Highlights
-
•
Expression of chitinase gene was studied by RT-PCR in response to Alternaria brassicae.
-
•
Chitinase gene is induced by Alternaria, wounding and by JA and not by SA. It shows the tissue specificity of the gene.
-
•
Pathogen-inducible 2.5 kb chitinase class IV promoter was isolated from B. juncea by Genome Walking.
-
•
Induction pattern of chitinase gene is also reflected in promoter validation studied in transgenic Arabidopsis leaf.
-
•
This will help in using this promoter discretely in developing fungus resistant transgenic plants.
Keywords: Brassica juncea, Alternaria brassicae, Chitinase promoter, Resistance
Abstract
Chitinases are the hydrolytic enzymes which belong to the pathogenesis-related (PR) protein family and play an important role not only in plant defense but also in various abiotic stresses. However, only a limited number of chitinase genes have been characterised in B. juncea. In this study, we have characterised B. juncea class IV chitinase gene (accession no EF586206) in response to fungal infection, salicylic acid (SA), jasmonic acid (JA) treatments and wounding. Gene expression studies revealed that the transcript levels of Bjchitinase (BjChp) gene increases significantly both in local and distal tissues after Alternaria infection. Bjchitinase gene was also induced by jasmonic acid and wounding but moderately by salicylic acid. A 2.5 kb class IV chitinase promoter of this gene was isolated from B. juncea by Genome walking (accession no KF055403.1). In-silico analysis of this promoter revealed a number of conserved cis-regulatory elements related to defense, wounding and signalling molecules like SA, and JA. For validation, chitinase promoter was fused to the GUS gene, and the resultant construct was then introduced into Arabidopsis plants. Histochemical analysis of T2 transgenic Arabidopsis plants showed that higher GUS activity in leaves after fungal infection, wounding and JA treatment but weakly by SA. GUS activity was seen in meristematic tissues, young leaves, seeds and siliques. Finally investigation has led to the identification of a pathogen-inducible, developmentally regulated and organ-specific promoter. Present study revealed that Bjchitinase (BjChp) promoter is induced during biotic and environmental stress and it can be used in developing finely tuned transgenics.
1. Introduction
B. juncea, an important oil seed crop of the country, contributes nearly 27% of vegetable oil requirements of the country. A. brassicae is an important necrotrophic pathogen and is a limitation for the productivity of crop. After infection host plants synthesizes a group of low molecular weight defensive proteins called (PR) proteins. These proteins are produced during pathological situations and are associated with host defense mechanism during incompatible interactions and help to prevent pathogen progress. Chitinase proteins are members of family PR3, PR4, PR8 and PR 11 of the PR proteins that are strongly induced, after the pathogen infection in the host plant. Chitinases are important weaponry of plants against pathogens and have ability to inhibit fungal growth. Chitinases are involved in catalysis of the compounds that constitute the integral part of plant cell wall like chitin, chitosan, lipochitooligosaccharides, peptidoglycan, arabinogalactan and glycoprotein containing N-acetyl glucosamine. PR-3 includes chitinases of classes Ia, Ib, II, IV, VI, and VII. PR-4 includes class I and II chitinases [1]. On the basis of sequence annotation and blast search analysis at TAIR database (http://www.arabidopsis.org/), the Arabidopsis genome appears to contain 25 chitinase or chitinase-like proteins put together from GH18 and GH19 families. The length of the chitinase proteins in Arabidopsis varies from 211 to 430 amino acids with the average length of 308 amino acids. The rice genome at TIGR database shows 49 chitinase or chitinase-like proteins put together from GH18 and GH19 families. Chitinases perform diverse physiological and ecological roles across the spectrum of living organisms [2]. It has been shown by in vitro studies with purified chitinases that chitinases act as antifungal elements directly by hydrolyzing chitin. Chitinases are induced after infection in several crops as reviewed by [2]. Fungal infections cause upregulation of chitinase gene in B. juncea and tall fescue grass [3], [4], [5].
Biotrophic pathogens generally induce SA defense signailling pathway while necrotrophic pathogen, induce JA signailling pathway. In most of the cases JA and SA interaction is antagonistic in nature. Chitinases and defensins have been shown to be induced by JA but not by SA while PR1 PR2 and PR5 are induced by SA but these two pathways are not totally independent and cross-talk at various points. There are lots of gaps in our knowledge of SA-JA interaction. Therefore the first objective of the present study was to gain insight into defense signailling pathway. We have analysed expression of chitinase in response to Alternaria, SA, JA, and wounding. Expression of PR gene like chitinase in a targeted manner has the potential appreciably to benefit the genetic improvement of B. juncea. The levels of Chitinases in rice cultivars are correlated with resistance to sheath blight pathogen Rhizoctonia solani [6]. A linear correlation was observed between chitinase activity and percentage inhibition of fungal growth or disease tolerance in transgenic grapevines [7]. Transgenic plants over expressing chitinases of diverse classes have been produced by employing a range of donor and recipient host plant species. Transgenic plants have been produced that over express chitinase singly or in combination with other antifungal proteins. The first document of transgenics for fungal resistance was developed by constitutively expressing bean chitinase in tobacco and B. napus [8]. Transgenics mustard plants have been produced that over expresses chitinases gene under 35 s promoter by Mondal et al. [9]. Transgenic cotton expressing bean endochitinase gene (chi) under the CaMV 35S promoter was developed [10]. Carrot plants co transformed with barley chitinase and wheat PR protein was found to be successfully resistant to necrotrophic, A. radicicola and B. cinerea, [11]. Tomato transformed with rice chitinase and the alfalfa defensin gene. Co- transformed plants were three fold times resistant to pathogen than control plants [12]. Transgenic rice plants expressing the rice thaumatin-like proteins individually or in combination with the rice chitinase gene can be more effective than their individual transgenics [13]. It is significant that most of the transgenic plants overproducing chitinases show enhanced resistance to bacteria and fungi.
It has been observed that the constitutive over-expression of transgene not only hampers the growth and productivity of plants [14], [15] but also compete for energy and building blocks for the synthesis of protein or RNA required for plant growth under normal conditions. So it is need of hour to generate transgenic plants that produce the transgenic products only during pathogenesis. Such pathogen- inducible promoters can be used in important crops to develop fungus resistant transgenics. These transgenics will be more specific for time and place of transgene induction. Therefore the second objective of the present study was isolation and characterization of pathogen-inducible chitinase promoter from B. juncea. Recognizing the important cis-regulatory elements involved in response to different treatment will help in generating more efficient transgenic plants.
2. Materials and methods
Plant materials used were B. juncea var.Varuna and Arabidopsis thaliana plants (ecotype Columbia for promoter validation studies). Brassica and Arabidopsis plants were raised in pots containing a mixture of soil and organic manure (2:1) in net house set at a temperature of 22 °C and illuminated with compact fluorescent lamps (light intensity of 12.5 μmol/m2/s1) for a 16 h/8 h Light/dark cycle. The seedlings were grown for 45 days until they reached the 4–8 leaf stage. Infected leaves of B. juncea with Alternaria blight symptoms were collected. A. brassicae was isolated from the infected leaves of B. juncea. Leaves having necrotic lesions or margins were cut and surface sterilized in 70% v/v solution of ethanol followed by 0.1% w/v solution of mercuric chloride and after washing were placed on PDA plates. Growing edges of mycelia were sub-cultured on PDA for several passages until pure cultures were obtained and fungal culture was identified at the Indian Type Culture Collection IARI (A. brassicae identification no: 8794.12) as shown in Fig. 1. Conidial suspensions were prepared by scraping mycelium from 21-day old cultures and suspending in sterilized distilled water. Concentration of conidia was adjusted to 5 × 103 conidia/ml.
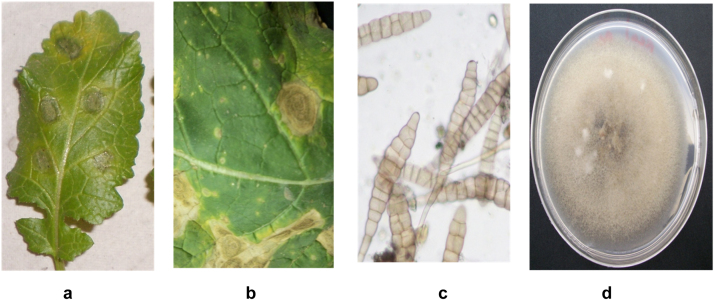
Fig. 1.
Leaves of B. juncea Infected with Alternaria blight caused by A. brassicae showing necrotic lesions with concentric rings. a and b) Infected leaves c) Direct scrapings obtained from an infected leave showing A. brassicae spores. d) Colonies of A. brassicae spores (on RDA medium).
2.1. Alternaria infection, hormonal and wounding treatment of Brassica juncea
Leaves of the B. juncea plants were sprayed with SDW and kept covered with transparent polythene bags at 22 °C for 24 h with 16 h light/8 h-dark cycle in order to maintain the turgidity. Drops (10 μl) of the spore suspension of A. brassicae were placed on to the scratched sites of each leaf. For control, leaves were treated with drops of 10 μl of sterile distilled water. Plants were covered with polythene bags with 100% interior relative humidity provided by spraying water inside the bag and placing water-soaked cotton balls and paper towels inside each bag. Plants were replaced back in a BOD incubator at 22 °C for symptoms to develop. The leaf samples were collected at different time intervals 0, 2, 4, 8, 12, 24, 48, 72 and 96 h of post inoculation. Only the green tissue, about 5 mm distance surrounding the spot/inoculation droplet was used for RT-PCR studies (for local leaves). The un-inoculated leaves were also collected at different time intervals as mentioned above to study the systemic gene induction. Disease development was monitored for up to 10 days after inoculation. The plants were sprayed with 2 mM salicylic acid, 100 μM jasmonic acid, and with sterile distilled water for control plants. Plants were covered with polythene bags with 100% interior relative humidity and samples were harvested at 0, 2, 4, 8, 12, 24, 48 and 72 h post treatment. For wounding, B. juncea leaves were gently wiped off with a moist swab to clean the dirt from the lamina and midrib of the ad-axial surface and then wounded with sterile needle. Samples were harvested at 0, 2, 4, 8, 12, 24, 48 and 72 h after wounding.
2.2. Isolation of RNA and RT- PCR
Total RNA was extracted from control and treated leaves at different times of intervals using Trizol reagent (Sigma USA). Semi RT-PCR was performed to measure the transcript levels of BjChp gene in response to Alternaria infection, jasmonic acid, salicylic acid, and wounding using gene specific primers. For RT-PCR analysis first-strand cDNA was synthesized from 2 μg of DNase- treated total RNA by reverse transcriptase (Fermentas, USA) in 20 μl reaction volume using oligo (dT) primer. PCR was performed with Taq DNA polymerase. For PCR PR3 gene specific primers were designed from cDNA sequences of B. juncea PR3 (EF586206.1). Primers for amplification were as follows. PR3F −5′ AAG TTC GGT GCT TCC ATC TC3‘ and Rev Primer- PR3R 5′ TCC GGT ACA CTC CCT ACT ATT C. Primers for α-tubulin (used to normalize the sample) were made from cDNA sequences of Arabidopsis (NM_100360.3). These were as follows Sense 5′-CTGGGAGCTGTACTGTCTTG-3′ Antisense 5′- CAACGGAGGTAGAGACCTGTG −3′. PCR Primers were designed using Primer3 software. PCR was performed using a thermocycler in 50-μl final volume including 1 μl of ten times diluted cDNA (100 ng/ul) template, PCR conditions included an initial denaturing step at 94 °C for 4 min, followed by 30 cycles of 94 °C for 30 s, 55–57 °C for 30 s, 72 °C for 1 min with a final extension at 72 °C for 10 min. PCR products were separated using 1% agarose gels, stained with ethidium bromide and observed in a gel doc system. Experiments with all the treatments were carried out at least for three times.
2.3. Isolation and characterization of the regulatory region of B. juncea
The 5′ upstream region of chitinase genes was obtained from the B. juncea genome by PCR walking using Universal Genome Walker kit (Clonetech USA). Total genomic DNA was extracted from young leaf tissues of B. juncea plants by CTAB method [16] and divided into four portions. Each portion was digested by one of four restriction enzymes (EcoRV, Dra1, Pvu1, Stu1,) overnight and ligated with the Genome Walker adaptor. The primary PCR reaction was performed using the digested and adaptor ligated genomic DNA as a template with an adaptor-specific primer (AP1- 5′GTA ATA CGA CTC ACT ATA GGG C 3′) and gene-specific outer reverse primer CHP O-5′ ATC TCC GGG ACC GCT GTT CTT GCA AGG TCC 3. Programme for the Primary PCR was as follows:7 cycles of 94 °C for 25 s, 72 °C for 3 min followed by 32 cycles of 94 °C for 25 s, 67 °C for 3 min, and an additional 7 min at 67 °C. The PCR products obtained were diluted 50 times and used as a template for nested PCR with an adaptor-specific primer (AP2; 5′ −5′ACT ATA GGG CAC GCG TGG T 3′) and a gene-specific inner reverse primer CHP I-5′ AGA GGC GAC GGG TTT GGA AAC GGT TAG G 3. Secondary PCR programme as follows: cycles 94 °C for 25 s, 72 °C for 3 min followed by 20 cycles of 94 °C for 25 s, 67 °C for 3 min and after the final cycle 67 °C for an additional 7 min. Amplified 2.5 kb regulatory region was cloned into TA-cloning vector (Promega). Plasmid DNA was isolated from Positive clones and got sequenced. Cloned fragment got sequenced using M13 forward and reverse primers. In silico analysis Search against Plant CARE (PC) and PLACE (P) databases was carried out.
2.4. Constructions of promoter-reporter construct
To analyze the ability of pathogen-inducible chitinase promoter to direct the expression of the Gus reporter gene, promoter-reporter construct was used. Promoter fragment (2.5 kb) of BjChp was amplified with fidelity proof reading Advantage polymerase mix (Clone tech) using ChampF − 5′ GCTGGTCCTAAGTTGTTTCAAAGTG3′3′ as a forward primer and ChampR −5′CTTTGTGTGTGAGGTAGATAGAGG3′ as reverse primer and then cloned into pORE R2 vector using blunt end cloning procedure. Vector was simultaneously linearized and dephosphorylated with Fast Digest Sma1 restriction enzyme and FastAP Thermo sensitive Alkaline respectively. The purified PCR product was phosphorylated using T4 Polynucleotide kinase and cloned upstream of ATG start codon of β Glucuronidase gene of Gus reporter vector through blunt end ligation. The recombinant clones were confirmed by PCR to check the correct orientation using Gus and promoter specific primers. Expression cassettes (promoter + Gus gene+ Terminator) were released and introduced into A. tumefaciens strain EHA 105. Confirmation of the clones was done by PCR and restriction analysis of plasmid DNA from A. tumefaciens.
2.5. Transformation of construct into Arabidopsis plants
Arabidopsis (ecotype Colombia) plants were transformed by A. tumefaciens strain EHA 105 harbouring BjChp promoter reporter construct by floral dip method [17]. Seeds from transformed plants were screened on MS medium with kanamycin (50 μg/ml). T2 lines were generated for GUS analysis.
2.6. Validation of promoter
Validation of the pathogen-inducible chitinase promoter and its ability to drive the expression of the Gus-encoding reporter gene was done on the leaves of T2 transgenic Arabidopsis plants infected by Alternaria, or treated with defense inducer molecules like SA, JA and wounding. Leaves of the treated plants were subjected to Gus staining after 48 h. Level of Gus gene driven by the above promoter was measured by intensity of Gus staining. The histochemical assay for Gus reporter gene expression was done as described by Jefferson [18] with some modifications.
3. Results
3.1. Sequence analysis of B. juncea chitinase
cDNA library constructions and cloning of Class IV chitinase gene had been done previously from total RNA isolated from JA treated leaves of B. juncea in our lab at NRCPB, IARI, New Delhi and sequence was submitted to NCBI (accession no EF586206). Deduced protein contained 278 amino acids with an open reading frame of 837 bp, similar to other proteins in this class. PROSITE database analysis of the Bjchitinase showed the presence of chitin binding domain signature between 33 and 68 amino acid residues (SQNCGCPPGLCCSTNGYCGTTDDYCGVGCKEGPCKN). On conserve protein domain search from 82 to 278 amino acid, B. juncea chitinase ABQ57389.1 was found to have similarity with chitinase belonging to family 19 in the classification of glycosylhydrolases. Chitinases of family 19 are enzymes from plants that function in the defense against fungal and insect pathogens by destroying their chitin-containing cell wall. From 38 to 61amino acid it has hevein or type1 chitin binding domain. The multiple sequence alignment of B.Juncea chitinase with chitinases from other plants was performed using the Clustal omega software. As shown in Fig. S1. Sequence is consistently similar to other plant chitinase proteins. The Cysteines are invariant among members of the plant chitinases and are included in the consensus sequence along with other amino-acid residues like leucine, phenylalanine, Valine, and other amino-acids as shown by Astrics *. The phylogenetic analysis of deduced amino acid sequences of B. juncea Chitinase (ABQ57389.1) and chitinases from other plants is shown in Fig. 2. The evolutionary tree was constructed by the neighbor-joining method using the MEGA 4.0 software. Multiple sequences were used to build up the phylogenic tree were as follow: basic endochitinase B. oleracea (XP 013633710.1), endochitinase B. napus (XP 013686014.1), endochitinase CHB4-like B. napus (NP001302762.1), chitinase class IV partial B. napus (gb AAB01665.1), endochitinase CHB4-like B. rapa (XP009142951.1), chitinase B. rapa (AGC73657.1), predicted basic endochitinase CHB4-like C. sativa (XP010517931.1), putative chitinase A. thaliana (NP181885.1) and PREDICTED basic endochitinase CHB4-like C. sativa (XP010506247.1).
Fig. 2.
The phylogenetic analysis of B. juncea class IV chitinase (ABQ 57389.1) and chitinases of various plants, Phylogenetic tree was generated by the neighbor-joining method using the MEGA 4.0 software. The amino acid sequences used to build up the phylogenic tree were as follows: basic endochitinase B. oleracea (XP 013633710.1), basic endochitinase B. napus (XP 013686014.1), basic endochitinase 3 CHB4-like B. napus (NP001302762.1), chitinase class IV partial B.napus gb (AAB01665.1), basic endochitinase CHB4-like B.rapa (XP009142951.1), chitinase B. rapa subsp. Pekinensis (AGC73657.1), PREDICTED basic endochitinase CHB4-like C.sativa (XP010517931.1), putative chitinase A. thaliana (NP181885.1), PREDICTED basic endochitinase CHB4-like C. sativa (XP010506247.1).
3.2. Expression analysis of B. juncea chitinase
Expression profiling of B. jchitinase gene was done by RT-PCR to see the changes in the transcript levels of chitinase gene relative to the reference gene α-tubulin at different time intervals after Alternaria infection, wounding and treatment with defense inducers such as SA and JA. It was observed that transcript levels of BjChitinase gene was strongly up regulated in response to necrotrophic fungus A. brassicae. Semi-quantitative PCR (Fig. 3) results showed that the transcript accumulation of BjChp upon A. brassicae challenge started as early as at 2 h and was significantly increased at 4 h, 8 h, 24 h and 48 h. Levels of transcript accumulation peaked at 12 h and 96hr post treatment in distal systematic leaves. In local leaves induction was not as strong as in distal leaves (Fig. 3a and b). Level of induction is same throughout the treatment but at later time interval no induction of gene was seen. Down regulation of BjChp transcript is seen at 72 h upon A. brassicae infection in local as well as in distal leaves which could be because of diurnal effect. Upon treatment with jasmonic-acid, BjChp transcript was not induced during early hour of treatment 2 h, 4 h,and 8 h but strongly induced at later time intervals at 12 h, 24 h, 48 h, and 72 h (Fig. 3c). SA treatment at 2 mM showed very slight induction of BjChp transcript at 2 h and at later time intervals 12 h, 24 h, 48 h, and 72 h (Fig. 3d). PCR results showed that there is upregulation of BjChp transcript upon wounding during latter time interval at 8 h, 12 h, 24 h, 48 h, 72 h (Fig. 3e). These results indicate that induction of B. juncea chitinase transcripts occurs upon A. brassicae inoculation, wounding and treatments with JA but not significantly with SA treatment.
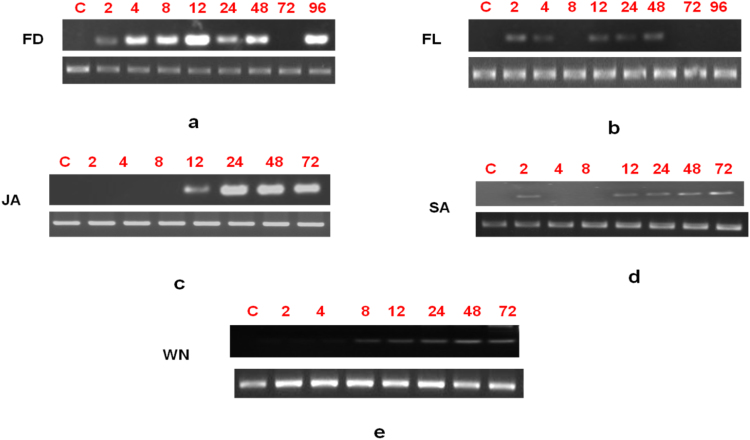
Fig. 3.
RT-PCR analysis of the Chitinase gene induction in B. juncea in response to A. brassicae challenge a) fungal distal (FD) b) A. brassicae challenge fungal local (FL) c) jasmonic acid (JA) d) Salicylic acid (SA), e) wounding. C: Control, 2–96: Hours post treatment/infection. α Tubulin was used to normalize the amount of template in PCR reactions.
3.3. Isolation and in silico analysis of the promoter
To study the upstream region of the gene, 2.5 kb promoter along with its cis regulatory elements was isolated from B. juncea and cloned in promoter less pORE R2 vector (NCBI with Gen Bank accession no KF055403.1). In silico analysis Search against Plant CARE (PC) and PLACE (P) databases revealed various putative Cis-acting regulatory elements in the promoter apart from a CAAT box, and a TATA box, which are necessary for the transcription activation of this promoter as shown in Fig. S2 and Table 1. Important cis-acting elements upstream of translation start site are highlighted as follows: RY-element required for in seed-specific regulation, TGACG-motif and CGTCA-motif for MeJA- response, ABRE site involved in the abscisic acid responsiveness, TCA-element involved in salicylic acid responsiveness, WUN-motif involved in wound-responsive element, TC-rich repeats- involved in defense and stress, MOTIFSkn-1motif- involved in endosperm expression, MBS MYB binding site for drought inducibility, ERE site ethylene-responsive element and TGA-element-for Auxins-responsiveness.
Table 1.
Putative Cis-acting regulatory elements identified in the BjChp promoter by in silico analysis Search against Plant CARE (PC) and PLACE (P) databases.
| S.No | Motif | Sequence | Position (upstream of ATG) | Function | Reference |
|---|---|---|---|---|---|
| 1 | TATA-BOX | TATAAAT | −69 to −75 | core promoter element around −30 of transcription start | PlantCARE |
| 2 | CAT −BOX | ACAAT | −462 to −465 | Element in promoter and enhancer regions | Shirsat et al. (1998) |
| 3 | ABRE site | TACGTG | −151 to −156 | involved in the abscisic acid responsiveness | PlantCARE |
| 4 | ABRE site | TACGGTC | −1402 to −1408 | involved in the abscisic acid responsiveness | PlantCARE |
| 5 | TGACG-motif | TGACG | −139 to − 143 −179 to − 183 |
Regulatory element involved in the MeJA- response | Despres (2003) |
| 6 | CGTCA-motif | CGTCA | −2140 to −2144 | Involved in the MeJA- response | Plant CARE |
| 7 | TCA-element | TCATCTTTTT | −360 to −369 | Element involved in salicylic acid responsiveness | Plant CARE |
| 8 | WUN-motif | TCCTTACGAAA | −434 to −444 | Element involved in wound-responsive element | Plant CARE |
| 9 | TC-rich repeats | ATTTTTTCCA | −939 to − 948 | Element involved in defense and stress | Plant CARE |
| 10 | MOTIFSkn-1motif | GTCAT | −1002 to − 998 —1580 to −1584 −1042 to −1046 |
Element involved in endosperm expression | Plant CARE |
| 11 | MBS | TAACTG | −1465 to −1470 | MYB binding site for drought inducibility | Plant CARE |
| 12 | TGA-element | AACGAC | −1747 to − 1752 | Auxin-responsive element | Plant CARE |
| 13 | ERE site | ATTTCAAA | −1494 to − 1501 | ethylene-responsive element | Plant CARE |
| 14 | RY-element | CATGCATG | −2177 to − 2184 | in seed-specific regulation | Plant CARE |
3.4. Validation of promoter
Transgenic Arabidopsis plants harbouring BjChp promoter were developed by floral dip method [17]. Validation of promoter was done in the leaves of the transgenic indicator Arabidopsis plants in response to A. brassicae, defense inducer molecules like SA and JA and upon wounding as shown in Fig. 4. To study the regulation of the promoter in response to Alternaria challenge, leaves of the T2 transgenic Arabidopsis plants harbouring B. juncea chitinase promoter were inoculated with A. brassicae and subjected to GUS staining after 48 h (Fig. 4b) Strong GUS activity was observed in the veins as well as surrounding of the necrotic lesions. Analysis of uninoculated control transgenic Arabidopsis leaves showed only basal level of GUS activity (Fig. 4a). GUS assay revealed strong GUS activity in the veins as well as marginal areas of the jasmonic acid treated leaves and a low level GUS activity in SA treated leaves showing that chitinase promoter is strongly upregulated by jasmonic acid and weakly by SA (Fig. 4d and c) In present study, GUS activity was seen in seeds as shown in Fig. 5a (although GUS staining has been masked by seed colour), Regarding developmental regulation, chitinase starts showing expression in young leaves and throughout maturity, whereas high activity of GUS promoter is seen at the base of the siliques as shown in Fig. 5(b and c)
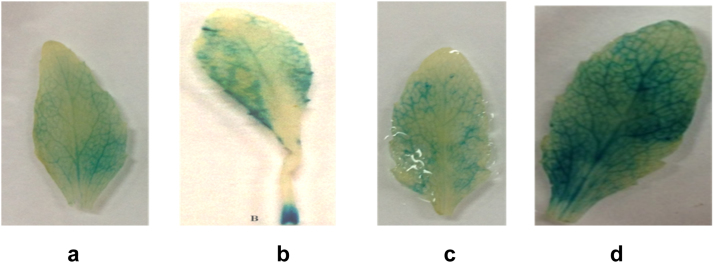
Fig. 4.
Gus expression in the leaf of transgenic Arabidopsis plants containing the BjChp promoter, 24 h after A. brassicae infection, treatment to SA and MeJA and wounding. a) Gus expression of control (untreated transgenic leaf) b) expression in A. brassicae inoculated transgenic leaf c) expression in the transgenic leaf after 2 mM SA treatment d) in the transgenic leaf after 100 μ MeJA treatment.
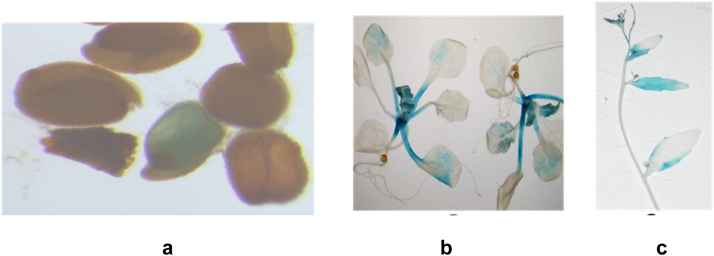
Fig. 5.
Histochemical Gus analysis of transgenic BjChpT2 Arabidopsis plants in different tissues and stages of plant development. a) Transgenic Arabidopsis seeds showing green colour, b) Gus expression in the leaves and meristematic tissues of transgenic seedlings c) Gus expression seen in the receptacle part of flower and seen in the base of the siliques and on the leaf.
4. Discussion
Alternaria blight caused by A. brassicae is a serious disease in Brassica. After infection host plants synthesizes a group of low molecular weight defensive (PR) proteins. Chitinase proteins belong to group PR3 of the PR proteins that are strongly induced in the host plant cell and are important weaponry against fungal pathogen. Plants seem to use mechanisms that effectively adjust their defense repertoires on the basis of the characteristics of their attackers. In defense against necrotrophic pathogens, the JA and ET signalling pathways synergize to activate a specific set of defense genes including plant defensins [28]. Interaction between A. Brassicae and B. juncea is not fully understood, therefore the present study was undertaken for isolation and molecular characterization of pathogen-inducible chitinase gene and its promoter in B. juncea. In this study we have analysed the expression of class IV chitinase gene (accession no EF586206) and regulatory region (accession no KF055403.1), in response to Alternaria infection both in local and distal tissues, upon treatment to defense signailling molecules like jasmonic acid (JA) and salicylic acid (SA) and upon wounding.
Results of RT-PCR and Gus visualisation studies revealed that B. juncea chitinase gene and its promoter were upregulated upon infection to A. brassicae. Earlier studies have also reported upregulation of chitinase upon infection in several crop plants as reviewed by Grover [2]. Lately also there are reports of induction of chitinase upon infection to sheath blight by pathogen R. solani [6]. Chitinases have been shown to impart resistance to fungal infection [5]. During the studies upregulation of chitinase gene and promoter was observed upon treatment to JA. Similar findings have been reported by [19], [20], [21], [22]. Phytohormones like salicylic acid and jasmonic acid are important compounds to induced defense responses or pathogen signalling pathways that regulates the expression of pathogen-related proteins [23], [24], [25]. In Arabidopsis MeJA induces several SA independent PR proteins PR3 and PR4 [26], [27], [28], [29], [30]. Previous studies have shown that MeJA signal transduction pathways plays important role in resistance to A.brassicola in model plant Arabidopsis Bart et al., 1998. Here we suggest that the induction of B. juncea chitinase gene and its promoters by MeJA could confer the long lasting resistance to A. brassicae in B. Juncea when over expressed.
Use of specific promoter instead of constitutive 35S promoter will result in localized and targeted gene expression. Use of such promoters to develop fungus resistant transgenics will be more specific for time and place of transgene induction. Therefore, second aim of the present study was isolation and molecular characterization of pathogen-inducible promoters with its cis-elements from pathogenesis-related –gene in B. juncea. Full length pathogen-inducible chitinase gene encoding 278 amino acid sequences and its 2.5 kb upstream promoter region was isolated from B. juncea. Bjchitinase gene upon blast shows similarity with lysozyme like super family 19 of glycoside hydrolyses family (2, E1). PROSITE database analysis of the deduced protein showed the presence of chitin recognition domain signature.
Insilico analysis of BjChp promoter revealed a copy of defense related cis-regulatory elements, TC rich repeats (ATTTTCTTCA), responsive to defense and stress. Validation of Bjchp promoter was done on in the T2 transgenic Arabidopsis plants by A. brassicae, upon treatment with defense inducers such as SA, JA, and upon wounding. Gus activity was observed in the veins as well as surrounding regions of leaves of the T2 transgenic Arabidopsis plants after inoculation with A. brassicae. However analysis of uninoculated transgenic Arabidopsis leaves showed only basal level of GUS expression. GUS assay revealed strong GUS activity in the veins and marginal areas of the jasmonic acid treated leaves and moderate GUS activity in SA treated leaves (could be because of wound produced during cutting), showing that BjChp promoters is upregulated by jasmonic acid. The intensity of GUS staining in water treated leaves was very low and basal similar to SA treated leaves. Further studies are required to understand the induction of GUS in veins which could be related to flow of some signal in the veins and also to clarify the molecular mechanisms underlying the expression of chitinase genes in B. Juncea.
Plants respond to wounding by inducing variety of genes both locally and systematically that contributes in healing of damaged tissues and prevent further invasion of pathogens [31]. Induction of genes has been reported by mechanical wounding in Arabidopsis [32]. To study wound signalling in plants, leaves have been used [33], [34], [35], [36]. Mechanical damage or wound signals can lead to increase resistance to insects [37], [38] or fungal pathogens [39], [40], [22]. GUS visualisation studies showed that the wound induced chitinase promoter in leaves of B. Juncea. Presence of various regulatory elements in the of Bjchp promoter, responsive to SA, JA, wounding and defense and stress further confirm the studies. GUS activity was seen in seeds although blue colour was masked by seed coat colour. Chitinase promoter seems to be active in meristematic tissues of seedlings, stem, and flower receptacles and at the base of siliques which might be to protect these vulnerable tissues to pathogen attack. Activity of chitinase promoter in seeds and meristematic tissue suggest its role during the process of the organogenesis [22]. Chitinases degrade the chitooligosaccharides on the cell walls of the seed thereby, facilitate the emerging radical to protrude out of seed hence have a role in germination of seed. Chitinases have a role in protecting inner tissues of the seed against pathogen [41]. Chitinase is expressed in the micropylar endosperms of tomato seeds prior to their radical emergence [42]. Indications are that chitinases present constitutively in storage tissues such as seeds, fruits and tubers might contribute a storage form of nitrogen [43]. Chitinases are induced in abscission zones [44], [45] may therefore be involved in defense of the scarified tissues to invasion by pathogens.
5. Conclusion
In the present study, we have isolated and characterised B. juncea class IV chitinase gene and its promoter. This gene was found to be pathogen-inducible and strongly induced both in local and distal tissues after Alternaria infection. Gene is also induced after the treatment with phytohormones such as jasmonic acid (JA) and upon wounding. Similar induction pattern is also reflected in promoter validation study in transgenic Arabidopsis. Such pathogen- inducible promoters can be used in important crops to develop fungus resistant transgenics. Further promoter characterization by making deletion constructs will throw light specifically about the promoter regions involved in Alternaria, JA and wound mediated expression.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgement
Author is thankful to Director NRCPB for all possible help and financial support during the tenure of the work.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.btre.2017.01.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Guevara-Morato M.A., de Lacoba M.G., García-Luque I., Serra M.T. Characterization of a pathogenesis-related protein 4 (PR-4) induced in Capsicum chinense L3 plants with dual RNase and DNase activities. J. Exp. Bot. 2010;61:3259–3271. doi: 10.1093/jxb/erq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grover A. Plant chitinases: genetic diversity and physiological roles. Crit. Rev. Plant Sci. 2012;31:57–63. [Google Scholar]
- 3.Wang J., Tian N., Huang X., Chen L.Y., Schlappi M., Xu Z.Q. Tall fescue turf grass class I chitinase is activated by fungal elicitors, dehydration ethylene and mechanical wounding. Plant Mol. Biol. 2009;27:305–314. [Google Scholar]
- 4.Wu X.F., Wang C.L., Xie E.B., Gao Y., Fan Y.L., Liu P.Q. Molecular cloning and characterization of the promoter for the multiple stress-inducible gene BjCHI1 from Brassica juncea. Planta. 2009;229:1231–1242. doi: 10.1007/s00425-009-0911-9. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed N.U. Identification and expression analysis of chitinase genes related to biotic stress resistance in Brassica. Mol. Biol. Rep. 2012;39:3649–3657. doi: 10.1007/s11033-011-1139-x. [DOI] [PubMed] [Google Scholar]
- 6.Shrestha C.L., Õna I., Muthukrishnan S., Mew T.W. Chitinase levels in rice cultivars correlate with resistance to the sheath blight pathogen Rhizoctonia solani. Eur. J. Plant Pathol. 2007;120:69–77. [Google Scholar]
- 7.Nookaraju A., Agrawal D.C. Enhanced tolerance of transgenic grapevines expressing chitinase and b-1, 3-glucanase genes to downy mildew. Plant Cell Tissue Organ Culture. 2012;111:15–28. [Google Scholar]
- 8.Broglie K., Chet I., Holliday M., Cressman R., Biddle P., Knowlton S., Mauvais C.J., Broglie R. Transgenic plants with enhanced resistance to the fungal pathogen R. solani. Science. 1991;22(254):1194–1197. doi: 10.1126/science.254.5035.1194. [DOI] [PubMed] [Google Scholar]
- 9.Mondal K.K., Chatterjee S.C., Viswakarma N., Bhattacharya R.C., Grover A. Chitinase-mediated inhibitory activity of Brassica transgenic on growth of A. brassicae. Curr. Microbiol. 2003;47(3):171–173. doi: 10.1007/s00284-002-3980-6. [DOI] [PubMed] [Google Scholar]
- 10.Tohidfar M., Mohammadi M., Ghareyazie B. Agrobacterium-mediated transformation of cotton (G. hirsutum) using a heterologous bean chitinase gene. Plant Cell Tissue Organ Culture. 2005;83:83–96. [Google Scholar]
- 11.Jayaraj J., Punja Z.K. Combined expression of chitinase and lipid transfer protein genes in transgenic carrot plants enhances resistance to foliar fungal pathogens. Plant Cell Rep. 2007;26:1539–1546. doi: 10.1007/s00299-007-0368-x. [DOI] [PubMed] [Google Scholar]
- 12.Chen S.C., Liu A.R., Wang F.H., Ahammed G.J. Combined overexpression of chitinase and defensin genes in transgenic tomato enhances resistance to Botrytis cinerea. Afr. J. Biotechnol. 2009;8:5182–5188. [Google Scholar]
- 13.Shah J.M., Singh R., Veluthambi K. Transgenic rice lines constitutively co-expressing tlp-D34 and chi11 display enhancement of sheath blight resistance. Biol. Plant. 2013;57(2):351–358. [Google Scholar]
- 14.Kasuga M., Liu Q., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999;17(3):287–291. doi: 10.1038/7036. [DOI] [PubMed] [Google Scholar]
- 15.Karim S., Aronsson H., Ericson H., Pirhonen M., Leyman B., Welin B., Mantyla E., Palva E.T., Van Dijck P., Holmstrom K.O. Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol. Biol. 2007;64(4):371–386. doi: 10.1007/s11103-007-9159-6. [DOI] [PubMed] [Google Scholar]
- 16.Murray M.G., Thompson W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8(October (19)):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clough S.J., Bent A. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 18.Jefferson R.A. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mo1. Biol. Rep. 1987;5:387–405. [Google Scholar]
- 19.Graham L.S., Sticklen M. Plant chitinases. Can. J. Bot. 1994;72:1057–1083. [Google Scholar]
- 20.Kasprzewska A. Plant chitinases – regulation and function. Cell. Mol. Biol. Lett. 2003;8:809–824. [PubMed] [Google Scholar]
- 21.Rakwal R., Yang G., Komatsu S. Chitinase induced by jasmonic acid, methyl jasmonate, ethylene and protein phosphatase inhibitors in rice. Mol. Biol. Rep. 2004;31:113–119. doi: 10.1023/b:mole.0000031407.18708.95. [DOI] [PubMed] [Google Scholar]
- 22.Van Loon L.C., Rep M., Pieterse C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez M.E. Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant Mol. Biol. 2000;44:429–442. doi: 10.1023/a:1026561029533. [DOI] [PubMed] [Google Scholar]
- 24.Balbi V., Devoto A. Jasmonate signalling network in A. thaliana: crucial regulatory nodes and new physiological scenarios. N. Phytol. 2008;177(2):301. doi: 10.1111/j.1469-8137.2007.02292.x. [DOI] [PubMed] [Google Scholar]
- 25.Leon-Reyes A., Spoel S.H., De Lange E.S., Abe H., Kobayashi M., Tsuda S., Millenaar F.F., Welschen R.A.M., Ritsema T., Pieterse C.M.J. Ethylene modulates the role of nonexpressor of pathogenesis-related genes1 in cross talk between salicylate and jasmonate signaling. Plant Physiol. 2009;149:1797–1809. doi: 10.1104/pp.108.133926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y., Chang P.F.L., Narasimhan M.L., Raghothama K.G., Hasegawa P.M., Bressan R.A. Plant defence genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell. 1994;6:1077–1085. doi: 10.1105/tpc.6.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durrant W.E., Dong X. Systemic acquired resistance. Ann. Rev. Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 28.Thomma Bart P.H.J., Eggermont Kristel, Penninckx Iris A.M.A., Mauch-Mani Brigitte, Vogelsang Ralph, Cammue Bruno P.A., Broekaert Willem F. Plant Biology Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei C., Qi M., Sheng G., Yang Y. Inducible over expression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression and host resistance to fungal infection. Mol. Plant-Microbe Interact. 2006;19(10):1127–1137. doi: 10.1094/MPMI-19-1127. [DOI] [PubMed] [Google Scholar]
- 30.Chung H.S., Koo A.J.K., Gao X., Jayanty S., Thines B., Jones A.D. Regulation and function of Arabidopsis Jasmonate ZIM-domain genes in response to wounding and herbivory. Plant Physiol. 2008;146:952–964. doi: 10.1104/pp.107.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durrant W.E., Rowland Owen, Piedras Pedro, Hammond-Kosack Kim E., Jones Jonathan D.G. CDNA-AFLP reveals a striking overlap in race-specific resistance and wound response gene expression profiles. Plant Cell. 2000;12:963–977. doi: 10.1105/tpc.12.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reymond P., Weber H., Damond M., Farmer E.E. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 2000;12:707–719. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farmer E.E., Ryan C.A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearce G., Strydom D., Johnson S., Ryan C.A. A polypeptide from tomato leaves induces wound-inducible proteinase-inhibitor proteins. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- 35.Seo S., Okamoto N., Seto H., Ishizuka K., Sano H., Ohashi Y. Tobacco map kinase—a possible mediator in wound signal-transduction pathways. Science. 1995;270:1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- 36.Niki T., Mitsuhara I., Seo S., Ohtsubo N., Ohashi Y. Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiol. 1998;39:500–507. [Google Scholar]
- 37.Leon J., Rojo E., Sanchez-Serrano J.J. Wound signalling in plants. J. Exp. Bot. 2001;52:1–9. doi: 10.1093/jexbot/52.354.1. [DOI] [PubMed] [Google Scholar]
- 38.Howe G.A. Jasmonates as signals in the wound response. J. Plant Growth Regul. 2004;23:223–237. [Google Scholar]
- 39.Chassot C., Buchala A., Schoonbeek H., Metraux J.P., Lamotte O. Wounding of Arabidopsis leaves causes a powerful but transient protection against Botrytis infection. Plant J. 2008;55:555–567. doi: 10.1111/j.1365-313X.2008.03540.x. [DOI] [PubMed] [Google Scholar]
- 40.Walters D.R., Cowley T., Weber H. Rapid accumulation of trihydroxy oxylipins and resistance to the bean rust pathogen Uromyces fabae following wounding in Vicia faba. Ann. Bot. 2006;97:779–784. doi: 10.1093/aob/mcl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez L., Allona I., Aragoncillo C. Seed chitinases. Seed Sci. 2002;12:217–230. [Google Scholar]
- 42.Wu C.T., Leubner-Metzger G., Meins F. Class I beta- 1,3-glucanase and chitinase are expressed in the micropylar endosperm of tomato seeds prior to radicle emergence. Plant Physiol. 2001;126:1299–1313. doi: 10.1104/pp.126.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peumans W J., Proost P., Swennen R L. The abundant class III chitinase homolog in young developing banana fruits behaves as a transient vegetative storage protein and most probably serves as an important supply of amino acids for the synthesis of ripening-associated proteins. Plant Physiol. 2002;130:1063–1072. doi: 10.1104/pp.006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts J.A., Whitelaw C.A., Gonzalez-Carranza Z.H., McManus M.T. Cell separation processes in plants— model, mechanisms and manipulation. Ann. Bot. 2000;86:223–235. [Google Scholar]
- 45.Agusti J., Merelo P., Cercos M., Tadeo F.R., Talon M. Ethylene induced differential gene expression during abscission of citrus leaves. J. Exp. Bot. 2008;59:2717–2733. doi: 10.1093/jxb/ern138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.