Abstract
At research institutions, isoflurane delivered by precision vaporizer to a face mask is the standard for rodent surgery and for procedures with durations that exceed a few minutes. Pure oxygen is often used as the carrier gas for isoflurane anesthesia, despite documented complications from long-term 100% oxygen use in humans and known occupational safety risks. We therefore examined the effect of anesthetic delivery gas on physiologic variables in mice and rats. Rodents were anesthetized for 60 min with isoflurane delivered in either 21% or 100% oxygen by means of a nose cone. We noted no difference between carrier gasses in physiologic variables in mice, including body temperature, respiratory rate, mean arterial pressure, surgical recovery time, pH, or PaCO2.However, blood gas analysis revealed evidence of a ventilation–perfusion mismatch in the 100% oxygen group. Pressure–volume hysteresis and histomorphometric analyses confirmed the presence of increased atelectasis in mice that received 100% oxygen. Unlike mice, rats that received isoflurane in 100% oxygen had acute respiratory acidosis and elevated mean arterial pressure, but atelectasis was similar between carrier gasses. Our data suggest that both 100% and 21% oxygen are acceptable for the delivery of isoflurane to mice. However, mice anesthetized for studies focused on lung physiology or architecture would benefit from the delivery of isoflurane in 21% oxygen to reduce absorption atelectasis and the potential associated downstream inflammatory effects. For rats, delivery of isoflurane in 21% and 100% oxygen both caused perturbations in physiologic variables, and choosing a carrier gas is not straightforward.
Abbreviations: A-a gradient, alveolar–arterial gradient; FiO2, fraction of inspired oxygen
At many research institutions, isoflurane delivered by precision vaporizer to a face mask is a standard anesthesia method for rodent surgery and for procedures that last longer than a few minutes. Although the specific carrier gas often is not described in the methods section of manuscripts, isoflurane typically is delivered in 100% oxygen. A review of published literature did not reveal an absolute recommendation pertaining to a carrier gas for the delivery of isoflurane to rodents.
In contrast, studies in human medicine have shown that induction of anesthesia with oxygen concentrations below 60% is beneficial. CT imaging of human patients during inhalant anesthesia has shown areas of atelectatic lung that are dependent upon oxygen concentration.7 Absorption atelectasis occurs because oxygen is extremely soluble in blood. When 100% oxygen is used to deliver isoflurane to a patient, the oxygen quickly diffuses across the alveoli into the blood stream, accelerating the rate of alveolar collapse. However, in room air (21% oxygen), oxygen shares alveolar space with less-soluble gases such as nitrogen, thus helping to retain alveolar patency. Although the benefits of decreased oxygen concentrations for isoflurane delivery are well documented in human literature, they seem less clear in veterinary species. For example, a study in horses showed no differences in respiratory variables when 50% oxygen rather than 100% oxygen was used to deliver isoflurane.8 However, studies in both dogs and cats have shown that a reduced fraction of inspired oxygen (FiO2) decreases atelectasis and improves lung aeration.14,15 The fraction of inspired oxygen is the percentage of oxygen in the space being measured, such that room air has an FiO2 of 0.21 and 100% oxygen has an FiO2 of 1.0.
Information on smaller species is sparse. Previous studies have used both 100% and 21% oxygen in rodents, but the carrier gases used were ancillary to the main research question being addressed.3,6 These studies did not specifically address isoflurane anesthesia by nose cone in nonmechanically ventilated rodents or assess physiologic variables associated with animal wellbeing. To our knowledge, no studies have directly evaluated how the delivery gas (100% oxygen or 21% oxygen) used to administer isoflurane to rodents via nose cone affects atelectasis or physiologic variables, such as mean arterial pressure, respiratory rate, temperature, PaO2, recovery time, and the alveolar–arterial (A-a) gradient.
The A-a gradient is a measure of the gas exchange efficiency between the lungs and the bloodstream. Normally, the A-a gradient should be approximately 1 mm Hg for every 1% of inspired oxygen. One common cause of an elevated A-a gradient is atelectasis. Atelectasis results in impaired gas exchange and decreased lung compliance. Severe atelectasis can alter microvascular permeability and lead to a proinflammatory state.6 In addition, the oxygen concentrations used to deliver isoflurane to rodents might influence the health of the animals after anesthesia and affect research studies involving lung physiology and architecture. We hypothesized that delivery of isoflurane in 21% oxygen would reduce atelectasis without significantly altering physiologic parameters in mice and rats.
Materials and Methods
Animals.
Male CD1 mice (age, 9 to 10 wk; Harlan, Indianapolis, IN) were housed at 5 per cage and male F344 rats (age, 9 to 10 wk; Charles River, Wilmington, MA) were housed at 2 per cage in static microisolation cages in an SPF barrier facility. Mice were negative for viruses including mouse hepatitis virus, minute virus of mice, mouse parvovirus, enzootic diarrhea of infant mice virus, ectromelia virus, Sendai virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, reovirus, lymphocytic choriomeningitis virus, mouse adenovirus, and polyomavirus as indicated by negative results from sentinels for these animals. Rats were negative for sialodacryoadenitis virus, Kilham rat virus, rat parvovirus, Sendai virus, pneumonia virus of mice, reovirus, Mycoplasma pulmonis, Theiler murine encephalomyelitis virus, lymphocytic choriomeningitis virus, and mouse adenovirus, as indicated by negative results from sentinels for these animals. Mice and rats had unrestricted access to food (Laboratory Rodent Diet 5001, PMI LabDiet, St Louis, MO) and water. The animal housing room was maintained on a 12:12-h light:dark cycle with constant temperature (72 ± 2 °F [22.2 ± 1.1 °C]). Mice and rats were acclimated for at least 7 d prior to experimental use. All procedures were approved by the University of Michigan's Animal Care and Use Committee.
Study design.
Mice (n = 10) were anesthetized for 60 min through a nose cone by using a nonrebreathing system with isoflurane delivered in either 100% oxygen or 21% oxygen. A femoral catheter was placed surgically to record blood pressure and facilitate blood collection. Respiratory rate and body temperature were taken immediately at onset of anesthesia. After 60 min of isoflurane anesthesia, body temperature, respiratory rate, and mean arterial blood pressure were measured. At the same time, an arterial blood sample was obtained to measure the pH, PaCO2, and PaO2. The animals were then allowed to recover and the recovery time calculated, after which they were immediately euthanized. In 2 additional experiments, mice were similarly anesthetized for 60 min with isoflurane in 21% or 100% oxygen before being used to quantify atelectasis according to either pressure–volume hysteresis (n = 10) or histology (n = 5).
The same study design was used with rats, but physiologic parameters and blood gas values were measured at 15, 30, 45, and 60 min after the induction of anesthesia (n = 10). Time to anesthetic recovery was then determined, after which the rats were immediately euthanized. For rats, atelectasis was quantified by using μCT imaging.
Anesthesia.
Rodents underwent chamber induction with 5% isoflurane delivered in either 100% oxygen or 21% oxygen from medical air tanks at a flow rate of 1 L/min. Waste anesthetic gas was scavenged by using activated charcoal (F/AIR canister, Braintree Scientific, Braintree, MA) placed within a chemical fume hood. Once the rodents no longer responded to an interdigital pinch, they were transferred to a temperature-controlled surgery plate, received a nose cone, and were maintained on 1.5% to 2.0% isoflurane for 60 min. After induction of anesthesia, a temperature transponder (IPTT-300, Biomedic Data Systems, Seaford, DE) was implanted subcutaneously on the lateral flank by using the needle provided by the manufacturer, to monitor temperature throughout the procedure.
Direct blood pressure.
The inguinal region of anesthetized mice was shaved, aseptically prepared, and draped, and the femoral artery was exposed and cannulated with a polyethylene 10 catheter (SIMS Portex, Keene, NH) as previously described.4 The catheters were flushed with heparinized (100 IU/mL) lactated Ringers solution before insertion. The catheter was connected through a transducer to a pressure analyzer (Micro-Med, Louisville, KY) to record the mean arterial blood pressure. The femoral catheter was removed at the end of the 60-min study, the area splashed with lidocaine, and the incision closed.
Arterial blood gas analysis.
Blood gas analysis was performed with blood from the femoral artery catheter using iSTAT CG8+ cartridges (Abaxis, Union City, CA) according to the manufacturer's guidelines. Prior to obtaining a sample for each analysis, 0.3 mL of blood was removed to prevent hemodilution. The unused blood was returned slowly through the femoral artery catheter.
Recovery time and euthanasia.
After 60 min, the anesthesia was discontinued, the animals were recovered on a heated surface, and recovery time was defined as the latency to voluntary movement in sternal recumbency. The mice were then immediately humanely euthanized by isoflurane overdose followed by cervical dislocation and bilateral pneumothorax. Rats were euthanized by isoflurane overdose followed by bilateral pneumothorax.
Oxygen concentration validation.
The oxygen concentration within the nose cone was validated by using a oxygen analyzer (Maxtec Handi + Scuba Analyzer, Maxtec, Salt Lake City, UT), per the manufacturer's instructions. The analyzer tubing was placed inside the nose cone at the level of the mouse or rat's nose. The device was calibrated per the manufacturer's instructions prior to each use.
A-a gradient.
The A-a gradient was calculated by using the equation
where Patm is the atmospheric pressure, and PH2O is the saturated vapor pressure of water at the prevailing atmospheric pressure The performance site has an elevation of 863 ft (www.usclimatedata.com), which corresponds to an atmospheric pressure of approximately 736 mm Hg. At room temperature, PH2O is 47 and the FiO2 concentration was either 0.21 or 1.
Pressure–volume hysteresis.
After 60 min of anesthesia by using isoflurane delivered in either 100% or 21% oxygen, anesthetized mice were euthanized by ketamine–xylazine overdose (200 mg/kg ketamine, 20 mg/kg xylazine). Once breathing ceased, the animals were exsanguinated through a midline laparotomy and transection of the inferior vena cava to prevent clotted blood from entering the trachea during testing. Without opening of the chest, an 18-gauge metallic cannula was inserted into the trachea through a midline cervical incision. Animals were then connected to a respiratory testing device (Flexivent, SCIREQ, Montreal, Canada). Immediate postmortem ventilation was performed according to parameters considered standard in mice: tidal volume, 10 mL/kg; respiratory rate, 150 breaths per minute, and positive end-expiratory pressure, 2 cm H2O. The ventilator obtains forced-oscillation measurements by analyzing pressure and volume signals acquired in reaction to input signals (perturbations) supplied by computer-controlled graphite pistons. The validity of these measurements has been described elsewhere and is comparable to that of other mechanical ventilator systems.17
Histology and atelectasis quantification.
After 60 min of anesthesia using isoflurane delivered in either 100% or 21% oxygen, the lungs were removed from the deeply anesthetized animals (to accurately represent the state of alveoli during respiration and prevent postmortem alveolar collapse) and placed in 10% neutral buffered formalin. The lungs were not inflated with fixative, to prevent expanding alveoli that might have collapsed under anesthesia. Tissue was fixed for a minimum of 5 d. After fixation, lungs were placed into cassettes for processing and embedding in paraffin blocks. After sectioning, 3 nonconsecutive sections (50 μm apart) were deparaffinized and stained with hematoxylin and eosin. Individual whole sections were scanned (Aperio, Leica Biosystems, Buffalo Grove, IL). Slides were assigned a number such that all quantitation analysis could be performed by an observer blinded to the treatment group. By using Image J software (http://rsbweb.nih.gov/ij/), the total area of lung was outlined manually. Within the lung section, the total area of tissue was calculated by using a thresholding technique that distinguished stained area (tissue) from unstained area (open airspace). The total lung area, the total area occupied by tissue, and the total area occupied by open airspace were calculated for the entire lung field from each slide (3 slides per animal, 5 animals per group). Large vessels and bronchi were excluded from the thresholded area.
μCT scanning and morphometric analysis for atelectasis quantification.
After 60 min of anesthesia with isoflurane delivered in either 100% or 21% oxygen, rat lungs were removed under deep anesthesia (to accurately represent the state of alveoli during respiration and prevent postmortem alveolar collapse) and fixed in 10% neutral buffered formalin. The lungs were not inflated with fixative to prevent expanding alveoli that may have collapsed under anesthesia. Fixed lungs were immersed in an aqueous solution of 1% (w/v) phosphomolybdic acid (Sigma-Aldrich, St Louis, MO) for 3 d. Lungs were scanned by using nanoCT (Nanotom-s, Phoenix X-ray, GE Measurement and Control, Wunstorf, Germany). Scan parameters included an 80-kV and 160-μA X-ray source and 1250-ms exposure time, for a total scan time of 1 h. Images were reconstructed at a 3-μm isotropic voxel size and converted to DICOM format by using VGStudio MAX 2.1 (Volume Graphics, Heidelberg, Germany) for analysis. Slides were assigned a number such that all quantitation analysis could be performed by an observer blinded to treatment group. μCT images were converted to 8-bit image files that contained a mean of 2000 slices. Three slices (depth: 1200, 2100, and 3000 μm) from 5 animals per group were quantitated. Lung parenchyma was outlined, and the thresholding tool was used to manually differentiate and quantitate the total area occupied by parenchyma compared with that occupied by open alveoli in each slide (Image J software; http://rsbweb.nih.gov/ij/). The total lung area, total lung area occupied by open airspace, and total lung area occupied by parenchymal tissue were quantitated.
Statistics.
Values were calculated and graphics were generated by using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Data are presented as mean ± SEM (n = 10 animals per group). For comparisons of 2 groups at a single time point, significance was determined by calculating a P value by using a Student t test. P values less than 0.05 were considered significant. For data comparing 2 groups over multiple time points, 2-way, repeated-measure ANOVA was used. When differences were significant, posthoc Bonferroni correction was performed. For flow–volume loops, the pressure–volume loop data were generated by using Flexivent software, exported, and analyzed by using Excel (Microsoft, Redmond, WA), and graphics were generated by using GraphPad Prism 5.0. Data are expressed as a mean lung volume for 10 animals per group. Statistical significance was determined by using the mean lung volume at 30 cm H2O.
Results
Effect of delivery gas for isoflurane in mice. Physiologic parameters.
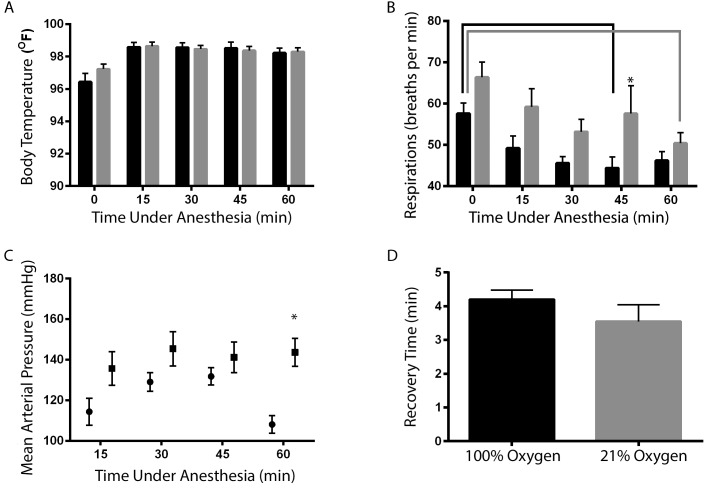
After 60 min of isoflurane anesthesia, none of the monitored parameters differed between animals receiving 100% oxygen compared with 21% oxygen as a carrier gas (body temperature: 100% oxygen, 96.4 ± 0.5 °F; 21% oxygen, 96.3 ± 0.9 100% oxygen; respiratory rate: 100% oxygen, 67.3 ± 9.2 breaths per minute; 21% oxygen, 63.6 ± 8.0 breaths per minute; mean arterial pressure: 100% oxygen, 104.4 ± 8.4 mm Hg; 21% oxygen, 88.1 ± 6.2 mm Hg; and recovery time: 100% oxygen, 2.9 ± 0.3 min; 21% oxygen, 3.2 ± 0.5). In addition, respiratory rate and body temperature were assessed at the onset of anesthesia. There was no difference between the onset of anesthesia and the 60-min time point in either the respiratory rate or temperature in either oxygen delivery group (respiratory rate: 100% oxygen at 0 min, 60.4 ± 12.1 breaths per minute, 100% oxygen at 60 min, 67.3 ± 9.2 breaths per minute; 21% oxygen at 0 min, 56.4 ± 11.9 breaths per minute; 21% oxygen at 60 min, 63.6 ± 8.0; body temperature: 100% oxygen at 0 min, 94.9 ± 0.9 °F; 100% oxygen at 60 min, 96.4 ± 0.5 °F; 21% oxygen at 0 min, 94.6 ± 0.7 °F; 21% oxygen at 60 min, 96.3 ± 0.9 °F).
Blood gas analysis.
There were no differences in pH or PaCO2 at either FiO2 (pH: 100% oxygen, 7.4 ± 0.0; 21% oxygen 7.4 ± 0.1; PaCO2: 31.6 ± -3.4 100% oxygen, ± 30.4 mm Hg; 21% oxygen, 29.4 ± 4.2 mm Hg) . Animals receiving isoflurane delivered in 21% oxygen were adequately oxygenated, because they had a mean SpO2 of 97%, which did not differ from that of the 100% oxygen group (P = 0.08, data not shown). However, mice that received isoflurane delivered in 21% oxygen had a lower PaO2 than did animals that received isoflurane in 100% oxygen (P < 0.0001; Figure 1 A). Mice provided 100% oxygen had an A-a gradient much higher than expected for their inspired oxygen concentration, whereas those given 21% oxygen had an A-a gradient consistent with the expected value for their inspired oxygen concentration (Figure 1 B).
Figure 1.
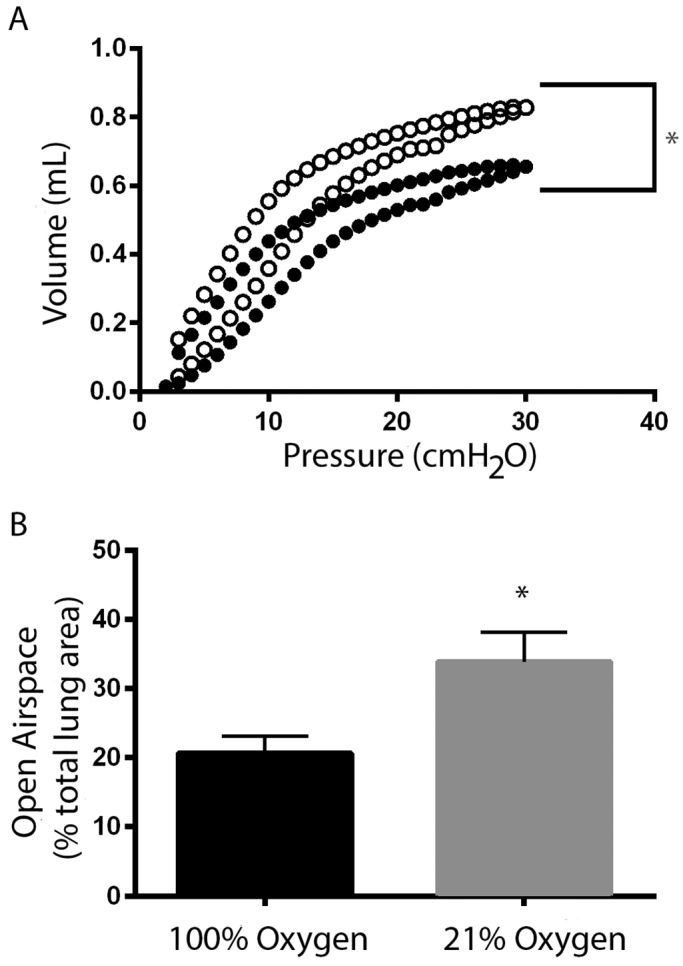
Respiratory mechanics. After 60 min of isoflurane anesthesia, animals that received isoflurane in 100% oxygen (solid circles) had more atelectasis than did those that received isoflurane in 21% oxygen (open circles), according to (A) flow–volume loop measurement (n = 10 per group) and (B) histology (n = 5 per group). *, Values significantly (P < 0.05) different between 21% oxygen and 100% oxygen at a given time point.
Pressure–volume hysteresis.
Hysteresis refers to the nonlinear nature of lung compliance. Transpulmonary pressure at a given volume during inhalation is less than the transpulmonary pressure at the same volume during exhalation. As such, each measurement on the pressure–volume curve has a lower point corresponding to inhalation and an upper point corresponding to exhalation. At each applied pressure, the 21% oxygen group had a greater lung volume (that is, alveoli expanded more easily) than did the 100% oxygen group (as illustrated by the fact that they are higher on the curve at each point). This pattern demonstrates that the use of 21% oxygen to deliver isoflurane to mice improved compliance curves compared with those of animals given isoflurane in 100% oxygen (Figure 1 A, n = 10 animals per group).
Histomorphometric analysis.
Histologic sections were evaluated for the total lung area, total lung area occupied by open airspace, and total lung area occupied by parenchymal tissue. The percentage of total lung area occupied by open airspace was lower in animals that had isoflurane delivered in 100% oxygen compared with delivery in 21% oxygen (P = 0.017; Figure 1 B, n = 5 mice per group).
Effect of isoflurane delivery gas on rats. Physiologic parameters.
Delivery gas had no effect on temperature (P = 0.276, Figure 2 A) or recovery time in rats (Figure 2 D, P = 0.270). In both groups, the body temperature was lower immediately after induction (time 0) than at all subsequent time points (15, 30, 45, and 60 min, Figure 2 A). Respiratory rate decreased between 0 and 45 min in the 100% oxygen group (P = 0.025) and between 0 and 60 min in the 21% oxygen group (P = 0.004, Figure 2 B). Respiratory rate at 45 min of anesthesia was lower in the 100% oxygen group than in the 21% oxygen group (P = 0.047, Figure 2 B). After 60 min of anesthesia, mean arterial pressure was higher in rats receiving isoflurane in 100% oxygen compared with 21% oxygen (P = 0.001, Figure 2 C). The mean arterial pressure of the 21% oxygen group was similar to previously reported values for isoflurane-anesthetized rats,10,11 whereas that of the 100% oxygen group was higher than expected for isoflurane-anesthetized animals.10,11
Figure 2.
Effects of isoflurane delivery gas on physiologic values (mean ± SEM; n = 10) in rats. (A) Body temperature at 15, 30, 45, or 60 min did not differ between 100% oxygen (black bars) compared with 21% oxygen (gray bars). (B) Respiratory rate was lower (*, P < 0.05) in the 100% oxygen group than the 21% oxygen group at 45 min of anesthesia. Respiratory rate differed between time points within both the 100% oxygen (black bracket) and 21% oxygen (gray bracket) groups. (C) Mean arterial pressure was significantly increased (*, P < 0.05) at 60 min in animals receiving isoflurane in 100% oxygen at 60 min compared with 21% oxygen. (D) Recovery time did not differ between the 2 groups.
Blood gas analysis.
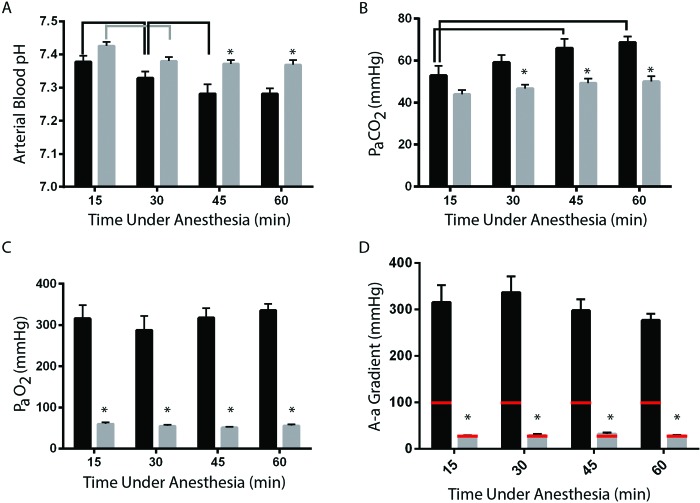
Unlike mice, rats had carrier-gas–associated differences in pH and PaCO2. In both oxygen-delivery groups, pH dropped between 15 and 30 min time points (21% oxygen, P = 0.015; 100% oxygen, P = 0.008; Figure 3 A), although both values were within physiologically normal range. In the 21% oxygen group, pH stabilized and did not drop after 30 min (Figure 3 A). In the 100% oxygen group, pH continued to decrease during anesthesia and was lower at 45 min than at 30 min (P = 0.012, Figure 3 A). pH was lower at 45 (P = 0.001) and 60 min (P = 0.002) in rats given isoflurane in 100% oxygen compared with 21% oxygen (Figure 3 A). At both of these time points, pH was below the normal range for isoflurane-anesthestized rats. PaCO2 was higher in animals given 100% oxygen than 21% oxygen at 30, 45, and 60 min (P = 0.025, P = 0.001, P = 0.0003, respectively; Figure 3 B). The 21% oxygen group showed no changes in PaCO2 over time (Figure 3 B), but in the 100% oxygen group, PaCO2 rose over time and was significantly higher after 45 and 60 min of anesthesia than it was after 15 min of anesthesia (P = 0.0011 and P < 0.0001, respectively; Figure 3 B). At every time point, the A-a gradient was higher in rats anesthetized by using isoflurane in 100% oxygen than in those provided isoflurane in 21% oxygen (P < 0.0001 for all comparisons; Figure 3 D). Animals exposed to 100% oxygen had an A-a gradient that was approximately 3 times higher than that expected for their inspired oxygen concentration, whereas rats given 21% oxygen had an A-a gradient that was approximately 1.5 times higher than expected.
Figure 3.
Effects of isoflurane delivery gas on blood-gas values in rats. (A) The pH at 45 and 60 min was significantly (*, P < 0.05) lower in animals in which isoflurane was delivered in 100% oxygen (black bars) compared with 21% oxygen (gray bars). Arterial blood pH differed between time points within both the 100% oxygen (black bracket) and 21% oxygen (gray bracket) groups. (B) PaCO2 at 30, 45, and 60 min was significantly (*, P < 0.05) higher (hypercapnia) in animals that received isoflurane in 100% oxygen compared with 21% oxygen but did not differ within groups (black bars, 100% oxygen). (C) PaO2 differed (*, P < 0.05) between the 100% oxygen and 21% oxygen groups at all time points. Hypoxemia was detectable in both groups by 15 min and was consistent within groups throughout the 60-min anesthesia period. (D) The A-a gradient was approximately 1.5 times the expected value (red bar) in animals that received isoflurane in 21% oxygen but was approximately 3 time the expected value in the 100% oxygen group.
Morphometric analysis.
To quantitate atelectasis, we calculated the total lung area, total lung area occupied by open airspace, and total lung area occupied by parenchymal tissue by using μCT images of the accessory lung lobe from rats that had been anesthetized with isoflurane delivered in 100% oxygen or 21% oxygen for 60 min. The percentage of total lung area occupied by open airspace did not differ between delivery gases (data not shown, P = 0.100).
Discussion
Although the benefit of decreased inspired-oxygen concentrations to deliver isoflurane is well-documented in human medicine, results are mixed in various veterinary species.8,14,15
In the current study, we sought to better define the effect of delivery gas (21% or 100% oxygen) on physiologic variables and atelectasis in mice and rats. To this end, we delivered isoflurane anesthesia through a nose cone without intubation or mechanical ventilation. We chose this approach in an effort to mimic the most likely anesthetic scenario in rodents. We selected the 60-min time point because it covers most surgical procedures performed in rodents at our institution.
In mice, the delivery gas did not affect physiologic parameters—including body temperature, respiratory rate, mean arterial pressure, anesthetic recovery time, pH, and PaCO2—during the 60-min period. The physiologic values that we measured are similar to those others have observed in isoflurane-anesthetized rodents2,3,9-11,16
Analysis of arterial blood showed that PaO2 was lower than expected for animals breathing 100% oxygen, demonstrating that they experienced relative hypoxemia. The clinical differential diagnoses for decreased PaO2 include diffusion impairment, anatomic right-to-left shunt, hypoventilation, decreased inspired oxygen concentration, and ventilation–perfusion inequality. Diffusion impairments and anatomic shunts are exceedingly rare in mice and can be eliminated as the cause of relative hypoxemia in multiple animals simultaneously. In addition, the lack of grossly or histologically apparent interstitial lung disease further argues against diffusion impairment. We also ruled out hypoventilation as a cause of hypoxemia, given that both groups (21% oxygen and 100% oxygen) were normocapnic. Although PaO2 is dependent on the inspired oxygen concentration, a normal PaO2 is approximately 5 times the inspired oxygen concentration.15 Therefore, the expected PaO2 is approximately 500 mm Hg in the 100% oxygen group and 100 mm Hg in the 21% oxygen group. To rule out the possibility of decreased oxygen concentration due to mixing of gasses in the nose cone, we measured the oxygen concentration at nose level throughout the 1-h period during which mice were anesthetized with isoflurane in 100% oxygen. When oxygen flow rate was set at 1 L/min, the oxygen concentration in the nose cone after 60 min was 96%. Although this concentration was lower than 100%, an abnormal PaO2 of 216 mm Hg could be achieved in a healthy animal only if the inspired oxygen concentration were approximately 50%. Given these data, we ruled out reduced oxygen concentration as the cause of the decreased PaO2 in our 100% oxygen group. Thus, the only remaining differential for this relative hypoxemia was a ventilation–perfusion mismatch.
Whenever ventilation–perfusion mismatch is suspected clinically, the A-a gradient should be calculated. We expected the A-a gradient to be higher in animals that received isoflurane in 100% oxygen compared with 21%, given that the A-a gradient depends on the inspired oxygen concentration (normal is 1 mm Hg per 1% inspired oxygen concentration). The animals given isoflurane in 21% oxygen had a normal A-a gradient (relative to the FiO2) of 19 mm Hg, but those that received 100% oxygen had an elevated A-a gradient (433 mm Hg). Therefore, we hypothesized that the cause of the relative hypoxemia in our 100% oxygen animals was a ventilation–perfusion mismatch due to atelectasis. We were able to quantitate decreased lung compliance (which is suggestive of atelectasis) in animals receiving isoflurane in 100% oxygen as compared with 21% by using quasi-static pressure–volume curves obtained from a ventilator system. The system in the current study uses a forced oscillation method to create pressure–volume curves to measure respiratory system mechanics.1,16 Static lung compliance is the ease with which the lungs can be expanded and is defined as the change in volume for any given applied pressure. Fibrosis and atelectasis are the most common causes of decreased compliance. Histologic evaluation revealed greater atelectasis but no evidence of fibrosis in the 100% oxygen group compared with the 21% animals. Therefore, the most likely cause of the reduced lung compliance in our 100% oxygen group is atelectasis.
The likely mechanism of the increased atelectasis in animals that received isoflurane in 100% oxygen is absorption atelectasis. Absorption atelectasis occurs in the 100% group because oxygen is extremely soluble, such that when 100% oxygen is used to deliver isoflurane to a patient, the oxygen quickly diffuses across the alveoli into the bloodstream, thereby accelerating the rate of alveolar collapse. However, in compressed room air (21% oxygen), 78% of the mixture is nitrogen, which is far less soluble than oxygen and remains in the alveoli longer, thus helping to preserve alveolar patency and slowing alveolar collapse.
To determine whether the response to isoflurane delivery gas showed species-associated differences, we also measured physiologic variables in rats anesthetized with isoflurane delivered in 100% or 21% oxygen. Similar to findings from mice, no differences were seen in the body temperature or recovery time between the 100% and 21% oxygen groups. Although statistical significance was not reached, the respiratory rates in rats given 100% oxygen showed a trend toward being lower than that in the 21% oxygen group at all time points (Figure 2 B). This finding is important because respiratory depression can lead to hypercapnia during anesthesia. Indeed, PaCO2 at 30, 45 and 60 min of anesthesia was higher in rats given 100% oxygen than in those that received 21% oxygen (Figure 3 B). In humans, high concentrations of inspired oxygen are well known to directly interact with breathing by decreasing the activity of the peripheral chemoreceptors at the carotid bodies and to modify central drive by decreasing the slope of the ventilatory CO2 response of the central chemoreceptors in the brainstem5. The resultant hypoventillation is the likely cause for respiratory acidosis seen on the blood gas analysis (Figure 3 A). Therefore, unlike mice, rats exposed to 100% oxygen developed respiratory acidosis by 30 min of isoflurane anesthesia. Given that rat physiologic variables showed changes by 15 min (and these values were not measured prior to anesthesia), we cannot exclude the possibility of differences between animals prior to the start of the experiment. Such differences are unlikely, however, because animals were randomly assigned to their treatment groups, were young, appeared healthy prior to experimentation, were free of several pathogens that might affect the lungs, and showed no gross lung pathology at the termination of the experiment.
In addition, rats showed much greater variation in mean arterial blood pressure than did mice. Delivery of isoflurane in 100% oxygen was associated with mean arterial pressures that trended toward being higher than those in the 21% oxygen group (at 15, 30, and 45 min) and that were consistent with hypertension at all time points (Figure 2 C). However, mean arterial pressure at the 60 min time point was increased in the 100% oxygen group (Figure 2 C). Although the mechanism is not well understood, hypercapnia can stimulate the sympathetic nervous system, leading to peripheral vasoconstriction and subsequent hypertension.12,13 Therefore, hypercapnia is a potential cause of the hypertension we observed in the 100% oxygen rats.
At all time points, rats given 21% oxygen had PaCO2 values that were consistent with previous reports from isoflurane-anesthetized rats, whereas PaCO2 values were higher than expected in the 100% oxygen group.10,11 Animals receiving isoflurane in 21% and 100% oxygen had a relative hypoxemia at every time-point (Figure 3 C). This was not due to lower than expected inspired oxygen concentration, as the direct measurement of oxygen concentration within the nose cone was at least 94% through the period of anesthesia for rats.
Relative hypoxemia was detectable in both groups of rats by 15 min and, unlike the scenario in mice, both groups had the same degree of relative hypoxemia (lower than expected PaO2 values) at all time points (Figure 3 C). The PaO2 for both oxygen delivery concentrations was 3 times the FIO2 (normal, 4 to 5 times FIO2). Therefore, unlike mice, in which only the use of 100% oxygen led to relative hypoxemia, both 21% and 100% oxygen led to relative hypoxemia (by 15 min) in rats. In addition, the hypoxemia in the rats that received isoflurane in 21% oxygen can be defined as true hypoxemia, because the PaO2 was less than 80 mm Hg. Unlike mice, both groups of rats had A-a gradients that were higher than expected, suggesting that ventilation–perfusion mismatch is present regardless of the delivery gas used, although the elevation was greater in the rats provided 100% oxygen compared with the 21% group.
Given that relative hypoxemia was similar in both groups of rats, it was unsurprising that morphometric quantitation of μCT images to compare total lung volume occupied by open airspace did not differ between the groups (data not shown, P = 0.1). However, because lung physiologic analysis was not performed in rats, we cannot exclude the possibility that subtle differences in lung compliance existed between groups but could not be detected by using μCT imaging. In addition, there were some limitations to the imaging methodology used for this study. For our analysis, we manually quantitated 3 slices of usable data from a single lung lobe for each animal. The development of a more sophisticated, automated image-analysis methodology might enable the detection of differences in atelectasis between the 2 groups, if these differences exist.
The reason for the differential physiologic responses to the isoflurane carrier gas between mice and rats is unknown. One possibility is that relative hypoxemia is apparent in both groups of rats but not mice due to the increased weight of the abdominal organs of rats. During anesthesia in dorsal recumbency, the weight of the abdominal organs in rats might cause increased compressional atelectasis (similar to the situation in horses). Therefore, absorption atelectasis might contribute less to the overall atelectasis during anesthesia in rats compared with mice. Alternatively, the oxygen percentage used to deliver isoflurane might have different effects on depth or rate of respiration between the 2 species, leading to the different responses observed.
In this study, we were able to demonstrate that mice—but not rats—exposed to isoflurane delivered in 100% oxygen had significantly more atelectasis than did animals given isoflurane delivered in 21% oxygen. The time point chosen for our evaluation was 60 min. Because absorption atelectasis is FiO2- and time-dependent, we suspect that this atelectasis would be less significant at earlier points and would continue to progress over time. Persistent atelectasis could lead to atelectrauma, which is typically related to shear stress as a result of cyclic opening and collapsing of the alveoli, potentially leading to damage of pulmonary capillaries and type I and type II pneumocytes.6 The damage to these cells can cause subsequent disruption of epithelial fluid transport and eventually edematous alveoli.6 Edema in alveoli triggers the recruitment of alveolar macrophages to the area, which then secrete inflammatory cytokines, which in turn can prompt neutrophil activation and release of additional inflammatory mediators.6 Thus, atelectasis during anesthesia can lead to a proinflammatory state in the lungs.
An additional consideration regarding the delivery gas for isoflurane is the safety concern related to its use in the operating room or laboratory. Fire has been identified as a potential danger in human operating rooms.12,18 In addition, the CompMed listserv hosted a lengthy discussion about the potential dangers associated with use of 100% oxygen in veterinary operating rooms. Therefore, the use of 21% oxygen to deliver isoflurane to rodents is not only physiologically beneficial to the anesthesia subjects but might also prevent potential fire-related injury to animals and humans.
Taken together, this information can be used to make recommendations regarding isoflurane carrier gas for rodents that are based solidly on physiologic data. Our results suggest that both 100% and 21% oxygen are acceptable for the delivery of isoflurane to mice. However, mice anesthetized for studies focused on lung physiology or architecture would likely benefit from the delivery of isoflurane in 21% oxygen, to limit alveolar atelectasis and the potential associated downstream inflammatory effects. For rats, the delivery of isoflurane in 21% and 100% oxygen both caused perturbations in physiologic variables, and the choice of carrier gas is less straightforward and may depend on the goals of the individual study.
References
- 1.Bates JHT, Suki B. 2008. Assessment of peripheral lung mechanics. Respir Physiol Neurobiol 163:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesarovic N, Nicholls F, Rettich A, Kronen P, Hässig M, Jirkof P, Arras M. 2010. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim 44:329–336. [DOI] [PubMed] [Google Scholar]
- 3.Constantinides C, Mean R, Janssen BJ. 2011. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J 52:e21–e31. [PMC free article] [PubMed] [Google Scholar]
- 4.Cotroneo TM, Hugunin KMS, Shuster KA, Hwang HJ, Kakaraparthi BN, Nemzek-Hamlin JA. 2012. Effects of buprenorphine on a cecal ligation and puncture model in C57BL/6 mice. J Am Assoc Lab Anim Sci 51:357–365. [PMC free article] [PubMed] [Google Scholar]
- 5.Dahan A, DeGoede J, Berkenbosch A, Olievier IC. 1990. The influence of oxygen on the ventilatory response to carbon dioxide in man. J Physiol 428:485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duggan M, McNamara PJ, Engelberts D, Pace-Asciak C, Babyn P, Post M, Kavanagh BP. 2005. Oxygen attenuates atelectasis-induced injury in the in vivo rat lung. Anesthesiology 103:522–531. [DOI] [PubMed] [Google Scholar]
- 7.Edmark L, Auner U, Enlund M, Ostberg E, Hedenstierna G. 2010. Oxygen concentration and characteristics of progressive atelectasis formation during anaesthesia. Acta Anaesthesiol Scand 55:75–81. [DOI] [PubMed] [Google Scholar]
- 8.Hubbell JAE, Aarnes TK, Bednarski RM, Lerche P, Muir WW. 2011. Effect of 50% and maximal inspired oxygen concentrations on respiratory variables in isoflurane-anesthetized horses. BMC Vet Res 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen BJA, De Celle T, Debets JJM, Brouns AE, Callahan MF, Smith TL. 2004. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol 287:H1618–H1624. [DOI] [PubMed] [Google Scholar]
- 10.Krafft P, Frietsch T, Lenz C, Piepgras A, Kuschinsky W, Waschke KF. 2000. Mild and moderate hypothermia (α-stat) do not impair the coupling between local cerebral blood flow and metabolism in rats. Stroke 31:1393–1401. [DOI] [PubMed] [Google Scholar]
- 11.Ori C, Dam M, Pizzolato G, Battistin L, Giron G. 1986. Effects of isoflurane anesthesia on local cerebral glucose utilization in the rat. Anesthesiology 65:152–156. [DOI] [PubMed] [Google Scholar]
- 12.Rinder CS. 2008. Fire safety in the operating room. Curr Opin Anaesthesiol 21:790–795. [DOI] [PubMed] [Google Scholar]
- 13.Somers VK, Mark AL, Abboud FM. 1988. Sympathetic activation by hypoxia and hypercapnia–implications for sleep apnea. Clin Exp Hypertens A 10 Suppl 1:413–422. [DOI] [PubMed] [Google Scholar]
- 14.Staffieri F, De Monte V, De Marzo C, Grasso S, Crovace A. 2010. Effects of 2 fractions of inspired oxygen on lung aeration and gas exchange in cats under inhalant anaesthesia. Vet Anaesth Analg 37:483–490. [DOI] [PubMed] [Google Scholar]
- 15.Staffieri F, Franchini D, Carella GL, Montanaro MG, Valentini V, Driessen B, Grasso S, Crovace A. 2007. Computed tomographic analysis of the effects of 2 inspired oxygen concentrations on pulmonary aeration in anesthetized and mechanically ventilated dogs. Am J Vet Res 68:925–931. [DOI] [PubMed] [Google Scholar]
- 16.Szczesny G, Veihelmann A, Massberg S, Nolte D, Messmer K. 2004. Long-term anaesthesia using inhalatory isoflurane in different strains of mice—the haemodynamic effects. Lab Anim 38:64–69. [DOI] [PubMed] [Google Scholar]
- 17.Vanoirbeek JAJ, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PHM, Verbeken E, Decramer M, Nemery B, Janssens W. 2010. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol 42:96–104. [DOI] [PubMed] [Google Scholar]
- 18.Yardley IE, Donaldson LJ. 2010. Surgical fires, a clear and present danger. Surgeon. 8:87–92. [DOI] [PubMed] [Google Scholar]