Abstract
Rodent health-monitoring programs based on sampling an IVC system's exhaust air dust (EAD) has enhanced and even replaced traditional sentinels for some rodent pathogens. EAD testing by qPCR assay is an optimal surveillance method for the rapid detection of Corynebacterium bovis-infected immunodeficient mice. Here we demonstrate that an active EAD surveillance program for C. bovis can be used to maintain nude mice C. bovis-free after the transition from historically enzootically infected colonies. During 3 events over 3 y, rapid detection of infection, elimination of infected mice, aggressive quarantine measures, and local decontamination prevented the spread of C. bovis within 2 barrier rooms. In total, 4 cages of infected nude mice were identified and removed, preventing the spread of infection to 469 other cages of immunodeficient mice. In addition, we present data regarding a refinement to EAD testing which enables row-specific surveillance of an IVC rack. This technique systemically decreases the amount of testing required to locate an individually infected cage. Due to our ability to rapidly detect and localize an infected cage, we were able to investigate the route of C. bovis introduction into our barrier rooms. Our epidemiologic investigation suggested that the transmission of C. bovis occurred through contaminated, cryopreserved, patient-derived xenograft tumor tissue. This previously unknown source of C. bovis can infect mice used to propagate these tumors. Together, these data demonstrate that a remediation program that combines rapid detection, test-and-cull, and local decontamination under quarantine conditions can eliminate C. bovis from a mouse colony.
Abbreviations: ATS, animal transfer station; EAD, exhaust air dust; HEM, horizontal exhaust manifold; PDX, patient-derived xenograft; qPCR, quantitative real-time PCR
The international distribution of Corynebacterium bovis within academic and industry research facilities is a testament to the bacteria's efficient transmission among susceptible mouse populations.3,5,6,12,20,22 In one report, infection spread rapidly among nude mice colonies housed in static caging, reaching 80% morbidity in a few days.20 The use of IVC systems, which supply cage-specific HEPA-filtered air, has been reported to prevent the spread of infection while mice remain in their cage.4 However, ample opportunity still exists for horizontal transmission of C. bovis through animal manipulations by both research and animal care staff.4 After infection, nude mice do not consistently present or fail to sustain clinical hyperkeratosis to alert researchers and staff to infection.3,5,8,17 Without the use of a surveillance program for C. bovis, asymptomatic mice remain as reservoirs for the transmission of infection.4,5
Environmental sampling for the detection of rodent pathogens is evolving to be a valuable adjunct to traditional sentinel monitoring programs.2,9,13,25 We have recently shown that qPCR evaluation of exhaust air dust (EAD) from an IVC system is a sensitive method for the detection of C. bovis.17 The objective of the current project was to demonstrate the utility of a C. bovis surveillance program based on EAD testing. Herein, we provide data regarding a refinement to EAD testing which, based on rack design, aids in identifying infected cage(s) through row-specific sampling followed by individual cage testing. Rapid detection followed by the identification of infected cages allowed for epidemiologic investigations into the route of entry. This investigation led to the discovery of a novel source of C. bovis contamination and infection transmission. We concluded that C. bovis was introduced through contaminated, cryopreserved, patient-derived xenograft (PDX) tumor tissue. Ultimately, our ability to rapidly detect and cull infected mouse cages, followed by local decontamination of research equipment and husbandry materials, prevented the spread of C. bovis within 2 colonies of nude mice carrying valuable and unique PDX tumors.
Materials and Methods
Routine husbandry and management of facilities.
The 2 primary rodent facilities at the University of Colorado Denver Anschutz Medical Campus opened in 2004 and 2008. A detailed description of the health status of each facility prior to July 2010 has been published previously.15 Both vivaria contain approximately 10,000 mouse cages each and are maintained by using the same standard operating procedures. To enter either facility, a hair bonnet, disposable gown, and shoe covers over personal clothing are required. Donning nitrile gloves and a surgical mask are required prior to opening cages. The majority of mice and all identified immunodeficient strains of mice are housed in JAG 75 cages on individually ventilated MicroVent racks (Allentown, Allentown, NJ), providing approximately 40 air changes hourly. All mouse cages are autoclaved with aspen-chip bedding (Teklad Aspen Sani-Chips, Envigo, Indianapolis, IN), a compressed cotton square, and a box or wire bar feeder. Reverse-osmosis–purified, hyperchlorinated water is provided from an automated watering system (Edstrom Industries, Waterford, WI) attached to the rack. Mice are fed irradiated rodent Teklad diets 2920X or 2919 (Envigo). Enrichment such as a Mouse Igloo (Bio-Serv, Frenchtown, NJ) or paper roles are autoclaved separately and added to cages in housing rooms. Rodent housing rooms are maintained at 22.0 ± 1 °C (72 °F), 30% to 40% humidity, with at least 12 fresh-air changes per hour and a 14:10-h light:dark cycle. All husbandry procedures are performed in a HEPA-filtered workbench (ATS or ATS2, Allentown) within animal housing rooms. During cage or rodent manipulation, all work surfaces and nitrile gloved hands are maintained moist with Clidox-S (Pharmacal, Naugatuck, CT) mixed at 1:18:1 (approximately 50 ppm ClO2) as a general disinfectant. Cage changes are performed on a standard 2-wk cycle. Rack and cage sanitation were performed as described previously.17
Animal housing rooms, dedicated procedure rooms, common areas, and corridor floors walls, and ceilings are disinfected on a set schedule by using a microfiber mop system (TAB Mops, CPI, Holland MI) to distribute disinfectant (Quatricide-PV, Pharmacal).23 Incoming supplies to the facilities are either disinfected by hand wiping with a germicidal detergent (Sani-Plex, Quip Laboratories, Wilmington, DE) or passed through a disinfectant tunnel that uses UV light (RGF Environmental Group, Riviera Beach, FL) or a mist tunnel (FPEC, Santa Fe Springs, CA) of ClO2-based disinfectant (100 ppm, MB10, Quip Laboratories).
A mouse health-surveillance program is uniformly performed across both facilities. A single cage of 2 female sentinel mice (Hsd:ICR, 5 to 6 wk) are housed on each single side of an IVC rack. At the time of cage change, sentinel mice are exposed to approximately 5 mL of soiled bedding from each cage on the rack and are screened quarterly. Sentinels are screened for pinworms and fur mites through inhouse microscopy of perianal tape impressions, fecal flotation, and fur plucks. Sentinel serum samples are evaluated by an independent laboratory (IDEXX Bioresearch, Columbia, MO) using multiplexed fluorometric immunoassays. Pathogens and pest targeted by the health surveillance program include mouse hepatitis virus, mouse parvovirus, minute virus of mice, mouse rotavirus, Theiler mouse encephalomyelitis virus, Ectromelia virus, lymphocytic choriomeningitis virus, mouse polyoma virus, mouse adenovirus type 1 and 2, Sendai virus, reovirus virus, Mycoplasma pulmonis, fur mites (Radfordia, Myocoptes), and pinworms (Aspiculuris, Syphacia). The rodent health-surveillance program that uses soiled bedding sentinels is not used for C. bovis surveillance.
C. bovis surveillance and remediation program.
Mouse barrier rooms and adjacent procedural space that contain C. bovis-free colonies are considered C. bovis-free space. All experimental equipment used in C. bovis-free spaces are decontaminated by facility staff prior to use. Experimental equipment was disinfected by hand wiping with a germicidal detergent (Sani-Plex, Quip Laboratories) followed by a 1:18:1 mixed ClO2 solution (Clidox-S, Pharmacal). The disinfection procedure is performed for all large and small equipment including animal transfer stations (ATS), gas anesthesia machines, circulating warm-water heating pads, computers, calipers, scales, and mouse restraint devices, to name a few examples. Autoclaving is used for all other materials that can be sterilized in that manner.
According to our recent publication, EAD sampling of the horizontal exhaust manifold (HEM) of IVC racks was implemented weekly for C. bovis surveillance of C. bovis-free nude mouse colonies.17 Racks monitored for C. bovis hold immunodeficient strains which are also susceptible to infection.14,21 The method for sampling the HEM for EAD testing has been described previously.9,13,17 Swabs were stored at ambient temperature for 24 to 48 h prior submission to the University of Colorado Denver Quantitative PCR Core for DNA isolation. Samples are collected on the first day of the standard work week, and results are typically obtained within 48 h.
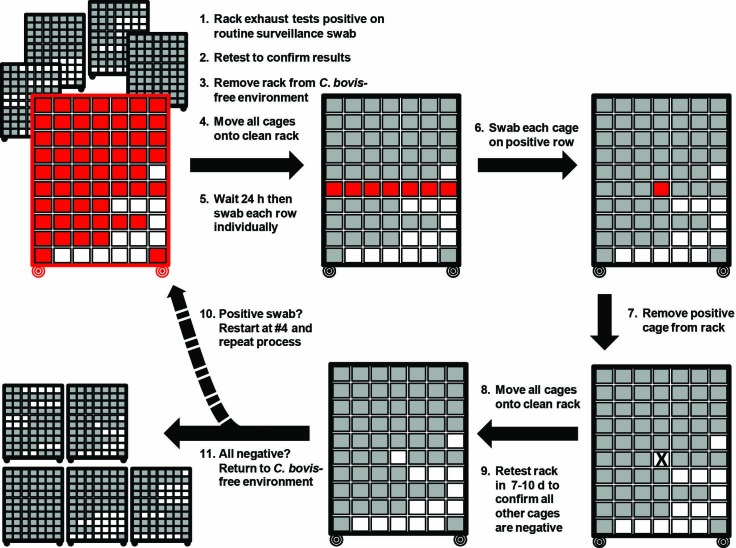
After the identification of a C. bovis-positive rack by EAD testing, we developed a standardized procedure to prevent the spread of C. bovis within the housing room and to identify individual positive cages (Figure 1). For each positive surveillance sample in the cases described following, several variations in the procedure occurred. In general, once a rack was identified as C. bovis-positive, results were confirmed, and the rack was moved to a housing room without immunodeficient mice within 72 h of the initial positive result. All husbandry and research equipment, including the ATS, were replaced or surface-cleaned by facility staff as described earlier. All consumables in the room such as nitrile gloves, rodent feed, facility forms, notepads and ink pens were discarded. By using the previously described mop system, Quatricide PV (Pharmacal) was applied to the walls, floor, ceiling, and door. In addition, any procedure room used by the investigator who had mice on the positive rack was decontaminated, including the contents of any storage cabinets. To identify the infected cages on the C. bovis-positive rack, all cages were moved to a newly autoclaved rack. After 24 h on the new rack, the 10 HEM of the rack were swabbed individually and submitted for C. bovis qPCR analysis. Once the positive row was determined, each cage on the positive row was tested by swabbing the inside of each cage lid (Figure 2). The inner cage lid was selected for swabbing in light of the design of the Allentown cage and rack system, in which intracage air escapes between the cage wall and lid at this location. Exhaust air is actively scavenged into the rack exhaust system at the rear of each cage slot (Figure 3). Positive cages were moved to known C. bovis-positive housing rooms. After C. bovis-positive cages had been removed from the rack, all remaining cages on the rack were again moved to a newly autoclaved rack. After 7 to 10 d, all HEM of the rack were retested for C. bovis DNA by using a single HEM swab. After confirmation that all rows had remained negative, all cages were changed in a HEPA-filtered ATS within the housing room without immunodeficient mice. Cages were then returned to the C. bovis-free barrier room and placed on a newly autoclaved rack. Routine weekly monitoring by HEM swabs was resumed. DNA was extracted from dry swabs (BBL Culture Swab EZ) and PDX tumor tissue (0.015 to 1 g) and the C. bovis qPCR assay was performed as described previously.16,17
Figure 1.
Illustration of the standard operating procedure to address a C. bovis-positive rack during routine EAD surveillance. Differential swabbing of the HEM of each row allows for diagnostic testing to localize the infected cage(s) on the rack. Autoclaving of racks removes C. bovis DNA to prevent false-positive results.
Figure 2.

Diagnostic samples from individual cages were collected from the inner cage lid at the rear of the cage. Individual cages on the row are sampled in sequence. The inner lid was sampled in an attempt to minimize the disturbance of C. bovis-contaminated particulates within the cage and thus decrease the potential for cross-contamination between cages.
Figure 3.

Horizontal exhaust air ports located at the rear of the cage slot (arrows) draw the cage-exhausted air into the horizontal exhaust plenum on an IVC rack (Allentown, Allentown, NJ).
Experimental design for the detection of HEM cross-contamination.
Male, nude mice (age, 8 to 24 wk) were housed in 2 cages with 4 mice per cage (n = 8). Mice were experimentally infected with C. bovis as described previously.17 Both cages of exposed mice were individually confirmed to be C. bovis-positive after exposure through qPCR analysis of a skin and oral swab. Each cage of C. bovis-infected mice was placed on a separate IVC rack at cage position A10, because it is closest to the HEM of row 10 and has been demonstrated to distribute C. bovis DNA into the rack exhaust plenum at a faster rate and higher copy number than the cage position most distant from the HEM.17 All other cage positions on the rack remained empty. At 24 h after placement of the infected cage and repeated weekly for 8 wk, a single swab was used to sample the HEM of rows 1 through 9, and a second swab used to sample the HEM of row 10. A rack with no cages was used as a control for environmental C. bovis contamination. For the control rack, a single swab was used to sample HEM 1 through 10 at the same interval as the 2 experimental racks. Due to the rack design, C. bovis-contaminated exhaust air entering the vertical exhaust plenum at HEM 10 must ascend the vertical exhaust plenum and pass by all other HEM prior to exiting the rack and entering the building exhaust system. Thus, the use of a common swab from HEM 1 through 9 will allow the detection of any cross-contamination that might occur between HEM that originated from HEM 10 only. To ensure optimal rack ventilation, both supply and exhaust air currents were tested every other week to confirm the rate of approximately 40 air changes hourly at the cage level. All animal studies were approved by the University of Colorado Denver IACUC.
Case Study
Surveillance detection of C. bovis.
The most extensive use of PDX models was performed by 2 research groups (A and B), whose laboratories have different nude mouse colonies in separate facilities. Shared use of PDX tumor models between labs does not occur.
Case 1.
In July 2013, 1 of 5 racks in a C. bovis-free barrier room tested positive for C. bovis by weekly rack surveillance. This rack was the first positive rack detected since the initiation of the rack-level C. bovis surveillance program in the fall of 2012. The barrier room, which contained only nude mice, and its associated procedural area had been C. bovis-free for the prior 10 mo. All 61 cages on the rack belonged to investigator A and contained nude mice carrying more than 40 unique PDX models. Four months prior to the positive sample, all PDX lines on the rack had been successfully transferred out of an enzootically infected in vivo tumor bank to establish a C. bovis-free in vivo tumor bank.16 To confirm the positive rack sample, a second swab was collected within 3 d of the initial sample and showed an 8-fold increase in C. bovis DNA copy number (Table 1). The rack was immediately moved into a housing room without immunodeficient mice. In contrast to the procedure outlined earlier in the Material and Methods section, a newly sanitized rack was not available to move all cages onto. As a result, the horizontal exhaust ports positioned at the rear of each cage slot were swabbed. All ports on the row were sampled by using a single swab (Figure 3). Rows 6 and 7 were both positive for C. bovis DNA, with copy number values of 16,599 and 370, respectively. According to the copy numbers, we suspected that row 6 contained the infected cage(s) and that the row 7 result was a false positive due to cross-contamination of fine particulate material falling from the row 6 sampling site. Individual swabs were collected from the inner lid margins of the 6 cages on row 6. These results confirmed the presence of a single C. bovis-positive cage at rack position A6. The cage of infected mice was removed and placed in a C. bovis-positive housing room. All 60 remaining cages on the positive rack were then transferred to a newly sanitized rack, and the rack was tested after 4 d. All rows tested negative, and all cages were returned to the C. bovis-free barrier room. Concurrently, the other 4 racks in the C. bovis-free room were retested and remained negative.
Table 1.
Case metrics regarding C. bovisinfections and room census summary
| Case | |||
| 1 | 2 | 3 | |
| Duration (mo) C. bovis-free before infection | 10 | 11 | 10 |
| DNA copy number from initial positive rack swab | 2007 | 2225 | 3040 |
| DNA copy number from confirmation rack swab | 17,299 | 4,726 | 2,531 |
| Time (d) from initial to confirmation swab | 3 | 7 | 3 |
| DNA copy number on new rack after 24 h | 16,599 | 167 | 1,252 |
| DNA copy number from infected cage(s) | 542,297 | 23,076 | 97,880 & 2093 |
| Number of C. bovis-positive cages identified | 1 | 1 | 2 |
| Number of cages on positive rack | 61 | 37 | 55 |
| Immunodeficient mouse cage census in room | 266 | 45 | 158 |
| Number of qPCR swabs to isolate or resolve infection | 43 | 40 | 26 |
| Duration (d) room veterinary quarantine | 15 | 19 | 12 |
To determine the source of infection, we traced the manipulation history of the positive cage. Husbandry logs showed that all cages on the rack had been changed 9 d prior to the positive rack swab. Due to the length of the C. bovis negative history of the room, dedicated traffic patterns, and experience of the animal care technician, the likelihood of C. bovis contamination of a single positive cage during a routine 2-wk cage change was unlikely. However, 27 d prior to the date of C. bovis detection, cryopreserved PDX tumor tissue, which had been harvested prior to the efforts to eliminate C. bovis from the colony, was inoculated into all the mice in the cage. Because we suspected that infection might have been transmitted through contaminated PDX tumor tissue, qPCR analysis was performed on a paired, cryopreserved, tumor sample of the same PDX model that was harvested and identically cryopreserved on the same day as the suspect tumor sample. The tumor tissue was positive for C. bovis DNA (Table 2). However, we were unable to diagnostically rule out the potential for C. bovis contamination through laboratory-owned experimental equipment, because it had all been decontaminated once the rack was confirmed to be C. bovis positive. Yet, prior to this positive result and for the 27 d after PDX tumor inoculation of the single infected cage, hundreds of other nude mice in the same C. bovis-free barrier room had been manipulated in the same procedural space, within the same biosafety cabinet, and by using the same gas anesthesia equipment for tumor measurements and experimental therapeutic administration. If experimental equipment had been the source of infection, other mice likely would have been infected during this time. Further communication with the lab revealed that 5 other cryopreserved PDX models had been implanted into nude mice in this room. These tissues were also harvested prior to efforts to eliminate C. bovis from the colony.Unfortunately, paired tumor samples from the previous 5 PDX models implanted were not available to be tested for C. bovis DNA by qPCR assay.
Table 2.
Epidemiology results of C. bovis-contaminated cryopreserved PDX tissue as the source of infection
| Case | Lab | Time (d) between Cryo-PDX and C. bovis detectiona | No. of C. bovis DNA copies (qPCR assay) in replicate Cryo-PDX | C. bovis incidence in surveyed Cryo-PDXb | No. of Cryo-PDX lines causing infection after implantationc |
| 1 | A | 27 | 370 | 60% | 1 of 6 (16%) |
| 2 | B | 11 | 568 | 100% | Unknown |
| 3 | B | 25 | 382 | 100% | 1 of 4 (25%) |
Detected by EAD testing after PDX implantation
Results previously reported in reference 16
Total represents only cryopreserved PDX tissues that were harvested prior to C. bovis elimination efforts
Case 2.
In July 2014, 1 of 5 racks in a C. bovis-free barrier room containing only nude mice belonging to investigator B tested positive for C. bovis. The room had been considered C. bovis-free for the prior 11 mo. Due to a delay in initial results, the confirmation swab was collected 7 d later and showed a doubling in C. bovis DNA copy number (Table 1). After confirmation, the procedure established to address a positive rack test was followed without incident. The positive rack held 37 cages of nude mice. Rows 3 and 10 were determined to be positive, with copy number values of 167 and 104, respectively. Seven cages of nude mice were present on row 3. Only the immunocompetent sentinel cage was present on row 10. The sentinel mice were euthanized, and individual swabs were collected from the inner lid margin of cages on row 3. Results confirmed the presence of a single C. bovis-positive cage on row 3 at cage position D4, which was removed and placed in a C. bovis-positive room. All 36 remaining cages on the positive rack were transferred to a newly sanitized rack and tested after 7 d. All rows tested negative, and all cages were returned to the C. bovis-free barrier room. Concurrently, all remaining racks within the C. bovis-free barrier room were retested and remained negative. All subsequent weekly rack surveillance samples remained negative within this barrier room until the presentation of case 3 (described following).
A retrospective evaluation of all the manipulations of the positive cage was performed. Husbandry records indicated that all cages were changed on the rack during the 7-d delay in results from the initial swab. This discovery helped to explain the C. bovis-positive status of the sentinel cage that had received soiled bedding from the infected cage. This finding also alerted us to the increased potential for cage-to-cage transmission, which was not observed with follow-up testing. We determined that cryopreserved PDX tumor tissue, harvested prior to efforts to eliminate C. bovis, was inoculated into all mice in the positive cage 11 d prior to the date of initial detection by routine rack surveillance. qPCR analysis was performed on a paired, cryopreserved, tumor sample of the same PDX model that was harvested and identically cryopreserved on the same day as the suspect tumor sample. The tumor tissue was positive for C. bovis DNA (Table 2). Although the researchers were unable to provide the number of (likely contaminated) cryopreserved PDX models implanted prior to this infection, no other cages of mice inoculated with PDX tumor tissue using the same equipment had tested positive before that point.
Case 3.
At 10 mo after case 2 (May 2015), 1 of 4 racks belonging to investigator B tested positive in the same C. bovis-free barrier room but on a different rack. A swab collected from the positive rack within 3 d of the initial sample showed a small decrease in C. bovis DNA copy number (from 3040 to 2531). After confirmation of these results, the procedure to address a C. bovis positive rack was followed. The positive rack held 55 cages of nude mice. Row 6 was determined to be C. bovis-positive, and individual cage swabs confirmed the presence of 2 C. bovis-positive cages at positions F6 and G6, with DNA copies of 97,880 and 2093, respectively. The cages of infected mice were removed and placed in a C. bovis-positive housing room. All 53 remaining cages were transferred to a newly sanitized rack and retested after 7 d. All rows tested negative, and cages were returned to the C. bovis-free barrier room.
The manipulation history of both positive cages was evaluated. Husbandry records indicated that all cages on the rack were changed on the same day as the collection of the initial positive rack swab. This finding indicates that the C. bovis-positive cage(s) on the rack was actively shedding C. bovis into the intracage environment, thus increasing the risk of cage-to-cage transmission.4 During cage changing, the cage at F6 was changed and then immediately followed by the cage at G6.The copy numbers and order of cage change suggested that cage F6 was the initial positive cage (index cage), and the infection most likely spread during the routine cage change to cage G6. Researchers stated that cage F6 received cryopreserved PDX tissue 25 d prior to the initial positive rack swab. Because of our continued suspicion that C. bovis infection could be transmitted by contaminated cryopreserved PDX tissue, qPCR analysis was performed on a paired tumor sample of the same PDX model and identically cryopreserved on the same day as the suspect tumor sample. As anticipated, the tumor tissue was positive for C. bovis DNA (Table 2). Decreasing the likelihood of contaminated instruments or equipment as the source of infection, 3 other cages of nude mice were inoculated with PDX tissue from different cryopreserved vials during the same session as the index cage. None of these cages developed C. bovis infections according to routine rack sampling.
Results
HEM cross-contamination experiment.
Swabs collected from HEM 10 on both racks were C. bovis-positive at 24 h after infected cage placement and for the duration of the study (n = 2). In contrast, the weekly swab collected from HEM 1 through 9 and from all HEM on the control rack remained negative for C. bovis DNA at each time point during the 8-wk study (data not shown).
Identification of C. bovis-positive cage and epidemiology investigation.
IVC EAD testing as described previously,17 in combination with differential HEM swabbing and individual cage sampling, effectively identified C. bovis-infected cages in each case reported. Individual cages containing infected mice were identified, with an average of 51.0 ± 12.5 uninfected cages cohoused on the rack. When C. bovis-positive cages were identified, rapid quarantine and removal of the infected cage(s) prevented the potential spread of infection to 469 other cages of susceptible mice in the same barrier rooms. An average of 36.3 ± 9.1 swabs was used to identify infected cages and confirm negative cages prior to the resolution of each event. The average length of time for the associated veterinary quarantine of the C. bovis-free barrier room after identification of a positive rack was 15.3 ± 3.5 d (Table 1). In all cases presented, cryopreserved PDX tumor tissues harvested prior to efforts and training to prevent C. bovis contamination during tumor harvest were involved. A summary of these investigations, including the qPCR assay results of replicate vials of the paired, cryopreserved PDX tumor used and the incidence of C. bovis contamination of the cryopreserved tumor stocks from each lab, is presented in Table 2.
Discussion
In the fall of 2012, C. bovis-free barrier rooms were established for 2 active research laboratories maintaining in vivo PDX tumor banks. All mice that entered these rooms were new arrivals from approved vendors and were confirmed to be C. bovis-negative according to qPCR analysis on arrival. Many procedural and physical barriers were implemented to prevent C. bovis entry. To provide rapid detection, we implemented a weekly C. bovis surveillance program that used EAD testing exclusively.17 For laboratories A and B, the prevalence of C. bovis DNA contamination of cryopreserved vials of PDX tissues harvested by using inadequate aseptic technique was 60% and 100%, respectively.16 The sources of the C. bovis infections presented in this case study were likely due to the implantation of contaminated cryopreserved PDX tissues, in light of qPCR tests of paired samples. However, we are unable to prove this hypothesis definitively, because the original tumor tissue and vials were not available for follow-up testing. To confirm the source of C. bovis entry into the barrier rooms, replicate vials of the same PDX models that were cryopreserved at the same time as the suspect tissues were obtained from each laboratory and tested for C. bovis DNA by qPCR assay. All replicate vials were positive for C. bovis (Table 2), but bacterial cultures of these replicate vials were not attempted. We acknowledge that culturing the organism would have further strengthened the link between contaminated PDX tissues and infected recipient mice.
Despite the high prevalence of C. bovis contamination among the cryopreserved tissue samples harvested by using inadequate technique, only 16% and 25% of these PDX tumor tissues resulted in infection. These findings are paradoxical, given that C. bovis is known to be efficiently transmitted by fomites,4,5 but there has been no investigation into transmission by biologic material. C. bovis is characterized as being exclusively a dermal infection in nude mice,3,5 and we hypothesize that skin inoculation with C. bovis occurs at the time of skin penetration by the trocar–needle system or forceps used to place tumor tissue. For this procedure, one barrier to infection is the method of skin disinfection. Our laboratories use 70% isopropyl alcohol with or without povidone–iodine for disinfection, combined with protocols that have been published on performing horizontal transfer of PDX tumors.7,10,11,16,18,24 However, alcohol is ineffective after evaporation, and povidone–iodine is rapidly inactivated by biologic fluids.19 In combination, these attributes may create an insufficiently persistent and residual barrier to C. bovis infection after initial skin preparation. A modification to these commonly used protocols may be the use of a chlorhexidine gluconate solution. Incorporation of chlorhexidine gluconate may further reduce C. bovis infections associated with contaminated tumor tissue, due to the agent's excellent persistent and residual antibacterial activity on skin.1 In addition, infection transmission may be diminished due to the relatively small amount of C. bovis that contaminates the cryopreserved tissues, as suggested by the low copy numbers per vial. In addition, we have demonstrated that C. bovis is not evenly distributed throughout the tumor sample.16 Thus, the piece of tumor selected for inoculation may not contain C. bovis. Nevertheless, even with a low incidence of infection from contaminated tissues, our findings underscore the necessity of preventing bacterial contamination during the tumor harvest procedure.
In light of our epidemiologic investigation into the source of C. bovis entry into these barrier rooms, a key procedural change was implemented to prevent contaminated cryopreserved tissues from causing infection in the future. After the first infection experienced by lab A (case 1), all cryopreserved tissues harvested prior to efforts to eliminate the infection were inoculated into mice housed in C. bovis-positive rooms. Although this measure practically ensured C. bovis infection of recipient mice, it eliminated a source of infection for the barrier rooms. Once tumors grew sufficiently large for horizontal transfer, they were harvested by using the enhanced aseptic technique procedure described previously.16 After the first infection experience by lab B (case 2), the lab forgot to implement this procedural change 10 mo later when presumed-contaminated cryopreserved stocks were used to reestablish several PDX models. Four presumed contaminated, cryopreserved PDX tumor models were inoculated into mice, with one resulting in infection. It is worth noting that for the prior 10 mo, the barrier room had been maintained C. bovis-free without the introduction of contaminated, cryopreserved PDX tumor material. After lab B's second infection (case 3) and the implementation of the described precautions, no C. bovis infections associated with PDX tumor tissues have occurred to date.
We have reported recently on the intracage infection kinetics and anticipated time-to-detection of C. bovis by using EAD surveillance. Mice with newly acquired infections were first detected by EAD testing in 7.3 ± 1.2 d (range, 7 to 10 d).17 As presented in the current case study, the time to infection detection by EAD testing averaged 21.0 ± 8.7 d (range, 11 to 27 d) after PDX inoculation. Our explanation for this difference is based on the dose of C. bovis exposure. Although the number of bacteria from the skin of infected mice has not been quantified, qPCR data collected from skin swabs by our group and others has shown that mice with established infections can yield 105 to 106 copies of C. bovis DNA per skin swab.8,17 According to the qPCR data obtained from the replicate cryopreserved PDX tissues, contaminated PDX tumor tissue showed a 3 to 4 log10 decrease in the copy number. Time-to-detection by EAD testing is central to establish a timeline for retesting racks holding presumed negative cages. In case 1, we waited only 4 d to retest. This decision was made prior to our discovery of the delay in time-to-detection with newly acquired infections.17 In cases 2 and 3, we elected to retest in 7 d rather than 10 d because of the amount of food remaining within each cage. Although waiting 10 d to retest presumed-negative cages would have been ideal, the requirement to open cages to replenish food to reach that point might compromise cages. All presumed-negative cages must remain closed until retesting, to eliminate the potential for cross-contamination.
The clinical presentation of hyperkeratotic dermatitis on infected nude mice is variable.5,8,17,20 From our observations obtained a year prior to the implementation of our C. bovis remediation program, only 38% of 1388 cages of nude mice presented with scaly skin in an enzootically infected housing room. Therefore, clinical signs are not a sensitive method for detecting C. bovis infections. In research institutions undergoing a transition to C. bovis-free mouse colonies, a surveillance program that supports rapid detection is crucial to prevent outbreaks. At our institution, EAD testing has been a tremendous advancement in establishing a reliable C. bovis surveillance program. As presented in this case study, our weekly C. bovis surveillance program identified the infection early and prevented widespread outbreaks.
Knowing exactly where an infectious agent exists on a 70-cage rack is still a diagnostic challenge. Because of the rack design, the ability to accurately isolate the infection to a single row (or column) decreased the cost of infected cage identification. Individual HEM swabbing has been proposed to aid in infection localization on a particular row of a rack.9,17
Our results demonstrate the lack of cross-contamination between HEM within the vertical exhaust plenum of the rack system for 8 wk with a single C. bovis-infected cage located on the rack. These data support the use of multiple HEM swabs to identify rack rows containing a C. bovis-infected cage. Without this ability, individually testing each cage on the rack would be much more costly and provide additional opportunity for cross-contamination between cages.
In November 2015, 29 mo after case 1, a break in the C. bovis-free barrier room of laboratory A occurred. While we addressed this event by using the same method as described earlier, we also performed a time-and-cost analysis. The duration of the veterinary quarantine placed on the room was 15 d. The infected cages of nude mice identified had not yet been inoculated with PDX tumor tissue. By using the cage manipulation history, the source of the infection was traced to contaminated tattooing equipment that was not decontaminated prior to entry. To eliminate C. bovis and its environmental contamination in the barrier room and dedicated procedure space, a total of 29.3 h was invested by researchers and vivarium staff. The total cost in personnel and research staff time and 26 diagnostic samples for C. bovis detection by qPCR analysis equaled approximately US$3600. For the cases presented in the current study, we did not perform a cost analysis but feel that the labor investment from this most recent event is similar to those in these other cases.
The reservoirs that cause the perpetuation of C. bovis infection within rodent housing facilities have been investigated previously. In one study, the infection was often sustained by subpopulations of immunodeficient mice that were housed in the same room and shed bacteria into the environment.4 According to our own experiences, we know that gram scales, digital calipers, tattooing equipment, biosafety cabinets, isoflurane vaporizers, and other research equipment can all be contaminated and result in the transmission of C. bovis. However, given the stringent procedural and physical barriers used to maintain these C. bovis-free barrier rooms, high frequency of C. bovis surveillance testing, rapid identification of infected cages, and confirmed presence of C. bovis DNA within paired tumor samples, our epidemiologic investigation indicated that the source of C. bovis infection was contaminated, cryopreserved PDX tumor tissue. As a result, we were able to identify a previously unknown source of C. bovis contamination that may result in infection. We continue to expand our surveillance program to include investigators that are transitioning to a C. bovis-free status yet are still maintaining C. bovis infected colonies. Similarly, we will continue to monitor well-established C. bovis-free colonies until this program systematically excludes C. bovis from our facilities.
Acknowledgments
We thank all staff members of the Office of Laboratory Animal Resources for their commitment to the elimination of C. bovis from our institution. Specifically, we thank Dr Derek Fong for his thorough review of this manuscript and Justin Laine for his technical assistance. We are also extremely grateful to Stacey Bagby, Julie Reisinger, and Drs Stephen Keysar and Todd Pitts for generously providing cryopreserved PDX tumor tissue for testing. We thank the University of Colorado Denver qPCR Core for their assistance in all swab and tissue sample preparation and processing. This project was supported by the University of Colorado Denver, Office of the Vice Chancellor for Research and Office of Laboratory Animal Resources. In addition, funding for the maintenance and management of the PDX in vivo tumor banks were provided in part by grants to SG Eckhardt (08-60-21-ECKH from AACR and W8IXWH-11-1-0526 from DOD), A. Jimeno (5R21DE019712-02 from NIDCR), JJ Arcaroli (W8IXWH-15-1-0173 from DOD), WA Messersmith (1RO1CA152303-01 from NCI), and the NCI Cancer Center Support Grant (P30CA046934-27, qPCR Core).
References
- 1.Anderson DJ, Podgorny K, Berríos-Torres SI, Bratzler DW, Dellinger EP, Greene L, Nyquist AC, Saiman L, Yokoe DS, Maragakis LL, Kaye KS. 2014. Strategies to prevent surgical site infections in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol 35:605–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer B, Besch-Williford CL, Livingston RS, Crim MJ, Riley LK, Myles M. 2016. Influence of rack design and disease prevalence on detection of rodent pathogens in exhaust debris samples form individually ventilated caging systems. J Am Assoc Lab Anim Sci 55:782–788. [PMC free article] [PubMed] [Google Scholar]
- 3.Burr HN, Lipman NS, White JR, Zheng J, Wolf FR. 2011. Strategies to prevent, treat, and provoke Corynebacterium-associated hyperkeratosis in athymic nude mice. J Am Assoc Lab Anim Sci 50:378–388. [PMC free article] [PubMed] [Google Scholar]
- 4.Burr HN, Wolf FR, Lipman NS. 2012. Corynebacterium bovis: epizootiologic features and environmental contamination in an enzootically infected rodent room. J Am Assoc Lab Anim Sci 51:189–198. [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford CB, Walton BJ, Reed TH, Coyle MB, White WJ, Amyx HL. 1995. Hyperkeratosis in athymic nude mice caused by a coryneform bacterium: microbiology, transmission, clinical signs, and pathology. Lab Anim Sci 45:131–139. [PubMed] [Google Scholar]
- 6.Debrue MC, Henderson KS, Lipman NS, Manuel CA, Mulder GB. 2015. Corynebacterium bovis: is scaly skin still keeping you down? p 47. Presented at the 66th Annual AALAS National Meeting Program, Phoenix, Arizona, 1–5 November 2015.In: 66th Annual AALAS National Meeting Program, Memphis (TN): American Association for Laboratory Animal Science. [Google Scholar]
- 7.DeRose YS, Gligorich KM, Wang G, Georgelas A, Bowman P, Courdy SJ, Welm AL, Welm BE. 2013. Patient-derived models of human breast cancer: protocols for in vitro and in vivo applications in tumor biology and translational medicine. Curr Protoc Pharmacol Chapter 14:Unit14.23 doi:10.1002/0471141755.ph1423s60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dole VS, Henderson KS, Fister RD, Pietrowski MT, Maldonado G, Clifford CB. 2013. Pathogenicity and genetic variation of 3 strains of Corynebacterium bovis in immunodeficient mice. J Am Assoc Lab Anim Sci 52:458–466. [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen ES, Allen KP, Henderson KS, Szabo A, Thulin JD. 2013. PCR testing of a ventilated caging system to detect murine fur mites. J Am Assoc Lab Anim Sci 52:28–33. [PMC free article] [PubMed] [Google Scholar]
- 10.Jin K, Teng L, Shen Y, He K, Xu Z, Li G. 2010. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol 12:473–480. [DOI] [PubMed] [Google Scholar]
- 11.Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. 2009. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc 4:1670–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim TH, Kim D, Han J, Chang S, Kim S, Seok S, Kim D, Park J, Park J. 2014. Detection of Corynebacterium bovis infection in athymic nude mice from a research animal facility in Korea. J Vet Sci 15:583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leblanc M, Berry K, Graciano S, Becker B, Reuter JD. 2014. False-positive results after environmental pinworm PCR testing due to rhabditid nematodes in corncob bedding. J Am Assoc Lab Anim Sci 53:717–724. [PMC free article] [PubMed] [Google Scholar]
- 14.Lepherd M, Redelsperger I, Lipman NS. 2013. False brush borders in the cecum of NSG Mice. Abstract presented at the AALAS National Meeting Baltimore, Maryland, 26–31 October 2013. J Am Assoc Lab Anim Sci 52:637. [Google Scholar]
- 15.Leszczynski J, Wallace M, Tackett J, Jiron U, Collins J, Warder C, Richardson L, Bell L, Russell C. 2014. Shutting down a working vivarium for decontamination. Lab Anim (NY) 43:283–290. [DOI] [PubMed] [Google Scholar]
- 16.Manuel CA, Bagby SM, Reisinger JA, Pugazhenthi U, Pitts TM, Keysar SB, Arcaroli JJ, Leszczynski JK. 2017. A horizontal transfer technique of PDX tumors to eliminate Corynebacterium bovis. J Am Assoc Lab Anim Sci. [In press]. [PMC free article] [PubMed] [Google Scholar]
- 17.Manuel CA, Pugazhenthi U, Leszczynski J. 2016. Surveillance of a ventilated rack system for Corynebacterium bovis by sampling exhaust air manifolds. J Am Assoc Lab Anim Sci 55:58–65. [PMC free article] [PubMed] [Google Scholar]
- 18.Morton CL, Houghton PJ. 2007. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc 2:247–250. [DOI] [PubMed] [Google Scholar]
- 19.Reichman DE, Greenberg JA. 2009. Reducing surgical site infections: a review. Rev Obstet Gynecol 2:212–221. [PMC free article] [PubMed] [Google Scholar]
- 20.Scanziani E, Gobbi A, Crippa L, Giusti AM, Giavazzi R, Cavalletti E, Luini M. 1997. Outbreaks of hyperkeratotic dermatitis of athymic nude mice in northern Italy. Lab Anim 31:206–211. [DOI] [PubMed] [Google Scholar]
- 21.Scanziani E, Gobbi A, Crippa L, Giusti AM, Pesenti E, Cavalletti E, Luini M. 1998. Hyperkeratosis-associated coryneform infection in severe combined immunodeficient mice. Lab Anim 32:330–336. [DOI] [PubMed] [Google Scholar]
- 22.Smith G, Field G, Peterson P, Reynolds RP, Wolf FR. 2010. Control and eradication of Corynebacterium-associated hyperkeratosis (CAH) in athymic nude mice, p 59. AALAS 61st National Meeting, Atlanta, Georgia, 10–14 October 2010.In: 61st Annual AALAS National Meeting Program, Memphis (TN): American Association for Laboratory Animal Science. [Google Scholar]
- 23.Wallace-Fields M, Tackett J, Richardson L, Leszczynski J. 2012. A novel approach to a mopping system to reduce labor and product costs and decrease chemical usage.Abstract presented at the AALAS National Meeting Minneapolis, Minnesota,4–9 November 2012. J Am Asso Lab Anim Sci 51:679–680. [Google Scholar]
- 24.Zhang X, Lewis MT. 2013. Establishment of patient-derived xenograft (PDX) models of human breast cancer. Curr Protoc Mouse Biol 3:21–29. [DOI] [PubMed] [Google Scholar]
- 25.Zorn J, Ritter B, Miller M, Kraus M, Northrup E, Brielmeier M. 2016. Murine norovirus detection in the exhuast air of IVCs is more sensitive than serological analysis of soiled bedding sentinels. Lab Anim. [DOI] [PubMed] [Google Scholar]