Highlights
-
•
Haloduracin and chloramphenicol exhibited synergistic effect against pathogens.
-
•
The synergy indicates possibility of reducing the effective dose of chloramphenicol.
-
•
Reduction of chloramphenicol dose minimizes its negative side effects.
-
•
This shows a new window of using antibiotics and bacteriocins in combination therapy.
Keywords: Haloduracin, Chloramphenicol, Combination therapy, Drug resistance, Topical antibiotics
Abstract
The emergence of drug-resistant pathogens has triggered the search for more efficient antimicrobial agents and formulations for treatment of infections. In recent years, combination therapy has become one of the effective clinical practices in treating infections. The present study deals with the effect of haloduracin, a lantibiotic bateriocin and chloramphenicol against clinically important bacteria. The combined use of haloduracin and chloramphenicol resulted in remarkable synergy against a spectrum of microorganisms including strains of Staphylococcus aureus, Enterococcus faecium, Enterococcus faecalis and different groups of Streptococcus. The synergy allowed using these antimicrobial agents at substantially reduced concentrations without compromising their efficiency. Use of lower doses of chloramphenicol can avoid the severity of its side effects. In addition to minimizing undesirable side effects of some drugs, this approach brings the possibility of using antibiotics that are no longer effective due to drug resistance. Furthermore, the observed synergy between haloduracin and chloramphenicol opens a new window of using bacteriocins and antibiotics in combination therapy of infections.
1. Background
Widespread use of antibiotics and the related non-compliance, improper medication and over-prescription have triggered resistance among bacteria against antimicrobial agents and today antimicrobial resistance is a growing global concern. Drug resistance is expanding at an alarming rate, for example, from 1987 to 2004, the percentage of penicillin resistance among Streptococcus pneumoniae infections that causes meningitis and pneumonia has grown from 0.02% to 20%, a staggering 1000 fold increase [1]. It has also been reported that in the United States the percentage of Vancomycin-Resistant Enterococci (VRE) strains has increased from 0.3 to 7.9% between1989 and 1993. In 2004, about 30% of the infections in hospital intensive-care units were caused by VRE strains (https://www.niaid.nih.gov/research/vre-overview). In recent years, the emergence of multidrug-resistant pathogenic strains have been reported. For instance, about 440,000 new cases of multidrug-resistant tuberculosis (MDR-TB) appears annually which causes at least 150,000 deaths [2], and extensively drug-resistant tuberculosis (XDR-TB) has been reported in 64 countries [3]. The emergence and spread of drug resistance among pathogens not only resulted in an increasing number of human casualties but also incurs an enormous economic loss. In the USA alone, drug resistant bacterial infection has brought an annual loss of $34 billion [4].
The alarming frequency of drug resistance among pathogens requires development of new drugs. However, the discovery and development of new drugs is a long and expensive process. Moreover, pathogens develop resistance against new drugs in a relatively short time. This possibly shortens the expected commercial life span (of the drugs) and therefore it has become less attractive for the big pharmaceutical companies to invest in search for and development of new antibiotics. Therefore, efficient and effective use of already existing antibiotics is essential. In this regard, combination antibiotics have been proven successful to increase drug efficiency and reduce the emergence of drug resistance [5]. Few studies have been focused on investigating the combined effect of non-antibiotic antibacterial agents and antibiotics against different pathogens [6]. However, studies on combined effects of bacteriocins and antibiotics on infectious microorganisms are still lacking.
Bacteriocins are non-antibiotic antibacterial agents and have been used in various applications. They have got use in biopreservation, shelf-life extension, control of fermentation microflora, etc. [7]. In recent years, bacteriocins are considered as alternative antibiotics and some of them have even been clinically studied [8], [9], [10]. It is known that microbial resistance to bacteriocin is relatively low. However, there are reports on resistance such as to nisin. The appearance of resistance and the possible mechanisms to develop the resistance against nisin, one of the most studied and commercialized bacteriocins have been reviewed by Zhou et al. [11]. Considering that nisin has been used for over 40 years, the reported microbial resistance is not significant and this property makes bacteriocins very interesting to use it in combination therapy. Haloduracin is a two-component novel lantibiotic bacteriocin produced by an alkaliphilic Bacillus [12], [13]. This lantibiotic is efficient against different Gram-positive bacteria even at very low concentration. Moreover, unlike many other bacteriocins such as nisin and lactostrepcins, it is operationally stable at physiological conditions, which makes it an interesting candidate for combination therapy with other antimicrobial agents.
Chloramphenicol has certain desirable properties such as it is a broad spectrum antibiotic, it diffuses efficiently in the body, and does not ionize at physiological condition [14]. However, the emergence of resistance to this antibiotic and its dose related toxicity that causes aplastic anemia, leukemia, bone marrow suppression and gray baby syndrome have restricted its use [14], [15], [16], [17]. Thus, formulations that increase the efficiency of chloramphenicol with concomitant reduction of its dose that can possibly avoid the severe side effects are important for efficient use of this remarkable antibiotic.
The present study deals with evaluation of the potential synergistic effect of haloduracin and chloramphenicol against Gram-positive pathogens with the aim of reducing the effective dose of chloramphenicol.
2. Materials and methods
2.1. Organisms
The haloduracin producing organism, Bacillus halodurans was isolated in our laboratory [12]. While the test organisms used in this study were obtained from different sources. Staphylococcus aureus (CCUG 2354), Staphylococcus aureus (CCUG 15915), Enterococcus faecium (CCUG 36804), Enterococcus faecalis (CCUG 9997), Group A Streptococcus Type12 (CCUG 4207) and Group B Streptococcus (CCUG 4208) were obtained from Culture Collection of University of Göteborg. Strains of Streptococcus group G (ATCC 12394), Streptococcus group C (ATCC 12388) and Bacillus subtilis (ATCC 168) were purchased from American Type Culture Collection.
2.2. The antimicrobial compounds
Haloduracin was produced as described previously [12]. It was produced using solid-state fermentation and purified to homogeneity by hydrophobic interaction chromatography. The purified substance was analysed with mass spectrometry and MS/MS to confirm that it was haloduracin. Chloramphenicol was purchased from AB Bergman Labora AB (Danderyd, Sweden).
2.3. Antimicrobial activity
The antimicrobial activities of haloduracin, chloramphenicol, and a combination of haloduracin and chloramphenicol have been studied against different clinically important microorganisms. S. aureus (CCUG 2354), S. aureus (CCUG 15915), E. faecium (CCUG 36804), E. faecalis (CCUG 9997), Streptococcus group G (ATCC 12394), Streptococcus group C (ATCC 12388), Group A Streptococcus Type12 (CCUG 4207) and Group B Streptococcus (CCUG 4208) were cultivated using tryptic soy broth (TSB). The overnight culture of each microorganism was diluted with fresh medium to final optical density (OD 600 nm, in 1 cm quvett) of 0.1. About 100 μl of the diluted culture was poured to microplate wells. Varying concentrations of chloramphenicol or haloduracin was then added to each well. The final volume of the culture and the antimicrobial agent solutions in the wells was 0.2 ml. Turbidity was measured by reading the cultures absorbance at 620 nm using microtiter plate-reader. The results given are averages of triplicate assays.
From a preliminary study it has been observed that haloduracin and ampicillin has no synergy against B. subtilis. Thus, a combination of haloduracin (5.4 μg/ml) and ampicillin (20 μg/ml) was used as a reference. The growth of the Bacillus cells in the presence of haloduracin (5.4 μg/ml) and 4 μg/ml of chloramphenicol was studied and the results compared to the reference. In addition, different concentrations of chloramphenicol ranging from 0.5 to 25 μg/ml were studied to determine the minimum concentration that can be used synergistically with haloduracin (5.4 μg/ml). To study the effect of haloduracin and chloramphenicol combination against pathogens, the minimum inhibitory concentrations were selected, after investigating the effect of a range of concentrations on each organism tested (Table 1).
Table 1.
Concentrations of chloramphenicol and haloduracin selected for the synergistic effect study.
| Organism | Chloramphenicol (μg/ml) |
Haloduracin (μg/ml) |
|---|---|---|
| Staphylococcus aureus (CCUG 2354) | 5 | 5.4 |
| Staphylococcus aureus (CCUG 15915) | 4 | 5.4 |
| Enterococcus faecium (CCUG 36804) | 10 | 4.3 |
| Enterococcus faecalis (CCUG 9997) | 4 | 6.5 |
| Group G Streptococcus (ATCC 12394) | 2 | 2.2 |
| Group C Streptococcus (ATCC 12388) | 1.5 | 2.5 |
| Group A Streoptococcus Type12 (CCUG 4207) | 1.5 | 2.2 |
| Group B Streptococcus (CCUG 4208) | 3 | 2.2 |
3. Results and discussion
A wide variety of clinically important drug resistant pathogens are members of the Gram-positive bacteria and among these pathogens, S. aureus, S. pneumonia and Enterococcus are known to be global health threats causing significant public health concern. Thus, there has been a need for efficient treatment of infections caused by such bacteria. This study focuses on evaluating the use of antibiotic and bacteriocin in combination against strains of S. aureus, Streptococcus and Enterococcus.
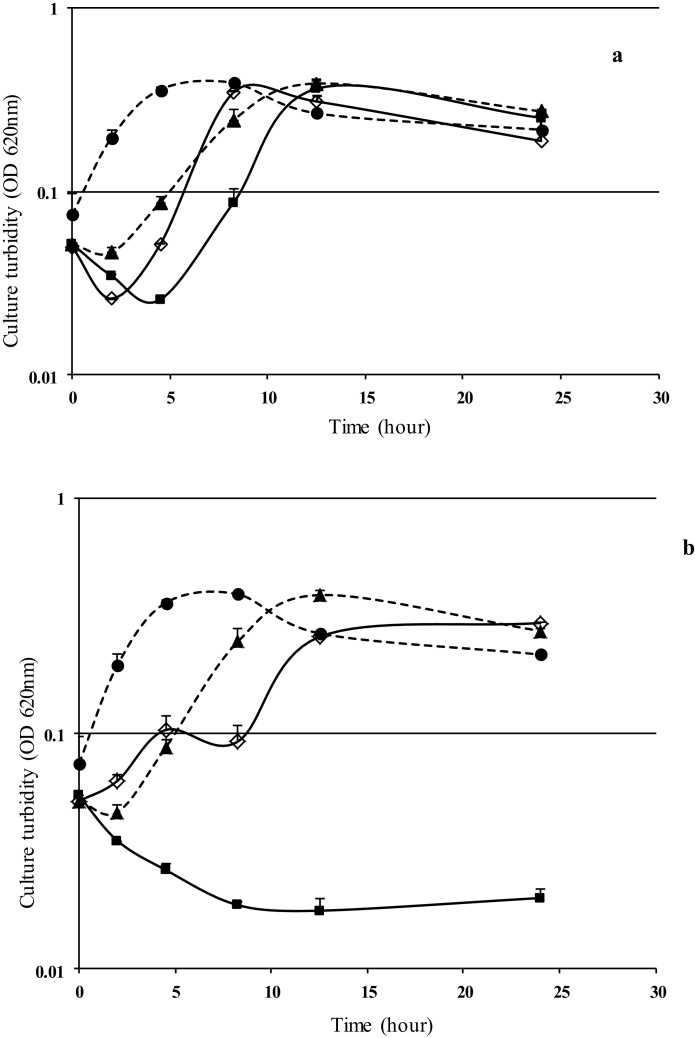
From a preliminary screening study, we have seen that ampicillin and haloduracin do not exert any synergistic effect neither against Gram-positive nor Gram-negative bacteria (data not shown). Fig. 1 shows the difference in the combined effect of ampicillin and haloduracin (which did not exhibit synergy) and that of chloramphenicol and haloduracin. As shown in Fig. 1a, after 8 h of cultivation the cultures grew to the same level of turbidity both in the absence or presence of the antimicrobial agents added separately or together. However, the cells did not grow even after 24 h of cultivation when haloduracin and chloramphenicol were used in combination (Fig. 1b), whereas the growth patterns in presence of haloduracin or ampicillin were similar to that observed for the ampicillin and/or haloduracin containing cultures. This shows the synergistic effect of haloduracin and chloramphenicol against B. subtilis cells.
Fig. 1.
The effect of haloduracin and antibiotics against B. subtilis. (a) The combined use of lantibiotic (5.4 μg/ml) and Ampicillin (20 μg/ml) which did not show any synergistic effect. (b) The combined use of lantibiotic (5.4 μg/ml) and chloramphenicol (4 μg/ml) which acted synergistically. Culture of B. subtilis (ATCC168) in only tryptic soy broth (●, dashed line), and in presence of haloduracin (▲, dashed line), in presence of ampicillin (◊) or chloramphenicol (◊) and in presence of both haloduracin and antibiotic (■).
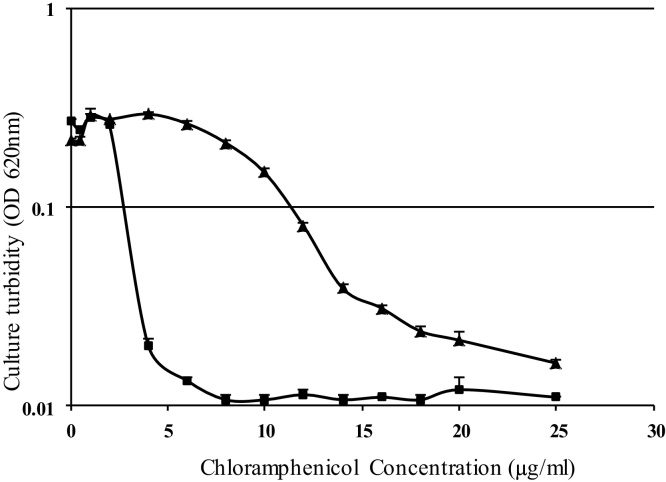
The results in Fig. 2 show the effect of varying concentrations of chloramphenicol alone or in the presence of 5.4 μg/ml haloduracin against B. subtilis. As shown in Fig. 1, 5.4 μg/ml haloduracin (alone) did not have any effect on the bacterial growth. There was no significant growth inhibition when chloramphenicol alone was used up to 10 μg/ml. However, the bacterial growth was significantly reduced when 4 μg/ml of chloramphenicol was used together with haloduracin and a comparable level of growth inhibition was achieved when 20 μg/ml chloramphenicol (alone) was used. This shows the possibility of using significantly reduced amounts of chloramphenicol with haloduracin to effectively prevent infections by Gram-positive pathogens. Treatments of infection caused by Gram-positive pathogens are becoming very challenging [18]. Among this group of organisms, strains of Staphylococcus, Streptococcus and Enterococcus are on the top of the list [19]. In this study, two strains of S. aureus, two species of Enterococcus and four strains of Streptococcus belonging to group A, B, C and D were included. Since the sensitivity of these strains to antimicrobial agents is different, it was necessary to determine the concentrations of haloduracin and chloramphenicol that are close to the minimum inhibitory concentrations for each organism tested. The effect of different concentrations of the antimicrobial agents was studied and the concentrations chosen for further studies are shown in Table 1.
Fig. 2.
The effect of haloduracin and different concentrations of chloramphenicol against B. subtilis (ATCC168). Culture turbidity of B. subtilis after 24 h of cultivation in presence of different concentration of chloramphenicol (▲) or in combined presence of haloduracin (5.4 μg/ml) and different concentration of chloramphenicol (■).
As summarized in Table 2, although the effect varies from strain to strain, there were synergistic effects with haloduracin-chloramphenicol combinations against all organisms tested. The synergy observed against E. faecium (CCUG 36804) was followed by that of Streptococcus group G (ATCC 12394) and Streptococcus (CCUG 4208) Group A strains. Relatively low synergistic effects were detected for S. aureus (CCUG 2354) and E. faecalis (CCUG 9997). In most cases the synergistic effect varied with the cultivation time. The efficacy decreased as the cultivation time was extended to 24 h, especially for S. aureus (CCUG 2354) and E. faecalis (CCUG 9997). However, the turbidity of the E. faecium was decreasing with increasing cultivation time.
Table 2.
The antimicrobial effects of haloduracin, chloramphenicol, and haloduracin-chloramphenicol combinations against clinically important bacteria. The concentrations of the antimicrobial agents used in this study are indicated in Table 1. The values in the table are the percentage figures generated from the ratio of the culture optical density containing the antimicrobial agent to the culture optical density of the negative control. Value = 100 × (Absof the sample/Absof the Negative control).
| Cultivation time (h) | Haloduracin |
Chloramphenicol |
Haloduracin plus chloramphenicol |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Microorganism | 9 | 12 | 24 | 9 | 12 | 24 | 9 | 12 | 24 |
|
Streptococcus (group A) Type 12 (CCUG 4207) |
83 ± 13 | 95 ± 20 | 126 ± 7 | 79 ± 4 | 74 ± 3 | 76 ± 7 | 29 ± 12 | 32 ± 18 | 36 ± 22 |
|
Streptococcus (group B) (CCUG 4208) |
97 ± 4 | 117 ± 1 | 107 ± 1 | 76 ± 3 | 90 ± 5 | 87 ± 3 | 32 ± 1 | 27 ± 2 | 30 ± 0.5 |
|
Streptococcus (group C) (ATCC 12388) |
111 ± 5 | 116.5 ± 3 | 121 ± 2 | 63 ± 3 | 63 ± 2 | 64 ± 4 | 19 ± 1 | 31 ± 5 | 46 ± 1 |
|
Streptococcus (group G) (ATCC 12394) |
78 ± 11 | 104 ± 5 | 107 ± 4 | 39 ± 2 | 51 ± 2 | 59 ± 2 | 12 ± 1 | 14 ± 3 | 26 ± 12 |
|
Enterococcus faecium (CCUG 36804) |
73 ± 10 | 82 ± 7 | 80 ± 6 | 89 ± 1 | 80 ± 2 | 79 ± 2 | 12 ± 2 | 6 ± 0.5 | 5 ± 1 |
|
Enterococcus faecalis (CCUG 9997) |
97 ± 7 | 102 ± 4 | 135 ± 3 | 76 ± 6 | 81 ± 4 | 101 ± 5 | 20 ± 3 | 27 ± 4 | 71 ± 11 |
|
Staphylococcus aureus (CCUG 15915) |
68 ± 1 | 84 ± 2 | 85 ± 2 | 68 ± 2 | 80 ± 0.1 | 79 ± 4 | 44 ± 2 | 49 ± 1 | 52 ± 4 |
|
Staphylococcus aureus (CCUG 2354) |
80 ± 6 | 87 ± 1 | 87 ± 6 | 71 ± 1 | 80 ± 3 | 80 ± 2 | 32 ± 3 | 37 ± 5 | 73 ± 9 |
One way of fighting the antimicrobial-resistance has been the use of antimicrobial agents in combination. In this study, the combined use of the relatively new active compound, haloduracin and the existing (phasing out) antibiotic, chloramphenicol has shown remarkable synergy against virulent strains of Streptococcus, Staphylococcus and Enterococcus. The observed synergistic effect implies that very low concentration of chloramphenicol can be used with haloduracin in topical applications.
The frequency of infections caused by S. aureus has reached an epidemic level and is expanding rapidly [20]. Meticillin-resistant S. aureus strains, which cause most of the skin and soft-tissue infections, are the major contributors for the epidemic [21]. On the other hand, enterococci are members of the commensal microflora in humans which often do not affect healthy individuals. However, enterococci are known for their opportunistic infections in immune compromised patients. Although most of these infections are caused by E. faecalis; E. faecium infection is a serious concern due to its multidrug resistance [22]. Pathogenic Streptococcus strains are known to cause different diseases. For example Streptococcus pyogenes (group A) is responsible for acute pharyngo-tonsillitis and the frequency of drug resistance among strains of these pathogens is increasing [23]. Streptococcus faecalis (group D) strains cause urinary tract and root canal infections. Streptococcus agalactiae (group B) is the common cause of meningitis and pneumonia in neonatal and elderly people. The major killer among the Streptococcus strains is the drug resistant S. pneumoniae which causes pneumonia [24]. The activity of haloduracin against these strains of microorganisms is interesting as it can potentially be used alone or in combination with any other antimicrobial agent to treat topical infections caused by Gram-positive pathogens.
Chloramphenicol is one of the most widely used topical antibiotics applied commonly to suture lines and skin grafts and other wounds. However, topical applications of chloramphenicol have been associated with different health problems [25], [26], [27], [28]. Thus, the synergistic effect of haloduracin and chloramphenicol can allow the use of haloduracin and low dose of chloramphenicol in topical applications. As most drug side effects are dependent on the dose, reduction of the cholramphenicol dose can help to minimize and limit its side effects.
As drug resistance among pathogens is becoming more prevalent against conventional antibiotic treatments, the use of non-conventional antimicrobial agents is being considered as alternatives. In this line, bacteriocins have shown great potential in the fight against antibiotic resistant pathogens [29], hence the observed haloduracin and chloramphenicol synergy is attractive for the treatment of infections caused by Gram-positive pathogens. Further studies on formulation and application of these two antimicrobial agents is expected to shed some light on treatment of drug-resistant pathogens and possibly open a new area of combined topical treatment using antibiotics and other antimicrobial peptides including bacteriocins.
Haloduracin has two components, Halα and Halβ, which work synergistically against Gram-positive bacteria [30]. Halα binds to lipid II and impairs the bacterial cell wall synthesis. Moreover, it is proposed that the binding of the Halα to lipid II facilitates the binding of Halβ and form a membrane pore forming trimeric complex that kills the bacteria. On the other hand, it is known that chloramphenicol irreversibly binds to bacterial ribosome thereby inhibiting protein synthesis. Although the exact mechanism of synergy between haloduracin and chloramphenicol is yet to be explained, it is tempting to propose that it may be partly due to the pore formation that leads to loss of amino acids that further slow down protein synthesis and a faster influx of chloramphenicol through the pore formed by the trimeric complex.
4. Conclusion
The combined use of chloramphenicol and haloduracin has shown remarkable synergistic effects against clinically important bacteria. The reduced concentration of chloramphenicol is expected to avoid the severity of administrating this antibiotic. These findings may open a new window of research on combined use of different antibiotics and bacteriocins against clinically important pathogens including drug resistant strains.
Competing interests
All the authors do not have any financial or non-financial competing interests.
Authors’ contributions
BM and GM designed the study. AD and ÅL conducted the experiment. GM and AD analyzed the data. AD, ÅL, BM, and GM wrote the manuscript, read and approved the submission.
Acknowledgement
The Ministry of Health and Medical Education of Iran is gratefully acknowledged for the financial support of A. Danesh.
References
- 1.Laxminarayan R., Malani A. Earthscan; 2007. Extending the Cure: Policy Responses to the Growing Threat of Antibiotic Resistance.http://www.cddep.org/publications/extending_cure_policy_responses_growing_threat_antibiotic_resistance [Google Scholar]
- 2.WHO . WHO; 2010. Multidrug and Extensively Drug-resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. [Google Scholar]
- 3.WHO . WHO; 2011. Antimicrobial Resistance.http://www.who.int/mediacentre/factsheets/fs194/en/ [Google Scholar]
- 4.Gerberding J. National Academic Press; Washington, D.C: 2003. The Resistance Phenomenon in Microbes and Infectious Disease Vectors: Implications for Human Health and Strategies for Containment; pp. 210–215. [PubMed] [Google Scholar]
- 5.Ejim L., Farha M.A., Falconer S.B., Wildenhain J., Coombes B.K., Tyers M., Brown E.D., Wright G.D. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat. Chem. Biol. 2011;7:348–350. doi: 10.1038/nchembio.559. [DOI] [PubMed] [Google Scholar]
- 6.Kristiansen J.E., Hendricks O., Delvin T., Butterworth T.S., Aagaard L., Christensen J.B., Flores V.C., Keyzer H. Reversal of resistance in microorganisms by help of non-antibiotics. J. Antimicrob. Chemother. 2007;59:1271–1279. doi: 10.1093/jac/dkm071. [DOI] [PubMed] [Google Scholar]
- 7.Balciunas E.M., Martinez F.A.C., Todorov S.D., Franco B.D.G., Converti A., Oliveira R.P.S. Novel biotechnological applications of bacteriocins: a review. Food Control. 2013;32:134–142. [Google Scholar]
- 8.Cotter P.D., Ross R.P., Hill C. Bacteriocins—a viable alternative to antibiotics? Nat. Rev. Microbiol. 2013;11:95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- 9.van Staden A.D., Brand A.M., Dicks L.M. Nisin F-loaded brushite bone cement prevented the growth of Staphylococcus aureus in vivo. J. Appl. Microbiol. 2012;112:831–840. doi: 10.1111/j.1365-2672.2012.05241.x. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein B.P., Wei J., Greenberg K., Novick R. Activity of nisin against Streptococcus pneumoniae, in vitro, and in a mouse infection model. J. Antimicrob. Chemother. 1998;42:277–278. [PubMed] [Google Scholar]
- 11.Zhou H., Fang J., Tian Y., Lu X.Y. Mechanisms of nisin resistance in Gram-positive bacteria. Ann. Microbiol. 2014;64:413–420. [Google Scholar]
- 12.Danesh A., Mamo G., Mattiasson B. Production of haloduracin by Bacillus halodurans using solid-state fermentation. Biotechnol. Lett. 2011;33:1339–1344. doi: 10.1007/s10529-011-0581-0. [DOI] [PubMed] [Google Scholar]
- 13.McClerren A.L., Cooper L.E., Quan C., Thomas P.M., Kelleher N.L., van der Donk W.A. Discovery and in vitro biosynthesis of haloduracin, a two-component lantibiotic. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17243–17248. doi: 10.1073/pnas.0606088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz S., Kehrenberg C., Doublet B., Cloeckaert A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004;28:519–542. doi: 10.1016/j.femsre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Wong S.H., Silva F., Acheson J.F., Plant G.T. An old friend revisited: chloramphenicol optic neuropathy. J. R. Soc. Med. 2013;4:20. doi: 10.1177/2042533313476692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz J., Capitano L., Nuneza L., Castro D., Sierra J.M., Hatha M., Borrego J.J., Vila J. Mechanisms of resistance to ampicillin, chloramphenicol and quinolones in multiresistant Salmonella typhimurium strains isolated from fish. J. Antimicrob. Chemother. 1999;43:699–702. doi: 10.1093/jac/43.5.699. [DOI] [PubMed] [Google Scholar]
- 17.Feder H.M., Osier C., Maderazo E.G. Chloramphenicol: a review of its use in clinical practice. review of infectious disease. Rev. Infect. Dis. 1981;3:479–491. doi: 10.1093/clinids/3.3.479. [DOI] [PubMed] [Google Scholar]
- 18.Woodford N., Livermore D.M. Infections caused by Gram-positive bacteria: a review of the global challenge. J. Infect. 2009;59:S4–S16. doi: 10.1016/S0163-4453(09)60003-7. [DOI] [PubMed] [Google Scholar]
- 19.Chambers H.F., DeLeo F.R. Waves of Resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundmann H., Aires-de-Sousa M., Boyce J., Tiemersma E. Emergence and resurgence of meticillinresistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 21.Moran G.J., Krishnadasan A., Gorwitz R.J., Fosheim G.E., McDougal L.K., Carey R.B., Talan D.A. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 22.Murray B.E. The life and times of the enterococcus. Clin. Microbiol. Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Passàli D., Lauriello M., Passàli G.C., Passàli F.M., Bellussi L. Group A Streptococcus and its antibiotic resistance. Acta. Otorhinolaryngol. Ital. 2007;27:27–32. [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds C.A., Finkelstein J.A., Ray G.T., Moore M.R., Huang S.S. Attributable healthcare utilization and cost of pneumoniae due to drug-resistant Streptococcus pneumoniae: a cost analysis. Antimicrob. Resist. Infect. Control. 2014;3:16. doi: 10.1186/2047-2994-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livingston R.J., Butterworth J.W., Belt P. Reaction or infection: topical chloramphenicol treatment. Ann. R. Coll. Surg. Engl. 2013;95:e20–e21. doi: 10.1308/003588413X13511609955418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doshi B., Sarkar S. Topical administration of chloramphenicol can induce acute hepatitis. BMJ. 2009;338:b1699. doi: 10.1136/bmj.b1699. [DOI] [PubMed] [Google Scholar]
- 27.Jankicevic J., Vesic S., Vukicevic J., Gajić M., Adamic M., Pavlović M.D. Contact sensitivity in patients with venous leg ulcers in Serbia: comparison with contact dermatitis patients and relationship to ulcer duration. Contact Dermat. 2008;58:32–36. doi: 10.1111/j.1600-0536.2007.01253.x. [DOI] [PubMed] [Google Scholar]
- 28.Moyano J.C., Alvarez M., Fonseca J.L., Francisco J.B., Bellido J.M. Allergic contact dermatitis to chloramphenicol. Allergy. 1996;51:67–69. [PubMed] [Google Scholar]
- 29.Hassan M., Kjos M., Nes I.F., Diep D.B., Lotfipour F. Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 2012;113:723–736. doi: 10.1111/j.1365-2672.2012.05338.x. [DOI] [PubMed] [Google Scholar]
- 30.Oman T.J., van der Donk W.A. Insights into the mode of action of the two-peptide lantibiotic haloduracin. ACS Chem. Biol. 2009;40:865–874. doi: 10.1021/cb900194x. [DOI] [PMC free article] [PubMed] [Google Scholar]