Abstract
Human patient-derived xenograft (PDX) tumors, propagated in immunodeficient mice, are rapidly growing in use as a model for cancer research. Horizontal transfer between mice, without in vitro cell culture, allows these tumors to retain many of their unique characteristics from their individual patient of origin. However, the immunodeficient mouse strains used to grow these tumors are susceptible to numerous opportunistic pathogens, including Corynebacterium bovis. At our institution, 2 in vivo tumor banks of PDX tumors had been maintained within nude mouse colonies enzootically infected with C. bovis. Elimination of C. bovis from these colonies required the aseptic harvest and horizontal transfer of tumor tissue between infected and naïve recipient mice without cross-contamination. Out of necessity, we developed a standard operating procedure using enhancements to traditional aseptic surgical technique with concurrent application of both procedural and physical barriers to prevent C. bovis transmission. By using these methods, all 61 unique PDX tumor models were successfully harvested from C. bovis-infected mice and transferred into recipient mice without transmission of infection. Our data demonstrate that, in situations where C. bovis-free colonies can be established and maintained, this procedure can successfully be used to eliminate C. bovis from an in vivo tumor bank of valuable PDX tumors.
Abbreviations: CHG, chlorhexidine gluconate; PDX, patient-derived xenograft; qPCR, quantitative real-time PCR
In recent years, the use of human patient-derived xenograft (PDX) tumor tissue grown in immunodeficient mice for cancer research has increased dramatically. The evolving interest in PDX tumor models reflects a better understanding of the importance of tumor heterogeneity in the human population. This diversity may not be mimicked by the limited number of immortalized cell lines in a designated panel of a given tumor type.6,34 Further investigation into PDX tumor models has led to the validation of their biologic stability, because these human tumor pieces grow and retain their architecture, receptors, and gene expression by exclusive horizontal transfer between immunodeficient mice without in vitro cell culture. In addition, the ability to expand PDX tumor tissue into large cohorts of mice allows for novel therapeutic development and a tool used in ‘precision medicine’ to create a patient's individually tailored cancer therapy regimen.28,33,35
Many of the immunodeficient mouse strains commonly used to propagate human PDX tumor tissues are susceptible to opportunistic pathogens, including Corynebacterium bovis.2,9,13 C. bovis is commonly associated with ‘scaly skin disease’ in athymic nude mice (Foxn1, nu/nu). However this characteristic clinical illness does not always manifest after infection and is believed to develop into life-long subclinical skin colonization.3,10,19 Clinical symptoms that can accompany scaly skin in nude mice include lethargy, dehydration, and decreased body condition.3,5 In our experience, these clinical symptoms cause delays in planned experimental procedures until spontaneous resolution of these clinical signs. In haired SCID and NSG (NOD.Cg-PrkdcscidIl2rgtm1Wgl/SzJ) mice, clinical symptoms of C. bovis infection vary but include rough hair coat and decreased body condition, with mildly scaly skin visible in areas of alopecia and periocular erythema.3,17,27
Reports from an international diagnostic laboratory indicate that C. bovis is a common contaminant of susceptible mouse populations,10,25 and continues to plague academic and industry research facilities.8,29 An early report characterizing a multifacility outbreak of C. bovis in Italy suggested that the sharing of tumor lines between facilities may be a potential route of cross-contamination between susceptible mouse colonies.26 Data recently presented by 2 international diagnostic laboratories showed that 4% and 13% of cultured cell lines or solid tumor tissue samples submitted for human or rodent pathogen testing by PCR assay were contaminated with C. bovis DNA.8,13 Although C. bovis does not penetrate the epidermis or cause sepsis of infected nude mice,3,5 failure to perform an aseptic surgical harvest of the tumor tissue is the most likely cause of contamination of solid tumor tissue. Published protocols on performing horizontal transfer between mice or for cryopreservation of PDX tissue harvested from mice recommend applying 70% ethanol to the tumor excision site9,15,21 or submerging the euthanized mouse carcass in 75% ethanol for 2 min14 or completely fail to address skin disinfection of the donor mouse.36 The majority of these protocols minimally address skin disinfection before harvest, but none take into consideration the disease status of the mice donating or receiving the tumor tissue. With the generation and transfer of biologic material between research institutions, cross-contamination of opportunistic pathogens such as C. bovis is a concern in regard to the preservation of the overall colony health status.24 In fact, we have recently presented data demonstrating the transmission of C. bovis to naïve, PDX-recipient nude mice from C. bovis-contaminated cryopreserved PDX tissue.20
In the fall of 2012, C. bovis needed to be eliminated from 2 large in vivo tumor banks of unique PDX models maintained within enzootically infected nude mouse colonies. The objective of this project was to develop and validate a protocol to prevent C. bovis contamination of PDX tumor tissue during tumor harvest and to prevent cross-contamination during direct horizontal tumor transfer between infected donor and naïve recipient mice.
Materials and Methods
Survey of cryopreserved PDX tissues for C. bovis.
Five vials, each containing a different PDX tumor model, were randomly selected from cryopreserved tissue banks of 2 independent research laboratories (labs A and B, n = 10). Both laboratories maintain in vivo and cryopreserved tumor banks and were pursuing C. bovis-negative status. However, these tumor tissues were harvested from enzootic colonies, prior to the implementation of the C. bovis remediation program. Cryopreserved (–80 °C) tissues were assayed for C. bovis DNA by qPCR analysis (as described following). This assessment was used to evaluate the aseptic technique that had been used previously for PDX tumor harvest and to estimate the extent of C. bovis contamination within these cryopreserved PDX tissue banks.
PDX donor and recipient mice.
All mouse manipulations and procedures were approved by the University of Colorado Denver IACUC. Female, athymic nude mice (Hsd:Athymic Nude-Foxn1nu; Envigo Laboratories, Indianapolis, IN) were obtained to receive horizontally transferred (mouse-to-mouse) PDX tumor tissue, with the goal of maintaining an in vivo PDX tumor bank. From the vendor, nude mice were documented to be free of endoparasites and ectoparasites by microscopy, free of lactate dehydrogenase elevating virus, Helicobacter spp., Corynebacterium bovis, Pneumocystis murina, and Streptobacillus moniliformis by PCR assay, and free of Bordetella bronchiseptica, Citrobacter rodentium, Corynebacterium kutscheri, Klebsiella oxytoca, K. pneumonia, Pasteurella multocida, P. pneumotropica, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella spp., Staphylococcus aureus, Streptococcus spp. group B , and Streptococcus pneumonia by culture. Serology from immunocompetent sentinel mice of the athymic nude stock confirmed the absence of lymphocytic choriomeningitis virus, minute virus of mice, mouse parvovirus, mouse hepatitis virus, mouse adenovirus type 1 and 2, mouse cytomegalovirus, mouse polyoma virus, mouse rotavirus, mouse thymic virus, murine norovirus, pneumonia virus of mice, respiratory enteric virus III, Sendai virus, Theiler murine encephalomyelitis virus, cilia-associated respiratory bacillus, Clostridium piliforme, Mycoplasma pulmonis, and Encephalitozoon cuniculi. All mice were confirmed by qPCR analysis to be C. bovis-negative on arrival and prior to distribution to researchers.
At the time of scheduled tumor harvest, PDX donor nude mice were enrolled in the study. By using the tissue harvest method described following, harvested tissues were evaluated for C. bovis DNA contamination. In light of historical diagnostic testing and clinical signs, all PDX donor mice in the in vivo tumor bank were considered to be C. bovis positive. To confirm, a dry sterile swab (BBL Culture Swab EZ, Becton Dickinson, Franklin Lakes, NJ) was used to sample both the oral cavity and skin and then assayed for C. bovis DNA by qPCR. All C. bovis-free, recipient nude mice were housed in dedicated housing rooms that were physically and procedurally separated from C. bovis-positive mice. Management of facilities; macroenvironmental parameters; biocontainment practices; sentinel monitoring programs; routine husbandry; intracage provisions; IVC rack system (Allentown, Allentown, NJ), IVC rack, and caging sanitation; and environmental sanitation performed to maintain a C. bovis-free environment are described in detail elsewhere.20
Aseptic tumor harvest and transfer procedure.
Aseptic surgical technique training by a veterinarian was provided to the researchers, who performed tumor harvests prior to the beginning of the study. The entire harvest and transfer procedure was designed to be performed by 2 people. One person harvested tissue from presumed or confirmed C. bovis-positive mice within procedural space that is considered to be C. bovis-contaminated. The second person received the aseptically harvested tumor tissue and performed the implantation procedure into naive mice in a procedural space considered C. bovis-free. PDX tumor models from donor mice were harvested when either of their bilateral flank tumors reached 2 cm in any plane, in accordance with humane endpoints established by the IACUC.
Tumor harvest.
Dependent on the discretion of the research laboratory, mice were euthanized by either cervical dislocation while under isoflurane anesthesia or by CO2 asphyxiation within the home cage by using a maximum of 20% displacement of cage air for 5 min, followed by cervical dislocation. After euthanasia, all carcass manipulations were performed within either a biosafety cabinet or an animal transfer station (ATS2; Allentown), both of which will be referred to as hoods from this point forward. A generic, clean, pipette tip box and lid for 200 -µL pipette tips was repurposed to hold 100 to 150 mL of 2% chlorhexidine gluconate solution (CHG; VetOne, Boise, ID). Immediately after euthanasia the intact carcass was completely submerged in CHG (Figure 1). After submersion, the carcass was manipulated with gloved fingertips for 15 to 30 s in the solution to ensure contact on all skin surfaces and then allowed to soak submerged for a minimum of 5 min.
While the carcass soaked in CHG, everything was removed from the hood, with the exception of the preheated glass bead-sterilizer (Germinator 500; CellPoint, Gaithersburg, MD), which is a burn hazard. The blower to the hood was turned off to allow presumed C. bovis-contaminated skin flakes to settle to the hood surface.2,4 All internal surfaces except the ceiling of the hood was sprayed with the ClO2 solution (mixed 1:18:1; Clidox-S, Pharmacal, Naugatuck, CT) and was wiped down with a Clidox-S-soaked disposable cloth (TERGOwipe by Rasco Aurora, CO). The hood blower was turned on and ran for a minimum of 5 min prior to reentering the work surface. This step allowed time for an acceptable air curtain to form and achieves a minimum 5 min contact-time for skin disinfection. Disposable nonsterile nitrile gloves were then changed.
Two sets of autoclaved instruments (2 forceps and 2 scissors), sterile gauze, 15- or 50-mL conical centrifuge tubes containing tumor transfer media (RPMI 1640 or DMEM F12 with 10% fetal calf serum and 1% penicillin and streptomycin), and the pipette tip box containing CHG and the submerged mouse were replaced inside the hood. The carcass was removed from the CHG bath, and excess solution was removed with sterile gauze. The sterile-tip technique was used for all sterile instrument manipulation. Nonsterile gloves were used to hold the handles of sterile instruments and C. bovis-contaminated skin. However, the ends of instruments that would touch sterile subcutaneous and tumor tissue were never touched by gloved fingers. One pair of scissors and forceps were used exclusively to touch and manipulate the exterior surface of the skin. These scissors were used to make a small cut in the skin, perpendicular to the dorsal midline of the carcass (Figure 1). From the small incision, the euthanized mouse's skin was stretched cranially and caudally to expose the subcutaneous tumors. The second set of instruments was then used exclusively to manipulate and cut subcutaneous tissues to free the tumor. Care was taken to ensure that the superficial surface of the mouse skin did not come into contact with the tumor tissue. Sections of the tumor that lay beneath ulcerated skin or that closely approximated skin were avoided and not harvested. By using the appropriate forceps, the freed tumor was placed into the transfer media.
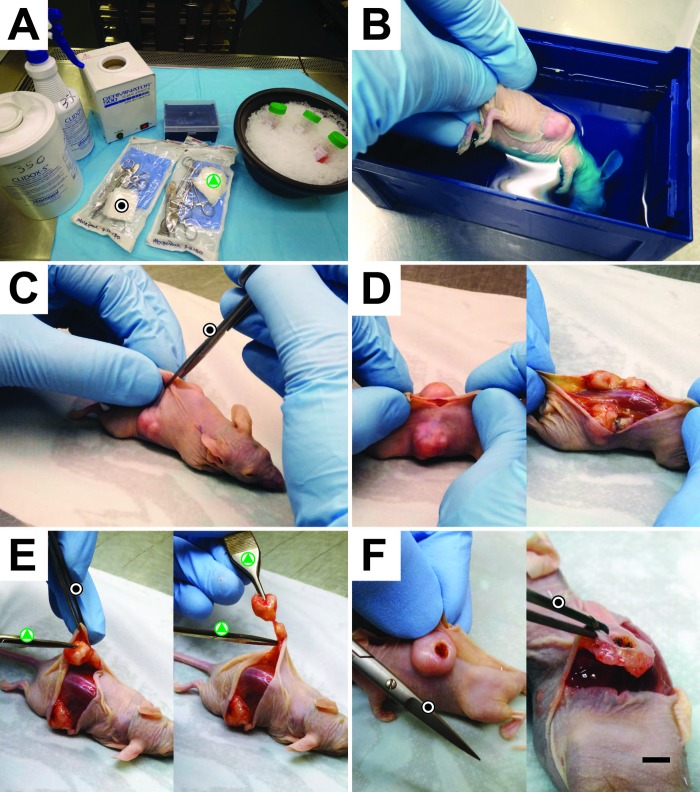
Figure 1.
PDX tumor harvest procedure. (A) Materials used during the harvest procedure. Black dot, sterile instruments used for skin contact only. Green triangle, sterile instruments used for subcutaneous tissue and tumor manipulation only. (B) Euthanized and cervically dislocated mouse was completely submerged into 2% CHG for a minimum of 5 min. (C) By using instruments dedicated for skin (black dot), a cut was made perpendicular to the dorsal midline, and (D) the skin was stretched rather than cut to expose the tumor tissue, to minimize instrument contact with the skin and subcutaneous tissues. (E) A second set of surgical instruments (green triangle) was used to free the tumor from subcutaneous tissue for extraction. (F) For tumors that are either adherent to the deep layers of the skin or have partial to full-thickness dermal ulcerations, a buffer zone of tumor tissue was left behind to ensure that tumor tissue was not contaminated with C. bovis. Scale bar, 4 mm.
In situations when more than one mouse carried the same PDX tumor model and required tumor harvest at the same time, a pipette tip box made for 200-µL tips was too small for all carcasses to be submerged concurrently. A generic pipette tip box for 1-mL pipette tips was used to accommodate all euthanized mice in 300 to 400 mL of CHG. In addition, the tips of instruments used to separately manipulate skin and tumor tissues were sterilized between mice by immersion in a bead sterilizer for 15 to 20 s, according to the manufacturer's instructions. Serial use of instruments did not exceed 5 mice, with the bead sterilizer used before each mouse after the first use.23
Tumor transfer.
Once tumor tissue was placed into transfer media and the cap closed, tubes were liberally sprayed with Clidox-S and wiped with a Clidox-S–soaked TERGO wipe. The tubes were then individually rolled in Clidox-S–soaked TERGO wipes for a minimum of 5 min prior to removal from the room. The person harvesting the tumors then brought the Clidox-S–wrapped tubes containing the tumor tissue to the C. bovis-free designated procedural area. Without entering the area, the harvester handed the tubes to the second person, who performed the horizontal tumor transfer into naïve recipient mice. The tubes were again liberally sprayed with Clidox-S and rewrapped in Clidox-S soaked TERGO wipes. The tumor tissue was then transferred into sterile petri dishes, and sterile instruments were used to mince the tissue into approximately 3 mm3 pieces within the transfer media. At this point, excess tumor tissue no longer needed for transfer was set aside for C. bovis DNA detection by qPCR assay.
Recipient mice were placed under isoflurane anesthesia. The procedure for inoculating recipient mice differs by laboratory. According to one procedure, the skin of the mouse was prepped with a single 70% alcohol wipe. Using autoclaved instruments, individual pieces of minced tumor were placed into the beveled end of a 12-gauge needle and trocar system (Cadence Science, Staunton, VA). The needle was introduced caudal to the scruff of the neck and advanced subcutaneously toward the hips (flanks) for tumor deposition. The skin was allowed to heal without intervention. For analgesia, one subcutaneous injection of meloxicam (2 mg/kg; Metacam, Boehringer Ingelheim Vetmedica, St Joseph, MO) was administered prior to anesthetic recovery. Alternatively, the skin of the mouse was prepared by using 3 rounds of alternating povidone–iodine and 70% isopropanol wipes. A 0.5-cm incision was made over each flank by using sterile iris scissors. A small subcutaneous pocket was made under the skin, and sterile forceps were used to grasp the skin; a second pair of forceps was placed the tumor tissue under the skin. The skin incision was then closed with a 7-mm wound clip. For analgesia, buprenorphine (0.05 mg/kg; Buprenex, Reckitt Benckiser Pharmaceuticals, Richmond, VA) was administered subcutaneously at the time of anesthetic induction and repeated every 12 h for 3 subsequent injections.
Surveillance of IVC rack exhaust air for C. bovis.
To ensure that PDX tumor-recipient mice maintained a C. bovis-negative status prior to and after horizontal tumor transfer, routine, weekly swabs were collected from the horizontal exhaust manifolds of the IVC rack for exhaust-air debris testing, as described previously.16,19 Briefly, a single dry, sterile swab was used to wipe the inside surface (60 cm2) of all 10 horizontal exhaust manifolds within the vertical exhaust plenum. Samples were stored at ambient temperature for 24 to 48 h prior to DNA isolation by the CU Denver Quantitative PCR Core.
DNA isolation and quantitative real-time PCR.
DNA was extracted from dry swabs (BBL Culture Swab EZ) used to collect samples from the horizontal exhaust manifold of IVC racks as well as oral and skin samples collected from athymic nude mice, as previously described.19 For DNA extraction from solid PDX tumor tissue, either 0.015 g or the entire tumor sample (0.5 to 1 g) was lysed in ATL buffer with proteinase K reagent (Qiagen, Germantown, MD). Lysis buffer was prepared according to the original sample weight, where 0.015 g of tissue was lysed in 0.2 mL buffer ATL and 20 μL proteinase K reagent (Qiagen). Larger tumor samples were lysed in 2 mL ATL with 200 μL proteinase K reagent (Qiagen). Tissues and lysis buffer were incubated at 56 °C until the tissue was completely lysed. DNA was extracted from 200 μL of digest supernatant by using the QIAamp DNA Mini Kit (Qiagen) according to the manufacturer's instructions. C. bovis qPCR reactions were performed on isolated DNA, by using all primers, parameters, and controls as previously described.19
Results
Cryopreserved tissues from PDX tumor bank.
Five randomly selected vials of cryopreserved PDX tumor tissue from 2 independent laboratories (A and B) were assayed by qPCR assay for C. bovis DNA (Table 1). These vials contained C. bovis-contaminated PDX tissue that was harvested prior to the use of the enhanced aseptic tumor harvest procedure and from enzootically infected colonies of nude mice. For laboratory A (n = 5), when a 0.015-g representative sample of solid tumor tissue was used from each vial, only 1 (20%) of the 5 vials was C. bovis-positive. The assay was then repeated by using the complete contents of each vial (solid tissue and media), and 3 (60%) of the 5 vials were C. bovis-positive. For laboratory B, the complete contents of each of the 5 vials were evaluated by qPCR assay; the results indicated that all 5 vials (100%) were C. bovis-positive.
Table 1.
Cryopreserved vials of PDX tissues evaluated by qPCR assay for C. bovis
| Lab | Sample | Sample sizea (g) | No. of vials positive/negative for C. bovis | Copy numberb,c |
| Ad | Tissue only | 0.015 | 1/5 (20%) | 1128 |
| Tissue & media | 0.5–1 | 3/5 (60%) | 1284 ± 664 | |
| B | Tissue & media | 0.5–1 | 5/5 (100%) | 1632 ± 817 |
0.5 to 1 g of tissue represents the entire contents of the cryopreservation vial
Data shown as mean ± 1 SD when appropriate
Copy number per gram of tissue was not calculated because results indicated that C. bovis is not evenly distributed in tumor samples
Results represent the same 5 vials of PDX tissue with 2 different sample sizes
Validation of tumor harvest procedure.
By using the described procedure, 61 unique PDX tumor models were harvested from 79 donor mice. For the donor mice of 34 of the different PDX tumor models, skin swabs and a representative sample of the harvested tumor tissue were assayed for C. bovis DNA. These data showed that 33 of 34 (97%) PDX tumor models tested were harvested from C. bovis-positive mice. The quantity of the tumor tissue digested for the qPCR assay for the first 17 tumor samples analyzed was 0.015 ± 0 g and contained solid tissue only. The sample weight of the second group of 17 tumor tissue samples analyzed was 0.14 ± 0.09 g, containing both solid tissue and culture media which represented all of the material provided. In total, all 34 harvested PDX tissue samples were negative for C. bovis DNA independent of the quantity or content of the sample digested for DNA isolation. After horizontal transfer of the aseptically harvested tumor tissue according to the described procedure, the 61 PDX tumor models were propagated into approximately 480 nude mice. For all 61 horizontal transfers, all recipient mice were monitored for C. bovis infection by routine, weekly, IVC rack exhaust-air surveillance swabs.19 According to this surveillance technique, no recipient mice have tested positive for C. bovis since the implementation of this procedure at our institution in the fall of 2012.
Sterilization of instruments.
All instruments used to cut skin or harvest PDX tissue were autoclaved prior to initial use. After the initial harvest of bilateral flank tumors from a single mouse, the distal tips of both sets of instruments were resterilized by using a bead sterilizer (Germinator 500). The need to use the bead sterilizer occurred when multiple mice carrying the same or different PDX tumor models required tumor harvesting on the same day. The method of instrument sterilization used immediately prior to harvest was tracked for each PDX tumor model, with 25 tumors harvested by using autoclaved instruments and 36 tumors harvested by using bead-sterilized instruments. Both methods of instrument sterilization were effective at preventing recipient mouse infection.
Discussion
Herein, we described a procedure used to successfully eliminate C. bovis from 2 in vivo tumor banks containing unique PDX tumor models. By using these methods, 100% of these PDX tumor models were salvaged from the enzootically infected colonies. The timeline for completion of this project reflected the growth rate of some of the PDX tumors in nude mice and the number of PDX tumors maintained within each in vivo tumor bank. Laboratories A and B were able to complete 44 and 17 tumor transfers within approximately 6 and 2.5 mo, respectively. This project was successful because of the procedural barriers used to prevent the inadvertent contamination of PDX tissue harvested from C. bovis-infected mice. In addition, physical barriers were used to separate and maintain infected and uninfected nude mouse colonies so that recipient mice could be maintained as C. bovis-free before and after inoculation of PDX tumor tissue.
Most published procedures that have described PDX tumor harvest and horizontal transfer minimally address skin disinfection at the site of tumor excision for the donor mouse. According to those procedures, 70% to 75% ethanol was the skin disinfectant of choice.9,14,15,21 However, we used CHG instead of ethanol because CHG will not evaporate during the procedure, is available at the desired concentration (compared with the 95% ethanol stock), and has demonstrated similar efficacy.1,12 We also selected 2% CHG as compared with other CHG concentrations (0.25% to 4%), because 2% CHG works more effectively than lower concentrations and is equivalent to higher concentrations during a 5- to 10-min exposure.11,30 C. bovis is a diffuse skin infection that is aerosolized through contaminated mouse dander.2,4 Because of this characteristic, we concluded that the entire carcass must be disinfected prior to tumor harvest. The easiest way to accomplish this goal was complete submersion of the carcass in disinfectant, in agreement with one of the previously published protocols.14 This practice is ideal for the use of athymic nude mice, because skin–disinfectant contact is unencumbered by the hair coat. We concede a variety of haired mouse strains that are routinely used to propagate xenograft tumors in preclinical research. In these situations, we recommend removing the hair from a generous 3- to 4-cm region around and on top of the tumor masses before complete submersion of the carcass in disinfectant.
For our study, we elected not to perform skin cultures after whole-carcass submersion in 2% CHG to quantify or identify the bacteria that were able to survive. Our rationale for this decision was that aseptic surgical technique would still be required, given that complete skin sterilization likely would not be achieved using this technique. Due to the lack of relevance of this method to the current standard of care for surgical skin preparation, we were not able to find literature describing the efficacy of 2% CHG with complete tissue submersion. Nevertheless, 2% CHG has been show in vitro to result in a 5 to 6 log10 reduction of a variety of relevant bacteria when applied in excess to a bacterial suspension test.7 As an in vivo correlate, when 2% CHG was applied to human skin in a less liberal manner, a site-specific 2 to 3 log10 decrease in bacterial skin burden was achieved in 10 min.11
Additional refinements to this procedure may emerge in the future. As an example, the combined use of 2% CHG in 70% isopropyl alcohol exceeds the skin disinfection of each individual component in this mixture and likely will provide even greater skin disinfection prior to tumor harvest.18,32 We did not use such a product because packages of 2% CHG in 70% isopropyl alcohol in volumes large enough for whole-carcass submersion are not yet available. At the beginning of the project, we also considered submersing the carcass in Clidox-S, but at that time no data were available regarding the tissue-penetrating properties of the active ingredient ClO2, and we therefore ruled out that possibility. Since then, aqueous ClO2 is an emerging skin antiseptic with limited tissue penetration and exceptional bactericidal and sporicidal properties.22,31 However, aqueous ClO2 products marketed for skin disinfection are not yet commercially available.
Another technique that might be implemented to reduce skin bacterial burden prior to harvest is the use of systemic antibiotic therapy. One study demonstrated that 78% of C. bovis-infected mice given amoxicillin impregnated chow were C. bovis culture-negative 3 wk after beginning treatment.3 Although we do not consider this method as one to completely clear nude mice of this organism, the use of preharvest antibiotic therapy may provide an additional barrier to C. bovis tumor contamination and horizontal transfer cross-contamination.
C. bovis DNA was not detected among the 34 representative tissue samples assayed from a subset of all the PDX tumor models harvested. It is noteworthy that only 2 of the 34 tumor tissues evaluated were harvested by a veterinarian, whereas the remaining samples were harvested by graduate students and research staff trained in aseptic surgical technique. This finding suggests that basic training in this enhanced aseptic surgical technique is sufficient to prevent skin–tumor tissue cross-contamination. The rationale for combining whole-body disinfection with enhanced aseptic technique was to reduce the quantity of C. bovis on the skin and free contaminated skin debris prior to surgery if a breakdown in aseptic technique occurred. We suspect that any breakdown in aseptic technique would leave detectable C. bovis DNA on the tumor tissue. However, because no C. bovis DNA was detected from representative tumor samples, whole-body skin disinfection may not be required, providing that strict aseptic technique is achieved. Regardless, we continue to feel that complete submersion of the carcass in a disinfectant provides a simple and additional level of protection from the potential contamination of tumor tissue with viable bacteria. In addition, the use of a representative sample of harvested tumor tissue may not accurately represent the C. bovis status of the entire harvested tumor. For example, when we used a representative, 0.015-g sample of solid PDX tissue from cryopreserved stocks, only 1 (33%) of the 3 vials was correctly identified as being contaminated with C. bovis, as compared with performing the assay on the entire sample. Therefore, we recommend the use of whole-body disinfection as an additional barrier to tumor contamination.
An essential component to the success of this procedure is the ability to create and maintain C. bovis-negative populations of mice. During this process, both C. bovis-positive and -negative colonies need to be maintained concurrently. C. bovis is known to cause diffuse environmental and equipment contamination with airborne dissemination.4 Attention to detail is necessary for the establishment of C. bovis-free dedicated equipment, traffic patterns for personnel, and even the division of labor within research and vivarium staff to prevent cross-contamination between colonies. We have found that the establishment of a C. bovis surveillance program using exhaust-air debris testing from IVC caging systems provides rapid detection within nude mouse colonies.19 In our experience, this surveillance program has been invaluable in the maintenance of C. bovis-free colonies while concurrently maintaining separate infected colonies.20
The protocol described here contains minor enhancements to traditional aseptic surgical technique in combination with bioexclusion principles used to eliminate C. bovis. Although this protocol was created to prevent C. bovis contamination of tumor tissue and cross-contamination during horizontal tumor transfer, this procedure could easily be implemented as a routine precaution during the transfer of any xenograft or allograft tissue from one rodent to another or before cryopreservation to reduce bacterial contamination from the skin.
Acknowledgments
We thank Dr Derek Fong for his thoughtful and thorough review of this manuscript. We are also grateful to the University of Colorado Denver qPCR Core for their assistance in sample preparation and processing. This project was supported by the University of Colorado Denver, Office of the Vice Chancellor for Research, and Office of Laboratory Animal Resources. In addition, funding for the maintenance and management of the PDX in vivo tumor banks was provided in part by grants to SG Eckhardt (08-60-21-ECKH from AACR and W8IXWH-11-1-0526 from DOD), A Jimeno (5R21DE019712-02 from NIDCR), JJ Arcaroli (W8IXWH-15-1-0173 from DOD), WA Messersmith (1RO1CA152303-01 from NCI), and the NCI Cancer Center Support Grant (P30CA046934-27, qPCR Core).
References
- 1.Adams D, Quayum M, Worthington T, Lambert P, Elliott T. 2005. Evaluation of a 2% chlorhexidine gluconate in 70% isopropyl alcohol skin disinfectant. J Hosp Infect 61:287–290. [DOI] [PubMed] [Google Scholar]
- 2.Besch-Williford CL, Franklin CL. 2007. Aerobic gram-positive organisms, p 389–406. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL, editors. The mouse in biomedical research. Amsterdam (Netherlands): Elsevier.
- 3.Burr HN, Lipman NS, White JR, Zheng J, Wolf FR. 2011. Strategies to prevent, treat, and provoke Corynebacterium-associated hyperkeratosis in athymic nude mice. J Am Assoc Lab Anim Sci 50:378–388. [PMC free article] [PubMed] [Google Scholar]
- 4.Burr HN, Wolf FR, Lipman NS. 2012. Corynebacterium bovis: epizootiologic features and environmental contamination in an enzootically infected rodent room. J Am Assoc Lab Anim Sci 51:189–198. [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford CB, Walton BJ, Reed TH, Coyle MB, White WJ, Amyx HL. 1995. Hyperkeratosis in athymic nude mice caused by a coryneform bacterium: microbiology, transmission, clinical signs, and pathology. Lab Anim Sci 45:131–139. [PubMed] [Google Scholar]
- 6.Cree IA, Glaysher S, Harvey AL. 2010. Efficacy of anticancer agents in cell lines compared with human primary tumour tissue. Curr Opin Pharmacol 10:375–379. [DOI] [PubMed] [Google Scholar]
- 7.DeBaun B. 2008. Evaluation of the antimicrobial properties of an alcohol-free 2% chlorhexidine gluconate solution. AORN J 87:925–933. [DOI] [PubMed] [Google Scholar]
- 8.Debrue MC, Henderson KS, Lipman NS, Manuel CA, Mulder GB. 2015. Corynebacterium bovis: is scaly skin still keeping you down? Panel discussions p 47. Presented at the 66th Annual AALAS National Meeting Program,Phoenix, Arizona, 1–5 November 2015. In: 66th Annual AALAS National Meeting Program, Memphis (TN): American Association for Laboratory Animal Science. [Google Scholar]
- 9.DeRose YS, Gligorich KM, Wang G, Georgelas A, Bowman P, Courdy SJ, Welm AL, Welm BE. 2013. Patient-derived models of human breast cancer: protocols for in vitro and in vivo applications in tumor biology and translational medicine. Curr Protoc Pharmacol Chapter 14: Unit 14.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dole VS, Henderson KS, Fister RD, Pietrowski MT, Maldonado G, Clifford CB. 2013. Pathogenicity and genetic variation of 3 strains of Corynebacterium bovis in immunodeficient mice. J Am Assoc Lab Anim Sci 52:458–466. [PMC free article] [PubMed] [Google Scholar]
- 11.Edmiston CE, Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. 2007. Comparative of a new and innovative 2% chlorhexidine gluconate-impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control 35:89–96. [DOI] [PubMed] [Google Scholar]
- 12.Hibbard JS, Mulberry GK, Brady AR. 2002. A clinical study comparing the skin antisepsis and safety of chloraprep, 70% isopropyl alcohol, and 2% aqueous chlorhexidine. J Infus Nurs 25:244–249. [DOI] [PubMed] [Google Scholar]
- 13.IDEXX BioResearch Resources. [Internet] 2017. PDX and tumor stocks: biosecurity risks webinar series. [Cited 04 February 2017]. Available at: https://www.idexxbioresearch.eu/resources-biological-testing/
- 14.Jin K, Teng L, Shen Y, He K, Xu Z, Li G. 2010. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol 12:473–480. [DOI] [PubMed] [Google Scholar]
- 15.Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. 2009. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc 4:1670–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leblanc M, Berry K, Graciano S, Becker B, Reuter JD. 2014. False-positive results after environmental pinworm PCR testing due to rhabditid nematodes in corncob bedding. J Am Assoc Lab Anim Sci 53:717–724. [PMC free article] [PubMed] [Google Scholar]
- 17.Lepherd M, Redelsperger I, Lipman NS. 2013. False brush borders in the cecum of NSG mice. Abstract presented at the AALAS National Meeting Baltimore, Maryland, 26–31 October 2013. J Am Assoc Lab Anim Sci 52:637. [Google Scholar]
- 18.Maiwald M, Chan ESY. 2012. The forgotten role of alcohol: a systematic review and meta-analysis of the clinical efficacy and perceived role of chlorhexidine in skin antisepsis. PLoS One 7:e44277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manuel CA, Pugazhenthi U, Leszczynski JK. 2016. Surveillance of a ventilated rack system for Corynebacterium bovis by sampling exhaust air manifolds. J Am Assoc Lab Anim Sci 55:58–65. [PMC free article] [PubMed] [Google Scholar]
- 20.Manuel CA, Pugazhenthi U, Spiegel SP, Leszczynski JK. 2017. Detection and elimination of Corynebacterium bovis from barrier rooms with an environmental sampling surveillance program. J Am Assoc Lab Anim Sci [In press]. [PMC free article] [PubMed] [Google Scholar]
- 21.Morton CL, Houghton PJ. 2007. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc 2:247–250. [DOI] [PubMed] [Google Scholar]
- 22.Noszticzius Z, Wittmann M, Kály-Kullai K, Beregvári Z, Kiss I, Rosivall L, Szegedi J. 2013. Chlorine dioxide is a size-selective antimicrobial agent. PLoS One 8:e79157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oppelt KA. 2013. Efficacy of glass bead sterilization for aerobic bacterial decontamination of surgical instruments in serial rodent surgeries. Presented at the ACLAM Forum, Williamsburg, Virginia 14–17 April 2013. In: ACLAM Forum conference program. http://www.aclam.org/Content/files/files/Forum2013/ACLAM_Forum_2013_Oppelt.pdf [Google Scholar]
- 24.Peterson NC. 2008. From bench to cageside: risk assessment for rodent pathogen contamination of cells and biologics. ILAR J 49:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173. [DOI] [PubMed] [Google Scholar]
- 26.Scanziani E, Gobbi A, Crippa L, Giusti AM, Giavazzi R, Cavalletti E, Luini M. 1997. Outbreaks of hyperkeratotic dermatitis of athymic nude mice in northern Italy. Lab Anim 31:206–211. [DOI] [PubMed] [Google Scholar]
- 27.Scanziani E, Gobbi A, Crippa L, Giusti AM, Pesenti E, Cavalletti E, Luini M. 1998. Hyperkeratosis-associated coryneform infection in severe combined immunodeficient mice. Lab Anim 32:330–336. [DOI] [PubMed] [Google Scholar]
- 28.Siolas D, Hannon GJ. 2013. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res 73:5315–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith G, Field G, Peterson P, Reynolds RP, Wolf FR. 2010. Panel discussion: control and eradication of Corynebacterium-associated hyperkeratosis (CAH) in athymic nude mice, p 59.AALAS 61st National Meeting,Atlanta, Georgia,10–14 October 2010. In: 61st Annual AALAS National Meeting Program. Memphis (TN): American Association for Laboratory Animal Science. [Google Scholar]
- 30.Stinner DJ, Krueger CA, Masini BD, Wenke JC. 2011. Time-dependent effect of chlorhexidine surgical prep. J Hosp Infect 79:313–316. [DOI] [PubMed] [Google Scholar]
- 31.Stratilo CW, Crichton MKF, Sawyer TW. 2015. Decontamination efficacy and skin toxicity of 2 decontaminants against Bacillus anthracis. PLoS One 10:e0138491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taha M, Kalab M, Yi Q-L, Landry C, Greco-Stewart V, Brassinga AK, Sifri CD, Ramirez-Arcos S. 2014. Biofilm-forming skin microflora bacteria are resistant to the bactericidal action of disinfectants used during blood donation. Transfusion 54:2974–2982. [DOI] [PubMed] [Google Scholar]
- 33.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, Arcaroli JJ, Messersmith WA, Eckhardt SG. 2012. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol 9:338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilding JL, Bodmer WF. 2014. Cancer cell lines for drug discovery and development. Cancer Res 74:2377–2384. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Claerhout S, Pratt A, Dobrolecki LE, Petrovic I, Lai Q, Landis MD, Wiechmann L, Schiff R, Giuliano M, Wong H, Fuqua SW, Contreras A, Gutierrez C, Huang J, Mao S, Pavlick AC, Froehlich AM, Wu M-F, Tsimelzon A, Hilsenbeck SG, Chen ES, Zuloaga P, Shaw CA, Rimawi MF, Perou CM, Mills GB, Chang JC, Lewis MT. 2013. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res 73:4885–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. doi: 10.1002/9780470942390.mo120140. Zhang X, Lewis MT. 2013. Establishment of patient-derived xenograft (PDX) models of human breast cancer.Curr Protoc Mouse Biol 3:21 –29. [DOI] [PubMed] [Google Scholar]