Abstract
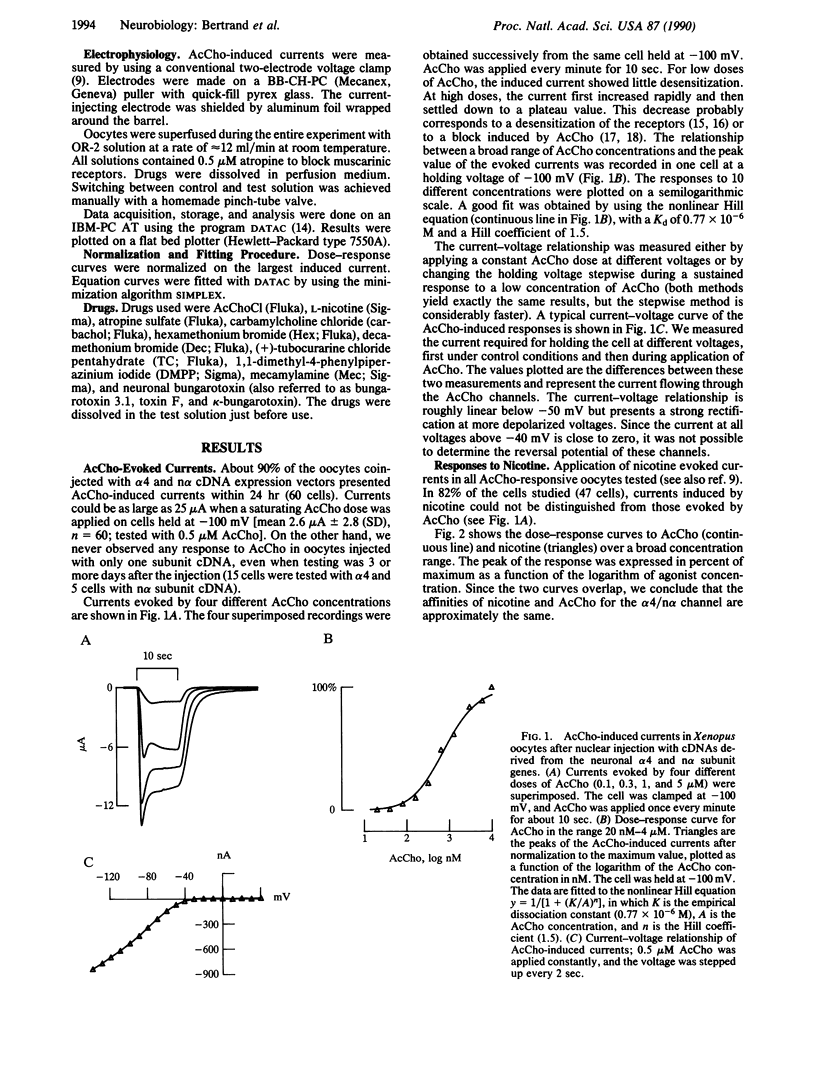
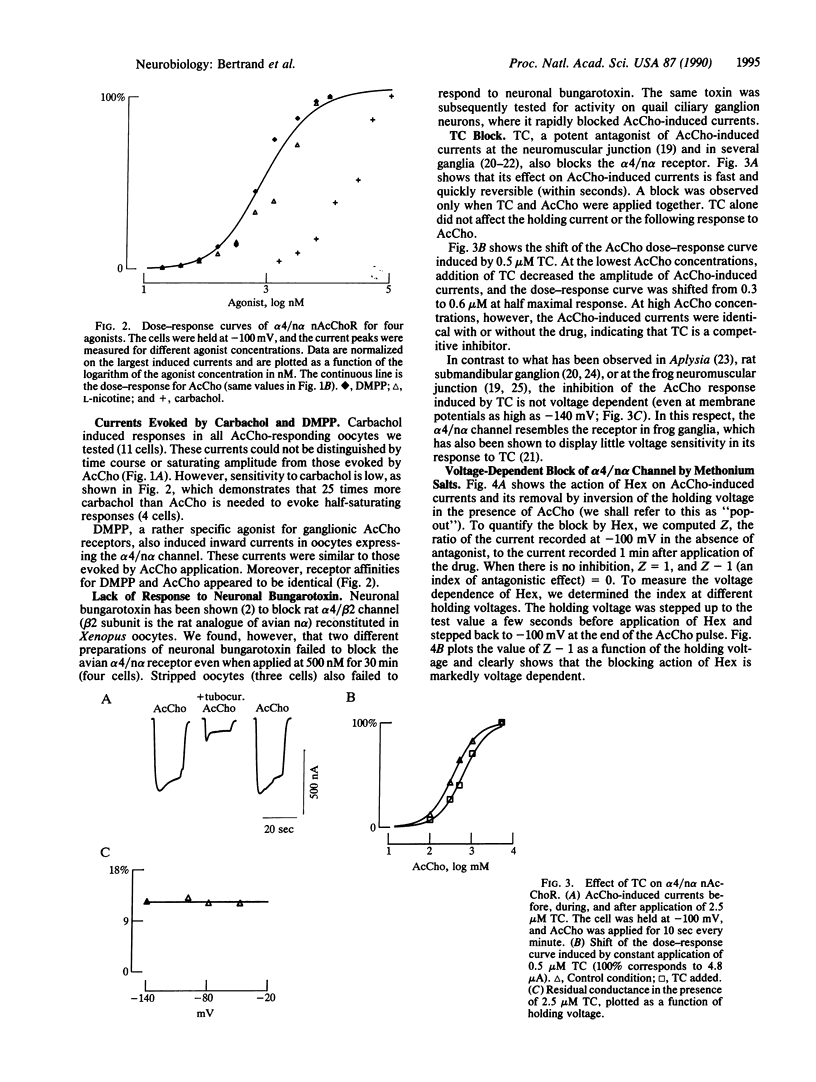
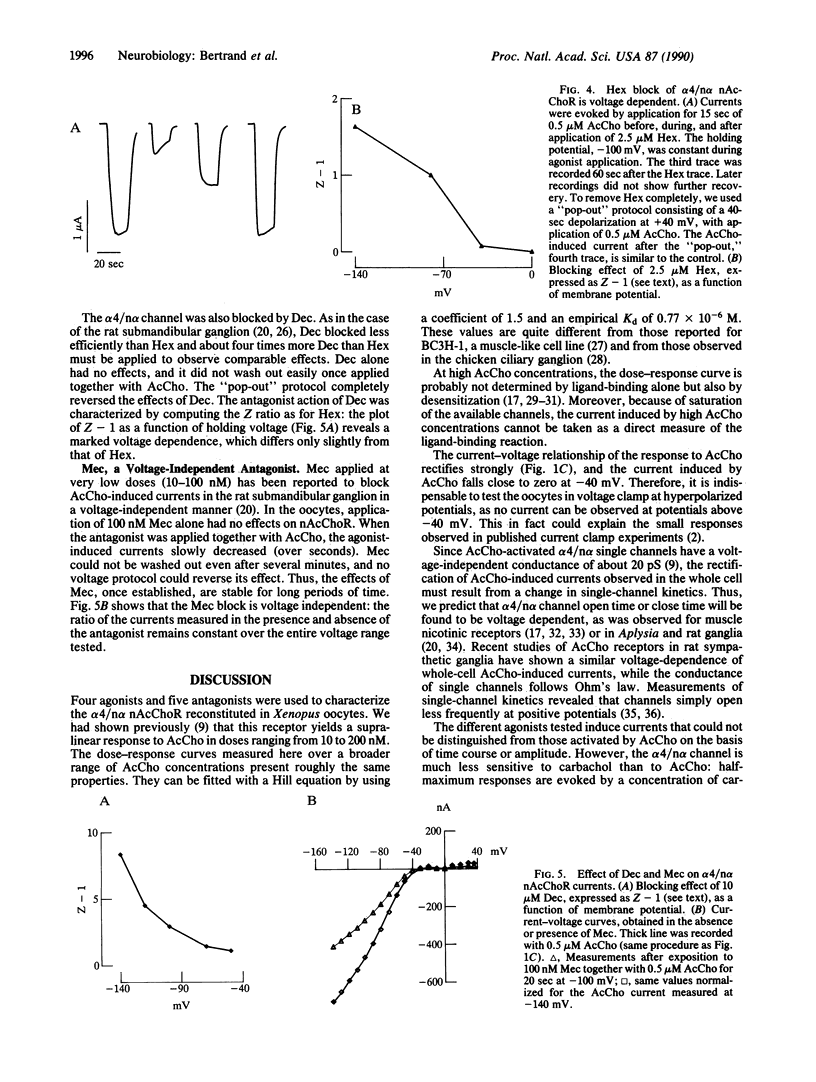
Neuronal nicotinic acetylcholine receptor of the alpha 4/non-alpha (alpha 4/n alpha) type was reconstituted in Xenopus oocytes after nuclear injection of cDNA expression vectors. Functional neuronal receptor was only formed when the two subunits alpha 4 and n alpha were coinjected, neither alpha 4 nor n alpha alone being effective. Responses to bath application of acetylcholine (AcCho) have been measured in voltage clamp. AcCho doses as low as 10 nM induce currents of up to 50 nA. Dose-response studies indicate a Kd of about 0.77 x 10(-6) M and a Hill coefficient of 1.5, thus predicting more than one AcCho binding site per receptor molecule. The current-voltage relationship of AcCho-induced currents presents a strong inward rectification. Responses to AcCho were compared to those of three other agonists: L-nicotine, carbachol, and 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP). Sensitivities to AcCho, nicotine, and DMPP are quite similar. Sensitivity to carbachol is much lower, but the currents are otherwise indistinguishable from those induced by AcCho. Five AcCho antagonists--neuronal bungarotoxin (kappa-bungarotoxin), tubocurarine (TC), hexamethonium bromide (Hex), decamethonium bromide (Dec), and mecamylamine (Mec)--have been tested. Neuronal bungarotoxin has no effect on the alpha 4/n alpha channel, whereas 2.5 microM TC reduces by half the current peak evoked by 1 microM AcCho. The block by TC is independent of membrane voltage. By contrast, the block of AcCho-induced currents by Hex or Dec is strongly voltage dependent, suggesting that these substances enter the channel. The block by Mec is detectable at concentrations as low as 100 nM when applied together with 1 microM AcCho and is voltage independent. Hex, Dec, and Mec are effective only when AcCho is present. While the effects of all other agents are fully reversible, the Mec block is persistent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. An analysis of the dose-response curve at voltage-clamped frog-endplates. Pflugers Arch. 1975 Oct 28;360(2):145–153. doi: 10.1007/BF00580537. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Kinetics of agonist conductance changes during hyperolarization at frog endplates. Br J Pharmacol. 1975 Feb;53(2):308–310. doi: 10.1111/j.1476-5381.1975.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Tokutomi N., Kijima H. Kinetic analysis of acetylcholine-induced current in isolated frog sympathetic ganglion cells. J Neurophysiol. 1989 Feb;61(2):283–290. doi: 10.1152/jn.1989.61.2.283. [DOI] [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballivet M., Nef P., Couturier S., Rungger D., Bader C. R., Bertrand D., Cooper E. Electrophysiology of a chick neuronal nicotinic acetylcholine receptor expressed in Xenopus oocytes after cDNA injection. Neuron. 1988 Nov;1(9):847–852. doi: 10.1016/0896-6273(88)90132-8. [DOI] [PubMed] [Google Scholar]
- Bertrand D., Bader C. R. DATAC: a multipurpose biological data analysis program based on a mathematical interpreter. Int J Biomed Comput. 1986 May;18(3-4):193–202. doi: 10.1016/0020-7101(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Boulter J., Connolly J., Deneris E., Goldman D., Heinemann S., Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R. T., Jacob M. H., Couturier S., Ballivet M., Berg D. K. Expression and regulation of neuronal acetylcholine receptor mRNA in chick ciliary ganglia. Neuron. 1988 Aug;1(6):495–502. doi: 10.1016/0896-6273(88)90180-8. [DOI] [PubMed] [Google Scholar]
- Chabala L. D., Gurney A. M., Lester H. A. Dose-response of acetylcholine receptor channels opened by a flash-activated agonist in voltage-clamped rat myoballs. J Physiol. 1986 Feb;371:407–433. doi: 10.1113/jphysiol.1986.sp015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris E. S., Connolly J., Boulter J., Wada E., Wada K., Swanson L. W., Patrick J., Heinemann S. Primary structure and expression of beta 2: a novel subunit of neuronal nicotinic acetylcholine receptors. Neuron. 1988 Mar;1(1):45–54. doi: 10.1016/0896-6273(88)90208-5. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Steckman M. L. Comparison of exogenous energy sources for in vitro maintenance of follicle cell-free Xenopus laevis oocytes. In Vitro. 1976 Mar;12(3):173–179. doi: 10.1007/BF02796439. [DOI] [PubMed] [Google Scholar]
- Goldman D., Deneris E., Luyten W., Kochhar A., Patrick J., Heinemann S. Members of a nicotinic acetylcholine receptor gene family are expressed in different regions of the mammalian central nervous system. Cell. 1987 Mar 27;48(6):965–973. doi: 10.1016/0092-8674(87)90705-7. [DOI] [PubMed] [Google Scholar]
- Gurney A. M., Rang H. P. The channel-blocking action of methonium compounds on rat submandibular ganglion cells. Br J Pharmacol. 1984 Jul;82(3):623–642. doi: 10.1111/j.1476-5381.1984.tb10801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Rang H. P. Nicotinic receptors of frog ganglia resemble pharmacologically those of skeletal muscle. J Neurosci. 1988 Sep;8(9):3258–3265. doi: 10.1523/JNEUROSCI.08-09-03258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Margiotta J. F., Berg D. K., Dionne V. E. The properties and regulation of functional acetylcholine receptors on chick ciliary ganglion neurons. J Neurosci. 1987 Nov;7(11):3612–3622. doi: 10.1523/JNEUROSCI.07-11-03612.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Neild T., Ascher P. Voltage sensitivity of acetylcholine currents in Aplysia neurones in the presence of curare. Nature. 1976 Jun 10;261(5560):501–503. doi: 10.1038/261501a0. [DOI] [PubMed] [Google Scholar]
- Mathie A., Cull-Candy S. G., Colquhoun D. Single-channel and whole-cell currents evoked by acetylcholine in dissociated sympathetic neurons of the rat. Proc R Soc Lond B Biol Sci. 1987 Nov 23;232(1267):239–248. doi: 10.1098/rspb.1987.0072. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Neely A., Lingle C. J. Trapping of an open-channel blocker at the frog neuromuscular acetylcholine channel. Biophys J. 1986 Nov;50(5):981–986. doi: 10.1016/S0006-3495(86)83538-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef P., Oneyser C., Alliod C., Couturier S., Ballivet M. Genes expressed in the brain define three distinct neuronal nicotinic acetylcholine receptors. EMBO J. 1988 Mar;7(3):595–601. doi: 10.1002/j.1460-2075.1988.tb02852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D. C., Colquhoun D. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc R Soc Lond B Biol Sci. 1985 Sep 23;225(1240):329–355. doi: 10.1098/rspb.1985.0065. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Colquhoun D., Rang H. P. The action of ganglionic blocking drugs on the synaptic responses of rat submandibular ganglion cells. Br J Pharmacol. 1982 Jan;75(1):151–168. doi: 10.1111/j.1476-5381.1982.tb08768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P. The characteristics of synaptic currents and responses to acetylcholine of rat submandibular ganglion cells. J Physiol. 1981 Feb;311:23–55. doi: 10.1113/jphysiol.1981.sp013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer R., Whiting P., Esch F., Blacher R., Shimasaki S., Lindstrom J. cDNA clones coding for the structural subunit of a chicken brain nicotinic acetylcholine receptor. Neuron. 1988 May;1(3):241–248. doi: 10.1016/0896-6273(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Skok V. I. Channel-blocking mechanism ensures specific blockade of synaptic transmission. Neuroscience. 1986;17(1):1–9. doi: 10.1016/0306-4522(86)90221-6. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Miledi R. Change in desensitization of cat muscle acetylcholine receptor caused by coexpression of Torpedo acetylcholine receptor subunits in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):367–371. doi: 10.1073/pnas.86.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann A. Tubocurarine, a partial agonist for cholinergic receptors. J Neural Transm Suppl. 1983;18:353–361. [PubMed] [Google Scholar]
- Voellmy R., Rungger D. Transcription of a Drosophila heat shock gene is heat-induced in Xenopus oocytes. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1776–1780. doi: 10.1073/pnas.79.6.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E., Wada K., Boulter J., Deneris E., Heinemann S., Patrick J., Swanson L. W. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jun 8;284(2):314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wada K., Ballivet M., Boulter J., Connolly J., Wada E., Deneris E. S., Swanson L. W., Heinemann S., Patrick J. Functional expression of a new pharmacological subtype of brain nicotinic acetylcholine receptor. Science. 1988 Apr 15;240(4850):330–334. doi: 10.1126/science.2832952. [DOI] [PubMed] [Google Scholar]
- Whiting P. J., Liu R., Morley B. J., Lindstrom J. M. Structurally different neuronal nicotinic acetylcholine receptor subtypes purified and characterized using monoclonal antibodies. J Neurosci. 1987 Dec;7(12):4005–4016. doi: 10.1523/JNEUROSCI.07-12-04005.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



