Abstract
Laboratories and vivariums typically are maintained at ambient temperatures of 20 to 24 °C, leading to cold stress in mice. When mice are inactive and sleeping during the light phase, their zone of thermoneutrality associated with a basal metabolic rate is 30 to 32 °C. If given a choice, mice will use thermoregulatory behavior to seek out thermoneutral temperatures during the light phase. The cold stress of a vivarium can be problematic to researchers requiring an animal model that is not stressed metabolically. However, it may not be practical or economically feasible to maintain an animal vivarium at thermoneutral temperatures. One problem with raising the ambient temperature of a vivarium is that personnel wearing protective equipment will be subject to considerable heat stress. Here we present plans for the construction and operation of a device that allows mice to access a heated floor that is maintained at an approximate thermoneutral temperatures (30 to 32 °C). The device is made of inexpensive, readily available materials and uses a disposable hand warmer as a heat source. One hand warmer provides a thermoneutral environment for approximately 12 h. This device is easily adapted to a standard mouse or rat cage and requires only brief daily maintenance to change the heating pad. With this device in a standard cage, mice can select a warmer environment associated with thermoneutral conditions during the light phase and cooler ambient temperatures during the dark phase.
Many studies suggest that the temperature of a typical vivarium (20 to 24 °C) is too cold for normal laboratory mice as well for animals with compromised health.2,3,6,10 This cool environment is maintained primarily for the thermal comfort of humans working in the vivarium, who must wear protective personnel equipment that restricts heat loss (that is, lab coats, gloves, and mask or respirator).
Mice adapt relatively quickly to behavioral thermoregulatory devices, such as temperature gradients and related types of behavioral systems. For example, metal tubes heated at one end and cooled at the other provide a simple conductive and convective temperature gradient, where mice will seek out relatively warm air and surface temperatures.3 In addition, groups of mice moved through a series of tubes to occupy standard cages maintained at ideal ambient temperatures.2 In a more complicated experimental device, mice seek out an ideal metal plate temperature (or avoid stressful temperatures) to operate an operant thermoregulatory device.9 We likewise developed a purely conductive gradient, where mice selected an optimal surface temperature that was independent of air temperature.7 All in all, mice appear to use their thermoregulatory behavior to locate thermal environments that are associated with ideal thermal comfort and minimize energy expenditure.6
In many circumstances, an investigator may want to maintain mice in a standard vivarium but give them an opportunity to select a warm, thermoneutral environment. For example, maintaining mice at a near-thermoneutral temperature improves the efficacy of immunologic responses to various tumors.8 Toxic chemicals and a variety of drugs elicit hypothermic responses in mice and rats housed under standard vivarium conditions.5 Providing a setting that allows mice treated with a drug or toxicant to behaviorally thermoregulate could improve animal health and wellbeing. Nude mice as well as young and aged mice are known to be particularly sensitive to cold temperatures and could exhibit increased susceptibility to the typically cool environment of a vivarium.4,6 Moreover, the plethora of genetically engineered strains—especially experimental models with altered thyroid, brown adipose tissue, or muscular thermogenesis—are additional examples of potentially increased susceptibility to cold stress. In addition, recovery from surgical procedures might be improved if the animals had access to a warm environment. To this end, we developed a simple system that will provide mice in a standard mouse cage with a source of heat that will allow them to maintain a thermoneutral environment for approximately 12 h.
Materials and Methods
Overview of device.
A commercially available, disposable hand warmer (HotHands, Philadelphia, PA) was used to provide a source of heat that would create a thermoneutral area in a small area of the cage. This idea was inspired by a previous publication.1 Different models of commercially available hand warmers provide various heating durations. The 10-h warmer generates heat through a reaction of nontoxic chemicals that are contained within the paper packaging. They can be purchased in bulk from the manufacturer at a cost of less than US$1.00 per warmer and are 5.1 × 8.9 × 0.5 cm. According to the manufacturer, the 10-h warmer has an average temperature of 135 °C with a maximum temperature of 158 °C. These warmers are designed for one-time use and are typically placed in gloves or shoes and are safe when held against bare skin. The warmers begin to heat immediately once the protective cellophane packaging is removed and the warmer is exposed to oxygen.
Preliminary studies.
In the development of a device to accommodate a hand warmer, we considered various designs of chambers made of acrylic that had either a heated floor or wall, or we attempted to warm a small chamber by convective heat exchange. However, a single warmer was ineffective at increasing the air temperature of a small chamber higher than 30 °C. We expected that mice would simply crawl into the device and settle into a warm part of the modified hut. The early designs were elevated by several centimeters off of the cage floor but the mice were unwilling to climb into these structures.
Current design.
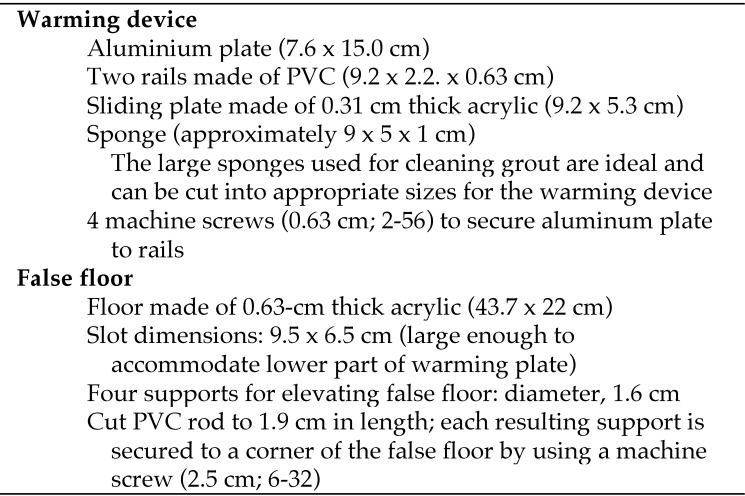
We determined that the hand warmer was most effective when it was used as a source of conductive and not convective heating. We eventually settled on a relatively simple design comprising a conductively heated aluminum plate that was positioned flush with the floor of a standard polycarbonate (that is, shoebox-style) mouse cage. With this design, the mice do not have to climb into the device; they can simply move to a heated part of the cage floor. A modified mouse hut was placed over the heated floor with the expectation that the mice would seek the covered refuge during the light phase. To achieve this design, we had to create a false floor that was positioned above the actual cage floor. The elevated false floor allows for the heated aluminum plate to sit flush with the cage floor, allowing the mice to easily move from the cage onto the warmed plate. The complete list of parts is given in Figure 1.
Figure 1.
Complete list of parts.
Design details.
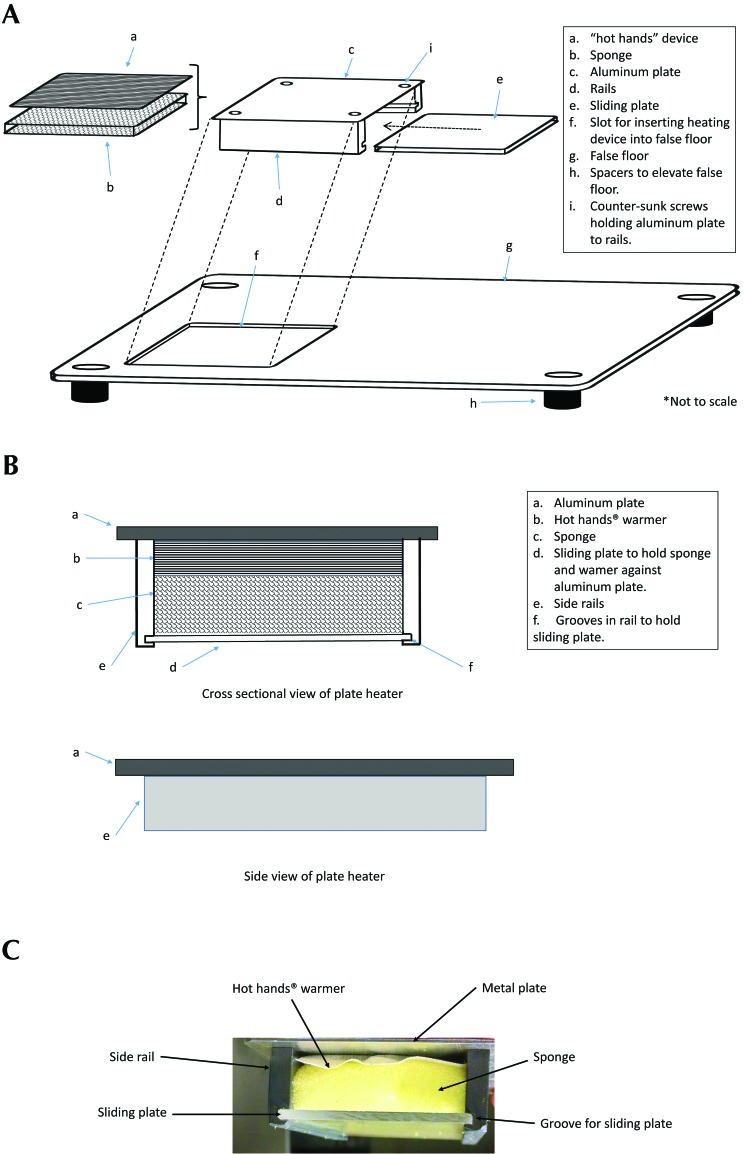
The heated floor device was constructed of a PVC plastic frame and an aluminum plate heated with a hand warmer (Figure 2 A and C). The edges and corners of the cut piece of aluminum were smoothed with a file to avoid any sharp edges that might lead to injury. The warmer was held in place against the bottom of the aluminum plate by using a section of cellulose sponge that was positioned between the warmer and the sliding plate, which was made of 0.31-cm thick acrylic. The thickness of the sponge was slightly larger than the space between the hand warmer and sliding plate to facilitate slight compression of the warmer against the metal plate. We discovered that the sponge was an excellent device for applying even pressure across the entire warmer, which has an uneven surface, resulting in excellent conduction of heat between the hand warmer and the aluminum plate. The sponge also allows for the circulation of oxygen to the hand warmer, resulting in ideal heating of the plate. We also believe that the sponge serves to impede heat loss of the warmer away from the plate and encourage effective conduction to the plate. It is important to note that, in our past experience, using a mechanical, spring-loaded plastic plate to hold the warmer resulted in inadequate heating of the plate. We speculate that the mechanical device either impaired air delivery to the hand warmer or led to conduction of heat away from the aluminum plate.
Figure 2.
(A) Diagram of the heating plate and false floor. (B) Diagram of the heating plate showing the hand warmer held against the aluminum plate with sponge and sliding plate. (C) Photograph of the end of the heating plate showing aluminum plate, sponge, and sliding plate. For complete list of dimensions of parts, see the Materials and Methods section.
We also discovered that the surface area of the aluminum plate was a critical determinant in the device's performance. When the surface area of the aluminum plate was too large, the heat from the hand warmer was dispersed over too large an area, resulting in a very modest rise in temperature of the aluminum plate. After experimenting with various sizes of aluminum plates, we decided on a 7.6 × 15.0-cm plate (105 cm2). This size was ideal in terms of temperature elevation and was large enough for at least 2 mice to share. We presume that a system could be designed for larger rodents by using multiple or larger hand warmers with an expanded aluminum plate. In addition, although not quantified in this study, we expect that the maximal plate temperature could be lowered by increasing the surface area of the heated aluminum plate. Therefore, depending on a species’ thermal preference, an ideal plate temperature could be achieved with this system.
The false floor was constructed of 0.63-cm thick acrylic (Figure 2 B) such that the top of the floor was elevated more than 2.0 cm above the bottom of cage floor, with 4 PVC supports. Because the standard polycarbonate mouse cages used in our facility have rounded corners, the corners of the false floor were milled to maintain a close fit between the walls of the cage and the false floor. This adjustment was necessary to avoid bedding slippage and to prevent an animal's tail or foot from getting lodged in a gap between the cage and false floor, and the false floor might be modified to fit a variety of cages. A rectangular hole was cut into the false floor to allow the warming device to be positioned. In our design, we prepared false floors to hold 2 plate warmers; a heated and unheated (that is, control) unit. A single warmer device per cage is sufficient, but additional units can be added depending on the needs of the researcher. The location of the heated plates was chosen so as not to interfere with the operation of the food bin or water bottle. We positioned the devices near the corners of the cage.
We expected that mice would be more inclined to use the warming device if a covered refuge was placed over the heated plate. Rectangular or circular red plastic enrichment devices for rats (Bio-Serv International, Flemington, NJ) were cut in half longitudinally, and each half was placed over a plate warmer. The mice could enter or exit the plate at either end. The inside width of the mouse hut was slightly larger than the width of the metal plate, allowing for a snug fit. The mice were unable to move the hut from its position over the heating plate. We believe that other types of mouse huts and igloos could be used alternatively.
Cost.
The materials used to construct the warming unit and hand warmer are relatively inexpensive. Depending on how much acrylic and aluminum are purchased in bulk, we estimate that the materials for each unit cost approximately US$10.00. The hand warmers can be purchased in bulk from the manufacturer at a discounted price of less than US$0.30 per warmer.
Temperature measurements.
In view of the manufacturer's specifications of the maximal temperatures generated by the hand warmer, we were concerned that the temperature of the aluminum plate would be excessive, leading to injury. However, once we decided on the optimal surface area for the aluminum plate, we found that the conduction of heat from the warmer to the plate resulted in nonhazardous temperatures, as assessed by using an infrared thermometer to measure plate temperature. To account for the low emissivity of bare aluminum in infrared measurements, a piece of adhesive tape was placed over the desired site of the aluminum plate, and temperature was measured by using a handheld infrared thermometer (model 561, Fluke, Everett, WA). We also measured the performance of the system under typical housing conditions by monitoring the plate temperature over a 24-h period; to this end, we placed a radiotransmitter that measures temperature (model F10 transmitter, Data Sciences International, St Paul, MN) directly on the aluminum plate. The experiment was repeated 5 times.
To test the performance of the device with mice, a thin layer of bedding (ALPHA-dri, Shepherd Specialty Papers, Watertown, TN) was spread throughout the cage. Two female C57BL6 mice were placed in a cage containing 2 plates, one with an active warmer and the other without a warmer. Although corncob bedding can be used with this system, we did not use wood shaving bedding because we did not want the mice to build a nest around the mouse hut and obstruct heat loss from the system.
We then observed the behavior of the mice throughout the day. In another test of the system, 2 groups of 4 female BALB/c mice were housed in standard cages with corncob bedding. The cages were fit with the heating plate system (one heated and one unheated) over a period of 28 d; the heating pads were replaced daily. This experiment was a part of a tumor growth study (results to be reported at a later time). All studies involving animals were approved by the appropriate IACUC.
Results
Plate heating performance.
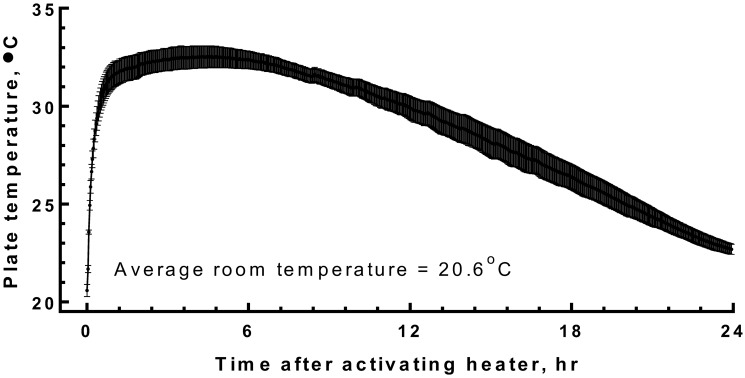
A time-course analysis of the plate temperature shows the maintenance of a steady warm temperature of 30 to 32 °C on the plate for approximately 10 h when a 10-h hand warmer was inserted (Figure 3). After the initial 10-h period, the temperature of the plate continued to remain well above room temperature. In this experiment, the device maintained a plate temperature greater than 30 °C for approximately 13 h, after which plate temperature decreased slowly between 11 to 24 h after the warmer was activated. The heat source does not affect the overall air temperature of the cage outside of the mouse hut. Acrylic is a poor conductor of heat, and we expected little warming of the false floor around the heating unit. We found that the temperature of the acrylic floor when measured at 1, 3, and 5 cm from the edge of the metal plate was 1.2, 0.7, and 0.5 °C warmer than that of the standard room temperature.
Figure 3.
Time-course analysis of the plate temperature (mean ± SEM, n = 5; 2-min interval) when a fresh warming pad is activated at 0 h and positioned under the warming plate.
Mouse behavior.
After placing the false floor containing heated and unheated aluminum plates into the mouse cage, we found that a pair of female C57BL6 mice remained huddled on the warmed plate throughout the light phase (Figure 4). They rarely spent any appreciable time within the unheated refuge. In the long-term study involving groups of 4 BALB/c female mice housed in cages with heated plates for 28 d, the mice spent the majority of time during the light phase huddled within the heated refuges (data not shown). We conclude that, depending on the size of the mice, a single heated plate unit could allow at least 4 mice to achieve a state of thermal comfort during the day.
Figure 4.
Photograph of 2 female C57BL6 mice huddled together in a heated refuge consisting of a red hut placed over an active plate in a standard cage with ALPHA-dri bedding (Shepherd Specialty Papers, Watertown, TN). The hut to the right sits over an inactive heating plate.
Discussion
The goal of this study was to develop a simple device that would allow mice in a standard vivarium maintained at 20 to 22 °C to use behavioral thermoregulation to maintain a state of thermal comfort. The device we present essentially meets the 24-h thermal requirements of mice as long as a new heating pad is inserted into the device early in the day, at the start of the light phase. That is, when a new heat pad was inserted in the morning, the device maintained a relatively warm floor temperature during the light phase. Mice tended to avoid the warmer temperatures during the dark phase, when they were active. Therefore, with a newly activated warmer at the start of 12:12-h light:dark cycle, mice are provided with a metal floor that is maintained at a relatively stable temperature in excess of 30 °C for at least 12 h. Moreover, the mice can roam throughout the cooler cage during the dark phase, or they can continue to remain on the warmed floor, which will be cooler than it was during the day, given that the heat output from the hand warmer declines. In addition, the mice can move away from the heat source at any time. Furthermore, this device might allow rodents to maintain thermal comfort during recovery from surgery and other experimental manipulations that might compromise autonomic thermoregulatory control. An additional advantage of this device is that it is amenable to use with radiotelemetry such as the system we use (Data Sciences International). That is, the metal plate does not interfere with the transmission of the radio signal from the transmitter.
The preferred ambient temperature of laboratory mice exhibits a circadian pattern, with mice preferring warm ambient temperatures during the light phase when they are inactive and sleeping and lower temperatures during the dark phase when they are active and eating.6 The preferred light phase temperature of most mice ranges from 30 to 33 °C, whereas the nighttime preferred temperature decreases to approximately 26 °C but varies throughout the dark phase.6 The preferred temperature is typically lowest during the early part of the dark phase and then rises gradually through the dark phase. Moreover, the paws and foot pads of mice are furless, and mice appear to prefer a relatively warm surface so that their furless skin remains relatively warm by means of conductive heat exchange.7 In addition, testing mice in a specialized temperature gradient involving a thermally conductive copper floor and independent measurement of air temperature showed that mice quickly selected a relatively warm floor of 30 to 35 °C. Providing a mouse cage with a heated floor therefore may be an ideal means of creating a source of heat for manifestation of their behavioral thermoregulatory reflexes.
Given the thermal performance of the hand warmer (Figure 3), activation of a new warming pad in the early morning (that is, at the start of the light phase) allows the mice to experience an essentially ideal thermal environment for 24 h because the decline in temperature of the warmer at night parallels the preference of mice for cooler temperatures during the dark phase. In addition, constructing duplicate sets of warming plates allows the rapid exchange of a newly activated device with the expired device, with minimal disturbance to the mice. The mice thus are provided with a relatively stable, warm surface throughout the entire light phase, such that the hand warmer likely does not need to be replaced more than once daily. A possible exception is if the device were used for animals recovering from surgery or those with abnormal temperature regulation. In these cases, a plate heated 24 h daily might be required.
Acknowledgments
We greatly appreciate Drs Katie Kokolus and Justin Brown for their review of the manuscript.
The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, US Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
References
- 1.Boily P. 2009. Role of voluntary motor activity on menthol-induced hyperthermia in mice. J Therm Biol 34:420–425. [Google Scholar]
- 2.Gaskill BN, Rohr SA, Pajor EA, Lucas JR, Garner JP. 2009. Some like it hot: mouse temperature preferences in laboratory housing. Appl Anim Behav Sci 116:279–285. [Google Scholar]
- 3.Gordon CJ. 1985. Relationship between autonomic and behavioral thermoregulation in the mouse. Physiol Behav 34:687–690. [DOI] [PubMed] [Google Scholar]
- 4.Gordon CJ. 1993. Temperature regulation in laboratory rodents. New York (NY): Cambridge University Press. [Google Scholar]
- 5.Gordon CJ. 2005. Temperature and toxicology: an integrative, comparative, and environmental approach. Boca Raton (FL): CRC Press. [Google Scholar]
- 6.Gordon CJ. 2012. Thermal physiology of laboratory mice: defining the limits of thermoneutrality. J Therm Biol 37:654–685. [Google Scholar]
- 7.Gordon CJ, Becker P, Killough P, Padnos B. 2000. Behavioral determination of the preferred footpad temperature of the mouse. Device to measure the preferred footpad temperature in mice. J Therm Biol 25:211–219. [Google Scholar]
- 8.Kokolus KM, Capitano ML, Lee CT, Eng JWL, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, Repasky EA. 2013. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA 110:20176–20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CH, Tokizawa K, Nakamura M, Uchida Y, Mori H, Nagashima K. 2012. Hyperosmolality in the plasma modulates behavioral thermoregulation in mice: the quantitative and multilateral assessment using a new experimental system. Physiol Behav 105:536–543. [DOI] [PubMed] [Google Scholar]
- 10.Lodhi IJ, Semenkovich CF. 2009. Why we should put clothes on mice. Cell Metab 9:111–112. [DOI] [PubMed] [Google Scholar]
- 11.Maloney SK, Fuller A, Mitchell D, Gordon CJ, Overton JM. 2014. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda) 29:413–420. [DOI] [PubMed] [Google Scholar]