group's total frequency of agonistic encounters during a 30-min period in relation to a management event, with repeated measures of the period 1) before the start of the event, 2) after the start of the event, or 3) after the end of the event. Fixed effects used in the model included reproductive season (which is known to affect rates of aggression in rhesus macaques40,42), the type of event that occurred, whether the interval overlapped with another event, the person who recorded the management event, the number of people involved in event, and the time of day at which the event occurred (morning compared with evening). Group ID was included in the model as a random effect. Associations between aggression counts and fixed effect variables were tested by using an Information Theoretical approach using Akaike Information Criteria (AIC).9 The simplest models (univariate and bivariate) were analyzed for goodness of fit and compared with the null model. Models with increased complexity were then assessed by using the best-fit simple models and remaining fixed effects. If model fit did not improve as model complexity increased, model building ceased, to prevent spurious results. Two-way interactions that were thought to be informative regarding animal management were evaluated by using the same criteria for the best-fit multivariate models only. For example, because reproductive season might have affected the way management events were performed, their interaction was considered. After all models were considered, the model with the lowest AIC was chosen for best fit.
Analysis of total daily aggression.
The total number of agonistic encounters occurring in each group was counted daily (that is, total daily aggression) and evaluated with the number of management events occurring each day. Only complete days of behavioral data (starting at 0900 and ending at 1600) and management events for those days were analyzed (total of 192 d). Feeding and morning health events were not counted in the total management events count because these occurred daily. Management counts were placed into 4 categories: no management events (not including feeding and morning health), one management event, 2 or 3 management events, and 4 or more management events. The total time spent in each cage was categorized as follows: short period of time (less than 1 h), moderate period of time (1 to 3 h), or long period of time (greater than 3 h).
Counts of total daily aggression were analyzed by using a generalized linear mixed model28 and Poisson distribution. The dependent variable was total daily aggression, and fixed effects included reproductive season and total extra management events (total number of management events minus feeding and morning health). During individual management analysis, a relationship between aggression and the presence of Technician 2 appeared, so the presence of this individual was evaluated in the multivariable daily aggression model as a fixed effect. Total amount of time spent in a cage by technicians was added to the multivariate model as a potential confounding factor. Group ID was included in the model as a random effect. Similarly to individual management event analysis, associations between aggression counts and fixed effect variables were tested by using an Information Theoretical approach and AIC to select the best fit model.9 Contrasts of interactions related to management events and aggression were evaluated by using pairwise comparisons and a Bonferroni-corrected significance level of 0.05.
Results
Individual management event model.
Pairwise comparisons of the 30-min intervals showed no significant difference (t802 = 1.431, P = 0.153) between the rates of aggression that occurred 30 min before a management event and the 30 min after the management event began. However, aggression rates in the 30 min after humans exited or left the cage were significantly higher than during the 30 min before a management event (t802 = 8.529, P < 0.0005) and the 30 min after starting a management event (t802 = 6.740, P < 0.0005; Figure 2).
Figure 2.
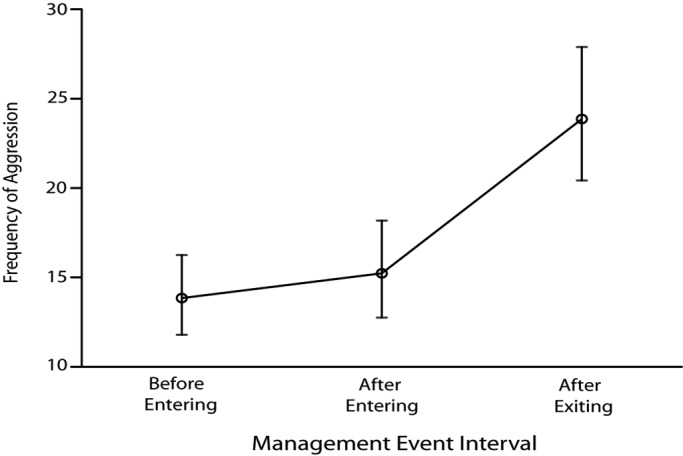
Estimated marginal means and 95% confidence intervals of conspecific aggression in rhesus macaques 30 min prior to a human entering the enclosure, after entering the enclosure, and after departure from the enclosure during a management event.
Because aggression during feeding may be related to competition1,8,32 rather than human intervention, similar analysis was run without including feeding as a management event. Again, pairwise comparisons of the 30-min intervals showed no significant difference (t453 = 0.451, P = 0.318) between the rates of aggression that occurred 30 min before a management event and the 30 min after the management event began. However, aggression rates in the 30 min after humans exited or left the cage were significantly higher than during the 30 min before a management event (t453 = 1.974, P < 0.0005) and the 30 min after starting a management event (t453 = 1.523, P = 0.006).
The model that best predicted aggression as it related to management events (based on an information theoretical approach to model selection) was the univariate model for type of management event (F8 = 1.986, P = 0.046, AIC = 1701.829; when compared with null (AIC = 1745.361; Table 1). All other models with a similar fit to the best-fit model contained the variable and did not improve model fit and therefore are not reported.
Table 1.
Best-fit linear mixed model based on AIC of agonistic encounters between rhesus macaques living in group-housed outdoor field cages as a result of individual management events performed by technicians
| 95% CI |
|||||||
| Coefficient | 1 SD | t | P | Lower limit | Upper limit | ||
| Intercept | 2.905 | 0.070 | 41.80 | <0.0005 | 2.769 | 3.042 | |
| Management event | |||||||
| Animal catch | −0.245 | 0.092 | −2.649 | 0.008 | −0.427 | −0.063 | |
| Approach cage | −0.131 | 0.069 | −1.914 | 0.056 | −0.266 | 0.003 | |
| Cage maintenance | −0.270 | 0.204 | −1.323 | 0.186 | −0.670 | 0.130 | |
| Minor cleaning | −0.147 | 0.087 | −1.688 | 0.092 | −0.318 | 0.024 | |
| Major cleaning | −0.330 | 0.187 | −1.761 | 0.079 | −0.697 | 0.038 | |
| Discharge | −0.142 | 0.118 | −1.200 | 0.231 | −0.374 | 0.090 | |
| Enrichment | −0.056 | 0.234 | −0.238 | 0.812 | −0.514 | 0.403 | |
| Morning health check | −0.252 | 0.102 | −2.470 | 0.014 | −0.452 | −0.052 | |
The baseline for this model is a cage during a feeding management event.
Total daily aggression.
Compared with the null model (AIC = 2044.109), the best-fit model for daily aggression (AIC = 1561.822) included the fixed effects reproductive season (F1,170 = 2.574, P = 0.110), the total number of extra events that occurred in the day (F3,170 = 18.768, P < 0.005), the total amount of time spent in cage (F2,170 = 22.873, P < 0.005), and whether Technician 2 was involved in events in the cage that day (F1,170 = 39.978, P < 0.005). All 2-way interactions were included in the model with the exception of the interaction between Technician 2 and reproductive season: reproductive season × extra events (F3,170 = 6.224, P < 0.005), reproductive season × time in cage (F2,170 = 12.610, P < 0.005), extra events × time in cage (F4,170 = 11.819, P < 0.005), extra events × Technician 2 presence (F3,170 = 6.154, P = 0.001), and time in cage × Technician 2 presence (F2,170 = 4.215, P = 0.016; Table 2).
Table 2.
Best-fit linear mixed model based on AIC of the total daily agonistic encounters between rhesus macaques in group-housed field cages as a result of human interactions and reproductive season
| 95% CI |
|||||||
| Coefficient | 1 SD | t | P | Lower limit | Upper limit | ||
| Intercept | 5.163 | 0.069 | 74.912 | <0.0005 | 5.027 | 5.299 | |
| Reproductive season | |||||||
| Breeding | −0.023 | −0.023 | −0.991 | 0.323 | −0.068 | 0.023 | |
| Extra management events (EME) | |||||||
| 1 | −0.023 | 0.022 | −1.074 | 0.284 | −0.067 | 0.020 | |
| 2 or 3 | −0.056 | 0.036 | −1.536 | 0.126 | −0.127 | 0.016 | |
| 4 or more | 0.125 | 0.125 | 2.427 | 0.016 | 0.057 | 0.551 | |
| Total time in cage (TIC) | |||||||
| 1–3 h | −0.463 | 0.114 | −4.074 | <0.0005 | −0.687 | −0.239 | |
| More than 3 h | −0.195 | 0.052 | −3.752 | <0.0005 | −0.297 | −0.092 | |
| Technician 2 | |||||||
| Present | 0.330 | 0.029 | 11.261 | <0.0005 | 0.272 | 0.388 | |
| Reproductive season × EME (Figure 3) | |||||||
| Breeding season and 1 EME | 0.114 | 0.035 | 3.276 | 0.001 | 0.045 | 0.183 | |
| Breeding season and 2 or 3 EME | 0.176 | 0.044 | 4.035 | <0.0005 | 0.090 | 0.263 | |
| Breeding season and ≥ 4 EME | 0.204 | 0.069 | 2.956 | 0.004 | 0.068 | 0.339 | |
| Reproductive season × TIC (Figure 5) | |||||||
| Breeding season and 1-3 h TIC | 0.041 | 0.039 | 1.015 | 0.295 | −0.036 | 0.117 | |
| Breeding season and >3 h TIC | −0.248 | 0.062 | −4.024 | <0.0005 | −0.369 | −0.126 | |
| Extra management events × TIC (Figure 4) | |||||||
| 1 EME and 1–3 h TIC | 0.346 | 0.111 | 3.104 | 0.002 | 0.126 | 0.566 | |
| Extra management events × TIC (Figure 4) | |||||||
| 2 or 3 EME and 1–3 h TIC | 0.381 | 0.111 | 3.413 | 0.001 | 0.160 | 0.601 | |
| 2 or 3 EME and >3 h TIC | 0.377 | 0.067 | 5.610 | <0.0005 | 0.244 | 0.510 | |
| Extra management events × TIC (Figure 4) | |||||||
| ≥4 EME and >3 h TIC | 0.022 | 0.127 | 0.171 | 0.865 | −0.228 | 0.271 | |
| Technician 2 present × TIC (Figure 6) | |||||||
| Technician 2 present and 1-3 h TIC | −0.073 | 0.041 | −1.769 | 0.079 | −0.155 | 0.009 | |
| Technician 2 present and >3 h TIC | −0.209 | 0.072 | −2.881 | 0.004 | −0.351 | −0.066 | |
| Technician 2 present × EME (Figure 7) | |||||||
| Technician 2 present and 1 EME | −0.160 | 0.042 | −3.849 | <0.0005 | −0.242 | −0.078 | |
| Technician 2 present and 2 or 3 EME | −0.034 | 0.047 | −0.717 | 0.474 | −0.127 | 0.059 | |
| Technician 2 present and ≥4 EME | −0.101 | 0.065 | −1.552 | 0.123 | −0.228 | 0.027 | |
The baseline for this model is a cage during birthing season with no extra management events, total time in cage less than 1 h, and without technician 2 present.
Extra management events.
A general trend of increasing daily aggression was noted as the number of extra management events during the day increased. Pairwise comparisons of interaction terms were conducted by using estimated marginal means. During birthing season, aggression rates were significantly greater (t170 = 4.034, P < 0.0005) when 2 or 3 extra management events occurred in a day as compared with one extra event. Results were more pronounced during breeding season, with significantly higher aggression rates (t170 = 4.660, P < 0.0005) when 2 or 3 extra events occurred in a day as compared with no extra management events. The rate of aggression was also significantly higher (t170 = 7.281, P < 0.0005) when 2 or 3 extra events occurred during the day compared with one extra management event. In addition, daily aggression was significantly higher when 4 or more extra events occurred in a day as compared with no (t170 = 3.464, P = 0.002) and 1 (t170 = 5.120, P < 0.0005) extra events. There was no significant difference in aggression between one extra event and no extra events for either the birthing (t170 = –0.067, P = 0.947) or breeding (t170 = 0.348, P = 0.728) season (Figure 3).
Figure 3.
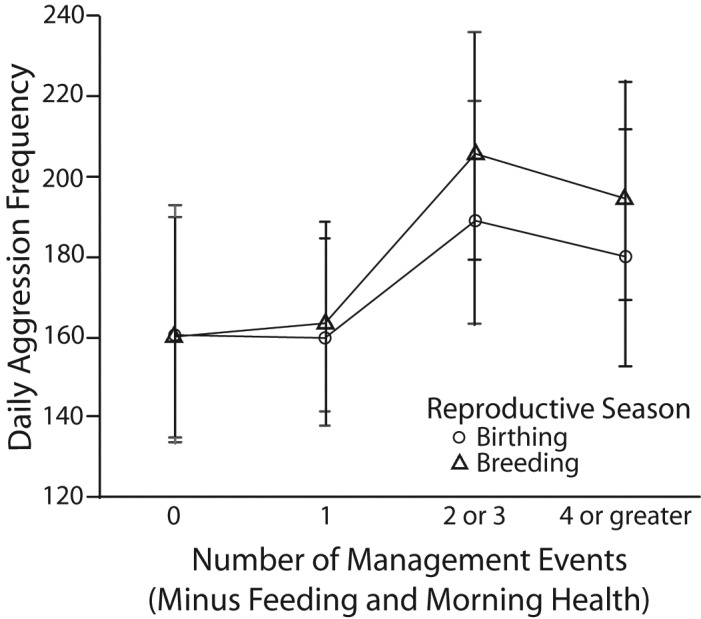
Estimated marginal means and 95% confidence intervals of daily aggression in rhesus macaques during birthing and breeding season in regard to whether 0, 1, 2 or 3, or 4 or more management events occurred in an enclosure (not including feeding and morning health check).
Similar effects were observed for the interaction of extra management events and the total amount of time spent in or around a cage (Figure 4). When a short period of time was spent in a cage, there was no significant difference in aggression between when one extra management event occurred and 2 or 3 extra events (t170 = 2.175, P = 0.062) or no extra events (t170 = 2.385, P = 0.055) occurred. There were no days where 4 or more extra management events occurred and a short period of time was spent in or around a cage. When a moderate period of time was spent in a cage, there was a significantly higher aggression rate when 4 or more management events occurred as compared with no extra events (t170 = 3.390, P = 0.004). There was also a significant difference in aggression when 2 to 3 extra management events was compared with 1 (t170 = 3.202, P = 0.007) or no (t170 = 4.082, P < 0.0005) extra management events. Finally, aggression rates were significantly greater when one extra management event occurred compared with no extra management events (t170 = 3.003, P = 0.009).
Figure 4.
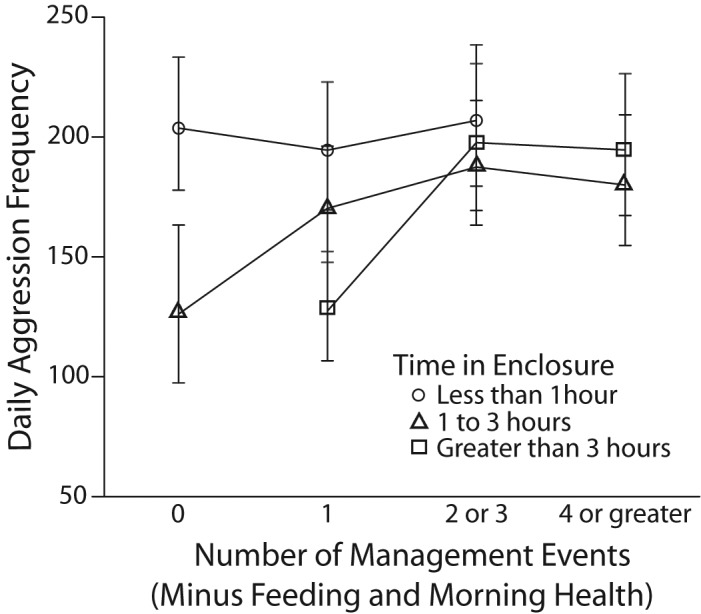
Estimated marginal means and 95% confidence intervals of daily aggression in rhesus macaques in regard to the total amount of time spent in an enclosure by technicians and the whether 0, 1, 2 or 3, or 4 or more management events occurred in an enclosure (not including feeding or morning health check).
When humans spent a long period of time in a cage, daily aggression was significantly greater when 4 or more management events occurred as compared with one extra event (t170 = 6.568, P < 0.0005) and when 2 or 3 extra management events occurred as compared with one extra management event (t170 = 6.385, P < 0.0005). There were no days when a long period of time was spent in or around a cage and no extra management events occurred.
Time spent in cage.
Descriptive statistics for the amount of time spent in a cage per management event can be observed in Table 3. Daily aggression showed a decreasing trend as the daily amount of time spent in or around a cage increased. Comparisons of time spent in the cage showed that during birthing season, when a short period of time was spent in a cage, aggression was significantly greater than when a moderate period of time was spent in a cage (t170 = 5.240, P < 0.0005) but not when a long period of time was spent in a cage (t170 = 1.803, P = 0.073). However, aggression rates were significantly less when a moderate period of time was spent in a cage as compared with a long period of time (t170 = 2.643, P = 0.018). During breeding season, the less time spent in the cage, the greater the aggression counts. Significantly more aggression occurred when a short period of time was spent in or around a cage compared with the moderate category (t170 = 5.522, P < 0.005) and the long category (t170 = 7.082, P < 0.0005) and when a moderate period of time was compared with the long period of time (t170 = 2.000, P = 0.047; Figure 5).
Table 3.
Descriptive statistics of time (min:s) spent in cage per management event performed and recorded by technicians working in or around captive group-housed rhesus macaque cages at the California National Primate Research Center from 2008 through 2009
| n | Mean | 1 SD | Minimum | Maximum | |
| Morning health checka | 21 | 21:46 | 28:18 | 0:05 | 2:18 |
| Feedinga | 150 | 01:18 | 03:25 | <0:01 | 0:39 |
| Minor cleaning | 28 | 32:51 | 22:20 | 0:05 | 1:30 |
| Major cleaninga | 5 | 34:12 | 17:28 | 0:08 | 0:50 |
| Animal catcha | 27 | 11:22 | 08:01 | 0:01 | 0:33 |
| Animal discharge | 22 | 03:43 | 04:56 | 0:01 | 0:22 |
| Cage maintenance | 5 | 28:12 | 24:24 | 0:01 | 0:55 |
| Enrichment | 3 | 17:00 | 17:05 | 0:01 | 0:35 |
| Approach cage | 58 | 45:22 | 45:47 | 0:01 | 3:51 |
Some occurrences removed from analysis due to missing data for time leaving the cage.
Figure 5.
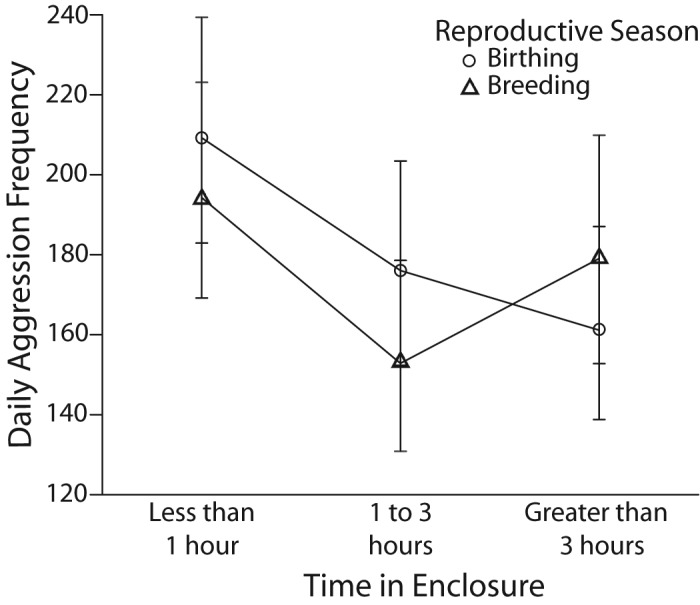
Estimated marginal means and 95% confidence intervals of daily aggression in rhesus macaques during birthing and breeding seasons in regard to the total amount of time spent in an enclosure by technicians.
When no extra events occurred in a cage, daily aggression was significantly greater when a short period of time was spent in the cage as compared with a moderate period of time (t170 = 5.109, P < 0.0005). When a single extra event occurred in a cage, aggression was significantly greater when a short period of time was spent in the cage as compared with a moderate period (t170 = 4.834, P < 0.0005) and a long period of time (t170 = 7.428, P < 0.0005). In addition, the aggression rate was significantly greater when a moderate period of time was spent in a cage as compared with a long period of time (t170 = 5.084, P < 0.0005). When 2 or 3 extra events occurred in a cage, aggression was greater when a short period of time was spent in a cage as compared with a moderate period of time (t170 = 3.349, P = 0.003). Finally, when 4 or more events occurred in a cage, there was no significant difference in aggression between a moderate period and a long period of time spent in or around a cage (t170 = 1.696, P = 0.092; Figure 4).
Technician 2.
Due to the effect found for Technician 2 in the individual event model, the presence of Technician 2 was analyzed to see whether daily aggression rates were affected. Technician 2 appeared to have a general increasing effect on aggression within animal groups. On days where the total amount of time spent in a cage by technicians was short, the presence of Technician 2 significantly increased aggression in those cages (t170 = 8.489, P < 0.0005). Also, when a moderate amount of time was spent in a cage by technicians, the presence of Technician 2 significantly increased aggression (t170 = 5.181, P < 0.0005). Technician 2 had no significant effect on aggression when technicians spent a long period of time in a cage during the day (t170 = 0.384, P = 0.701; Figure 6).
Figure 6.
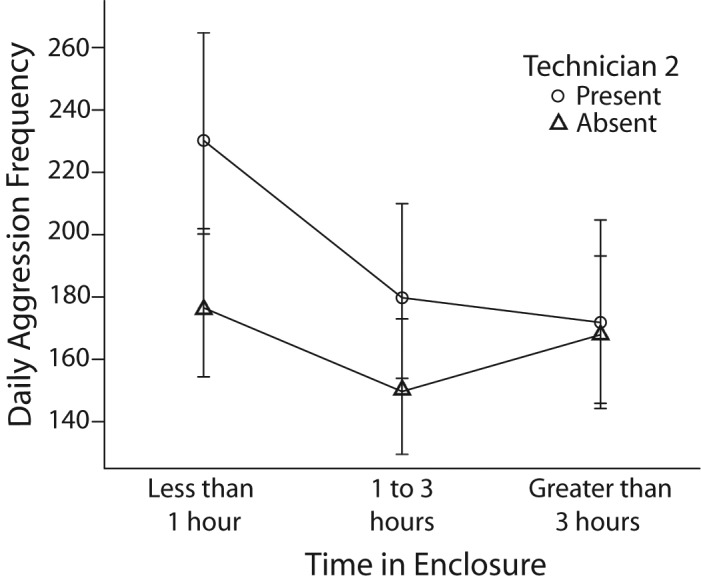
Estimated marginal means and 95% confidence intervals of daily aggression in rhesus macaques in regard to the total amount of time spent in an enclosure by technicians and whether Technician 2 was present.
Even when daily management events were at their lowest (that is, no extra management events), there was a significant increase in aggression when Technician 2 was present (t170 = 5.977, P < 0.0005). When 2 or 3 extra management events occurred in a day, the presence of Technician 2 during some of those events caused a significant increase in aggression (t170 = 5.582, P < 0.0005) as well. There were no significant increases in aggression when Technician 2 was present on days when one extra management occurred (t170 =1.798, P = 0.074) or 4 or more extra management events occurred (t170 = 1.890, P = 0.060; Figure 7).
Figure 7.
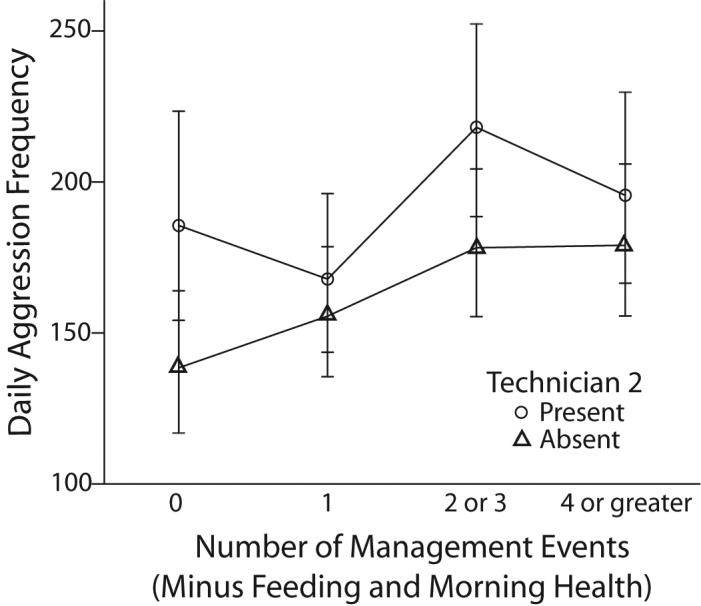
Estimated marginal means and 95% confidence intervals of daily aggression in rhesus macaques in regard to whether 0, 1, 2 or 3, or 4 or more management events occurred in an enclosure (not including feeding or morning health check) and whether Technician 2 was present.
Discussion
Comparisons between management data and behavioral observations in this study provided insight into the effects that human management events have on captive rhesus macaque aggression. Initial univariable analysis showed a significant increase in conspecific aggression almost immediately after a management event occurred. In addition, as extra management events increased, so did agonistic encounters, indicating that human activity affected aggressive behaviors in captive rhesus macaques. Past research has shown that the effects of human presence in captivity may have deleterious effects on primate behavior in a variety of species and settings. For example, levels of wounding in research chimpanzees were higher on weekdays when human activity was greater than on weekends.19,22 Furthermore, large numbers of visitors to primate zoo exhibits have been associated with increased aggression and stereotypical behavior in multiple NHP species, including cotton-top tamarins, Diana monkeys, ring-tailed lemurs, mandrills.10 In addition, mangabeys moved from cages with low visitor densities to high visitor densities showed increased intragroup aggression.29
The type of management event appeared to be a significant predictor of aggression, with feedings being significantly greater predictor of conspecific aggression than were animal catch and morning health check. One possible reason for this effect may be that placing resources in a concentrated area (such as a feeding platform) caused animal concentrations to increase, such that feeding competition, resource guarding, and displacement behaviors became more prominent.1,8,24,31,43 This concentration of resources in a single area such as a feeding platform is typical in primate research facilities.
Although increased encounters with humans predicted an increase in overall daily aggression, the amount of time spent in or around an enclosure was inversely related to the amount of aggression in that enclosure during the day. That is, as the amount of time humans spent in a cage increased, rates of conspecific aggression decreased. If aggression increases only after a management event concludes and this aggression rate persists throughout the rest of the day, then it makes sense that total aggression counts would be lower when more time is spent in the cage. However, why isn't this trend seen on days with many events, and why is there no increase in aggression during a management event? One explanation may be that events that take less time, such as discharging macaques back into the cage and animal catching, are likely more stressful and disruptive to a group's social structure than are events that take more time, such as cage cleaning and maintenance, during which humans do not directly interact with the animals. In squirrel monkeys, management events such as the introduction or removal of animals in social groups were associated with increases in aggression.41 The stress associated with capturing a macaque for removal or replacement into a social group may induce redirected aggression, where arousal or aggression elicited by one individual is directed toward another.44 Redirected and displaced behaviors are commonly seen in macaques and are perceived as a response to anxiety and stress caused by conspecifics.23,39,44 In addition, these events might have caused changes in social structure, which in turn can affect overall cage stability in captive rhesus macaques. 4,5,30 However, no significant differences were seen between specific management events in the individual management event model. This result may be due to small sample sizes of individual management events or too short of an interval to capture these effects, both of which can be addressed in future studies.
Another possible explanation for the decreased aggression as time spent in or around the cage increased is that the presence of humans actually prevented aggression. Possible explanations for why humans might have decreased conspecific aggression include a focus on the humans rather than on conspecifics, strengthening of alliances in the presence of an outsider, or increased policing by the human individual. Multiple studies of primate species in zoo environments have shown either no difference or a decrease in conspecific behavior as visitor density increases, possibly due to focus on the human individuals.13,15,18,20 In addition, relationships between animals and their caretakers in the laboratory environment can develop,3,11 and prolonged human presence may positively affect NHP behavior, possibly due to past positive reinforcement such as feeding and enrichment.
An unexpected finding of this study was the effect that specific humans had on conspecific primate aggression within groups. Rates of aggression, both for individual management events and on a daily basis, were higher when Technician 2 was present compared with other staff members. The characteristics, actions, and circumstances that caused these increases in aggression are not known. Studies on the behaviors and attributes of daily caretakers may provide insight into the specific characteristics that promote aggression in the animals that they care for.
The results of this study demonstrate that management activities as well as individual human characteristics increase conspecific aggression between macaques and that highly stimulating events (for example, feeding, discharge, animal catch) or people (for example, Technician 2) appear to correlate with this aggression. One likely explanation is that this increased stimulation is a source of anxiety and stress for the animals, leading to an increase in agonistic behavior. Stress has been correlated with aggression in many species of NHP.2,17,21,34,35 For indoor-housed macaques, decreased predictability of management events can increase physiologic markers of stress.16 Because only 3 events occurred consistently on a daily basis (2 feedings and morning health check) in these cages in the present study, the number of daily management events directly correlated with the number of spontaneous management events. Further research is indicated to determine whether spontaneous and stressful events are determining factors for overall group aggression.
Another important factor that predicted aggression in rhesus monkeys in our study was reproductive season. There was a significant increase in aggression during the breeding season as compared with birthing season. This result is consistent with findings in previous studies on breeding behavior in rhesus macaque.40,42
Because the collection of aggression and management data were performed by separate groups of personnel (observers compared with technicians), data sets did not completely overlap. Behavioral observations began at 0900, whereas morning feedings (the first management event of the day) occurred at 0600 to 0700. This situation decreased the usable sample size for specific management events, and therefore significant differences between management events could not be assessed. Future research on the effect of various management events should schedule observations at least 30 min prior to the first management of the day to get a complete picture of how aggression changes throughout the day.
In this study, we demonstrated that human management events at research facilities that house rhesus macaques affect levels conspecific aggression. This information is important from both a management perspective and an animal-wellbeing perspective. Continued research into the assessment of feeding techniques is indicated to minimize daily trauma rates. In-depth analysis of other management techniques is also warranted to assess and either modify or eliminate unnecessary stressful events. In addition, assessment of human characteristics and behaviors that increase primate aggression might be fruitful in determining how to approach and interact with primate groups. By better understanding these factors, personnel can take steps to prevent unnecessary trauma, decrease costs to the facility, promote good research practices, and provide a healthy home environment for the animals that live at the facility.
Acknowledgments
We thank Dr Eliza Bliss-Moreau, Gilda Moadab, Dr Mary Christopher, and the students of the MPVM program for editing assistance. We also thank the animal care staff and staff research associates at the California National Primate Research Center for providing research support for the project.
References
- 1.Balasubramaniam KN, Dunayer ES, Gilhooly LJ, Rosenfield KA, Berman CM. 2014. Group size, contest competition, and social structure in Cayo Santiago rhesus macaques. Behaviour 151:1759–1798. [Google Scholar]
- 2.Barrett GM, Shimizu K, Bardi M, Asaba S, Mori A. 2002. Endocrine correlates of rank, reproduction, and female-directed aggression in male Japanese macaques (Macaca fuscata). Horm Behav 42:85–96. [DOI] [PubMed] [Google Scholar]
- 3.Bayne K. 2002. Development of the human–research animal bond and its impact on animal wellbeing. ILAR J 43:4–9. [DOI] [PubMed] [Google Scholar]
- 4.Beisner BA, Isbell LA. 2011. Factors affecting aggression among females in captive groups of rhesus macaques (Macaca mulatta). Am J Primatol 73:1152–1159. [DOI] [PubMed] [Google Scholar]
- 5.Beisner BA, Jackson ME, Cameron A, McCowan B. 2012. Sex ratio, conflict dynamics, and wounding in rhesus macaques (Macaca mulatta). Appl Anim Behav Sci 137:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beisner BA, Jackson ME, Cameron AN, McCowan B. 2011. Detecting instability in animal social networks: genetic fragmentation is associated with social instability in rhesus macaques. PLoS One 6:e16365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein I, Williams L, Ramsay M. 1983. The expression of aggression in Old World monkeys. Int J Primatol 4:113–125. [Google Scholar]
- 8.Blois-Heulin C, Martinez-Cruz B. 2004. Influence of food dispersion on feeding activity and social interactions in captive Lophocebus albigena and Cercocebus torquatus torquatus. Primates 46:77–90. [DOI] [PubMed] [Google Scholar]
- 9.Burnham KP, Anderson DR.2002. Model selection and multimodel inference: a practical information-theoretic approach. New York (NY): Springer. [Google Scholar]
- 10.Chamove AS, Hosey GR, Schaetzel P. 1988. Visitors excite primates in zoos. Zoo Biol 7:359–369. [Google Scholar]
- 11.Chang FT, Hard LA. 2002. Human–animal bonds in the laboratory: how animal behavior affects the perspective of caregivers. ILAR J 43:10–18. [DOI] [PubMed] [Google Scholar]
- 12.Cross HA, Harlow HF. 1965. Prolonged and progressive effects of partial isolation on the behavior of macaque monkeys. Journal of experimental research in personality 1:39–49. [Google Scholar]
- 13.Fa J. 1989. Influence of people on the behavior of display primates. p 270–290. In: Segal EF.Housing, care, and psychologic wellbeing of captive and laboratory primates. New York (NY): Noyes Publications. [Google Scholar]
- 14.Flack JC, de Waal FB, Krakauer DC. 2005. Social structure, robustness, and policing cost in a cognitively sophisticated species. Am Nat 165:E126–E139. [DOI] [PubMed] [Google Scholar]
- 15.Glatston AR, Geilvoet-Soeteman E, Hora-Pecek E, Van Hooff J. 1984. The influence of the zoo environment on social behavior of groups of cotton-topped tamarins, Saguinus oedipus oedipus. Zoo Biol 3:241–253. [Google Scholar]
- 16.Gottlieb DH, Coleman K, McCowan B. 2013. The effects of predictability in daily husbandry routines on captive rhesus macaques (Macaca mulatta). Appl Anim Behav Sci 143:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honess P, Marin C. 2006. Behavioural and physiologic aspects of stress and aggression in nonhuman primates. Neurosci Biobehav Rev 30:390–412. [DOI] [PubMed] [Google Scholar]
- 18.Hosey G, Melfi V. 2014. Are we ignoring neutral and negative human–animal relationships in zoos? Zoo Biol 34:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Hosey GR. 2005. How does the zoo environment affect the behaviour of captive primates? Appl Anim Behav Sci 90:107–129. [Google Scholar]
- 20.Hosey GR, Druck PL. 1987. The influence of zoo visitors on the behaviour of captive primates. Appl Anim Behav Sci 18:19–29. [Google Scholar]
- 21.Keverne E, Meller RE, Martinez-Arias A. 1978. Dominance, aggression, and sexual behaviour in social groups of talapoin monkeys. p 533–547. In: Herbert J, Chivers D.Recent advances in primatology, vol 1. New York (NY) Academic Press. [Google Scholar]
- 22.Lambeth SP, Bloomsmith MA, Alford PL. 1997. Effects of human activity on chimpanzee wounding. Zoo Biol 16:327–333. [Google Scholar]
- 23.Maestripieri D. 1993. Maternal anxiety in rhesus macaques (Macaca mulatta). Ethology 95:19–31. [Google Scholar]
- 24.Mathy JW, Isbell LA. 2001. The relative importance of size of food and interfood distance in eliciting aggression in captive rhesus macaques (Macaca mulatta). Folia Primatol (Basel) 72:268–277. [DOI] [PubMed] [Google Scholar]
- 25.McCowan B, Anderson K, Heagarty A, Cameron A. 2008. Utility of social network analysis for primate behavioral management and wellbeing. Appl Anim Behav Sci 109:396–405. [Google Scholar]
- 26.McCowan B, Anderson K, Heagarty A, Cameron A. 2008. Utility of social network analysis for primate behavioral management and wellbeing. Appl Anim Behav Sci 109:396–405. [Google Scholar]
- 27.McCowan B, Beisner BA, Capitanio JP, Jackson ME, Cameron AN, Seil S, Atwill ER, Fushing H. 2011. Network stability is a balancing act of personality, power, and conflict dynamics in rhesus macaque societies. PLoS One 6:e22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCullagh P. 1984. Generalized linear models. Eur J Oper Res 16:285–292. [Google Scholar]
- 29.Mitchell G, Herring F, Obradovich S, Tromborg C, Dowd B, Neville LE, Field L. 1991. Effects of visitors and cage changes on the behaviors of mangabeys. Zoo Biol 10:417–423. [Google Scholar]
- 30.Oates-O'Brien RS, Farver TB, Anderson-Vicino KC, McCowan B, Lerche NW. 2010. Predictors of matrilineal overthrows in large captive breeding groups of rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 49:196–201. [PMC free article] [PubMed] [Google Scholar]
- 31.Pruetz JD, Isbell LA. 2000. Correlations of food distribution and patch size with agonistic interactions in female vervets (Chlorocebus aethiops) and patas monkeys (Erythrocebus patas) living in simple habitats. Behav Ecol Sociobiol 49:38–47. [Google Scholar]
- 32.Richter C, Gras P, Hodges K, Ostner J, Schulke O. 2015. Feeding behavior and aggression in wild Siberut macaques (Macaca siberu) living under low predation risk. Am J Primatol 77:741–752. [DOI] [PubMed] [Google Scholar]
- 33.Sade DS. 1972. Sociometrics of Macaca mulatta. I. Linkages and cliques in grooming matrices. Folia Primatol (Basel) 18:196–223. [DOI] [PubMed] [Google Scholar]
- 34.Sapolsky RM. 1982. The endocrine stress response and social status in the wild baboon. Horm Behav 16:279–292. [DOI] [PubMed] [Google Scholar]
- 35.Sapolsky RM. 1987. Stress, social status, and reproductive physiology in free-living baboons. p 291–322. In: Crews D.Psychobiology of reproductive behavior: an evolutionary perspective. Englewood Cliffs (NJ): Prentice Hall. [Google Scholar]
- 36.Teas J, Feldman HA, Richie TL, Taylor HG, Southwick CH. 1982. Aggressive behavior in the free- ranging rhesus monkeys of Kathmandu, Nepal. Aggress Behav 8:63–77. [Google Scholar]
- 37.Thierry B. 1985. Patterns of agonistic interactions in 3 species of macaque (Macaca mulatta, M. fascicularis, M. tonkeana). Aggress Behav 11:223–233. [DOI] [PubMed] [Google Scholar]
- 38.Thierry B, Singh M, Kaumanns W. 2004. Macaque societies: a model for the study of social organization. Cambridge (United Kingdom): Cambridge University Press. [Google Scholar]
- 39.Troisi A. 2002. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress 5: 47–54. [DOI] [PubMed] [Google Scholar]
- 40.Vandenbergh JG, Vessey S. 1968. Seasonal breeding of free-ranging rhesus monkeys and related ecological factors. J Reprod Fertil 15:71–79. [DOI] [PubMed] [Google Scholar]
- 41.Williams LE, Abee CR. 1988. Aggression with mixed age-sex groups of Bolivian squirrel monkeys following single-animal introductions and new group formations. Zoo Biol 7:139–145. [Google Scholar]
- 42.Wilson AP, Boelkins RC. 1970. Evidence for seasonal variation in aggressive behaviour by Macaca mulatta. Anim Behav 18:719–724. [DOI] [PubMed] [Google Scholar]
- 43.Wrangham R. 1981. Drinking competition in vervet monkeys. Anim Behav 29:904–910. [Google Scholar]
- 44.Zumpe D, Michael RP. 1979. Relation between the hormonal status of the female and direct and redirected aggression by male rhesus monkeys (Macaca mulatta). Horm Behav 12:269–279. [DOI] [PubMed] [Google Scholar]



