Highlights
-
•
Fungal strains having the potential of high cell density accumulation to be used for textile azo dyes bioremediation.
-
•
The optimization of growth conditions for enhancing the biomass accumulation and protein production by six fungal strains was studied in 7 liter fermentor.
-
•
The maximum biomass accumulation and protein production were achieved in molasses containing.
-
•
The molasses as a cheaper carbon source was successfully used to promote fungal biomass and protein accumulation by the azo dye removing fungal strains.
Keywords: Fungal cultivation, Azo textile dye, Molasses, Controlled pH
Abstract
This work aims at optimizing the high cell density fungal cultivation for producing large quantities of fungal biomass to be used in azo dye residues bioremediation. In our previous studies the efficacy of using certain fungal strains to decolorize a range of commercial textile dyes of different structures (azo, disazo) were investigated. Several promising fungal strains belonging to Aspergillus tubigenesis, Aspergillus niger, Aspergillus terreus, and Aspergillus fumigates demonstrated high capacity in decolorizing various azo dyes. This study focuses on the high cell density cultivation of the fungal strains identified as potential bioremediation agents. The study includes the optimization of all parameters involved in bioprocess development for high cell density cultivation of six promising fungal strains. The growth of the fungal strains was tested on the sucrose medium in 7 l-fermenter. The growth of these fungal strains having the capacity to accumulate large quantities of biomass was also tested in medium containing molasses as a cheap substrate. The residual molasses, biomass dry weight and protein content of the six fungal strains showed that the strains 20 and 2 were marked by the highest protein content. In this study a comparative analysis between the results of dry weight, residual molasses and protein content of geowth of the strains 20, 5 and 2 under uncontrolled and controlled pH of media in batch fermentation was studied to follow the accumulation of biomass and protein production in the growth media. The results indicate that the dry weight accumulated by strains No. 20, 5 and 2 grown on molasses was better than those of strains grown on sucrose. Fungal strain No. 5 had the highest biomass dry weight accumulation. The study shows that the molasses as cheaper sugar sources were better than sucrose for growing fungal biomass.
1. Introduction
The filamentous fungi are naturally excellent protein secretors and can produce industrial enzymes in sufficient amounts [14]. Abd-El Rahim [20] assessed the textile dyes bioremediation using different fungal biomass as compared with some abiotic agents. Abd-El Rahim et al. [19] studied enhancing the bioremoval of textile dyes by biomass of fungal strains from media supplemented with gelatine wastes and sucrose. In another work Abd-El Rahim [19] studied the enhancment of fungal growth and biomass accumulation by several promising strains. The biomass was tested for rapid dye removal. Filamentous fungi represent attractive bioflocculating agents because of their self-pelletization capacities. Fungal self-pelletization has been observed for numerous filamentous strains and can be explained by coagulative and non-coagulative mechanisms [7], [24], [10], [23]. The coagulative mechanism observed in representatives of Aspergillus spp., Basidiomycete spp., Phanerochaete spp. that involves spore coagulation leading to developments of aggregates/pellets. As a result fungi produce dense spherical aggregates [24], [7]. The non-coagulative mechanism involves spores germinating into hyphae, which then intertwine into pellets. Representatives of several fungal species: Rhizopus spp., Mucor spp. and Penicillium spp. display the non-coagulative mechanism [24], [7] which play role in reducing the cost of biomass harvesting that requires additional energy input. Such property can play significant role in high cell density cultivation of fungi particularly for easy harvesting of the fungal biomass. The aim of this study is to grow several selected fungal strains having the potential of high cell density accumulation to accumulate large biomass to be used for textile azo dyes bioremediation.
2. Material and methods
2.1. Fungal strain and inocula preparation
The six highly efficient fungal strains identified in our previous laboratory testing as promising bioremediation agents were included in this study for further high biomass accumulation in the growth media. The six fungal strains previously isolated from an industry effluent damping site and tested for azo dyes bioremediation were maintained in potatoes agar slants medium and kept in the refrigerator. The strains were sub-cultured every 4 months. The composition of the culture medium used for inoculum preparation and biomass cultivation was: 10 g/l sucrose, 0.5 g/l H2PO4, 0.2 g/l MgSO4 7H2O, 0.1 g/l NaCl [19].
A spore suspension was used for inoculation of the flask cultures. Agar plates with the sporulation medium (24 g L−1 potato dextrose broth with 20 g L−1 agar) were plated out with spores from a refrigerated stock and incubated for 6 days at 28 °C. Spore suspensions were harvested by adding 10 ml of sterilized water onto the surface of agar plates to release spore from the aerial mycelium.
2.2. Batch fermentation
Biomass production experiments were performed in a 7.5L BioFlo 310 bench top Fermenter/Bioreactor (New Brunswick scientific Co., Inc.) with a working volume of 5L. The fermenter and media were sterilized by autoclaving at 121 °C for 20 min. The spore suspensions were used to inoculate the fermenter.
Samples were regularly withdrawn from the fermenter every 24 h. and analyzed for residual sugar (using hand Refractometer), accumulated biomass and total protein. The pH was recorded throughout the course of batch process. Foam was controlled by adding silicon oil antifoam (Fluka). The fermenter was aerated at an air flow rate of 2.5 l/min, corresponding to 0.5 v/v/min. The temperature was controlled at 28 °C. The process continued for 5 days (120 h).
2.3. Evaluation of biomass accumulation
Samples were collected from fermenter at certain intervals of incubation (24, 48, 72, 96 and 120 h, either was mentioned). The samples were passed through filter Watman paper No. 1. and the biomass was measured by drying of the biomass collected from the volume taken to constant weight at 65 °C.
2.4. Residual sugar readings
Sucrose consumption was monitored as the intake percentage of sucrose were quantified by Atago hand Refractometer.
2.5. Growth of fungal strains for biomass accumulation on sucrose and molasses
The fungal biomass accumulation was evaluated at intervals of incubation. Accumulation of biomass by six fungal strains were assessed in sucrose and molasses media. The changes in pH, biomass dry weight, residual sugars and protein content in the culture media were determined at all sampling intervals. Then specific growth, specific substrate consumption and specific protein production rates were calculated.
3. Results
3.1. Performance of the potential fungal strains in relation to sucrose consumption, biomass accumulation, and protein production
Change in sucrose containing media inoculated with the 6 fungal strains were tracked in the culture media throughout the experimental time. The selection of the strains no. 2, 5, 6, 11, 20, and 21 was based on the efficiency of such strains in bioremediation of textile dye residues either by bio-removal and/or bio-degradation.
In the medium containing 1% sucrose, strain 2 gradually consumed sucrose up to 50% after 72 h of incubation. Sampling after 96 h showed that, 80% of sucrose was used from the medium. The pH decreased from 3.5 to 2.2 at the end of the incubation. The biomass accumulation increased as the time of incubation increased and reached 8.05 g/l after 96 h. The protein content in the growth medium reached the maximum after 96 h of incubation being 13.01 μg/ml (Fig. 1).
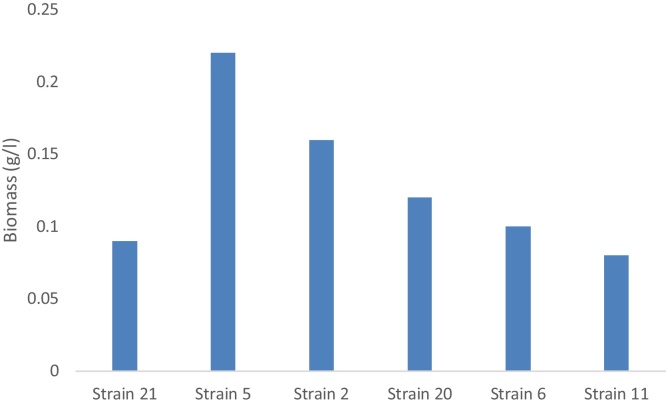
Fig. 1.
The cell mass produced by the six fungal strains grown on sucrose after 96 h incubation period.
In Fig. 2, growth of fungal strain No. 5 in the sucrose medium was increased as the time of incubation increased. At 48 h of incubation the biomass accumulation by strain 5 was less than that of strain 2 being 5.55 g/l compared with 7.25 g/l in case of strain 2. At the end of incubation period strain 5 had more biomass accumulation (8.55 g/l) than strain 2 (8.05 g/l). The pH of the growth media decreased at the end of incubation period. Increase in protein production in the growth media was noted with strain 5 being the highest producer at 72 h and amounted 13.86 μg/ml (Fig. 1).
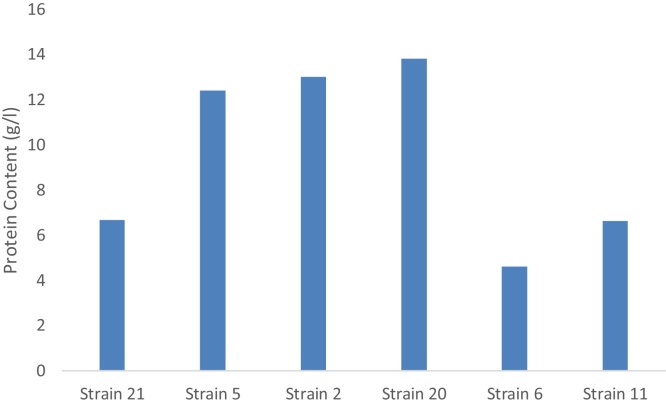
Fig. 2.
The protein content produced by the six fungal strains grown on sucrose after 96 h incubation period.
As regards strain 6 the pH changes showed decrease from 3.5 to 2.85 within 48 h of growth, and then the pH did not change markedly till the end of the incubation period. Fig. 1 illustrated that 40% of sucrose was consumed in the first 48 h and at the end of incubation. The residual sucrose was 0.2% of the initial sucrose amendment. The dry weight accumulation by this strain was low in comparison with the previous strains. It reached only 2.19 g/l at the end of the experiment (120 h) which is almost one fourth of the biomass accumulation by strain 2 and 5 at the same sampling time. The protein content of growth medium was also low amounting to 5.41 μg/ml at the end of incubation period.
The fungal strain 11 growing on sucrose medium showed similar trends in pH changes, sucrose consumption, dry weight accumulation and protein content in the growth medium. While pH and sucrose content decreased incubation time, the dry weight and protein content increased. Maximum biomass accumulation and protein content was reached after 96 h of incubation being 12 g/l of dry weight and 8.62 μg/ml of respectively. This strain was marked by highest biomass accumulation among all tested strains in sucrose medium. The conversion of the carbon source (sucrose) to biomass was distinguished by high efficiency. The protein content of the growth medium amounted 13.81 mg/l at the end of incubation being the highest among all studied fungal strains growing on sucrose mediu (Fig. 3)
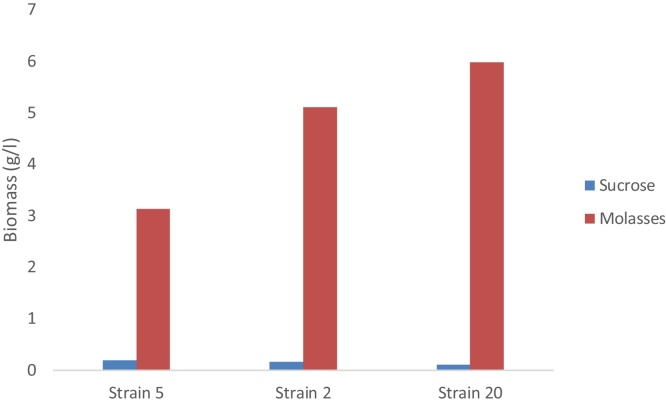
Fig. 3.
The cell mass produced by the three promising fungal strains grown on sucrose and molasses after 72 h incubation period.
The fungal growth of strain 21 on sucrose medium gradually decreased pH and extensively consumed the sucrose where the sugar was totally consumed after 96 h of incubation (Fig. 1). This strain was fast grower and consumed 80% of the sucrose in 48 h. Consequently the biomass accumulation was high at early growth phase (48 h of incubation). At this sampling time the strain accumulated 5.1 g dry weight per liter. However, the dry weight accumulation and released protein was much less than strains 5 and 20. In general the results show that strains 11, 20 and 5 I respectively order were the best biomass accumulators (Fig. 2).
3.2. Performance of the potential fungal strains in relation to molasses consumption, biomass accumulation, and protein production
In the medium containing 1% molasses, strain 2 gradually consumed molasses up to 60% after 72 h of incubation. The pH decreased gradually from 3.5 to 2.65 at the end of the incubation (72 h). The biomass accumulation increased as the time of incubation increased and reached 5. 40 g/l after 72 h. The protein content in the growth medium reached the maximum after 72 h of incubation being almost10.00 μg/ml.
The fungal growth of strain 5 in the molasses medium increased as the biomass accumulation increased with increasing the incubation period. At the end of the incubation, the biomass accumulation was less than that of strain 2 being 3.4 g/l. Strain 5 consumed molasses almost totally after 72 h of incubation. The pH of the growth media decreased and reached 3.0 at the end of incubation. The increase in protein content was gradual and reached the maximum at 72 h being 3.5 μg/ml.
As regards fungal strain 6 the pH changes showed decrease from 3.5 to 2.8 within 72 h of growth. Fig. 4 illustrates that the 80% of molasses was consumed at the end of incubation period (72 h) by strain No. 5. The dry weight accumulation by this strain in Fig. 5 was comparatively low. It reached only 3.0 g/l at the end of the experiment (72 h) which is almost one third of the biomass accumulation by strain 2 and at the same sampling time. The protein content of growth medium was consequently low amounting 3.8 μg/ml at this sampling time. The fungal strain No. 20 growing on molasses medium showed almost similar trends in pH changes, molasses consumption and dry weight accumulation. While pH and molasses decrease with increasing sampling time, the dry weight and protein content increased. Maximum biomass accumulation and protein content was reached after 72 h of incubation being 5.9 g/l for dry weight and 12.0 μg/ml for protein. This strain was marked by the highest protein content among all tested strains in molasses medium. The utilization of 55% of the added molasses by this strain after 72 h of incubation gave 5.9 g of dry weight. The results show that the fungal strain 20 did not change pH significantly throughout the incubation period. The above results indicate that the fermentation is almost ceased after 48 h.
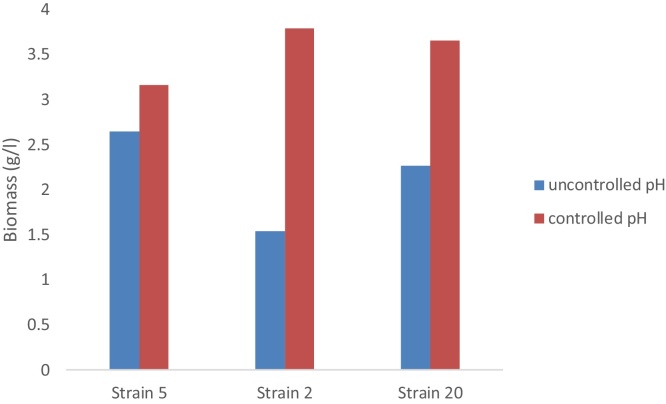
Fig. 4.
The cell mass produced by the three promising fungal strains grown on molasses at controlled and uncontrolled pH after 30 h incubation period.
By comparing the results for residual molasses, biomass dry weight and protein content of the six tested strains, it was found that fungal strains 20 and 2 have the highest protein content after 72 h of incubation, while strain11 has the highest biomass dry weight after 72 h of incubation. It was also found that all the strains gave almost similar values of dry weight after 48 h incubation, whereas the strain 5 gave the lowest values for residual molasses.
Strain No. 11 is the best strain since it gave the highest biomass although it consumed only about 60% of molasses.
3.3. Comparison of three fungal strains performance on sucrose and molasses
It was obvious from the results of the dry weight accumulation for most potential fungal strains (20, 5 and 2) that molasses is a better substrate than sucrose for fungal growth. The results show that the obtained dry weight of fungal strain 20 using molasses was 59 times more than its value using sucrose. The dry weights of fungal strains 5 and 2 using molasses were approximately 15 times more than its values using sucrose.
3.4. Performance of the three promising fungal strains: (No. 5, 2, 20) on molasses medium at controlled pH 3 in batch fermenter
Three promising azo dyes removing fungal strains 5, 2, 20 were grown on molasses as substrate with controlled pH 3. The biomass dry weight, residual molasses and protein content were measured throughout the incubation period. Paralel to that the specific growth rate, specific substrate consumption rate and specific production rate were calculated.
The fungal strains 5, 2, 20 grown on a molasses medium at controlled pH3 extensively consumed the molasses leaving zero molasses after 30 h of incubation. The strains were fast growers and consumed 90% of the molasses in less than 24 h. After 48 h the dry weight accumulation reached 3.2, 3.9, 3.2 g/l. The increase in protein released in the growth media followed the same trend observed in biomass accumulation with strain 2 being more protein producer than the other two strains.
The calculated specific growth rate, specific substrate consumption rate and specific protein production rate of fungal strains 2, 5, and 20 showed that the specific growth rate for fungal strain 2 was at its highest value 0.05 h−1 after 20 h of the incubstion, and then decreased at 24 h to approximately 0.04 h−1 then declined to zero throughout the remaining time of the incubation. This indicates that the fungal growth has ended after 20 h of incubation.The specific growth rate for fungal strain 5 had however its highest value (0.035 h−1) at the beginning of the incubation, and then dropped after 20hr to approximately 0.015 h−1 then resumed the decline through the remaining incubation time. These results indicate that the fungal strain 20 has come to the maximum growth rate after 20 h of incubation, whereas the specific growth rate of strain 20 reached the maximum (0.08 h1) after 20 h of incubation, and then dropped slightly at 24 h to 0.07 h−1 and declined to zero throughout the remaining time of the incubation.This supports the results indicating that the growth of these fungal strains has ceased after 20 h of incubation.
The data show that the specific substrate consumption rate by strain 2 followed the same pattern of specific growth rate,which was at its highest value (0. 17 h−1) after 20 h of incubation.The consumption decreased gradually during the remaining time of the incubation. The specific substrate consumption rate of strain 5 can be considered a mirror image for the specific growth rate and was at its highest value (0. 35 h−1) at the beginning of the incubation, then dropped after 20 h to 0.15 h−1 then resumed the decline throughout the remaining time of the incubation. The specific substrate consumption rate of strain 20 had the same trend of strain 5 being 0.325 h−1 at 20 h then the decreased throughout the remaining time of the incubation. The specific protein production rate of strain 2 increased gradually during the first 24 h to reach its highest value (0.225 h1), then stabilized for the rest of the incubation period. The specific production rate of strain 5 followed the same trend as the previous strain with its highest value (0.06 h−1) after 24 h, and then stabilized for the rest of the incubation period. The results show that the specific production rate of strain 20 increased in a straight line pattern during the incubation time to reach its highest value after 30 h (0.16 h−1). Most of the above results support the concept that the growth and multiplication of the fungal strains have ceased after 24 h.
3.5. Comparison of three fungal strains performance on molasses media at uncontrolled and controlled pH
A comparison between the results of dry weight, residual molasses and protein content for strains 20, 5 and 2 under uncontrolled and controlled media pH is illustrated in Fig. 4.
The results show that controlling the pH at 3 throughout the incubation period increased the biomass significantly over that in the uncontrolled pH media. The biomass accumulation was doubled for fungal strains 20 and 2 after 30 h of incubation, while the dry weight of fungal strain 5 increased from 2.5 to 3 g/l at the same incubation period.
The protein released by strain 20 in the growth media at controlled pH was 2.25 times its value at uncontrolled pH, whereas, strain No. 2 produced protein at controlled pH 2.5 times its value at the uncontrolled pH process. In the mean time isolate 5 doubled its production of protein in the controlled pH3 media after 30 h of incubation (Fig. 4).
In general, the molasses in the growth media has been totally utilized in the pH controlled media after 30 h of incubation due to the higher consumption of the substrate to build up and accumulate the biomass indicating the efficient conversion of substrate to biomass and other products such as protein (Fig. 4).
4. Discussion
This work aims at optimization of high biomass accumulation by six fungal strains.The strains were isolated in previous studies and tested for growth on sucrose and molasses media to obtain high biomass. These six fungal strains were selected based on their capacity to accumulate large amounts of biomass in a short time. The medium containing molasses as a cheap substrate was used for growing the fungal strains in 7.5-l fermenter. In this study three strains were grown on medium containing molasses at a concentration of 1% at starting pH 3.0. The growth of fungal strains after incubation period of 2 days at 28 °C gave the best biomass accumulation. The study was focused on process development and optimization of process controlling parameters in 7.5 l bench fermentor to study the accumulation of the fungal biomass and the total protein in the broth media. This work addressed two aspects: the first is the fungal biomass accumulation required for bioremediation of textile dye residues and the second, is the production of protein including enzymes participating in the breakdown of the dye residues.
The study showed that the molasses (the cheaper source of sugar) is better than sucrose for growing fungal biomass particularly when the pH was controlled throughout the incubation period at 3. This has resulted in a dramatic increase in biomass accumulation. Many authors have previously reported that the filamentous fungus Trichoderma reesei produces a variety of extracellular cellulases and hemicellulases that hydrolyse plant-derived polysaccharides into monomeric sugars which in turn are used as a source of carbon and energy by the fungal cells [1], [12], [13]. The most abundant of these enzymes are cellobiohydrolases and endoglucanases, with cellobiohydrolase I (CBHI) to comprise the major part of the total extracellular protein produced by the fungus [8]. Abd-El Rahim [20] and Abd-El Rahim et al. [19] studied the biomass production by different fungal strains for further using in textile dyes removal.
Production of extracellular proteins by filamentous fungi, such as α-amylase in Aspergillus oryzae [2], [17] and glucoamylase in Aspergillus niger [11], [16], [22], has been shown to be growth-associated in many cases. However, examples of non growth associated production are also known, such as recombinant protein production by Fusarium venenatum [21]. Taking into account the data from the wide range of growth rate studies, the results in this study as well as those obtained by other scientists [3], [4], [15] indicate that production of the hydrolytic enzymes that constitute the major part of the extracellular proteins produced by T. reesei is not directly growth-rate associated.
In terms of specific protein production rate as well as the yield of extracellular protein per amount of carbon source consumed, the production of extracellular proteins was maximal at the specific growth rate of 0.031 h−1, however, at higher growth rates a significant reduction in production was observed [18].
The study showed that the type of substrate is a crucial factor in the cell mass cultivation and product formation by several fungal strains. Also, controlling the pH of the broth media in the fermenter throughout incubation time is crucial. In our study using molasses as substrate increased the cell mass cultivation and protein production than using sucrose. Controlling the pH at 3throughout the incubation period also, increased the cell mass cultivation and protein production as compared with the uncontrolled growth media pH. This was in agreement with other results obtained by Elshafei et al. [5] who improved the fungal growth of T. reesei by 63% and the exoglucanase activity by 15 fold by controlled media pH. Hussein et al. [6] reported that controlling pH at 6 enhanced catalase production by P.chrysogenum and the growth by about 2–3 folds. The shorter exponential phase and the earlier stationary phase of the growth in the pH controlled batch also indicated that pH control enhanced the growth cycle of fungi. It was also noticed that pH control, reduced the time course of the batch by 24 h since the carbon substrate was almost totally exhausted by the sixth day rather than the seventh day in batches without controlled pH. The same phenomenon was recorded in this study by growing the three fungal strains in molasses containing media with controlled pH 3 throughout the incubation period.
5. Conclusion
The optimization of growth conditions for enhancing the biomass accumulation and protein production by six fungal strains was studied in 7.5 l fermentor. The maximum biomass accumulation and protein production were achieved in molasses media comperd to sucrose. The study shows that the molasses as a cheaper carbon source was successfully used to promote fungal biomass and protein accumulation by the azo dye removing fungal strains.
Acknowledgements
Authors wish to thank the Program of the National Strategy for Biotechnology and Genetic Engineering, administered by the Science and Technology Center (STC) at the Academy of Scientific Research and Technology funded for sponsoring the project on “Process development for maximized cultivation of three fungal strains used in bioremediation of textile azo dyes residues” which supported partially this research.
References
- 1.Biely P., Tenkanen M. Enzymology of hemicellulose degradation. In: Harman G.E., Kubicek C.P., editors. Vol. 8197. Taylor & Francis Ltd.; London, Bristol: 1998. pp. 25–47. (Trichoderma and Gliocladium). [Google Scholar]
- 2.Carlsen M., Nielsen J., Villadsen J. Growth and α-amylase production by Aspergillus oryzae during continuous cultivations. J. Biotechnol. 1996;45:81–93. [Google Scholar]
- 3.Castillo F.J., Blanch H.W., Wilke C.R. Lactase production in continuous culture by Trichoderma reesei Rut-C30. Biotechnol. Lett. 1984;6:593–596. [Google Scholar]
- 4.Chaudhuri B.K., Sahai V. Comparison of growth and maintenance parameters for cellulase biosynthesis by Trichoderma reesei-C5 with some published data. Enzyme Microb. Technol. 1994;16:1079–1083. [Google Scholar]
- 5.Elshafei A.M., Hussein F., El-Mahalawy A., Kahil T., Mostafa Enas M., Abd Eltawab B. Upgrading of exoglucanase production by trichoderma reesei NRC 210. Int. J. Eng. Res. Dev. 2014;10(8):68–73. [Google Scholar]
- 6.Hussein Fatma, Hamed Ragaa R., El-beih Fawkia, Mostafa Enas M., El-shershaby Asmaa. Enhanced production of catalase by Penicillium chrysogenum in benchtop bioreactor. Int. J. Sci. Eng. Res. 2015;6(8):900–904. [Google Scholar]
- 7.Gultom S.O., Hu B. Review of microalgae harvesting via co-pelletization with filamentous fungus. Energies. 2013;6:5921–5939. [Google Scholar]
- 8.Keränen S., Penttilä Y. Production of recombinant proteins in the filamentous fungus Trichoderma reesei. Curr. Opin. Biotechnol. 1995;6:534–537. doi: 10.1016/0958-1669(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 10.Luo S.S., Dong Z.J., Wu X.D., Liu Y.H., Ruan R. Pelletization behavior of fungal Chlorella sp symbiosis system. Res. J. Biotechnol. 2013;8:56–59. [Google Scholar]
- 11.Pedersen H., Beyer M., Nielsen J. Glucoamylase production in batch, chemostat and fed-batch cultivations by an industrial strain of Aspergillus niger. Appl. Microbiol. Biotechnol. 2000;53:272–277. doi: 10.1007/s002530050020. [DOI] [PubMed] [Google Scholar]
- 12.Penttilä M. Heterologous protein production in Trichoderma. In: Harman G.E., Kubicek C.P., editors. Vol. 8197. Taylor & Francis Ltd.; London: 1998. pp. 365–382. (Trichoderma and Gliocladium). [Google Scholar]
- 13.Penttilä M., Limón C., Nevalainen H. Molecular biology of Trichoderma and biotechnological applications. Mycology. In: Arora D.K., editor. Handbook of Fungal Biotechnology. 2nd edn. Marcel Dekker; New York, Basel: 2004. pp. 413–427. [Google Scholar]
- 14.Bergquist Peter, Te'o Valentino, Gibbs Moreland, Curach Natalie, Nevalainen Helena. Recombinant bleaching enzymes from thermophiles expressed in fungal hosts. Appl. Enzymes Lignocell. 2009:438–453. Chapter 24. [Google Scholar]
- 15.Schafner D.W., Toledo R.T. Cellulase production in continuous culture by Trichoderma reesei on xylose-based media. Biotechnol. Bioeng. 1992;39:865–869. doi: 10.1002/bit.260390808. [DOI] [PubMed] [Google Scholar]
- 16.Schrickx J.M., Krave A.S., Verdoes J.C., van den Hondel C.A., Stouthamer A.H., van Verseveld H.W. Growth and product formation in chemostat and recycling cultures by Aspergillus niger N402 and a glucoamylase overproducing transformant, provided with multiple copies of the glaA gene. J. Gen. Microbiol. 1993;139:2801–2810. doi: 10.1099/00221287-139-11-2801. [DOI] [PubMed] [Google Scholar]
- 17.Spohr A., Carlsen M., Nielsen J., Villadsen J. Amylase production in recombinant Aspergillus oryzae during fed-batch and continues cultivation. J. Ferment. Bioeng. 1998;86:49–56. [Google Scholar]
- 18.Pakula Tiina M., Salonen Katri, Uusitalo Jaana, Penttilä Merja. The effect of specific growth rate on protein synthesis and secretion in the filamentous fungus Trichoderma reesei. Microbiology. 2005;151(1):135–143. doi: 10.1099/mic.0.27458-0. [DOI] [PubMed] [Google Scholar]
- 19.Abd-El Rahim Wafaa M., Moawad H., Khalafallah M.A. Enhancing the growth of fungal promising strains for rapid dye removal. Fresenius Environ. Bull. (FEB) 2003;12(7):764–770. [Google Scholar]
- 20.Abd-El Rahim Wafaa M. Assessment of textile dyes remediation using biotic and abiotic agents. J. Basic Microbiol. 2006;46(4):318–328. doi: 10.1002/jobm.200510076. [DOI] [PubMed] [Google Scholar]
- 21.Wiebe M.G., Robson G.D., Shuster J., Trinci A.P. Growth-rate-independent production of recombinant glucoamylase by Fusarium venenatum JeRS 325. Biotechnol. Bioeng. 2000;68:245–251. doi: 10.1002/(sici)1097-0290(20000505)68:3<245::aid-bit2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Withers J.M., Swift R.J., Wiebe M.G., Robson G.D., Punt P.J., van den Hondel C.A. Optimization and stability of glucoamylase production by recombinant strains of Aspergillus niger in chemostat culture. Biotechnol. Bioeng. 1998;59:407–418. [PubMed] [Google Scholar]
- 23.Xia C.J., Wei W., Hu B. Statistical analysis and modeling of pelletized cultivation of Mucor circinelloides for microbial lipid accumulation. Appl Biochem. Biotech. 2014;172:3502–3512. doi: 10.1007/s12010-014-0759-8. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J.G., Hu B. A novel method to harvest microalgae via co-culture of filamentous fungi to form cell pellets. Bioresour. Technol. 2012;114:529–535. doi: 10.1016/j.biortech.2012.03.054. [DOI] [PubMed] [Google Scholar]