Abstract
Environmental enrichment items such as running wheels can promote the wellbeing of laboratory mice. Growing evidence suggests that wheel running simulates exercise effects in many mouse models of human conditions, but this activity also might change other aspects of mouse behavior. In this case study, we show that the presence of running wheels leads to pronounced and permanent circling behavior with route-tracing in a proportion of the male mice of a genetically distinct cohort. The genetic background of this cohort includes a mutation in Arhgap19, but genetic crosses showed that an unknown second-site mutation likely caused the induced circling behavior. Behavioral tests for inner-ear function indicated a normal sense of gravity in the circling mice. However, the levels of dopamine, serotonin, and some dopamine metabolites were lower in the brains of circling male mice than in mice of the same genetic background that were weaned without wheels. Circling was seen in both singly and socially housed male mice. The additional stress of fighting may have exacerbated the predisposition to circling in the socially housed animals. Singly and socially housed male mice without wheels did not circle. Our current findings highlight the importance and possibly confounding nature of the environmental and genetic background in mouse behavioral studies, given that the circling behavior and alterations in dopamine and serotonin levels in this mouse cohort occurred only when the male mice were housed with running wheels.
Abbreviation: DOPAC, 3,4-dihydroxyphenylacetic acid
Providing environmental enrichment items, such as running wheels, has become common as an effort to improve laboratory animal wellbeing. Numerous species of wild, domestic, and laboratory animals demonstrate voluntary wheel running. Wheel running is beneficial to many physiologic systems, including but not limited to the brain, heart, and digestive system and has an advantageous effect on metabolism and aging (reviewed in reference 33). In addition, wheel running is increasingly being used to simulate the effects of exercise in models of various human conditions.3,8,42 Recent studies have connected voluntary wheel running to the delay of experimental autoimmune encephalomyelitis symptoms in a mouse model of multiple sclerosis,2 decreased depression and anxiety behaviors in a sand rat model of seasonal affective disorder,40 decreases in brain oxidative stress in mice with phenylketonuria,25 decreased retinal degeneration and vision loss in a mouse model of retinitis pigmentosa,11 and changes in cecal microbiota in ovariectomized rats as a model for menopause.17 Nevertheless, under some circumstances, wheel running in rodents can lead to body changes (tail hyperflexion and arching of the back), increased aggression, and possible addiction-like behavior (reviewed in reference 33).
A commonly reported benefit of environmental enrichment for mice, including wheel running, is the attenuation of stereotypic behavior,10,31 defined as a behavior that is highly repetitive, invariant, and has no obvious function.24 Common stereotypic behaviors in mice include bar-chewing, cage-top twirling, circling, and route-tracing. Voluntary wheel running itself has been considered an elective behavior, given that wild mice in nature have been shown to use running wheels, when provided in a natural setting, to approximately the same extent as do laboratory mice.27 Voluntary wheel running as a form of environmental enrichment significantly reduces stereotypic behavior in laboratory mice,13,32 although wheel running did not affect the degree of stereotypic behavior in deer mice, as measured in a separate cage without the wheel.30 Other studies have shown that the presence of a wheel can lead to increased aggression in male mice.39 Increased aggression and stereotypic behavior resulting from competition over environmental enrichments, including a running wheel, have also been reported to occur in socially housed female mice.1
Circling behavior, where a mouse repeatedly turns in circles on the top or floor of the cage, may be a type of stereotypic behavior but is often attributed to defects in vestibular function of the inner ear, thus affecting the ability to sense gravity. Inner-ear defects are seen in mice deficient in SLC26A4,20 caspase 3,23 and Dmp1,21 as well as in the stargazer, turning, and waltzing rat strains,18 all of which display circling behavior. Some circling rodent models with inner-ear defects also show defects in the dopamine signaling pathway,15,19 and induced vestibular dysfunction results in alterations in the dopamine system.7,35,38 These results suggest that circling behavior due to vestibular dysfunction may act—at least in part—through secondary changes in the dopamine system.
Although voluntary wheel running is associated with a growing list of benefits in rodent models of human disorders, several reviews have cautioned that this activity may have some negative side effects.33,37 In addition, aggression and stereotypy vary significantly among strains.28 In the current case study, we show how the introduction of igloo-style running wheels induced a pronounced and permanent stereotypic circling behavior in a proportion of a specific cohort of male mice (herein referred to as FVB;129/Hemc) but not in other mice in the colony. Circling mice also showed significant alterations in the levels of the biogenic amines dopamine, noradrenaline and serotonin, and the dopamine metabolite 3,4-dihydroxyphenylacetic acid (DOPAC). The underlying genetic predisposition that resulted in these changes would not have been evident without exposure to the running wheels.
Case Study
Mice.
Approval from the Animal Care and Use Committee of the University of Alberta was obtained for all experiments involving mice (University of Alberta AUP 00000094). Mice were housed in IVC (31.8 cm × 16.5 cm × 12.7 cm; IVC Blue Line, Tecniplast, Buguggiate, Italy) containing aspen chips as bedding (Nepco, Riverside, RI) and cotton fiber squares (NES3600, Ancare, Bellmore, NY). All mice were exposed to a 14:10-h light:dark cycle and an ambient temperature of 22 ± 2 °C. Mice were fed Laboratory Rodent Diet 5001 (LabDiet, St Louis, MO) without restriction, except for breeders, which were fed Mouse Diet 9F 5020 (LabDiet) without restriction. Tap water was provided from a water bottle without restriction. For environmental enrichment, all mice were provided with cotton squares and either a cardboard house (standard housing; rectangular tent-shaped Shepherd Shacks, Shepherd Specialty Papers, Watertown, TN) or an igloo-style running wheel attached to a dome (S3174 and S3175, Innodome and Innowheel, BioServe, Flemington, NJ) for housing. The colony is monitored for 18 pathogens by using the MFIA Mouse Assessment Serology Profile from Charles River Research Animal Diagnostics Services (Wilmington, MA). Serology has been negative except for low levels of murine norovirus.
The FVB;129-Arhgap19Gt(YHD020)Byg/Hemc mice used in this study carry the gene trap mutation Arhgap19Gt(YHD020)Byg. The founder male mouse was generated (BayGenomics, San Francisco, CA) as previously described.16 Genotyping for the Arhgap19 mutation involves primers for the wildtype allele (2i-F, 5′ GAT GTT GAC TGG CTC GGT CT 3′; 2i-R, 5′ CCC AAT ACT GTC AGC CTG GT 3′) and the inserted construct that identifies the mutant allele (En2-1F, 5′ AAC AAA CTT GGC CTC ACC AG 3′; BGeo-R, 5′ AAA TTC AGA CGG CAA ACG AC 3′).16 A pedigree showing the generation of the line is shown in Figure 1. The chimeric founder male mouse was crossed with FVB/N female mice (Charles River Laboratories) to generate progeny heterozygous for the Arhgap19Gt(YHD020)Byg allele. These progeny were then backcrossed to FVB/N mice or interbred to produce homozygotes. The FVB;129-Arhgap19Gt(YHD020)Byg/Hemc mice resulted from a single breeder pair that was homozygous for the Arhgap19Gt(YHD020)Byg allele. The genetic background of this line consists of 81.25% FVB/N and 18.75% 129P2 (from the original 129P2 embryonic stem cell line containing the Arhgap19 mutation). Because we present genetic evidence that Arhgap19 is not associated with the circling phenotype described in this case study, we use the abbreviation FVB;129/Hemc for these mice hereafter.
Figure 1.
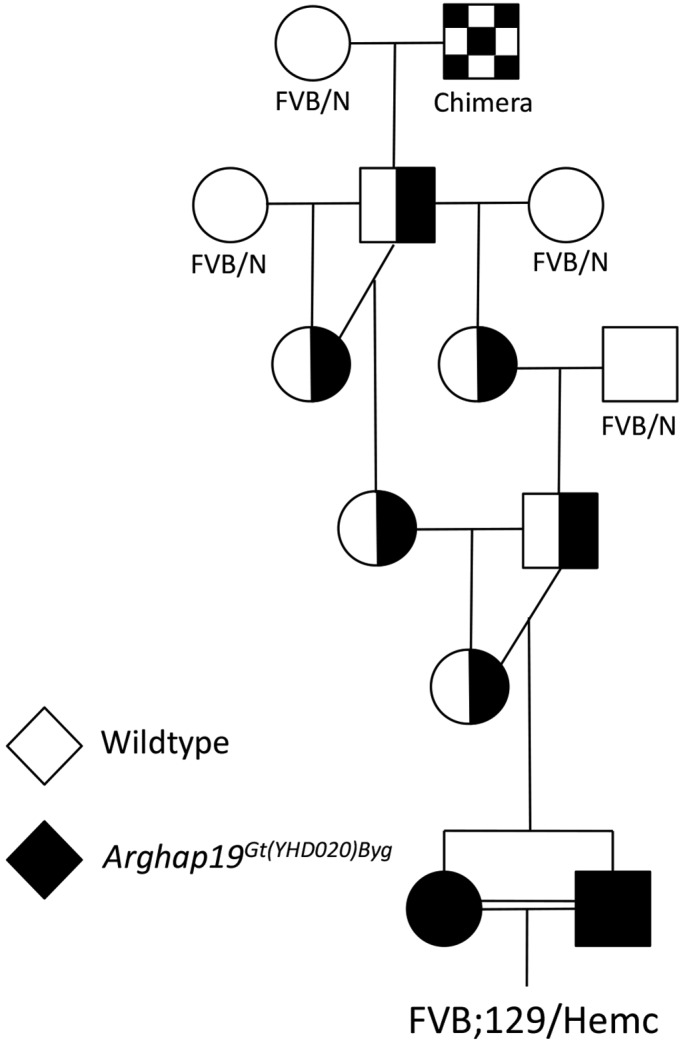
Mouse pedigree showing the ancestry of the FVB;129/Hemc line. The male chimera (checkered box) was a mosaic of 129P2 and DBA/2J:C57BL/6J hybrid cells. 129P2 germ cells were heterozygous for the Arhgap19Gt(YHD020)Byg mutation; therefore, Arhgap19Gt(YHD020)Byg was transmitted to progeny 50% of the time, and all of the chimera's progeny were 50% FVB and 50% 129P2.
Phenotyping of circling trait.
Male mice in our colony are housed in social groups of 2 to 5 animals per cage. In June 2011, we introduced igloo-style running wheels for environmental enrichment to all weaned male and female nonbreeder cages in our mouse colony, regardless of age, and continued to provide wheels to newly weaned mice. Our colony contains BALB/cCrl, FVB/N, and C57BL/6 strains, some of which carry mutations in either Cecr2, Smarca1, Smarca5, or Arhgap19. Arhgap19 was exclusively on the FVB background for all experiments in the current study. In November 2011, we noted the first circling FVB;129/Hemc male mouse and eventually determined that a percentage of the FVB;129/Hemc male mice housed with running wheels developed circling behavior. Circling was not observed in male mice of other strains.
To look for circling behavior in mice, cages were removed from the ventilated rack, and the awakened mice were observed in their cages for approximately 5 min. However, once the behavior was established, mice engaging in circling behavior could easily be seen without removing the cage from the ventilated rack. Circling behavior was first noted to occur in short bursts and with a tendency to turn in the same direction. However, within approximately 1 wk of the behavior first being noted, circling was predominantly or solely unidirectional and very obvious when the mice were active. All mice in this study were checked at least weekly for circling from weaning at 3 wk until 3 mo of age. More circling mice might have been identified had they been observed longer than 3 mo, but this factor was not tested.
Once we suspected a link to the running wheels, we removed them, and no new cases of circling behavior were identified. We then reintroduced the wheels for all of the described experiments, in which mice were arbitrarily given either wheels at weaning (n = 284, all experiments described), cardboard houses at weaning (n = 57), or cardboard houses at weaning followed by wheels at 6 to 12 wk of age (n = 40). Prior to weaning, all mice were housed with cardboard houses (standard housing). After weaning, mice were housed with either a running wheel or with standard housing and were housed either singly or with their littermates in groups of 2 to 5, depending on the number of littermates. A mouse was scored to have developed circling behavior when it demonstrated unidirectional circling behavior when active and when it preferentially or always turned in one direction. Once established in a mouse, the phenotype was obvious and permanent, and we did not quantitate the behavior. Significant differences in penetrance of the circling phenotype between different groups of mice were calculated by using the 2 × 2 Fisher exact test of independence.4
In addition, cages of male FVB;129/Hemc group-housed with wheels showed an increase in exposure to fighting, unlike other mouse strains with or without wheels (Balb/c with and without Cecr2, Smarca 1, or Arhgap19 mutations; FVB/N with and without Cecr2 mutations; and C57BL/6J with and without Smarca5 mutation). This effect was originally noted as wounding, particularly to the hind trunk or tail. Mice in these cages were separated and singly housed with wheels, which was the same housing type as they had when they were weaned. To avoid injury to subsequent animals housed with wheels, all socially housed male mice were observed daily and particularly watched after cage changing. If the mice fought, all were housed singly with wheels before wounding occurred. We made no attempt to quantify fighting or determine which cage mates were the aggressors, victims, or spectators; therefore, fighting was not scored for individual mice. However, a mouse was considered as exposed to fighting when it was in a cage that required separation due to observed fighting.
We also observed female mice for abnormal circling behavior and fighting. However, due to very low numbers of female mice presenting with these phenotypes, we did not pursue this behavior by performing more extensive experiments.
Phenotyping to identify inability to sense gravity due to inner-ear defects.
Two rounds of tests were done, about 4 y apart. Three circling and 4 noncircling male siblings, all exposed to wheels, underwent a series of behavioral tests to screen for vestibular dysfunction by using an established phenotyping protocol.12 A second set of male mice (4 circling and exposed to wheels and 3 noncircling without wheels), all from the same litter, underwent the same tests approximately 4 y after the first group. In brief, the mice underwent the trunk curl test, in which mice with vestibular defects, when held by the tail, preferentially curl their trunks rather than reach for a presented surface; the contact righting test, where mice with vestibular defects will not right themselves when turned onto their backs while in a plastic tube; and the swim test, in which mice with vestibular defects demonstrate underwater tumbling. All behavioral tests were videorecorded and reviewed to calculate time spent engaging in each type of behavior scored. For the trunk curl test, each mouse was held by the tail, and the first 18 s were scored for the amount of time spent curling the trunk forward, or sideways, or hanging straight down, or reaching toward the presented surface. To be considered as curling, the trunk had to be bent by 90° or the forepaws and hindpaws clasped. For the contact-righting test, mice were placed inside a clear plastic tube (diameter, 2. 8 cm) that was open at both ends. Once the mouse was inside, the tube was turned so that the mouse was on its back. If the mouse righted itself, then the tube was turned again so that the mouse was once again positioned in its back. The time spent with the head upside down, right side up, or sideways was scored over a total of 20 s, starting when the mouse was turned onto its back. For the swim test, mice were placed in a tank of water (50.8 cm × 40.6 cm × 20.3 cm; water temperature, 25 °C) and allowed to swim for 2 min. Time spent swimming normally, swimming in circles, or immobile and floating during the first minute was scored. The amount of time spent underwater tumbling was not scored, because the mouse was immediately rescued when in this situation. During the second minute, only underwater tumbling was monitored. Analysis of the data consisted of performing a series of one-way ANOVA followed by Bonferroni correction. At each age point, each mouse was tested only once, during the day, in the containment hood in the room in which they were housed. The order of testing was as described earlier.
Analysis of biogenic amines and amino acids in left and right brain hemispheres.
Samples of brain tissues were collected from 6 male FVB;129/Hemc mice that circled (housed with wheels), 5 FVB;129/Hemc male mice that did not circle (housed with wheels), and 6 FVB;129/Hemc male mice that did not circle and were housed with cardboard houses. All mice were 90 d of age at the time of sample collection but were otherwise chosen arbitrarily. Mice were socially housed (2 to 5 animals per cage) but were separated and housed singly when fighting was seen. All 6 circling mice housed with wheels were separated from the group due to fighting, where 3 of the 6 circling mice spent their last 21 d singly housed, and the other 3 mice spent their last 9 d singly housed. Except for one mouse, all noncircling mice that were housed with wheels had to be separated due to fighting, and 2 of the circling mice spent their last 45 d individually housed, one spent his last 21 d singly housed, and one spent his last 9 d singly housed. The sole noncircling mouse housed with a wheel remained socially housed with one circling sibling for the entire 90 d. All mice with cardboard houses did not fight and were socially housed for the entire 90 d.
Mice were euthanized by cervical dislocation, and whole brains were removed and cut into left and right hemispheres. The brain samples were then flash frozen in isopentane cooled on dry ice and stored at –80 °C until further use. Left and right brain hemispheres were independently homogenized in ice-cold water and then each was divided into 2 portions. To study biogenic amines, a 10% volume of 1 N perchloric acid was added to a portion of brain homogenate, and the precipitated protein was removed by using centrifugation. Concentrations of biogenic amines and their metabolites (dopamine; 3,4-dihydroxyphenylacetic acid; noradrenaline: 3-methoxytyramine; homovanillic acid; serotonin; and 5-hydroxyindoleacetic acid) and the amino acids tyrosine and tryptophan were measured by using HPLC with electrochemical detection, as previously described.29 To study additional amino acids, methanol was added to the other portion of homogenate, and after the removal of precipitated protein by centrifugation, concentrations of amino acids (aspartate, glutamate, l-serine, d-serine, glutamine, glycine, arginine, taurine, alanine, and γ-aminobutyric acid) were measured by using HPLC with fluorimetric detection after derivatization, as previously described.9 Statistically significant differences in biogenic amines, amino acids, and metabolites between mice were calculated by averaging concentrations from the left and right hemispheres and then performing a series of one-way ANOVA followed by Bonferroni correction with a P value of 0.05 used to define statistical significance (SYSTAT, SYSTAT Software, San Jose, CA).
Results
Circling behavior in FVB;129/Hemc male mice.
Running wheels were introduced to all nonbreeding mice in our colony as environmental enrichment for 11 mo. Both sexes of all mouse strains were observed to use the running wheels, but wheel use was not recorded for individual mice. During this time, unidirectional circling behavior manifested at 2 to 3 mo of age in a proportion of male mice of a particular cohort of mice (FVB;129/Hemc). Once a mouse adopted circling behavior, it exhibited this behavior for the rest of its life (usually culled at approximately 3 mo but as long as 294 d), regardless of whether the mouse was housed with a running wheel or not, and regardless of whether the mouse was housed with other mice or alone. The majority of circling mice did not use the wheel for the purpose of running during bouts of circling, and the circling behavior was considered stereotypic because route-tracing was evident. Route-tracing is a type of stereotypy that occurs when an animal follows an identical, repeated route. One example in the FVB;129/Hemc circling mice involved a mouse that ran on the wheel for approximately 1 s, ran off the wheel, circled around the cage, and then repeated this cycle over and over. Another example involved a mouse that repeatedly ran through the doors of the house beneath the wheel as part of its circling cycle. Circling behavior appeared to be more pronounced when the mouse was more alert or aggravated, such as during a cage change. Circling behavior did not noticeably interfere with general health or reproduction, as exemplified by 2 circling male mice that were placed in successful breeding pairs. These 2 male mice, which lived for 249 and 294 d, were exposed to wheels for 9 and 14 wk before being placed in breeder pairs without a wheel. Both mice sired at least 4 successful litters despite their circling behavior.
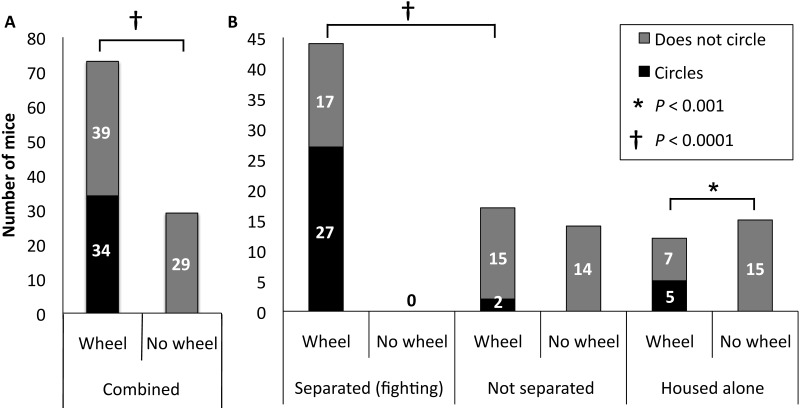
Once it was clear that this circling behavior was limited to a single genetic cohort of mice in the colony, we sought to characterize the phenotype and its relationship to the presence of the running wheels. A total of 75 FVB;129/Hemc male mice were weaned into social groups of 2 to 5 siblings (depending on the number of male mice available for weaning at any one time) with either a running-wheel dome house (61 animals) or a cardboard house (14 animals). An additional 27 male mice were weaned into individual housing with either a running wheel (12 animals) or a cardboard house (15 animals). Because circling penetrance was not 100%, we assigned additional animals to the running-wheel group. Only mice housed with running wheels showed circling behavior (34 of 73, 47%). None of the 29 mice housed without running wheels showed circling behavior. This difference was statistically significant (P = 8.70 × 10–7, 2 × 2 χ2 test; Figure 2 A).
Figure 2.
Circling in FVB;129/Hemc male mice. (A) Circling behavior was observed only in male mice for which a running wheel was present in the cage. (B) Mice represented under ‘separated (fighting)’ were initially weaned into social housing with 2 to 5 males per cage and then separated to individual housing when signs of fighting, such as wounding and fighting after cage change, were observed. Mice represented under ‘not separated’ were weaned into social housing with 2 to 5 males per cage and were never separated. Mice represented under ‘housed alone’ were housed alone from weaning. Mice were housed with either running wheels or cardboard houses (no wheel). Overt signs of fighting were observed exclusively in mice housed with wheels.
Circling behavior was first noted in male mice approximately 4 to 11 wk after receiving running wheels at approximately 3 wk old (weaning). When wheels were introduced to 9 adult male mice that ranged from 6 to 12 wk old, 6 of these mice required separation due to fighting, 2 of which developed circling behavior approximately 6 to 7 wk after wheel introduction. The timeframe between wheel introduction at 4 to 11 wk and the subsequent circling manifestation is similar to that of mice weaned with a wheel at 3 wk. This pattern implies that circling behavior is dependent on the presence of a running-wheel regardless of the age at which it was introduced.
A large proportion of socially housed FVB;129/Hemc male mice exposed to running wheels required separation due to fighting within a cage, as compared with all other males from all other lines in our colony, both with and without wheels. Among groups of 2 to 5 male mice, 72.1% (44 of 61) of socially housed male mice with running wheels were in cages where fighting was noted, compared with none of the 14 socially housed males without wheels (P = 4.73 × 10–7, Figure 2 B). Circling behavior in cages with running wheels manifested in socially housed FVB;129/Hemc male mice in cages with fighting and separation (27 of 44, 61.4%) significantly more often than in socially housed FVB;129/Hemc male mice that were not exposed to fighting (2 of 17, 11.8%; P = 0.00054, Figure 2 B). The proportion of singly housed male mice showing circling behavior (5 of 12, 41.7%) did not significantly differ from that of socially housed males exposed to fighting and separation showing circling behavior (27 of 44, 61.4%; P = 0.325). On average, socially housed male mice were separated due to fighting before circling behavior was noted, although fighting was detected from 51 d before to 30 d after circling (mean, 15.5 d prior to circling; median, 14 d prior to circling; n = 24). Among the 24 mice for which the dates of circling and separation due to fighting were noted, 19 developed circling behavior after being exposed to fighting, 1 mouse developed circling behavior on the same day that the brothers were separated, and 4 mice exhibited circling behavior before having to be separated. The level of or participation in fighting was not measured.
Circling behavior and the Arhgap19GT(YDH020)Byg mutation.
The FVB;129/Hemc cohort of mice are all homozygous for a GeneTrap mutation in Arhgap19 (Arhgap19GT(YDH020)Byg). We therefore performed a genetic analysis to assess whether circling behavior is associated with Arhgap19GT(YDH020)Byg homozygosity. We crossed the FVB;129/Hemc mice homozygous for Arhgap19GT(YDH020)Byg with FVB/N mice to create heterozygotes. We then crossed the heterozygotes, thereby generating all 3 Arhgap19GT(YDH020)Byg genotypes in the progeny: 25% homozygous mutant, 50% heterozygous for the mutation, and 25% wildtype. If the Arhgap19GT(YDH020)Byg mutation is associated with circling, then the phenotype would only occur in the homozygous mutant progeny. All progeny were given running wheels at weaning. Circling was seen in male mice of all 3 genotypes: 1 of 15 wildtype mice, 3 of 24 heterozygous mice, and 2 of 14 homozygous mutant male mice developing circling behavior. These results eliminated the Arhgap19GT(YDH020)Byg mutation as the cause of the circling. This finding suggests that circling was due to a novel independent mutation in a gene other than Arhgap19; in addition, the novel mutation would not be closely linked to Arhgap19. If this novel recessive mutation was also homozygous in the FVB;129/Hemc mice, then the progeny examined are expected to be approximately 25% homozygous for this mutation. The penetrance of circling behavior in the FVB;129/Hemc mice exposed to running wheels was only 46.6% (34 of 73 mice). Therefore, a circling penetrance of 11.3% (6 of 53 mice) in the overall progeny of the heterozygous male mice is approximately the expected incidence (6.17 of 53; 46.6% of the approximately 25% of homozygotes). This finding supports a genetic basis for the circling behavior in the presence of a running wheel, but in a gene other than Arhgap19.
In addition, we backcrossed the Arhgap19Gt(YHD020)Byg heterozygotes with the FVB/N strain for 10 generations to create a congenic line for Arhgap19Gt(YHD020)Byg on the FVB strain. None of the 51 congenic Arhgap19Gt(YHD020)Byg FVB male mice provided with running wheels developed circling behavior. Because the mice were selected at each backcross generation for the presence of Arhgap19Gt(YHD020)Byg, an independent mutation most likely would be lost after 10 generations, thus explaining the loss of the circling behavior.
Circling behavior in female mice.
Female FVB;129/Hemc mice housed with running wheels show a very low penetrance of circling behavior (1 of 33, 3.0%). This pattern is also true of the female progeny of the Arhgap19Gt(YHD020)Byg heterozygous crosses, which showed a circling incidence of 2 of 28 (7.1%). None of the socially housed FVB;129/Hemc female mice demonstrated any fighting that would result in their separation. Although 3 of the 28 (10.7%) female progeny from the heterozygous cross were exposed to fighting and separated, none of them developed circling behavior. Unlike male congenic Arhgap19Gt(YHD020)BygFVB mice, of which none circled, 3 of 46 (6.5%) female congenic mice housed with wheels acquired circling behavior, none of which was exposed to fighting. Of the 13 female congenic Arhgap19Gt(YHD020)BygFVB mice housed without wheels, 1 developed circling behavior; we did not evaluate this mouse further.
Inner ear defects as a potential cause of circling behavior.
Circling behavior in rodents is often associated with inner ear defects.6,21 We therefore tested 7 circling male mice by using a series of behavioral tests designed to look for the inability to sense gravity due to vestibular dysfunction; 7 noncircling mice (4 housed with wheels, 3 housed with cardboard houses) were tested as controls. One of the 7 circling mice was the male mouse that parented the FVB;129/Hemc line; 2 of his brothers served as controls. In addition, 2 circling mice and 2 controls were F1 progeny of the breeder pair that founded the FVB;129/Hemc line; the remaining mice tested were from a later generation. According to one-way ANOVA, results of the trunk curl test did not significantly differ between groups, and both circling mice and noncircling mice would occasionally curl their trunks as well as reach for a presented surface. Circling mice were capable of righting themselves in the contact-righting test and showed no significant difference from the controls. All mice were able to swim, although circling mice spent more time swimming in circles than did noncircling mice. The amounts of time spend swimming normally and in circles differed significantly (normal swimming: P = 0.0041, F = 9.0147; circular swimming: P = 0.0293, F = 4.8070; one-way ANOVA). Bonferroni-corrected pairwise tests identified a significant decrease in normal swimming in the circling mice (P < 0.05). This difference resulted from the fact that 5 of the 7 circling mice continued to circle while swimming. However, none of the mice displayed underwater tumbling at any time during the 2 min of swimming. Because none of the 7 circlers showed any indication of an inner ear defect, additional circling mice were not tested.
Dopamine and serotonin biochemistry in circling FVB;129/Hemc male mice.
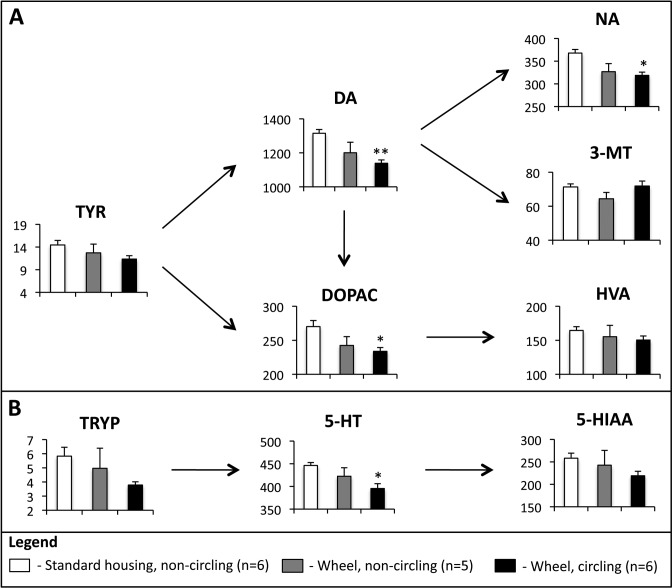
Changes in some amino acids and biogenic amines have been shown to be associated with circling in mice.5,15,34 We therefore compared the levels of biogenic amines, amino acids, and metabolites in the left and right brain hemispheres of male FVB;129/Hemc mice housed with wheels that did or did not develop circling behavior with those of male FVB;129/Hemc mice in standard housing conditions. Some asymmetries between brain hemispheres were noted but occurred in both circling and noncircling mice, and no definitive pattern in circling mice could be discerned. Therefore, left and right hemispheres were treated as technical replicates and averaged to obtain concentrations for the brain overall. Levels of the biogenic amines dopamine, noradrenaline, and the metabolite DOPAC were significantly altered (dopamine: P < 0.01, F = 6.5; noradrenaline: P < 0.05, F = 5.9; DOPAC: P < 0.05, F = 4.5; one-way ANOVA). Bonferroni-corrected pairwise tests identified significantly decreased levels of dopamine, noradrenaline, and DOPAC in circling mice housed with wheels compared with male mice housed under standard conditions (P < 0.01, P < 0.05, and P < 0.05, respectively; Figure 3 A). The biogenic amine metabolic pathway for serotonin was affected also (P < 0.05, F = 4.6): serotonin levels were lower in circling mice housed with wheels compared with standard-housed male mice (P < 0.05; Figure 3 B). None of the other amino acids, biogenic amines, and their metabolites we tested showed a significant difference between any of the 3 groups.
Figure 3.
Male FVB;129/Hemc mice that circle have altered brain biochemistry. (A) Male circling mice have significantly lower dopamine (DA), noradrenaline (NA), and 3,4-dihydroxyphenylacetic acid (DOPAC) concentrations compared with male mice with standard housing, all of which did not circle. (B) Male circling mice have significantly lower serotonin (5-HT) levels compared with male mice in standard housing. Arrows denote the metabolic pathway, with only intermediates measured by HPLC shown. Values that differ significantly (*, P < 0.05; †, P < 0.01) from those of mice in standard housing are indicated. The y axis displays total brain concentration, with tyrosine (TYR), and tryptophan (TRYP) as μg/g of tissue and the remaining compounds as ng/g of tissue. Data are given as mean ± SEM (error bars). 3-MT, 3-methoxytyramine; HVA, homovanillic acid; 5-HIAA, 5-hydroxyindoleacetic acid.
Discussion
Running wheels are commonly used as environmental enrichments to improve the wellbeing of laboratory mice. Our case study is the first report in which the presence of running wheels induced stereotypic behavior in male mice, and in particular, the first to connect running wheels to pronounced circling behavior. No male mouse in our colony, housed either socially or solitary, showed circling behavior without the presence of a wheel. This predisposition to circling behavior in the presence of running wheels is specific to the FVB;129/Hemc cohort in our colony, because none of the FVB/N, BALB/cCrl, and C57BL/6 male mice housed with wheels showed any circling. It should be noted that we did not test multiple styles of wheels, and doing so would be interesting. More importantly, because the igloo-style running wheel includes a shelter, future experiments should test either a free-standing wheel or give the control mice the igloo shelters without the wheels, to assess any confounding housing difference between the circlers and noncirclers. The igloo-style shelter of the circlers and the cardboard houses of the controls might have affected the circling outcome, given that they represent different types of enrichment (the cardboard houses being more amenable to chewing and manipulation). For instance, igloo shelters are associated with low levels of aggression in male mice,39 whereas flat Utrecht shelters were associated with increased aggression in male mice.41
FVB;129/Hemc mice are homozygous for a gene trap mutation in Arhgap19, whose product is a Rho-GAP protein expressed in the mouse embryo16 and several human fetal but not adult tissues.22 However, we have shown genetically that the circling behavior is not associated with the Arhgap19Gt(YHD020)Byg mutation. When we created Arhgap19GT(YDH020)Byg heterozygotes and crossed them, the circling phenotype was present in expected numbers for Mendelian inheritance with reduced penetrance but did not follow the Arhgap19GT(YDH020)Byg genotype. Furthermore, our BALB/cCrl substrain contains a homozygous nonsense mutation in Arhgap19,16 and none of these mice demonstrated any circling behavior when running wheels were introduced for the same 11-mo period. Therefore, mice with a homozygous gene trap mutation in Arhgap19 have no detectable abnormal phenotype. In addition, the circling phenotype was no longer evident in Arhgap19Gt(YHD020)Byg FVB congenic male mice after 10 generations of backcrossing, suggesting that predisposition to circling is caused by a second-site mutation that was not selected for and therefore lost. The second-site mutation may have originated from the 129P2 parental strain or as a spontaneous mutation in one of the mice used to generate the FVB;129/Hemc line. Such a mutation would be difficult to identify and would require a technique such as linkage analysis coupled with whole exome or whole-genome sequencing. Another possible source of a de novo mutation is the introduction of a second gene trap insertion during the production of the Arhgap19Gt(YHD020)Byg ES cell line. A full second insertion is unlikely, given that our genotyping protocol uses primers within the gene trap to identify the mutation. Therefore, a second-site gene trap insertion, unless linked to Arhgap19, would have been apparent as a skew in the Mendelian inheritance of the gene trap, which was not observed. A second full insertion tightly linked to the Arhgap19 gene is not likely responsible for the circling phenotype because the circling phenotype did not follow Arghap19 inheritance in the heterozygous crosses and likely would not have been lost during production of the congenic line. A fragment of the gene trap inserted elsewhere in the genome could still be responsible for the circling phenotype.
Circling behavior often manifests in mice with vestibular dysfunction. If the FVB;129/Hemc line had vestibular defects, we would expect that circling behavior would manifest regardless of housing conditions; however, only mice housed with wheels developed circling behavior. Regardless, trunk curl, contact-righting, and forced swim tests of 7 circlers demonstrated that FVB;129/Hemc circling mice were able to sense gravity. It is still possible that their inner ears and ability to sense gravity are subtly affected.
We showed that FVB;129/Hemc circling mice have significantly lower levels of dopamine and its metabolite DOPAC in whole-brain extracts. Some mice with vestibular dysfunction and circling behavior also have alterations in dopamine biochemistry,7,35,38 suggesting a common pathway with different primary causes. Dopamine and serotonin (5-hydroxytryptamine) have been proposed to be agonistically linked in rodents, meaning that reduction in dopamine levels leads to reduction in serotonin levels.14 Brain serotonin levels are altered in partial bilateral striatal 6-hydroxydopamine-lesioned rats, which demonstrate unilateral circling behavior, but these rats also have perturbations in brain dopamine levels.36 Therefore, one possibility is that alteration in serotonin levels in our FVB;129/Hemc mice is a response mechanism to alterations in dopamine or noradrenaline signaling or vice versa. Further experimentation is required to shed light on the mechanisms and significance of the biogenic amine alterations in circling mice.
Much like running wheels, social housing is established as an environmental enrichment. However, in some strains of mice, social housing coupled with the provision of running wheels can induce aggressive behavior in male mice.13 Although we did not measure levels of fighting in specific mice, signs of fighting requiring separation, such as wounding and observation of fighting after cage change, were common in cages of FVB;129/Hemc male mice with running wheels and was absent in FVB;129/Hemc male mice without running wheels (Figure 2 B). In addition, the majority of FVB;129/Hemc male mice with running wheels that developed circling behavior experienced the presence of fighting in the cage, although not necessarily directly, whereas few FVB;129/Hemc male mice with running wheels acquired circling behavior when signs of fighting were absent. In these latter cases, observation might have missed low levels of fighting without wounding. The presence of an igloo-style running wheel appears to act as an environmental trigger for circling behavior in the genetically predisposed FVB;129/Hemc line. The fighting may act as a stressor and amplify the acquisition of circling behavior; however, unlike the presence of the running wheel, fighting is not necessary for circling behavior to develop in these mice, given that singly housed mice with wheels also showed high penetrance of circling behavior. Therefore additional stressors, such as being housed alone or fighting, likely increase the susceptibility to circling in these mice but are insufficient to trigger the behavior on their own. This mechanism may explain the lack of circling in female mice, which showed no evidence of fighting and were exclusively socially housed, suggesting that stressors may not have reached a threshold in female mice. We did not test this hypothesis. In another study, female mice exposed to igloo-style running wheels showed no significant increase in aggression.39
Our results indicate that mice with a specific genetic background can be predisposed to developing pronounced and permanent circling behavior when exposed to an environmental trigger. We conclude that in mice with some specific genotypes, the provision of igloo-style running wheels acts as an environmental trigger that allows these abnormalities in behavior and brain biochemistry to manifest. Without the trigger (here, the running wheel), the behavior is unlikely to manifest. Therefore, based on our results and similar cases reported in the literature,1,13,26 running wheels should be introduced with careful monitoring. In our case, the circling phenotype and its underlying genetic cause would have gone completely undetected without the environmental trigger.
Acknowledgments
This study was funded by an operating grant from The Women and Children's Health Research Institute (WCHRI) of Alberta, Canada. We would like to thank Kacie Norton, Carol Crandle, Annette Morin, Sarah Collard, and Simmone Kerswell for assistance with the mice and helpful discussions. We thank Dr. Sung Hyun Kang for help with biostatistics.
References
- 1.Akre AK, Bakken M, Hovland AL, Palme R, Mason G. 2011. Clustered environmental enrichments induce more aggression and stereotypic behaviour than do dispersed enrichments in female mice. Appl Anim Behav Sci 131:145–152. [Google Scholar]
- 2.Benson C, Paylor JW, Tenorio G, Winship I, Baker G, Kerr BJ. 2015. Voluntary wheel running delays disease onset and reduces pain hypersensitivity in early experimental autoimmune encephalomyelitis (EAE). Exp Neurol 271:279–290. [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Nakagawa S, An Y, Ito K, Kitaichi Y, Kusumi I. 2016. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front Neuroendocrinol. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RA. 1934. Statistical methods for research workers. Edinburgh.Oliver and Boyd. [Google Scholar]
- 5.Fitzgerald LW, Miller KJ, Ratty AK, Glick SD, Teitler M, Gross KW. 1992. Asymmetric elevation of striatal dopamine D2 receptors in the chakragati mouse: neurobehavioral dysfunction in a transgenic insertional mutant. Brain Res 580:18–26. [DOI] [PubMed] [Google Scholar]
- 6.Friedman LM, Dror AA, Avraham KB. 2007. Mouse models to study inner ear development and hereditary hearing loss. Int J Dev Biol 51:609–631. [DOI] [PubMed] [Google Scholar]
- 7.Giardino L, Zanni M, Pignataro O. 1996. DA1 and DA2 receptor regulation in the striatum of young and old rats after peripheral vestibular lesion. Brain Res 736:111–117. [DOI] [PubMed] [Google Scholar]
- 8.Goh J, Ladiges W. 2015. Voluntary wheel running in mice. Curr Protoc Mouse Biol 5:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant SL, Shulman Y, Tibbo P, Hampson DR, Baker GB. 2006. Determination of D-serine and related neuroactive amino acids in human plasma by high-performance liquid chromatography with fluorimetric detection. J Chromatogr B Analyt Technol Biomed Life Sci 844:278–282. [DOI] [PubMed] [Google Scholar]
- 10.Hadley C, Hadley B, Ephraim S, Yang M, Lewis MH. 2006. Spontaneous stereotypy and environmental enrichment in deer mice (Peromyscus maniculatus): Reversibility of experience. Appl Anim Behav Sci 97:312–322. [Google Scholar]
- 11.Hanif AM, Lawson EC, Prunty M, Gogniat M, Aung MH, Chakraborty R, Boatright JH, Pardue MT. 2015. Neuroprotective effects of voluntary exercise in an inherited retinal degeneration mouse model. Invest Ophthalmol Vis Sci 56:6839–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardisty-Hughes RE, Parker A, Brown SD. 2010. A hearing and vestibular phenotyping pipeline to identify mouse mutants with hearing impairment. Nat Protoc 5:177–190. [DOI] [PubMed] [Google Scholar]
- 13.Howerton CL, Garner JP, Mench JA. 2008. Effects of a running wheel-igloo enrichment on aggression, hierarchy linearity, and stereotypy in group-housed male CD-1 (ICR) mice. Appl Anim Behav Sci 115:90–103. [Google Scholar]
- 14.Kaariainen TM, Garcia-Horsman JA, Piltonen M, Huotari M, Mannisto PT. 2008. Serotonergic activation after 2-week intrastriatal infusion of L-dopa and slow recovery of circling in rats with unilateral nigral lesions. Basic Clin Pharmacol Toxicol 102:300–307. [DOI] [PubMed] [Google Scholar]
- 15.Kincaid AE. 2001. Spontaneous circling behavior and dopamine neuron loss in a genetically hypothyroid mouse. Neuroscience 105:891–898. [DOI] [PubMed] [Google Scholar]
- 16.Kooistra MK, Leduc RY, Dawe CE, Fairbridge NA, Rasmussen J, Man JH, Bujold M, Juriloff D, King-Jones K, McDermid HE. 2011. Strain-specific modifier genes of Cecr2-associated exencephaly in mice: genetic analysis and identification of differentially expressed candidate genes. Physiol Genomics 44:35–46. [DOI] [PubMed] [Google Scholar]
- 17.Liu TW, Park YM, Holscher HD, Padilla J, Scroggins RJ, Welly R, Britton SL, Koch LG, Vieira-Potter VJ, Swanson KS. 2015. Physical activity differentially affects the cecal microbiota of ovariectomized female rats selectively bred for high and low aerobic capacity. PLoS One 10:e0136150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loscher W. 2010. Abnormal circling behavior in rat mutants and its relevance to model specific brain dysfunctions. Neurosci Biobehav Rev 34:31–49. [DOI] [PubMed] [Google Scholar]
- 19.Loscher W, Richter A, Nikkhah G, Rosenthal C, Ebert U, Hedrich HJ. 1996. Behavioral and neurochemical dysfunction in the circling (ci) rat: a novel genetic animal model of a movement disorder. Neuroscience 74:1135–1142. [DOI] [PubMed] [Google Scholar]
- 20.Lu YC, Wu CC, Shen WS, Yang TH, Yeh TH, Chen PJ, Yu IS, Lin SW, Wong JM, Chang Q, Lin X, Hsu CJ. 2011. Establishment of a knock-in mouse model with the SLC26A4 c.919-2A>G mutation and characterization of its pathology. PLoS One 6:e22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv K, Huang H, Lu Y, Qin C, Li Z, Feng JQ. 2010. Circling behavior developed in Dmp1 null mice is due to bone defects in the vestibular apparatus. Int J Biol Sci 6:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv L, Xu J, Zhao S, Chen C, Zhao X, Gu S, Ji C, Xie Y, Mao Y. 2007. Sequence analysis of a human RhoGAP domain-containing gene and characterization of its expression in human multiple tissues. DNA Seq 18:184–189. [DOI] [PubMed] [Google Scholar]
- 23.Makishima T, Hochman L, Armstrong P, Rosenberger E, Ridley R, Woo M, Perachio A, Wood S. 2011. Inner ear dysfunction in caspase-3 deficient mice. BMC Neurosci 12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason GJ. 1991. Stereotypies: a critical review. Anim Behav 41:1015–1037. [Google Scholar]
- 25.Mazzola PN, Bruinenberg V, Anjema K, van Vliet D, Dutra-Filho CS, van Spronsen FJ, van der Zee EA. 2015. Voluntary exercise prevents oxidative stress in the brain of phenylketonuria mice. JIMD Rep 27:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McQuaid RJ, Audet MC, Anisman H. 2011. Environmental enrichment in male CD-1 mice promotes aggressive behaviors and elevated corticosterone and brain norepinephrine activity in response to a mild stressor. Stress 15:354–360. [DOI] [PubMed] [Google Scholar]
- 27.Meijer JH, Robbers Y. 2014. Wheel running in the wild. Proc Biol Sci 281:pii:20140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nevison CM, Hurst JL, Barnard CJ. 1999. Strain-specific effects of cage enrichment in male laboratory mice (Mus musculus). Anim Welf 8:361–379. [Google Scholar]
- 29.Parent M, Bush D, Rauw G, Master S, Vaccarino F, Baker G. 2001. Analysis of amino acids and catecholamines, 5-hydroxytryptamine and their metabolites in brain areas in the rat using in vivo microdialysis. Methods 23:11–20. [DOI] [PubMed] [Google Scholar]
- 30.Pawlowicz A, Demner A, Lewis MH. 2010. Effects of access to voluntary wheel running on the development of stereotypy. Behav Processes 83:242–246. [DOI] [PubMed] [Google Scholar]
- 31.Powell SB, Newman HA, McDonald TA, Bugenhagen P, Lewis MH. 2000. Development of spontaneous stereotyped behavior in deer mice: effects of early and late exposure to a more complex environment. Dev Psychobiol 37:100–108. [PubMed] [Google Scholar]
- 32.Richter H, Ambree O, Lewejohann L, Herring A, Keyvani K, Paulus W, Palme R, Touma C, Schabitz WR, Sachser N. 2008. Wheel-running in a transgenic mouse model of Alzheimer's disease: protection or symptom? Behav Brain Res 190:74–84. [DOI] [PubMed] [Google Scholar]
- 33.Richter SH, Gass P, Fuss J. 2014. Resting is rusting: a critical view on rodent wheel-running behavior. Neuroscientist 20:313–325. [DOI] [PubMed] [Google Scholar]
- 34.Russell VA, Sagvolden T, Johansen EB. 2005. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct 1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schirmer M, Kaiser A, Lessenich A, Lindemann S, Fedrowitz M, Gernert M, Loscher W. 2007. Auditory and vestibular defects and behavioral alterations after neonatal administration of streptomycin to Lewis rats: Similarities and differences to the circling (ci2/ci2) Lewis rat mutant. Brain Res 1155:179–195. [DOI] [PubMed] [Google Scholar]
- 36.Scholtissen B, Deumens R, Leentjens AFG, Schmitz C, Blokland A, Steinbusch HWM, Prickaerts J. 2006. Functional investigations into the role of dopamine and serotonin in partial bilateral striatal 6-hydroxydopamine lesioned rats. Pharmacol Biochem Behav 83:175–185. [DOI] [PubMed] [Google Scholar]
- 37.Sherwin CM. 1998. Voluntary wheel running: a review and novel interpretation. Anim Behav 56:11–27. [DOI] [PubMed] [Google Scholar]
- 38.Stiles L, Zheng Y, Darlington CL, Smith PF. 2012. The D2 dopamine receptor and locomotor hyperactivity following bilateral vestibular deafferentation in the rat. Behav Brain Res 227:150–158. [DOI] [PubMed] [Google Scholar]
- 39.Swetter BJ, Karpiak CP, Cannon JT. 2011. Separating the effects of shelter from additional cage enhancements for group-housed BALB/cJ mice. Neurosci Lett 495:205–209. [DOI] [PubMed] [Google Scholar]
- 40.Tal-Krivisky K, Kronfeld-Schor N, Einat H. 2015. Voluntary exercise enhances activity rhythms and ameliorates anxiety- and depression-like behaviors in the sand rat model of circadian rhythm-related mood changes. Physiol Behav 151:441–447. [DOI] [PubMed] [Google Scholar]
- 41.Van Loo PLP, Kruitwagen CLJJ, Koolhaas JM, Van de Weerd HA, Van Zutphen LFM, Baumans V. 2002. Influence of cage enrichment on aggressive behaviour and physiologic parameters in male mice. Appl Anim Behav Sci 76:65–81. [Google Scholar]
- 42.Zigmond MJ, Smeyne RJ. 2014. Exercise: is it a neuroprotective and if so, how does it work? Parkinsonism Relat Disord 20 Suppl 1:S123–S127. [DOI] [PubMed] [Google Scholar]