Abstract
The α2 adrenergic agonist xylazine produces a sedative effect and is typically combined with ketamine and used for anesthesia or chemical restraint of laboratory mice. Xylazine's sedative effect—and its undesirable side effects of bradycardia, hypotension, and poor tissue perfusion—can be reversed by administration of α2 antagonists, such as atipamezole or yohimbine. Although atipamezole and yohimbine dosing guidelines are available for mice, no controlled comparison has been performed to guide the lab animal community in the selection of one over the other. This study is a single-dose crossover comparison of these 2 antagonist drugs, given intraperitoneally at clinically recommended doses, to determine which results in more rapid recovery of mice from xylazine–ketamine anesthesia. Time to return of righting reflex was used as the primary outcome measure. Mice were anesthetized with xylazine (10 mg/kg IP) and ketamine (80 mg/kg IP), followed 15 min later by injection of an α2 antagonist or saline (control). Time to return of righting reflex differed significantly among groups, with mice recovering in an average of 10.3 min after administration of atipamezole (1 mg/kg IP) as compared with 21.3 min after yohimbine (1.5 mg/kg IP) and 38.2 min after saline. When rapid recovery of mice after xylazine–ketamine anesthesia is desirable, administration of an antagonist to reverse the effects of the xylazine is indicated. When injection of the antagonist by the technically simple intraperitoneal route is desirable, our data indicate that (at the doses evaluated) atipamezole is more effective than yohimbine.
Xylazine is an α2 agonist commonly used in veterinary medicine as a sedative and as a component in balanced anesthesia combinations. The sedative effects of the α2 agonists are believed to be caused by stimulation of α2A adrenoreceptor subtypes in the locus ceruleus of the brainstem, thus decreasing norepinephrine release.23,34,42 Various studies across many species either compare or individually explore the relative merits of the α2 agonists clinically available to veterinarians, including xylazine, detomidine, medetomidine, and the purified active enantiomer of medetomidine, dexmedetomidine.3-5,10,11,18,21,41 Xylazine has an α2:α1 selectivity ratio of 160, in addition to lesser affinity for β-adrenergic, dopaminergic, cholinergic, serotoninergic, H2 histamine, and opioid receptors.21,29
Although the newer drugs medetomidine and dexmedetomidine have higher affinity and specificity for the α2 receptor, xylazine continues to be used extensively in laboratory animal medicine for the anesthesia of rodents.5,10,37,42,43 A study comparing 8 injectable anesthetic protocols for mice concluded that cocktails containing xylazine and ketamine were preferable over similar mixtures containing medetomidine in terms of both safety and surgical tolerance; however even the optimal combination induced significant hypoxia, hypercapnia, and acidosis.5 These side effects, which are common to all α2 agonists, are due primarily to the activation of α2 receptors as well as lesser activation of α1 and β receptors. Documented side effects of xylazine are transient hypertension, peripheral vasoconstriction, prolonged hypotension, 30% to 50% decrease in cardiac output, diuresis, hypothermia, hyperglycemia, cerebral hypoxia, and decreased gut motility lasting for hours.10,17,19,20,26,38,41
Despite these recognized undesirable side effects, a major advantage of α2 agonists is the availability of antagonists, which anesthetists can administer to negate these effects. Effective reversal of xylazine sedation with an antagonist results in prompt awakening of the patient, as well as normalization of most of the earlier listed side effects.4,6,12,15,16,17,26,38,39 The anesthetist must consider several factors in the decision to administer an antagonist. An antagonist should not be administered until the sedative, anxiolytic, and analgesic effects of the agonist are no longer needed. In most species, ketamine, if administered alone, results in unacceptable excitement and agitation during awakening from anesthesia. These ‘emergence reactions’ are commonly managed by concurrent use of an adjunct sedative, typically a benzodiazepine, phenothiazine, or, an α2 agonist.40 Caution should therefore be exercised in removing the effect of the α2 agonist from a ketamine–xylazine or ketamine–dexmedetomidine anesthetic event before sufficient time has elapsed for ketamine metabolism.6,28 If both a phenothiazine, such as acepromazine, and an α2 agonist are included in the combination, as previously recommended,5 reversal of the α2 agonist should result in improved hemodynamic function but not prompt awakening or ketamine-associated emergence reaction, because the sedative properties of the phenothiazine remain in effect.5,6,7
The selected dose of antagonist should counteract the amount of unmetabolized agonist remaining in the subject, accounting for the time needed for metabolism.40 When given alone to unsedated subjects, both atipamezole and yohimbine are used as research models to create anxiety and are expected to cause similar effect when dosed in excess of that necessary to counteract the remaining agonist.24,32 Resedation, in which antagonist is metabolized faster than agonist, has occasionally been reported with this class of drugs.6,40 Due to the relatively long half-life of dexmedetomidine as compared with xylazine and the relatively short half-life of atipamezole as compared with yohimbine, resedation, possibly manifested as ‘prolonged recovery’ is more likely with dexmedetomidine–atipamezole than with other combinations.33,40 An antagonist should be administered in the event of life-threatening anesthetic complications believed to be a result of the agonist. Judicious use of an antagonist at the end of an anesthetic event, giving consideration to all of the previously mentioned factors, is expected to result in prompt normalization of physiologic function, most importantly perfusion and tissue oxygenation. Minimizing the duration of hypoperfusion and hypoxia will likely improve anesthetic outcomes and overall animal health. In the case of regulatory mandates that animals must be attended by an anesthetist until fully awake, decreasing unnecessary anesthesia time may also decrease the time that staff spend observing sleeping mice.
Yohimbine and atipamezole are 2 α2 antagonists currently available to veterinarians. Tolazoline is a mixed α2α1 antagonist that is FDA-approved for use by veterinarians to reverse the effects of xylazine, but it was temporarily unavailable at the time of this study. In contrast to the agonists, less has been published directly comparing the clinical utility of the different antagonists for the purpose of reversing the effects of xylazine across species. We found no controlled studies comparing atipamezole to yohimbine for reversal of xylazine in rodents.1,4,20,25,27,38
Yohimbine, with an α2:α1 affinity ratio of 40, was the prototypic α2 antagonist used for pharmacologic exploration of adrenoreceptors and has been in clinical use since the early 1980s. However, FDA approval for yohimbine is limited to the reversal of xylazine in dogs and deer. Atipamezole, which was FDA-approved in 1996, is far more selective for the α2 receptor, with an α2:α1 affinity ratio of 8526, and its affinity for the α2 receptor is 100 times greater than that of yohimbine.21,29 Atipamezole was marketed and FDA-approved only for reversal of medetomidine, and later dexmedetomidine, in dogs; it is not labeled for the reversal of xylazine in any species.35 Therefore veterinarians, educated with dogs as the basis of their studies and encouraged to follow drug labels in private practice, continue to frequently choose or recommend yohimbine as the reversal agent of choice for xylazine across species and often fail to consider using atipamezole. Neither xylazine, atipamezole, nor medetomidine are familiar to physicians, because none is FDA-approved for use in humans. Dexmedetomidine is FDA-approved for anxiolysis and sedation in humans, but although atipamezole safely reverses the sedative effects of dexmedetomidine, it is not approved for any purpose in humans.24 Therefore physicians performing research on animals likely will not typically even think of reversing xylazine in an anesthetic protocol, much less to question which available drug is most effective.
In veterinary specialties such as exotic pet medicine and zoo animal medicine, where ‘extra-label drug use’ as defined by the Animal Medicinal Drug Use Clarification Act of 1994 is routine and where anecdotal evidence and clinical judgement are sufficient to guide drug choice, atipamezole has been commonly used to reverse xylazine with good effect.1,2,21,22,26,27 Extra-label drug use is similarly common in laboratory animal medicine, and it is the role of the veterinarian to advise physicians on the selection of drugs for use in animals. However, anecdotes without supporting controlled studies are rarely sufficient to justify a change to an established model, particularly when that model predates the availability of newer drugs.
Doses for comparison in this study were chosen from formularies and texts available to practicing lab animal veterinarians. References cited by these sources were explored to determine the context of doses originating in research literature and propagated by collection into reference material. For this study the most commonly recommended atipamezole dose was chosen. A higher yohimbine dose than typically recommended was intentionally chosen.
A 1992 booklet noted specifically that “Atipamizole can be used to reverse the effects of either xylazine or medetomidine” and “Yohimbine seems to be less effective than atipamezole as an antagonist.”35 The atipamezole dose recommended in the rodent section of this reference is 1 mg SC or IM per 10 mg xylazine, with a note that “It is advisable to use rather low doses of atipamezole as very rapid and complete reversal may induce excitement.” There was no recommended yohimbine dose for rodents, and no reference to a controlled study comparing atipamezole to yohimbine.35 A more recent reference states that atipamezole is preferable to yohimbine for reversal of both xylazine and medetomidine, claiming fewer side effects, but does not cite a specific study comparing them.13,14 On different pages within this same text, 3 dose ranges applicable to mice are given for atipamezole. Specifically dose ranges of 0.5 to 1 mg/kg (route not specified) and 0.1 to 1 mg/kg IM, IP, SC, or IV are listed respectively for “small laboratory animal species” and in a table without citations for “rabbits and rodents.”13,14 These represent the lowest recommended doses found in the literature, and the 0.1- to 1-mg range is often repeated in other formularies, with reference back to the earlier publication.13,14 The third atipamezole dose given in this same text, in the paragraph specifically addressing ketamine xylazine anesthesia in mice and therefore recommended for this specific purpose, is 1 mg/kg Sc.13,14 For the purpose of reversing medetomidine, a more potent agonist than xylazine, other authors used 5 mg/kg SC.6 Also for medetomidine reversal, another group recommended atipamezole 1 to 2.5 mg/kg IP in mice, with female Swiss Webster mice requiring more than males.11 These doses represent the upper limits of recommended atipamezole doses for mice.
A 1991 study compared 4 doses of yohimbine (1, 5, 10, and 20 mg/kg IP) with 3 doses of tolazoline (10, 20, and 50 mg/kg IP) for the reversal of xylazine–ketamine sedation in rats. The authors concluded that the optimal dose of yohimbine was 1 mg/kg due to adverse effects at the higher doses but that yohimbine was less effective than tolazoline (20mg/kg IP). The authors specifically stated that although yohimbine was effective at reversing respiratory depression and reducing the appearance of corneal and pedal reflexes, “only tolazoline was effective in reducing time to appearance of the crawl reflex and recovery time.”25 A ‘rabbits and rodents’ table in the preceding text13,14 lists yohimbine doses of 0.2 mg/kg IV and 0.5 mg/kg IM for “any regimen using xylazine or medetomidine” but also notes “not recommended.” The rodent sections of 2 other formularies8,30 provide yohimbine doses of 0.2 mg/kg IP for “rabbits and rodents” and 0.5 to 1 mg/kg IV for “all species.” As stated earlier, the dose of yohimbine that we chose for the current study was intentionally slightly higher than the “optimal” dose in a previous study,25 although still less than the next higher dose, which showed adverse effects, and was significantly higher than all other recommended rodent doses.
Despite the availability of a choice in antagonists, it is common practice to administer no antagonist to mice and rats sedated with ketamine–xylazine, leaving the animals to remain sedated until the drugs are metabolized, therefore prolonging hypotension, hypoperfusion, and hypoxia unnecessarily. For researchers to consider the addition of a reversal agent to their anesthetic protocols, the lab animal veterinarian must present data regarding the effectiveness of the proposed change, and, when more than one option is available, comparative data to support the recommendation of a specific drug. Furthermore, for the proposed change to be willingly adopted by the research team, the additional step must not be technically difficult and must be consistently repeatable. The purpose of the current study is to encourage researchers who use ketamine and xylazine in mice to add an antagonist to their anesthetic protocol. Although yohimbine or atipamezole might be given to mice by the intravenous, intramuscular, or subcutaneous routes, the intraperitoneal route is the least technically demanding method of parenteral drug administration commonly used in mice and therefore the one most likely to be accepted by a research lab. Although the outcomes of this study might have been different had other routes of administration been used, we believe that the comparison of these 2 drugs at recommended doses by the easiest route of administration is the most clinically relevant.
We compared the 2 α2 antagonists yohimbine and atipamezole and saline (as a control) to determine which is more clinically effective when injected IP for reversing the sedative effects of xylazine in male C57BL\6J mice anesthetized with ketamine and xylazine. For the purpose of this experiment, a relatively low ketamine dose and a relatively high xylazine dose were chosen to best determine the effect of xylazine antagonism.5,15,31,37 Time from administration of the antagonist to return of righting reflex, defined as ability of the mouse to roll from dorsal to sternal recumbency 3 times within 60 s, was the primary endpoint. The hypothesis was that this variable would differ significantly among these 3 regimens, with the expectation, in light of experience in other species, that atipamizole at the most commonly recommended dose for mice would produce shorter recovery times than yohimbine, despite the use of a yohimbine dose higher than commonly recommended. We also monitored heart rate because clinical experience in other species suggests that correction of bradycardia indicates effective antagonist delivery and impending awakening of the animal from anesthesia.
Materials and Methods
Mice.
Male C57BL\6J mice (n = 18; Jackson Laboratory, Bar Harbor, ME) were used for this study. The strain was chosen because they are commonly used in research as an inbred strain and are a common background strain for genetically engineered models. Mice were all born on the same day and arrived in the vivarium at 7 wk of age. Ages at time of experiments ranged from 10 to 21 wk. On arrival, mice were randomly housed 3 per box in IVC (Optimice IVC, Animal Care Systems, Centennial CO.); each cage of 3 mice became a cohort that subsequently were anesthetized together on 3 occasions. Mice were individually identified by tail tattoo (Labstamp system, Somark Innovations, San Diego CA.) to ensure that each mouse received all 3 treatments overall.
All animal work was performed within the University of Pittsburgh's AAALAC-accredited animal program and was reviewed and approved by the IACUC. Animals were housed on a 12:12-h light:dark cycle at 22 ± 1 °C and 45% ±10% humidity. Animals were maintained SPF for Myobia, Mycoptes, Radfordia, Aspicularis, Syphacia, Mycoplasma pulmonis, CAR bacillus, Encephalitozoon cuniculi, mouse parvovirus, minute virus of mice, mouse hepatitis virus, Theiler murine encephalomyelitis virus, murine rotavirus, Sendai virus, pneumonia virus of mice, reovirus, lymphocytic choriomeningitis virus, mouse adenovirus types 1 and 2, ectromelia virus, mouse pneumonitis virus (K virus), polyoma virus, mouse cytomegalovirus, Hantaan virus, mouse thymic virus, and lactate dehydrogenase elevating virus. Mice had unrestricted access to an irradiated diet (Purina ISO Pro Rodent 3000, LabDiet, St Louis, MO). Bedding was certified coarse-grade Aspen Sani-Chip (PJ Murphy Forest Products, Montville, NJ.) Standard enrichment was a cotton square and a Shepherd Shack (PharmaServ, Framingham, MA.) Water was reverse-osmosis–purified, hyperchlorinated to 3 to 5 ppm, and delivered without restriction through rack-mounted valves (Edstrom, Waterford WI).
Drug dilution and dosage.
Mice were anesthetized with ketamine (80 mg/kg IP) and xylazine (10 mg/kg IP); atipamezole was administered at 1 mg/kg IP, and yohimbine was administered at 1.5 mg/kg IP. All injections were administered in the lower right quadrant of the abdomen.
The anesthetic cocktail and the reversal drugs were formulated in house, mixed, and diluted in 0.9% sterile saline to provide for consistent dosing volume of 0.01 mL per gram of body weight. All components were pharmaceutical grade injectable solutions, were transferred to sterile multiuse vials by using aseptic technique, handled to maintain sterility, protected from light, and assigned an expiration date of 2 mo after dilution.
The anesthetic cocktail, administered to all mice, included 0.8 mL ketamine (100 mg/mL, lot 110803B, Butler Animal Health Supply) and 0.5 mL xylazine (20 mg/mL, lot LA31309, TranquiVed) into 8.7 mL of 0.9% sterile saline for a final volume of 10 mL and concentration of 8 mg/mL ketamine and 1 mg/mL xylazine. Mice were weighed and dosed at 0.01 mL of cocktail per gram of mouse, resulting in doses of 80 mg/kg ketamine and 10 mg/kg xylazine.
We added 0.75 mL yohimbine (2 mg/mL, lot LB 09510, Lloyd Laboratories) into 9.25 mL sterile saline (lot C856909, Abbott) for a concentration of 0.15 mg/mL. This solution was administered at 0.01 mL per gram of mouse for a dose of 1.5 mg/kg. We diluted 0.2 mL atipamizole (5 mg/mL, lot 1330519 or 1372314, Pfizer) into 9.8 mL saline for a final concentration of 0.1 mg/mL. This solution was administered at 0.01 mL per gram of mouse for a dose of 1 mg/kg. Saline was administered, as control for the antagonists, at the same 0.01 mL per gram.
Study design.
In this crossover study using Latin squares randomization, 18 mice of identical age were randomly assigned into groups of 3 and then tail-tattooed with sequential numbers. Each group of mice was anesthetized on 3 occasions by using the same anesthetic cocktail and arranged on a heating pad (Deltaphase Isotherm Pad, Braintree Scientific, Braintree, MA) in order of tattoo number from left to right. In most cases, at 15 min after anesthetic injection, the 3 mice were each injected with a different reversal drug; on one occasion, a single cohort was inadvertently injected after 10 min. After a washout period of at least 1 wk, mice were again anesthetized, arranged in the same order, and the reversal drugs rotated one position right, such that each mouse received a different drug from the previous experiment. The process was repeated a third time, completing the crossover such that each mouse received each reversal drug on only one occasion.
Data collection.
Time from injection of the reversal agent until “return of righting reflex” was the primary dependent variable. Return of righting reflex was defined as ability to roll from dorsal to sternal recumbency 3 times within a 60 second period. Time was kept by inserting time stamps for significant events into the data stream from 3 pulse-oximeters (MouseSTAT or PhysioSuite, Kent Scientific, Torrington CT). Serial data from the oximeters was logged on a PC using Free Serial Port Terminal 1.0.0.710 per the manufacturer's recommendation. Oximeters were set to sample SPO2, HR and Perfusion at 5 second intervals, and annular probes were used on either tails or paws. At minimum the time of xylaxine + ketamine injection, time of antagonist injection, and time of return of righting reflex 3× within 60 seconds were noted. In addition, other observations such as incomplete anesthesia, paddling, first attempt at righting, or an abrupt onset of whisker twitch were noted on some animals.
Data analysis and statistics.
Data generated by the oximeters were saved as .log text files, which were imported into a spreadsheet program (Excel, Microsoft, Redmond, WA) as fixed-width data fields. The interval between time stamps at ‘administration of reversal agent’ and ‘return of righting reflex 3 times within 60 s’ was calculated. Preliminary statistics were run in Excel, and further statistical analysis run in Minitab (Minitab, State College, PA.) Repeated-measures ANOVA was run on the complete dataset. To confirm that the accidental early administration of the reversal agents to 3 mice on the same day did not affect the study conclusions, the dataset was also analyzed by using single-factor ANOVA with those data points included and excluded. Tukey post hoc tests and 95% confidence intervals were obtained to confirm significant differences between groups.
Results
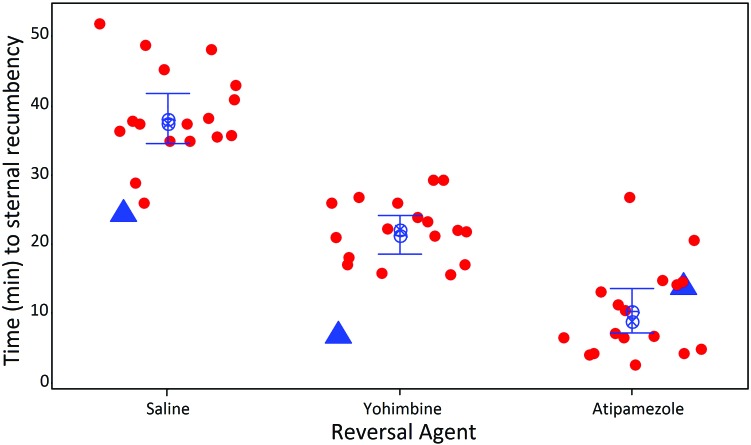
After the injection of xylazine–ketamine, mice lost righting reflex after 3.0 ± 1.3 min (mean ± 1 SD). Mice treated with saline 15 min after ketamine–xylazine administration remained anesthetized for an additional 38.2 ± 7.5 min, for an approximate total of 50 min of lateral recumbency. Mice recovered 21.3 ± 5.6 min after intraperitoneal administration of yohimbine, and mice treated with intraperitoneal atipamezole recovered in 10.3 ± 6.4 min. Statistical analysis confirmed a significant main effect of reversal drug on recovery time (F2,34 = 134.98, P < 0.001) and that the means of all groups differed significantly with each other (Figure 1). These data indicate that recovery time after intraperitoneal administration of atipamezole (1 mg/kg) is significantly faster than that after yohimbine (1.5 mg/kg IP), which is still faster than the time to recovery after no antagonist.
Figure 1.
Time to return of the righting reflex in mice anesthetized with the α2 agonist xylazine, in combination with ketamine, after intraperitoneal administration of either of the α2 antagonists atipamezole (1 mg/kg IP) and yohimbine (1.5 mg/kg IP) or saline (control). At these dosages, atipamezole (mean ± 1 SD, 10.3 ± 6.5 min) resulted in the most rapid recovery of the mice to sternal recumbency, allowing for prompt return of the mice to the home cage after anesthesia, compared with yohimbine (21.3 ± 5.6 min) and saline (38.2 ± 7.5 min). The 3 data points designated by triangles indicate a technical error, in which the antagonist was given at 10 min, rather than 15 min, after the anesthetic combination.
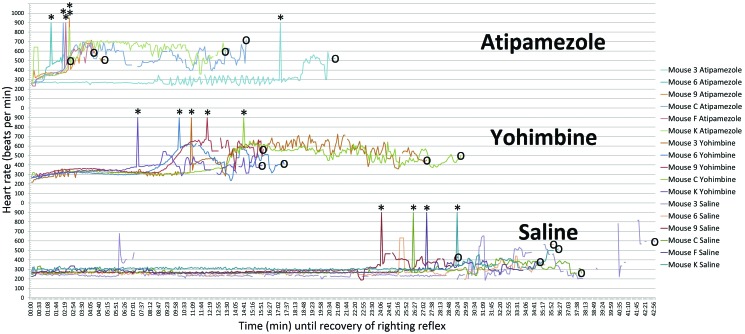
Heart rate data from 2 of the 3 oximeters contained many gaps due to use of too-large annular probes on tails and paws; the sole oximeter with an appropriately fitting probe generated high-quality data (Figure 2). From the data collected, we made the following generalizations. Heart rates of mice anesthetized with ketamine–xylazine were typically in the bradycardic range of 250 to 350 beats per minute. Heart rates of mice injected with saline remained in this same range for an additional 25 min and then increased only slightly as mice awakened. The groups treated with yohimbine and atipamezole both showed increases in heart rate, with similarly shaped curves, to a physiologically normal range of approximately 600 beats per minute shortly before waking. The difference between the yohimbine and atipamezole groups is that, after atipamezole treatment, this effect on heart rate began within 2 min and was complete within 4 min, whereas after yohimbine treatment there was a 7-min lag before the effect was first noted. Abrupt onset of a characteristic whisker twitch was noted in most animals, including the saline controls. It typically coincided with the steep upslope in heart rate in mice treated with reversal agents and with the subtle increase in heart rate seen in the controls and is a useful indicator of animal awakening when advanced monitoring equipment is unavailable.
Figure 2.
Stacked graph of heart rates of mice anesthetized with the α2 agonist xylazine, in combination with ketamine, from the time of intraperitoneal administration of either of the α2 antagonists atipamezole and yohimbine or saline (control) until return of righting reflex. The lower graph represents mice given saline, which remain anesthetized for an additional 38.0 ± 7.46 min and are bradycardic for most of that time. The heart rate of mice given atipamezole (1 mg/kg IP; top graph) begins to rise almost immediately and is normal or mildly tachycardic within 4 min; some mice begin to recover the righting reflex shortly thereafter. Yohimbine (1.5 mg/kg IP, middle graph) causes a similar rise in heart rate as that of atipamezole, but there is a delay of 7 to 10 min before the first effect is seen. The end of each tracing (circled) represents the return of the righting reflex, removal of the oximeter probe, and return of the mouse to its home cage. The upward spikes (asterisks) are artificial and were placed in the data stream to mark the time of onset of a characteristic rapid whisker twitch that was observed in all groups and which seemed to be an easily noted sign of impending recovery.
Discussion
Although very effective and used frequently, α2 adrenergic agonist sedatives, including xylazine, inherently produce undesirable side effects due to the activation of α2 receptors throughout the body. Animal wellbeing can be improved by minimizing the duration of this derangement and returning the animal to a normal physiologic state through the administration of an α2 adrenergic antagonist as soon as anesthesia is no longer required. Here we compared the 2 available α2 antagonists, atipamezole and yohimbine, at doses recommended in rodent formularies and administered by the technically simple intraperitoneal route, to determine which agent produced more rapid arousal of mice anesthetized with a commonly used ketamine– xylazine combination. The clinically relevant conclusion of this study is that mice anesthetized with xylazine and ketamine become ambulatory much sooner when the effects of xylazine are reversed by using an antagonist and that, at the doses and route of administration used, atipamezole returns the heart rate to normal and awakens the mice more quickly than yohimbine. We consider this effect to be desirable, provided that the factors mentioned in the Introduction are considered.
The normal resting heart rate of a mouse has been reported to be 450 to 500 beats per minute.36 The same reference states that the normal heart rate of a sleeping mouse is 350 to 450 beats per minute, 600 to 650 beats per minute after light activity, and 750 to 800 beats per minute during hand restraint or treadmill exercise. The recorded heart rate of all our anesthetized mice was in the range of 250 to 350 beats per minute, confirming the bradycardia expected with use of an α2 agonist. After the administration of atipamezole, heart rate began to increase within 2 min and was fully elevated after 4 min. In contrast, the same initial effect was not seen with yohimbine until at least 7 min after administration, with full effect about 2 min later. In both cases, the peak elevated heart rate was less than 725 beats per minute and therefore less than the tachycardia associated with the stress of handling, indicating that we did no harm but also suggesting that we might explore lower doses of atipamezole.
Atipamezole is labeled for intramuscular injection, and there is pharmacokinetic data to show that the agent reaches its peak plasma concentration in dogs within 15 min after intramuscular injection.30,33 In addition, atipamezole is rapidly absorbed after subcutaneous injection.21,29 Pharmacokinetic data for yohimbine distribution and excretion after intravenous injection are published, but data regarding the absorption and distribution of yohimbine after intramuscular or intraperitoneal injection have not yet been reported. Yohimbine is labeled only for intravenous injection in all species. We believe that the mice would have recovered more quickly had the yohimbine been given intravenously as labeled. However, the administration of intravenous injections to mice is technically demanding due to the small size of the veins, and although many researchers successfully inject mice intravenously, many others are uncomfortable with the technique. The intent of this study was to encourage the use of an antagonist as an anesthetic reversal agent by researchers who are not currently using one and to evaluate which of 2 readily available drugs is more efficacious when given by a route of administration with which most researchers are proficient.
A similar study in which medetomidine was reversed with atipamezole (5 mg/kg SC) recommended against administering the α2 antagonist too soon after administration of the ketamine–medetomidine combination.6 The authors noted vocalization and a delay in return to full ambulation in mice given atipamezole after 10 min as compared with 40 min. The vocalization can be accounted for by the premature administration of the antagonist, leaving the ketamine as a sole agent, as the authors surmised. However, we also suggest that the 5-mg/kg dose of atipamezole may have been excessively high to counter the 1-mg/kg medetomidine dose and might have led to anxiogenesis as a direct result of the α2 antagonism at the brainstem and resulting norepinephrine release.40 Other authors, using yohimbine and atipamezole as an established research model for anxiogenesis, demonstrated that atipamezole given at 3 mg/kg to an unsedated mouse caused a measurable increase in startle.40 From this finding, we speculate that any atipamezole given in excess of the minimum necessary to reverse the sedative effect may add undue anxiety. We make this suggestion to encourage further refinement by tailoring both dose and time of administration of the antagonist to the sedation protocol used and the type of procedure performed. Regarding the delay in return of ambulation observed in a previous study,6 we note that although the mice that received atipamezole prematurely took longer to ambulate than those given the antagonist later, both groups regained the ability to ambulate more than 2 h sooner than the control group, which was not given any antagonist. We interpret this result as clear evidence that the use of an α2 antagonist is beneficial and indicated and agree with the authors of the previous study6 that the ideal time of administration must be carefully weighed. Finally, we note that the half-life of medetomidine is much longer than that of xylazine, and the receptor affinity much greater; therefore, although parallels can be drawn between our current and the previous study,6 direct comparisons may not.
In conclusion, we recommend that when mice are sedated or anesthetized with a drug combination containing xylazine for a procedure of short duration, xylazine-associated effects should be reversed at the end of the procedure by the administration of an antagonist, unless a specific contraindication is present. The effects of all other medications that have been administered to the animal should be considered in the decision to remove the effects of the α2 agonist, with particular attention given to the time allowed for metabolism of ketamine and to the adequacy of pain control. The dose of antagonist should be minimized, to avoid anxiogenic effects. At the doses and route (intraperitoneal) tested, atipamezole safely awakened ketamine–xylazine-anesthetized mice more quickly than yohimbine. We believe that lower doses of atipamezole should be explored, in light of the mild tachycardia that occurred. The use of yohimbine by alternative routes of administration or at higher doses might be explored, but in light of published data from rats25, higher doses by the intraperitoneal route might be expected to cause adverse effects without improved time to recovery. We also recommend the comparison of atipamezole with tolazoline.
Acknowledgments
We thank the ACLAM Foundation (with support from Pfizer) for funding this study. We also to thank Philana Ling Lin and JoAnne L Flynn for assistance in manuscript preparation.
Pfizer is the United States distributor for atipamezole, but the potential conflict of interest was managed because the authors did not learn about the Pfizer support until after all data had been collected and analyzed.
References
- 1.Abdel-Wahed R, El-Kammar M, Abu-Ahmed H, El-Khenany H. 2011. Antagonism of xylazine– butorphanol combination in donkeys using tolazoline–atipamezole. Alexandria J Vet Sci 34:11–20 [Google Scholar]
- 2.Adamcak A, Otten B. 2000. Rodent therapeutics. Vet Clin North Am Exot Anim Pract 3:221–237 [viii]. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht M, Henke J, Tacke S, Markert M, Guth B. 2014. Effects of isoflurane, ketamine–xylazine, and a combination of medetomidine, midazolam, and fentanyl on physiologic variables continuously measured by telemetry in Wistar rats. BMC Vet Res 10:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrisko TD, Hikasa Y. 2003. The antagonistic effects of atipamezole and yohimbine on stress-related neurohormonal and metabolic responses induced by medetomidine in dogs. Can J Vet Res 67:64 –67. [PMC free article] [PubMed] [Google Scholar]
- 5.Arras M, Autenried P, Rettich A, Spaeni D, Rulicke T. 2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med 51:443–456. [PubMed] [Google Scholar]
- 6.Baker NJ, Schofield JC, Caswell MD, McLellan AD. 2011. Effects of early atipamezole reversal of medetomidine–ketamine anesthesia in mice. J Am Assoc Lab Anim Sci 50:916–920. [PMC free article] [PubMed] [Google Scholar]
- 7.Buitrago S, Martin TE, Tetens-Woodring J, Belicha-Villanueva A, Wilding GE. 2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J Am Assoc Lab Anim Sci 47:11–17. [PMC free article] [PubMed] [Google Scholar]
- 8.Carpenter J, Marion CJ, editors. 2013. Exotic animal formulary, 4th ed. St Louis (MO): Elsevier Saunders. [Google Scholar]
- 9.Charles River Laboratories 2017. Mouse serology profiles. [Cited 06 February 2017]. Available at: http://www.criver.com/files/pdfs/research-models/mouse-serology-profiles.aspx
- 10.Ciobanu L, Reynaud O, Uhrig L, Jarraya B, Le Bihan D. 2012. Effects of anesthetic agents on brain blood oxygenation level revealed with ultra-high–field MRI. PLoS One 7:e32645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz JI, Loste JM, Burzaco OH. 1998. Observations on the use of medetomidine–ketamine and its reversal with atipamezole for chemical restraint in the mouse. Lab Anim 32:18–22. [DOI] [PubMed] [Google Scholar]
- 12.Dittmar MS, Fehm NP, Vatankhah B, Horn M. 2004. Ketamine–xylazine anesthesia for radiologic imaging of neurologically impaired rats: dose response, respiratory depression, and management of complications. Comp Med 54:652–655. [PubMed] [Google Scholar]
- 13.Flecknell PA. 2016. Basic principles of anaesthesia, p 52. In: Flecknell PA, editor. Laboratory animal anaesthesia, 4th ed. Waltham (MA): Elsevier. [Google Scholar]
- 14.Flecknell PA. 2016. Anaesthesia of common laboratory species: special considerations, p 201–206 In: Flecknell PA. editor. Laboratory animal anaesthesia, 4th ed.Walham (MA): Elsevier. [Google Scholar]
- 15.Flecknell P, Lorgren JLS, Dyson MC, Maini RP, Swindle M, Wilson RP. 2015. Preanesthesia, anesthesia, analgesia and euthanasia.p 1136–1147. In: Fox JG, Anderson LC, Oton G, Pritchett-Corinig KR, Whary MT, editors. Laboratory animal medicine. San Diego (CA): Academic Press. [Google Scholar]
- 16.Gentili F, Pigini M, Piergentili A, Giannella M. 2007. Agonists and antagonists targeting the different α2 adrenoceptor subtypes. Curr Top Med Chem 7:163–186. [DOI] [PubMed] [Google Scholar]
- 17.Gross ME, Tranquilli WJ. 1989. Use of α2 adrenergic receptor antagonists. J Am Vet Med Assoc 195:378–381. [PubMed] [Google Scholar]
- 18.Henke J, Astner S, Brill T, Eissner B, Busch R, Erhardt W. 2005. Comparative study of 3 intramuscular anaesthetic combinations (medetomidine–ketamine, medetomidine–fentanyl–midazolam, and xylazine–ketamine) in rabbits. Vet Anaesth Analg 32:261–270. [DOI] [PubMed] [Google Scholar]
- 19.Hikasa Y, Takase K, Emi S, Ogasawara S. 1988. Antagonistic effects of α-adrenoceptor blocking agents on reticuloruminal hypomotility induced by xylazine in cattle. Can J Vet Res 52:411 –415. [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu WH. 1982. Xylazine-induced delay of small intestinal transit in mice. Eur J Pharmacol 83:55–60. [DOI] [PubMed] [Google Scholar]
- 21.Jalanka H. 1989. The use of medetomidine, medetomidine–ketamine combinations, and atipamezole at Helsinki Zoo—a review of 240 cases. Acta Vet Scand Suppl 85:193–197. [PubMed] [Google Scholar]
- 22.Jurczynski K, Flugger M. 2005. Experiences with the use of atipamezole–yohimbine combination for antagonization in blackbucks (Antilope cervicapra). In: Proceedings of the Institute for Zoo and Wildlife Research, Berlin, Germany: 04–08 May 2005. [Google Scholar]
- 23.Kable JW, Murrin LC, Bylund DB. 2000. In vivo gene modification elucidates subtype-specific functions of α2 adrenergic receptors. J Pharmacol Exp Ther 293:1–7. [PubMed] [Google Scholar]
- 24.Karhuvaara S, Kallio A, Salonen M, Tuominen J, Scheinin M. 1991. Rapid reversal of α2 adrenoceptor agonist effects by atipamezole in human volunteers. Br J Clin Pharmacol 31:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komulainen A, Olson ME. 1991. Antagonism of ketamine–xylazine anesthesia in rats by administration of yohimbine, tolazoline, or 4-aminopyridine. Am J Vet Res 52:585–588. [PubMed] [Google Scholar]
- 26.Langoi DL, Mwethera PG, Abelson KS, Farah IO, Carlsson HE. 2009. Reversal of ketamine–xylazine combination anesthesia by atipamezole in olive baboons (Papio anubis). J Med Primatol 38:404–410. [DOI] [PubMed] [Google Scholar]
- 27.Miller BF, Muller LI, Doherty T, Osborn DA, Miller KV, Warren RJ. 2004. Effectiveness of antagonists for tiletamine–zolazepam–xylazine immobilization in female white-tailed deer. J Wildl Dis 40:533–537. [DOI] [PubMed] [Google Scholar]
- 28.Muir WW, Hubell JAE, Skarda RT, Bednarski RM, editors. 2000. Handbook of Veterinary Anesthesia, 3rd ed. Phildelphia (PA): Mosby. [Google Scholar]
- 29.Pertovaara A, Haapalinna A, Sirvio J, Virtanen R. 2005. Pharmacologic properties, central nervous system effects, and potential therapeutic applications of atipamezole, a selective α2 adrenoceptor antagonist. CNS Drug Rev 11:273–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plumb DC. 2015. Plumb's veterinary drug handbook 8th ed. Ames (IA): Wiley–Blackwell. [Google Scholar]
- 31.Richardson CA, Flecknell PA. 2005. Anaesthesia and postoperative analgesia following experimental surgery in laboratory rodents: are we making progress? Altern Lab Anim 33:119–127. [DOI] [PubMed] [Google Scholar]
- 32.Risbrough VB, Geyer MA. 2005. Anxiogenic treatments do not increase fear-potentiated startle in mice. Biol Psychiatry 57:33–43. [DOI] [PubMed] [Google Scholar]
- 33.Salonen S, Vuorilehto L, Vainio O, Anttila M. 1995. Atipamezole increases medetomidine clearance in the dog: an agonist–antagonist interaction. J Vet Pharmacol Ther 18:328–332. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz DD, Clark TP. 1998. Selectivity of atipamezole, yohimbine, and tolazoline for α2 adrenergic receptor subtypes: implications for clinical reversal of α2 adrenergic receptor mediated sedation in sheep. J Vet Pharmacol Ther 21:342–347. [DOI] [PubMed] [Google Scholar]
- 35.Short CE. 1992. α2 agents in animals. Sedation analgesia and anesthesia. Santa Barbra (CA): Veterinary Practice [Google Scholar]
- 36.Singleton GR, Krebs CJ. 2007. The secret world of wild mice, p 35. In: Fox JG, Davisson MT, Quimby Fw, Barthold SW, Newcomer CE, Smith AL, editors. The mouse in biomedical research, 2nd ed, vol 1: History, wild mice , and genetics. Walham (MA): Elsevier. [Google Scholar]
- 37.Stokes EL, Flecknell PA, Richardson CA. 2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43:149–154. [DOI] [PubMed] [Google Scholar]
- 38.Talukder H, Hikasa Y, Matsuu A, Kawamura H. 2009. Antagonistic effects of atipamezole and yohimbine on xylazine-induced diuresis in healthy dogs. J Vet Med Sci 71:539–548. [DOI] [PubMed] [Google Scholar]
- 39.Talukder MH, Hikasa Y, Takahashi H, Sato K, Matsuu A. 2009. Antagonistic effects of atipamezole and yohimbine on medetomidine-induced diuresis in healthy dogs. Can J Vet Res 73:260 –270. [PMC free article] [PubMed] [Google Scholar]
- 40.Tranquilli W, Thurmon J, Grimm K, editors. 2007. Lumb and Jones’ veterinary anesthesia and analgesia, 4th ed. Ames (IA): Wiley–Blackwell. [Google Scholar]
- 41.Uhrig L, Ciobanu L, Djemai B, Le Bihan D, Jarraya B. 2014. Sedation agents differentially modulate cortical and subcortical blood oxygenation: evidence from ultra-high–field MRI at 17.2 T. PLoS One 9:e100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wellington D, Mikaelian I, Singer L. 2013. Comparison of ketamine–xylazine and ketamine–dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J Am Assoc Lab Anim Sci 52:481–487. [PMC free article] [PubMed] [Google Scholar]
- 43.Wells S, Trower C, Hough TA, Stewart M, Cheeseman MT. 2009. Urethral obstruction by seminal coagulum is associated with medetomidine–ketamine anesthesia in male mice on C57BL/6J and mixed genetic backgrounds. J Am Assoc Lab Anim Sci 48:296–299. [PMC free article] [PubMed] [Google Scholar]