Highlights
-
•
Catastrophe of heavy metal pollution in environment is discussed in terms of remediation through bacterial Exopolysaccharide.
-
•
Biosynthesis of polymer, mechanism of action and metal remediation through polymer has been expounded deeply.
-
•
Wide range of bacterial cells and their EPS in diverse forms have been critically analyzed.
-
•
Challenges that still lie in the path of commercialization has been scrutinized.
Keywords: Exopolysaccharide (EPS), Heavy metals, Biosorption, Bioremediation
Abstract
Heavy metal contamination has been recognized as a major public health risk, particularly in developing countries and their toxicological manifestations are well known. Conventional remediation strategies are either expensive or they generate toxic by-products, which adversely affect the environment. Therefore, necessity for an environmentally safe strategy motivates interest towards biological techniques. One of such most profoundly driven approach in recent times is biosorption through microbial biomass and their products. Extracellular polymeric substances are such complex blend of high molecular weight microbial (prokaryotic and eukaryotic) biopolymers. They are mainly composed of proteins, polysaccharides, uronic acids, humic substances, lipids etc. One of its essential constituent is the exopolysaccharide (EPS) released out of self defense against harsh conditions of starvation, pH and temperature, hence it displays exemplary physiological, rheological and physio-chemical properties. Its net anionic makeup allows the biopolymer to effectively sequester positively charged heavy metal ions. The polysaccharide has been expounded deeply in this article with reference to its biosynthesis and emphasizes heavy metal sorption abilities of polymer in terms of mechanism of action and remediation. It reports current investigation and strategic advancements in dealing bacterial cells and their EPS in diverse forms – mixed culture EPS, single cell EPS, live, dead or immobilized EPS. A significant scrutiny is also involved highlighting the existing challenges that still lie in the path of commercialization. The article enlightens the potential of EPS to bring about bio-detoxification of heavy metal contaminated terrestrial and aquatic systems in highly sustainable, economic and eco-friendly manner.
1. Introduction
Heavy metals (Pb2+, Cr2+/Cr3+, Cd2+, Ni2+/Ni4+, Zn2+, Cu2+, Hg2+ etc.) are natural elements with relatively high density compared to water [94]. In trace amounts some of the heavy metals such as Cu2+, Zn2+, Fe2+/Fe3+ etc. are essential for numerous vital biological processes in human physiology. However, toxicological manifestations arise at even slightly higher concentrations. Owing to their large scale industrial applications (pharmaceuticals, pesticides, plastics, rubbers, tanneries, organic chemicals and wood products) [46], occurrence of free forms of heavy metals in terrestrial as well as in aquatic system is on rise. Moreover, the non-biodegradable nature of heavy metals ensures its prolonged presence in the environment. Their high aquatic solubility triggers bioaccumulation and biomagnifications which eventually leads to insidious and irreversible health hazard (including potential carcinogenicity) even in very minimal concentration range of about 1 mg/L. Further they result in adverse environmental impacts because of their recalcitrant and non-biodegradable nature. Besides, certain environmental conditions favor their transformation from lower to higher toxic state like in case of mercury [99]. While talking about accessibility, precious metal ions in utilizable form are depleting day by day so their regeneration and extraction from all the potential sources is highly desired.
1.1. Toxicological manifestations of some heavy metals
Different heavy metal ions have different mechanisms of toxic infestations. Oxidative stress out of free radical imbalance (lead), harmful thiol or methyl derivative formation (mercury, arsenic, chromium), cofactor or metal ion replacement (aluminium, cadmium), cell membrane permeation, disturbance in ion channels, DNA and protein damage (chromium VI), binding to vital proteins and causing further discrepancies (cadmium), corrosive effects, saturation, organ penetration and lipid peroxidation (iron) are some of the predominant ones [35]. Recommended arsenic maximum concentration level by WHO in drinking water is 10 μg/L [102]. Beyond this the inorganic arsenic ion accumulation in living tissues instigates bronchitis, cancer, dermatitis, hypo 1 kerotosis, livercirrohosis, mental disturbance, ulcer etc in individuals [3], [77]. Cadmium ions can cause chronic toxicity leading to proteinurea and lung emphysema upon air borne exposure. In higher dosage it causes acute toxicity with headaches, nausea, diarrhoea and osteomalasia. Chromium ion toxicity is recognized in its hexavalent form, its incursion in body at occupational level can cause skin ulcers, while inhalation can lead to dermatitis. Chronic exposure causes asthma and can possibly result in cancer too. Cobalt is one of the essential elements required for functioning of vitamin B12 whose recommended dietary intake itself is 6 μg. Aerial intake of cobalt ions at concentration beyond 0.005 mg/m3 may lead to chronic lung ailments like asthama, pneumonia etc. Whereas dietary intake beyond 0.14–0.15 mg/kg of body weight results in heart complications and exposure to gamma rays can be lethal and carcinogenic. Overexposure to copper ions (beyond systemic excretion limit) results in hazards of immune system, kidney and liver distress, gastrointestinal ailments, anemia and even indirect onset of oxidative stress. Effects of lead toxicity are varied and widespread from hematopoietic, hepatic and renal system to even nervous system. Exposure at about 400–600 μg/L can also lead to chronic toxicity which is characterized by persistent vomiting, encephalopathy, lethargy, delirium, convulsions and coma if not attended timely [22].
So far, the mainstay treatment regimes for remediation of heavy metals ions include methods like coagulation, chemical precipitation, electrodialysis, evaporative recovery, floatation, flocculation, ion exchange, nanofilteration, reverse osmosis, ultrafiltration etc. [46], [98]. Although effective, these methods are usually expensive due to high energy and reagent requirements. Moreover, they generate large amount of toxic sludges and byproducts, which pollutes the environment. Many a times they may result in incomplete and unpredictable (quantitative removal cannot be accurately estimated, depends on several factors dealt later in the article) metal ion removal [5], [27]. Hence, there is an imperative need to devise effective, efficient, economic and environmentally safe strategies which can minimize the heavy metal ion concentration from toxic to safe limits in environment.
Sorption with specific reference to metal ions can be defined as any phenomenon that involves their association (ranging from electrostatic to covalent) with peripherally available one or more functional groups on sorbent material. When the sorbent involved in such reaction is a biological agent, the phenomenon is defined as biosorption. Prokaryotic as well as eukaryotic microbial biomasses (living or dead cells), like bacteria, fungi, yeast and few microalgae are such emerging candidates of biosorption which can uptake and reduce heavy metal ion concentration from contaminated water sources in eco-friendly manner [3], [15], [78].
Talking specifically of bacterial cells, heavy metal ions in both particulate as well as in soluble form can potentially be accumulated by intact bacterial cells (live or dead) and their byproducts. Extracellular polymeric substances are such complex blend of high molecular weight microbial biopolymeric secretory byproducts. Basically they contain proteins, polysaccharides, uronic acids, humic substances, lipids etc. Their most essential constituent, having ion sequestration capability, is extracellular polysaccharides or exopolysaccharide (EPS). Primarily it is composed of complex high molecular weight organic macromolecules like polysaccharide along with smaller proportions of protein and uronic acid [12], [48].
These biosynthetic polymers can exist either as attached capsular polysaccharide (CPS) or as slime upon microbial surfaces [105].They are produced out of self defense against environmental stress which not only protect cells against dewatering or toxic substances but serves as a carbon and energy source as well [88]. Compositionally they are often seen to be polymerized of hexose sugar moieties and exist as both homo or heteropolysaccharide (Table 1) Czaczyk and Myszka, 2007.
Table 1.
Homo and hetero-exopolysaccharides.
| EPS Class | Secreted product | Monomers | Linkage | Example Microorganisms | Reference |
|---|---|---|---|---|---|
| Homopolysaccharide | Dextran (α-D Glucans) |
Glucose | α-1,6 |
Leuconostoc mesenteroids, Streptococcus and Lactobacillus |
[25], [44], [52], [57], [59], [83], [86] |
| Mutan (α-D Glucans) |
Glucose | α-1,3 | |||
| Alternan (α-D Glucans) |
Glucose | α-1,3 and α-1,6 | |||
| Reuteran (α-D Glucans) |
Glucose | α-1,4 | |||
| Curdlan (β-d-glucans) |
Glucose | β -1,3 and β-1,2 |
Agrobacterium Biovar, Cellulomonas sp., Rhizobium, sp., Bradirhizobium japonicum, Streptococcus pneumoni Pediococcus sp. |
[14], [57] | |
| Levan(fructans) | Fructose | β -2,6 osidic bonds; |
B. subtilis,Zymomonas mobilis, Streptococcus salivarius and Streptococcus mutans |
[59],[14] | |
| Inulin-type (fructans) | Fructose | β -2,1 osidic bonds | S. mutans, L. reuteri | ||
| half row | |||||
| Heteropolysaccharide | Alginate | β −d-mannurosyl and α-l-guluronosyl units |
1–4 bonds |
Pseudomonas aeruginosa,Azotobacter vinelandii |
[14] |
| Xanthan | Glucose backbone, linked with trisaccharide side chain of glucuronic acid, mannose, pyruvil and acetyl residues |
2–5 and 2–4 bonds | Xanthomonas campestris |
[19] [25] |
|
| LAB(Lactobacillus)-EPS | Glucose,galactose, rhamnose, fructose | 1–2; 1–3; 1–4; 1–6 bonds |
Lactobacillus spp. | [19], [64]) | |
| Hyaluronan | Glucuronic acid and N-acetylglucosamine | Alternate β -1.4 and β -1,3 bonds |
Pseudomonas aeruginosa and Streptococci attenuated strains |
[25] | |
| Sphingans (Gellan, welan, rhamsan and diutan are) |
Rhamnose, glucose, glucoronic acid | 1–3 and 1–4 bonds | Sphingomonas spp. | [11] | |
These water-soluble glycopolymeric biomolecules display exemplary physiological, rheological and physio-chemical properties, that make them suitable for several clinical, industrial and environmental applications [12]. One such prospective application emerges from their ability of exhibiting physical defense [43]. Bacterial cell finds a way to protect itself from the infiltration of toxic metal ions by covering its peripheral surface with a shield of EPS. Structural and compositional makeup of EPS favors the sequestration of metal ions and hence obstructs them from penetrating the cell surface. Because of this property, in past few years the polymer has been comprehensively investigated as a treatment regime for reduction of heavy metal contamination.
The attempt of this article is to focus upon the defensive action of EPS that prevents microbial cell from toxic heavy metal ions. The article therefore discusses biosynthesis of the polymer, which determines its chemical composition that in turn facilitates its metal ion adsorption property. It then highlights microbial metal ion interaction mechanisms with special reference to EPS metal ion interaction. Finally, the article explores distinct strategies of metal remediation through microbial EPS. In order to understand the mechanism behind metal ion uptake through EPS it’s essential to get the picture of how the bacterial cells biosynthesize these incredible polymeric substances
2. Bacterial eps and its biosynthesis
Ever since the adsorption ability of the bacterial EPS has been recognized, numerous studies have reported about an array of bacterial species and range of diverse EPS possessing desired (net negative charge) feature. Apart from basic polysaccharide backbone, EPS can undergo multiple structural variations by altering the polymeric length or through combinatorial arrangement of various side chains, functional groups, non-carbohydrate substituent and diverse bondings and linkages [105]. The variations in polysaccharide makeup hugely rely upon the type and amount of carbon source available, abiotic stress factors like temperature and pH and the growth phase of bacterium during which synthesis occurs [14], [88]. The strategies for achieving heavy metal remediation through bacterial EPS must be focused on utilizing the non neutral, negatively charged EPS (EPS packed with abundant anionic functional groups) to be incorporated as a suitable biosorbent. Some of the reported commercial bacterial EPS with the required anionicity are alginate (Pseudomonas aeruginosa, Azotobacter vinelandii,), gellan (Sphingomonas paucimobilis), hyaluronan (Pseudomonas aeruginosa, Pasteurella multocida, Streptococci attenuated strains), xanthan (Xanthomonas campestris), galactopol (Pseudomonas oleovorans), fucopol (Enterobacter A47) [24], [25], [26], [69].
Although rigorous efforts are required to unravel the detailed science behind EPS biosynthesis, what reported literature suggests, has been reviewed here. The synthesis procedure can be categorized depending upon the mechanism and the site of synthesis either as intracellular or extracellular [81].
2.1. Extracellular and intracellular synthesis
Homopolysaccharides are generally synthesized extracellularly when the responsible enzymes involved in process transfer the activated precursor monosaccharides from substrate to growing polysaccharide. The polysaccharides then assemble in a particular fashion, linkage pattern and degree of branching. Dextran, levan and mutan are the common examples that require dextran sucrase and levan sucrase as enzymes and sucrose as a substrate respectively [7], [105]. Some of the gram positive cocci have been reported to produce extracellular EPS [105].
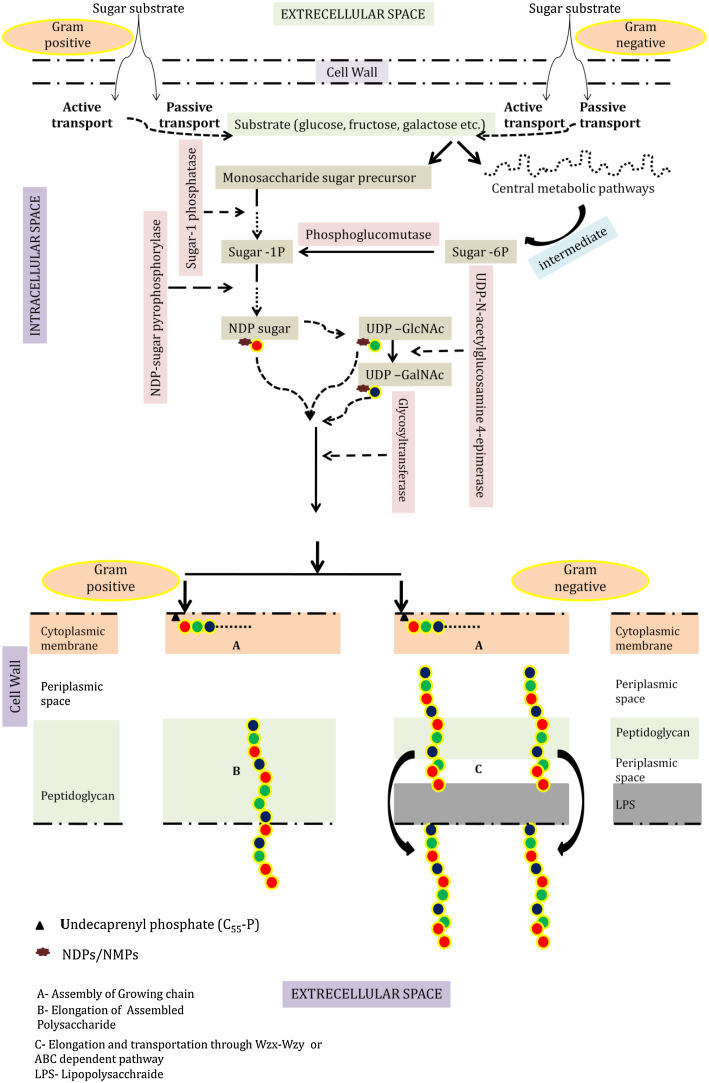
Intracellular synthesis of polysaccharides (homo or hetero) is a comparitively complex process and proceeds via intracellular assembly of sugar nucleotide precursors after which it is transported outside the cell. Different enzymes and regulatory molecules involved in several metabolic pathways participate in EPS biosynthesis. It begins with the entry of the substrate (any form of sugar) in bacterium, actively or passively, which is catabolized by periplasmic oxidation or intracellular phosphorylation [25]. Some of the precursors which do not take part in the central metabolic pathways, act as a raw material for EPS manufacture. Synthesis of biomolecule cannot proceed through simple sugar molecules instead the intracellular machinery requires charged and energy rich precursor monosaccharides in the form of nucleotide diphosphate/monophosphate sugar (NDP/NMP-sugar).This crucial step is governed by an independent pathway, where phosphorylated sugar, often in the form of sugar-1P and rarely in form of sugar-2P or sugar- 6P, serves as an activated primary residue [51], [105]. Metabolic pathway intermediates like Fructose-6P or Glucose-1P, in majority of cases leads to synthesis of UDP-GlcNAc (Uridine di-phosphate-N acetyl glucosamine), UDP-GalNAc (Uridine di-phosphate-N acetyl galactosamine) and dTDP-rhamnose precursor molecules for EPS synthesis [7]. Phosphoglucomutase enzyme catalyses the conversion of sugar-6P to sugar-1P (the central glycosyl donar for NDP/NMP sugar synthesis) and eventually UDP-glucosepyrophosphorylase and dTDP-glucosepyrophosphorylase catalyse conversion of this sugar-1P to UDP-glucose and dTDP-glucose [51]. UDP-N-acetylglucosamine 4-epimerase has been found to catalyse the conversion of UDP-GlcNAc to UDP-GalNAc in Lactobacillus rhamnosus [7]. An investigation also reported four genes responsible for dTDP-l-rhamnose NDP-sugar molecule synthesis in Lactobacillus rhamnosus strains. Thereafter with the help of enzyme glycosyltransferase, the sugar molecules from activated NDP/NMP-sugar moieties gets transferred to undecaprenyl phosphate (C55-P), an isoprenoid lipid carrier located at cytoplasmic membrane [93]. Studies have revealed that assembly of heptasaccharide units consisting of rhamnose moieties in L. rhamnosus involves six different glycosyltransferase genes [7]. The existence of lipid carrier molecule probably ensures the structured assembly of monomeric precursors and regulation of extracellular transport of growing or polymerized chain [105]. Although lipid intermediate pathway has also been sometimes utilized by gram positive bacterium for EPS assembly but it plays an important role in gram negative bacterial EPS assembly and transport, because of the obvious restriction posed by distinct cell wall structure of gram negative bacteria. Upon assembly of repeating moieties at the lipid carrier, the backbone chain gets simply translocated to the cell surface in the gram positive bacterium where its elongation will proceed. However, gram negative bacteria are generally believed to follow either Wzx-Wzy dependent or ABC transporter dependent pathway [25] with some exceptions (Ex- Synthase-dependent pathway followed by Pseudomonas aeruginosa).
In Wzx-Wzy pathway the lipid linked intermediate assembles in cytoplasmic space, after which both the assembly and elongation of growing polysaccharide takes place within priplasm and the completely synthesized polymer is then transported to extracellular surface. E coli-k a gram negative bacterium has been observed to assemble and transport its CPS through this mechanism [105]. Enzymes are supposed to be participating in overall process of polymerization and export, chain length control and detachment from lipid as well. For example flippase for transportation of lipid linked units while polymerase for precursor coupling [51]. Supplementary non-carbohydrate substituent (pyruvate, acetate, hydroxybutyrate, sulphate, succinate etc.) are incorporated at this stage in the growing chain at lipid carrier interface where acetyl CoA, phosphoenol pyruvate etc. act as donor molecules [13]. Fig. 1 schematically displays the entire biosynthesis mechanism of bacterial EPS. However several studies present contrasting evidences where EPS biosynthesis was observed to occur solely by NDP-sugar monomers without involvement of lipid intermediates, in the inner cytoplasmic region. Cellulose production by Acetobacter xylinum is one such example [4]. Post-synthetic modification has also been found in some EPS biosynthesis where polysaccharides grow as homopolysaccharides but enzymatic modification takes place after its translocation to cell surface. Synthesis of Alginate by A. vinelandii where mannuronan-5′epimerase converts mannuronic acid residue from polymannuronic acid molecule at cell surface to glucuronic acid, depicts one such example [105]. Finally the completely assembled and exported EPS (through one of the several explained mechanism) either attaches to cell surface as capsular polysaccharide or is secreted as slime.
Fig 1.
Biosynthesis of EPS in gram negative and gram positive bacteria, beginning with substrate diffusion, multiple array of enzymatic actions and substrate conversion within intracellular environment, eventual assembly of polysaccharide and export to external surface.
3. Microbial metal interactions
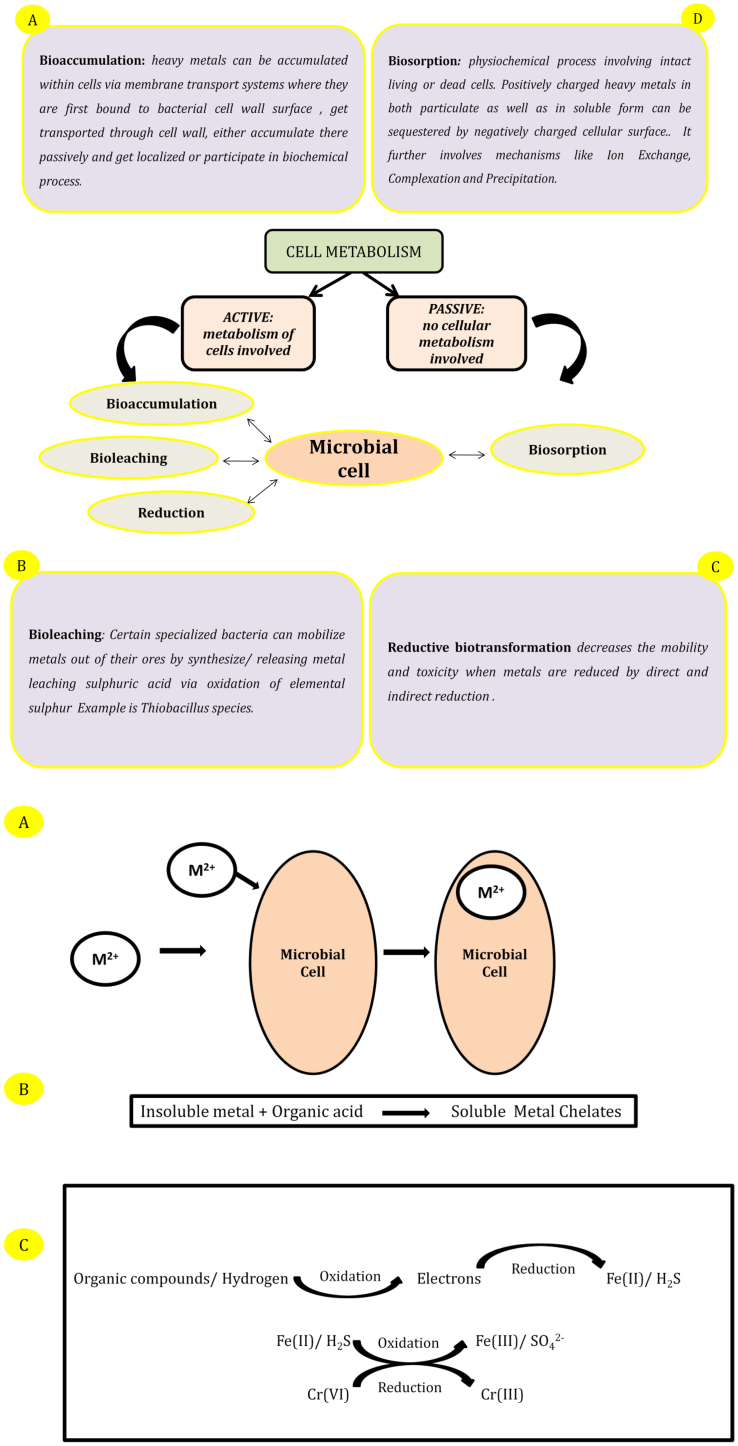
Reducing bioavailability or mobility of heavy metal ions is important for reducing hazards caused by their uptake and accumulation. The major drawbacks associated with conventional physiochemical methods (high cost and generation and disposal of toxic by-products) motivate interest towards cost effective, in situ operable and environmentally gentle biological methods. Earlier it was believed that metals only exhibit toxic effects on microbial system. But later it was revealed that they are not merely toxic, instead their presence can induce varied metal resistance mechanisms in microorganisms. They defend against the infilteration of heavy metal ions by actively or passively interacting with them and subsequently developing protecting means. Microbe and metal ion interaction can occur by various mechanisms which could be classified based on involvement of metabolism for example active and passive uptake of metal ions depending upon the interaction [3]. These interactions biologically transform them either into less toxic or less available (less utilizable) form or immobilize them to prevent their breach into bio-systems. Moreover microbial metal resistance or tolerance can be either specific or nonspecific. The specific resistance involves inducible mechanism of tolerance for example transformation of mercury via methylation, but such methyl mercury is highly toxic and volatile in nature. Non specific resistance might be inducible or constitutive. Production of low weight cystine rich proteins and peptides called metallothioneins by certain microbes like Cyanobacterium synococcus, E.coli, Pseudomonas putida falls under non specific inducible resistance [77]. Constitutive nonspecific mechanism involves production of slime layers (exopolysachharides). Fig. 2 illustrates the mechanism and modes of microbial metal ion interaction and their biotransformation.
Fig. 2.
Brief picture of metal microbial interactions taking place through two major mechanisms: Active and Passive. Bioaccumulation, Bioleaching, Biosorption and Redox mediated transformations are the major modes by which microbial cell interacts with and uptakes the heavy metal ion.
3.1. Microbial biosorption, advantages and disadvantages
Amongst various interactive modes bioaccumulation and biosorption have been studied broadly and hold significant importance as compared to other biotransformation modes [53]. Microbial biosorption has emerged as a rather more promising method owing to the inherent advantages, favorability and applicability over bioaccumulation. Unlike redox mediated or accumulation mediated metal ion sequestration, microbial biosorption works passively in a metabolism independent manner where live as well as dead biomass both can uptake metal ions through several physiochemical mechanisms [16]. Ion exchange, complexation, precipitation, chelation etc. are some such examples [97]. While talking about adsorption and cell viability, live cells can utilize an array of possible mechanisms to transform metal ions but beyond a particular concentration, maintaining their viability will be challenging. Moreover, the nutrient requirement and supplementation will be an additional liability. Dead cells are devoid of any such constraint. Consequently, the storage, metal accumulation and regeneration capacity is higher and requires milder operating conditions of pH, temperature etc. [2], [97]. Their excellent, sensitivity, versatility, rate and degree of accumulation enhance the uptake and sequestration even at lower initial metal ion concentrations. Moreover, dead cells are free from possible hazards posed by living cells, they can be recycled by desorption of adsorbed ions for further cycle of adsorption. Most importantly the metal ions recovered can be further exploited. In a way they act as rapid and reversible ion exchangers. The overall advantages and disadvantages of pure and cell bound EPS mediated metal remediation have been summarized in Table 2.
Table 2.
Major Advantages and Disadvantages of Pure and Cell bound EPS mediated heavy metal adsorption.
| SN. | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| 1. | Environmentally safe and cost effective adsorbent | Low efficiency of biosorption in real industrial samples |
| 2. | No generation of toxic byproducts | Absence of metal ion selectivity or specificity |
| 3. | Both live and dead cell bound EPS can be used for metal adsorption, dead cells are devoid of hazards posed by living cells | Adsorption is extremely sensitive to physiochemical operation parameters like pH, temperature, ionic strength, presence of other biological ligands which makes real sample application difficult. |
| 4. | Requires milder operating conditions compared to conventional physiochemical methods | Maintaining the viability of live cells at higher metal or toxicant concentration, is challenging |
| 5. | Sensitive and can carry out sequestration even at lower metal ion concentrations | Reusability requires use of weak desorption chemicals which alternatively result in reduced desorption efficiency |
| 6. | Can be recycled after desorption of adsorbed metal ions and the metal ions recovered can be further exploited | A maximum consecutive reusability is limited to 5–10 cycles of sorption-desorption |
Although the attractive advantages together with its cost economy makes microbial biosorption, an interesting tool for heavy metal remediation [2], [97], [99], [100] yet overcoming the apparent drawbacks needs to be worked upon.
3.2. Mechanism of EPS metal sorption
Bio-sorption is a surface phenomenon, devoid of any involvement of microbial metabolism. EPS or intact microbial cell (live or dead), mediated biosorption occurs by interaction between positively charged metal ions and negatively charged EPS and cell surfaces. Abundant active and ionisable functional groups and non-carbohydrate substituents like acetamido group of chitin, structural polysaccharides of fungi, amine, sulfhydral and carboxyl groups in proteins, phosphodiester (techoic acid), phosphate, hydroxyl groups in polysaccharide imparts overall negative charge to the polymer [103]. On contrary to homopolysaccharides, extracellular heteropolysaccharides are often polyanionic due to association of some of such functional groups with polysaccharide backbone [74]. The sorption and immobilization again occurs via different mechanisms like ion exchange, complexation, precipitation etc. [18], [103].
4. Different strategies of EPS mediated metal ion remediation
Heavy metal ions (arsenic, cadmium, chromium, copper, lead, manganese, mercury, nickel, zinc, selenium) are not only needed to be removed from ecosystem but also required to be recovered from every possible source given their important commercial applications. Biosorption (plant or microbial cells and cellular product mediated) and biotransformation (enzymes or metabolites mediated metal ion removal) are probably the most widely explored and reported biological metal abatement strategies in recent times [33]. Intact microbial cells as well as cell bound EPS have found extensive applications for metal remediation in industrial as well as environmental waste water sources [47]. Among microorganism – microalgae, bacteria, fungi all have been scientifically established as exopolysaccharide producers. However this article would focus on bacterial EPS and its application towards heavy metal sequestration, therefore the later part of the article will be dealing with different strategies of metal ions uptake by bacterial EPS
4.1. Homogenous consortial EPS
Remediation of metals through pure bacterial cultures and their EPS dates back to early eighties [45], [66], [67]. EPS produced from a methylotrophic bacterium Methylobacterium organophilum was reported to exhibit copper and lead ion removal nonspecifically within half an hour of reaction incubation at a pH Optimum of 7 [41]. A gram negative bacterium Herminiimonas arsenicoxydans has been found to not only initiate or induce biofilm formation in response to arsenic exposure but also were found to scavenge arsenic ions through exopolysaccharide with a tolerance limit of upto 5 mM metal ion concentration [54], [62], [63], [104]. Another betaproteobacteria Thiomonas sp. CB2 was also reported to secrete biomolecules to formulate surface biofilms in response to arsenic induced stress in the environment and trapped arsenic ions in its exopolysaccharide [55]. These reports indicate the fact that arsenic can not only induce bacterial exopolysaccharides synthesis in response to heavy metal stress but also the produced exopolysaccharides can appreciably adsorb this metal.
Activated sludge has always been a rich source of several metal ion resistant bacterial species [48], [73]. Cell biomass and capsular EPS of sludge isolated Pseudomonas were compared for their metal ion uptake capability through dye displacement method. Interestingly whole cells unveiled greater copper ion adsorption as compared to pure EPS [48]. Not only sludge but also distinct sources have been found to be the source of EPS producing bacterium.
In this regard EPS from marine bacterium has been of special significance given to their higher uronic acid content which imparts them enhanced anionicity. EPS of Marinobacter sp. has been investigated quite a few times against metals like lead and copper. Bhaskar et al. confirmed that EPS from Marinobacter adsorbed copper ions more than lead ions under neutral pH [6]. EPS from another marine bacterium Enterobacter cloaceae demonstrated substantial metal ion chelating ability. It was found to reduce the initial cadmium and copper ion load of 100 ppm by 65% and 20% respectively and hence exhibited adsorption of 2.05–16.00 mg of cadmium and 3.11–6.60 mg of copper ions per gram EPS. While unlike cadmium and copper, the bacterial EPS was found to just marginally uptake cobalt ions with an efficiency of 8% [34]. E.cloaceae was also observed to exhibit continuous growth and propagation on chromium exposure. Moreover, it displayed enhanced chromium ion uptake and tolerance via EPS synthesis. Cell and polysaccharide collectively sequestered 75% of ions at maximum initial metal ion concentration of 100 ppm, where EPS was a higher contributor in metal ion uptake compared to cells [33]. Marine micro-alga associated Halomonas sp. was reported to chelate calcium, silicate, iron, magnesium and aluminium metal ions. The intrinsic association of metal ions to EPS especially iron, as reported, was said to be playing a major role in making iron available to phytoplanktons [30].
Cyanobacterial EPS have also been widely reported for their promising metal ion chelation ability. EPS of Anabaena spiroides a cyanobacteria was reported to display maximum affinity for Mn2+ among different metals tested. EPR (Electron Paramagnetic Resonance) study was performed where two models (Langmuir and Freundlich) confirmed the maximum manganese ion complexation of 8.52 mg/g EPS [23]. In case of Gloeocapsa gelatinosa, abundance of acidic sugar in capsular polysaccharides so produced resulted in much higher lead ion sequestration around 82–86 mg Pb2+/g CPS as compared to whole cell surface. The structural and compositional makeup of EPS varied according to phase of bacterial growth and thus higher lead ion removal was seen during stationary phase due to high net acidic sugar incorporation in EPS [80]. Similarly another cyanobacteria Calothrix marchica was found to depict similar pattern of metal ion adsorption where higher acidic sugar content of CPS was responsible for the enhanced lead ion complexation of almost 65 mg Pb2+/g CPS. But the sole difference was the acidic sugar content, which displayed maximum production at peak of polysaccharide accumulation ie. after 30 days [82]. While in G.gelatinosa, the proportion of acidic sugar moieties amplified in stationary phase of growth itself. Capsular polysaccharides of cyanobacteria Cyanospira capsulata and Nostoc species depicted greater copper ion adsorption in comparison to nickel and zinc ions when cells were employed with intact CPS for adsorption. Langmuir isotherm in case of C. Capsulata confirmed 115 mg copper removal/g of protein while pure polysaccharide complexation quotient was around 20 mg/g polysaccharide. Removal efficiency of Nostoc species was much lower in contrast to C.capsulata [17]. EPS of Gloeocapsa calcarea and Nostoc punctiforme both illustrated appreciable chromium removal, while the EPS production was higher in G.calcarea, the metal sequestration capacity was higher in N.punctiforme. The adsorption maxima was reported to decline at initial metal concentration higher than 20 mg/L,till this range 90–91% metal reduction was reported at pH optimum of 2 [87]. Yet another cyanobacterium Lyngbya putealis exopolysaccharides revealed excellent metal uptake of 157 mg/g EPS at 40 ppm of initial metal load. Temperature and pH optima predicted through RSM (response surface methodology) were 45 °C and pH 2. Langmuir and BET (Brunauer Emmett Teller) models described the sorption to be multilayer with coefficient of determination 0.9925 [42]. Data suggests that metal ion sorption on polysaccharide is a single phase phenomena and the sorption reached equilibrium within few hours of reaction initiation but immobilized EPS could be presumably quicker in the task. A comparative study was carried out between live cell bound EPS, dead cell bound EPS and isolated EPS of cyanobacterium Cyanothece sp. CCY 0110 for copper, cadmium and lead ion removal. The isolated EPS displayed highest metal ion removal efficiency, reportedly 38% of copper ion reduction from an initial load of 10 mg/L [61].
Literature is evident of several other bacterial species which have shown remarkable metal chelation properties. The study conducted in University of Notre Dame dealt with some such bacterial species for exploring their metal ion uptake capacity. EPS and whole cells of Pseudomonas putida were reported to sequester around 80% of cadmium ions from initial load of 10 ppm but at higher pH around 9. Moreover, strains of Shewenella oneidensis, Agrobacterium species, Rhizobium tropici have also been found to produce polysaccharides which displayed substantial cadmium ion adsorption competence. S. oneidensis and R. tropici demonstrated around 80% reduction in initial cadmium ion load of 10 ppm working in a higher pH range between 8 and 9 but Agrobacterium species exhibited a peculiar behavior and adsorption was found to increase with decrease in pH below 4. Above this pH, the adsorption regained and reached its maximum at around pH8 which indicates that Agrobacterium can display metal ion adsorption at typically lower pH also, apart from higher pH (around 8) [39]. Alternatively same Pseudomonas putida isolated EPS displayed non significant metal ion adsorption relative to adsorption through biomass associated EPS. However EPS was successful in significantly enhancing the cell viability and tolerance towards cadmium ions [95]. Several distinct studies also have been carried out on Shewanella and Rhizobium species. In another report, comparative examination between wild and mutated types S.oneidensis through potentiometric titration revealed that wild type was superior EPS producer and hence enhanced zinc and lead ion adsorber. While in mutated types, the interaction of metal ions were stronger towards phosphoryl group indicating an important point that EPS reduction led the protonic diffusion to cell wall rich in phosphoryl group [31]. Rhizobium radiobacter EPS was explored for biosorption capacity of lead and zinc ions through kinetic studies and adsorption isotherms. Biosorption was found to be pseudo first order and fitted Langmuir model. Optimum pH for adsorption of Lead and zinc ions were found to be 5 and 6 respectively and the system effectively displayed five subsequent adsorption desorption cycle without affecting cells and EPS [101]. EPS like succinoglycan and galactoglucan from another Rhizobium species Sinorhizobium meliloti illustrated arsenic and mercury ion resistance. An interesting feature called rescuing was highlighted wherein EPS secreting (metal tolerant) strains displayed a protective effect towards non EPS secreting sensitive strain in co-culture experiments [65]. The study suggested that presence of even a single EPS producing strain in mixed consortia can also suffice the defensive needs of other microbial species. In order to develop resistance to higher metal ion exposure, such microbial strains might show increased induction towards EPS production.
In a recent study a local stream isolated strain Stenotrophomonas maltophilia EPS depicted a remarkable 69.1% and 43% of copper and chromium ion removal from initial concentration of 25 mg/L respectively. Quantitative EPS production was reported to be 0.74 g/l and 1.05 g/l respectively upon copper and chromium ion exposure [40]. The greater proportion of copper ion removal over other metal ions by bacterial EPS was also demonstrated by Yang et al. When both the ions were subjected to a binary system it was found that copper ions (1.77 mmol/g) was preferentially adsorbed over zinc ions (1.36 mmol/g) by Klebsiella sp. J1 EPS [106]. The reason behind this competitive adsorption behavior might be because the metal ion was presumably acting as complexation agent of protein or protein like substances for respective bacterial strains, as per Yang et al. suggested [106].
Soil isolated bacterium Bacillus firmus conveyed highest Pb2+ uptake amongst other metal ions like lead, copper and zinc under investigation. The adsorption was described to be a function of pH where acidic side amplified metal ion uptake. Langmuir and Freundlich isotherm confirmed 1103, 860 and 722 mg of Pb2+, Cu2+ and Zn2+ sorption per gram EPS which corresponds to 98.3%, 74.9% and 61.8% removal from an initial metal ion concentration and with a polysaccharide concentration of 1000 mg/L respectively and reached equilibrium within ten minutes of reaction initiation [84]. Researches on another soil isolated bacterium Azotobacter chroococcum suggest that EPS chelated lead and mercury ions in pH dependent manner. And at lower pH of metal solution nearly between 4 and 5 the maximum adsorption obtained was around 40–47% of initial metal ion concentration in solution [79]. In case of bacterium Ensifer meliloti, the polysaccharide illustrated enhanced metal ion uptake capacity relative to dried cells. Langmuir isotherm confirmed a maximum of 89, 85 and 66% of lead, nickel and zinc ion reduction respectively, from initial 50 ppm loaded solutions. EPS dosage was 0.02 g which achieved equilibrium adsorption within 20 min of reaction initiation [47].
GRAS (Generally Recognized as Safe) status bacteria have also been found offering tremendous potential in metal chelation. Paenibacillus jamilae one such bacterium, has exhibited significant lead ion sorption property, reportedly around 200–300 mg/g of EPS. Several other metal ions were tested like cadmium, nickel, zinc, copper but apart from lead maximum uptake was revealed in case of cadmium ion notably 21 mg/g of EPS [60], [75]. Another species of Paenibacillus i.e . P.polymyxa a gram positive GRAS status bacterium, displayed eminent EPS production under hyperosmotic stress which further increased copper ion adsorption ability of whole cell associated EPS system. Langmuir model deduced the biosorption capacity as 150 mg copper ions/g whole cells, whereas purified EPS at a concentration of 0.1 mg/ml displayed substantially higher adsorption of 1602 mg/g [1]. Yet another impressive EPS producing, fermented food isolate Lactobacillus plantarum has been studied in terms of various physiochemical parameters like temperature, pH, initial metal ion concentration, EPS dosage and contact time for examining metal ion uptake capacity. While the adsorption was found to be dependent on initial metal ion and adsorbent concentration, the pH optima, observed for maximum adsorption, was slightly lower i.e. 5 and contact time required to reach equilibrium was slightly higher i.e. 6 h [21]. Many such safe bacterial species are scattered in our environment unexplored. There is a need to investigate such EPS producers for metal ion chelation abilities as they might come up with potent polysaccharide rich in anionic moieties. Table 3 summarizes all the parameters and strategies currently been undertaken in EPS mediated heavy metal remediation
Table 3.
Remediation strategies and efficiencies.
| Remediation Strategy | Consortial Source and Specifics | Bacterial species | Heavy Metals Adsorbed | Remediation Efficiency | Reference |
|---|---|---|---|---|---|
| HOMOGENOUS CONSORTIAL EPS | Methylobacterium organophilum | Copper,Lead | 21% Cu2+,18% Pb2+ removal from 0.04 ppm initial metal load | [41] | |
| Herminiimonas arsenicoxydans | Arsenic | Upto 5 mmol/L metal ion uptake | [54] | ||
| Activated sludge isolates | Pseudomonas sp | Copper | N.A | [48] | |
| Marine bacteria | Enterobacter cloaceae | Cadmium,Copper, Chromium | 65% Cd2+,20% Cu2+; 75% Cr6+ reduction from 100 ppm initial metal load | [30], [34] | |
| Shewenella oneidensis | Cadmium | 80% Cd2+ removal | [31] | ||
| Cyanobacteria | Anabaena spiroides | Manganese | 8.52 mg Mn2+/g EPS | [23] | |
| Gloeocapsa gelatinosa | Lead | 82.22 ± 4.82 mg Pb2+/g CPS | [80] | ||
| Calothrix marchica | Lead | 65 mg Pb2+/g CPS | [82] | ||
| Cyanospira capsulate (Cells + EPS) | Copper | 115 mg Cu2+/g EPS at 12.3 ppm initial metal load | [17] | ||
| Nostoc PCC7936 | Copper | 85.0 ± 3.2 mg Cu2+/g EPS at 12.3 ppm initial metal load | |||
| Gloeocapsa calcarea | Chromium | 36 mg Cr6+/g EPS at 20 ppm initial metal load | [87] | ||
| Nostoc punctiforme | Chromium | 90.05 mg Cr6+/g EPS | |||
| Lyngbya putealis | Chromium | 157 mg/g of EPS Cr6+at 30 ppm initial load |
[42] | ||
| Pseudomonas putida | Cadmium | 80% Cd2+ at 10 ppm initial | [39] | ||
| Rhizobium. tropici | Cadmium | 80% Cd2+ at 10 ppm initial | |||
| Soil isolates | Bacillus firmus | Lead, Copper, Zinc | 1103 mg Pb2+/g EPS (98.3%,), 860 mg Cu2+/g EPS (74.9%), 722 mg Zn2+/g EPS (61.8%) |
[84] | |
| Azotobacter chroococcum | Lead,Mercury | 40.48% Pb2+(33.5 mg Pb2+/g of EPS); 47.87% Hg2+ (38.9 mg of Hg/g EPS) |
[79]. | ||
| Ensifer meliloti | Lead, Nickel, Zinc | 89% Pb2+, 85% Ni2+, 66% Zn2+ reduction from 50 ppm initial load |
[47] | ||
| GRAS status | Paenibacillus jamilae | Lead, Cadmium | 200–300 mg Pb2+/g EPS, 21 mg Cd2+/g of EPS |
[60], [75] | |
| Paenibacillus polymyxa | Copper | 1602 mg Cu2+/g EPS | [1] | ||
| Lactobacillus plantarum | Lead | 276.44 mg Pb2+/g EPS, at 1000 ppm initial metal load | [21] | ||
| HETEROGENUS CONSORTIAL EPS | Activated sludge mixed consortia | Zinc, Copper, Chromium Cadmium | 85 to 95% reduction from initial metal load of 10–100 ppm | [50] | |
| Lead Cadmium | N.A | [29] | |||
| Gram negative microbial consortia | Zinc, lead, Chromium, Nickel, Copper, Cadmium Cobalt |
75 to 78% reduction in metal load | [28] | ||
| Hydrocarbon contaminated water microbial consortium | Zinc, Copper, Cadmium | 87.12% Cd2+; 19.82% of Zn2+; 37.64% of Cu2+ reduction from 1 ppm initial metal load | [56] | ||
| DEAD BIOMASS EPS | Activated sludge isolate | Ochrobactrum anthropi | Chromium, Cadmium, Copper | 57.8 mg Cr6+/g EPS at initial metal load of 280 ppm, 26 mg Cu2+/g EPS at initial metal load of 91.6 ppm 29.5 mg Cd2+/g EPS at 100.6 ppm initial metal load |
[71] |
| Bacillus cereus | Chromium | 89.87% reduction from initial metal load of 50 ppm | [92] | ||
| Bacillus pumilus | Chromium | 89.23% reduction from initial metal load of 50 ppm | |||
| Pantoea agglomerans | Chromium | 85.5% reduction from initial metal load of 50 ppm | |||
| IMMOBILIZED EPS | Alginate bead immobilized | Chryseomonas luteola | Cadmium, Cobalt, Copper, Nickel |
64.10 mg Cd2+/g EPS 55.25 mg Co2+/g of EPS 1.989 mmol Cu2+/g EPS 1.224 mmol Ni2+/g EPS |
[72], [73] |
| Agar Beads immobilized | Paenibacillus polymyxa | Lead | 111.11 mg Pb2+/g EPS | [32] | |
| MODIFIED EPS | Phosphorylated bacterial EPS (cellulose) | Acetobacter | Lead, Copper, Manganese, Zinc, Cobalt | 90% reduction from initial metal load of 0.1 mmol/dm3 (Fe3+ > Cu2+ > Mn2+ > Zn2+; Co2+) |
[70] |
4.2. Heterogenous consortial EPS
Use of mixed culture bacterial consortia has achieved profound success for heavy metal remediation. Activated sludge has always been the abundant source of bioremediation grade microorganisms. EPS of many pure isolates or mixed consortia from sludge have been reported to be capable of metal chelation. Y. Liu et al. investigated the efficiency of mixed microorganisms instead of pure culture in terms of their heavy metal ion adsorption affinity. Exopolysaccharides from activated sludge mixed cultures were noted to reduce almost 85–95% of 10–100 ppm initial zinc, copper, chromium and cadmium ion load while cobalt and nickel ion removal efficiency was comparatively lower as confirmed by Freundlich and Langmuir isotherms [50]. Similarly the EPS of seven different bacterial species isolated from activated sludge depicted lead and cadmium ion scavenging in a pH dependent manner as confirmed by Kurbatov’s model, but with reasonably identical binding efficiency. It indicates that wide range of bacterial EPS might display analogous binding behavior towards a particular metal ion species when sorption is considered [29].
In a recent work, gram negative bacterial consortia confirmed around 75–85% of initial metal ions like zinc, lead, chromium, copper, cadmium and cobalt removal efficiency in less than two hours of contact duration [28]. EPS of microbial consortium isolated from hydrocarbon contaminated water source was observed to reduce 87.12% of Cd2+; 19.82% of Zn2+; and 37.64% of Cu2+ load [56]. Recently use of mixed consortial EPS from local stream was seen in an investigation where focus was on nickel, copper and chromium ion removal. Findings disclosed significant removal efficiency of 32%, 75.7%, and 51.1% of respective metal ions in molasses based medium [40]. The remarkable figures encourage the utilization of heterogeneous consortial EPS from natural waste and dump sites in a purposeful manner. It will resolve the dual purpose − remediation of hazardous metal toxicants at one end and resourceful utilization of residual waste masses at other.
4.3. Dead biomass EPS
Dead biomass exopolysaccharides have also been well accepted in the avenue of remediation as per the reasons already discussed in previous section. Exopolysaccharides of pure culture dead biomass, isolated from activated sludge has been one such example. EPS of dead biomass of this floc forming bacterium Ochrobactrum anthropi removed cadmium ions along with other toxic metals under specific influence of pH and initial metal ion concentration. The organism not merely exhibited peripheral metal ion adsorption but also revealed high metal tolerance (upto 30 mg/L of cadmium ion concentration). Maximum uptake capacity of the biosorbent was found to be 29.5 mg/g at 100.6 ppm initial load [71]. It depicted chromium ion tolerance upto a limit of 16 ppm and adsorption maxima of 57.8 mg/g of EPS from maximum initial load of 280 ppm within two hours of reaction. The adsorption optimum was contrastingly observed at acidic pH of 2. Freundlich and Langmuir adsorption models established that dead biomass exopolysaccharides, chelated copper ions maximally at acidic pH of 2 and reached absorption equilibrium within two hours with a capacity of 26 mg/g EPS at initial metal load of 91.6 ppm [71]. However, a comparative metal ion adsorption analysis between dead and immobilized biomass associated EPS from electroplating effluent (heavy metal resistant) isolates, stated dead biomass to be little lagging than immobilized, for copper, cadmium and lead ion uptake [78]. In a recent study live and dead biomass bound EPS of three different bacteria were evaluated for their chromium ion sorption capacity as a function of pH, temperature and initial metal ion concentration. Dead biomass EPS of Bacillus cereus, Bacillus pumilus and Pantoea agglomerans illustrated slightly superior uptake tendency at 37 °C each showing more than 85% reduction in initial metal load. The pH optimum was reported to be again between 2 and 3 for the said species [92]. The peculiar trend was repeatedly observed in several investigations where the pH range was lower for chromium and several other metal ion adsorption irrespective of type of bacterial species. The reason (for chromium ions) being the repulsion between negatively charged chromate ions (HCrO−4, CrO−7, Cr4O132−, Cr4O102−) and negatively charged groups on EPS and cell surface. The lowering of pH creates overall positive charge on account of functional group protonation and increased hydronium ion concentration [92].
4.4. Immobilized EPS
Advancements in immobilization techniques have excelled the biological reaction kinetics exclusively in terms of reaction rate and specificity enhancement. Numerous reports also verify that attachment of bacterial cells to solid surfaces, stimulates the exopolysaccharide production without altering the specific growth rate [96]. This was evidently demonstrated by Chryseomonas luteola immobilized in alginate bead along with its EPS for examining cadmium, cobalt, nickel and copper ion adsorption. Investigators paired up Chryseomonas luteola and it’s EPS with calcium salt of natural polymer – alginate in form of beads in different combinations. Interestingly the combination of bacterial EPS immobilized in calcium alginate resulted in maximum cadmium and cobalt ion sequestration from aqueous solutions reportedly 64.10 mg/g and 55.25 mg/g of EPS beads respectively [73]. Langmuir isotherm also depicted adsorption capacity of 1.989 mmol of Cu2+/g and 1.224 mmol of Ni2+/g dry weight of alginate immobilized culture EPS. While alginate bead alone displayed comparatively low metal ion adsorption efficiency reportedly 1.505 mmol of Cu2+/g dry weight and 0.996 mmol Ni2+/g dry weight. The reaction reached saturation within one hour of initiation at pH and temperature optima of 6 and 25 °C respectively [72]. Studies in the area of immobilization have been reported with GRAS status bacterial EPS too. Utility of GRAS Paenibacillus Polymyxa EPS immobilized in agar beads was evaluated for uptake of lead ions regulating solution pH, initial metal ion concentration etc. Maximum adsorption was found to be 111.11 mg/g immobilized EPS as per Langmuir model and adsorption saturation was reached within 120 min of reaction commencement. The pH optimum was found to be 5 [32].
4.5. Modified EPS
Chemical modifications like acetylation, carboxymethylation, methylation, phosphorylation, sulphonylation modifies the biological activities of EPS potentially enhancing the applicability of polymer. Modification in EPS structure has been found in quite a few studies. Phosphorylation of cellulose [91], oversulfated, acetylated and phosphorylated derivative of carrageenans [107], sulfated, phosphorylated, carboxymethylated, acetylated and sulfonylated derivatives of neutral polysaccharide from Polygonatum cyrtonema Hua. [49] esterified as well as phosphorylated derivatives of polysaccharide from Portulaca oleracea L [8] are few such examples. But employment of such modified polysaccharides for metal remediation has been barely reported. One such study explains the use of modified extracellular bacterial cellulose. This modified polymer with increased degree of phosphorylation displayed appreciable metal ion sorption capacity. Phosphorylated bacterial cellulose prepared from Acetobacter, tested with various heavy metal ions and lanthanides depicted higher sorption in comparison to unmodified bacterial cellulose [70].
The following table (Table 4) gives a brief picture about the patents filed in the area of EPS and heavy metal remediation
Table 4.
Patents in the field of microbial EPS involved in heavy metal remediation.
| EPS producing microorganism | Important Experimental Parameters | Metal ion removed | Remarks | Patent No. | Reference |
|---|---|---|---|---|---|
| Hyphomonas MHS-3, Hyphomonas sp. | Adsorbent system was effective over wide range of pH and temperature range of 1–11 and 0 °C –200 °C respectively | Cu2+,Hg2+, Pb2+,Cd2+,Zn2+ | The marine strains were able to remove the metal ions from an initial concentration of 50–100 ppb to US EPAa drinking water standards | WO1998030503 A1 | [9] |
|
Zoogloea ramigera 115SL Zoogloea ramigera |
Identification and mixing of genes responsible for polysaccharide synthesis from wild strains for manipulation and control of the exopolysaccharide biosynthetic pathway at the genetic level in modified strain |
N.A. | New modified bacterial strains were designed from wild type producing a novel (EPS) with several desirable characteristics that were absent from the parent strain including metal ion chelation | US4948733 A | [20] |
|
Pseudomonas vesicularisl Zoogloea ramigera, pseudomonas sp, Achromobacter georgi opolizanum, Pseudomonas mendocina |
Immobilized on water permeable biodegradable, nontoxic support system stable within −15 °C to +65 °C temperature range | N.A | A novel filter devise was designed as a replacement to slow sand filters for the water treatment to render it potable | US5264129 A | [90] |
| Arlhrobacler viscosus | Supported on faujasite (FAU) Zeolite | Cr6+ Removal | Devised for industrial applications for hexavalent chromium removal, through the retention of metal ions in the biofilms, in solutions with concentrations between 50 and 250 mg/L | EP1912905 A1 | [37] |
EPA–Environment protection agency.
5. Challenges and prospects
Bacterial biomass and their ubiquitous exopolysaccharide have been much explored and appreciated as an alternative biological metal remediation strategy given its rapid, efficient and sensitive response towards metal ion removal as well as for being economical. But as stated in the article, biosorption through EPS itself is a complex phenomenon and has distinct dynamics and controlling factors. Physiochemical and environmental factors in which the EPS producing cell flourishes, governs the basic structure and composition of synthesized polymer (neutral or negatively charged). The ionic nature of metal, its size and charge density in turn regulates its interaction with negatively charged EPS. Use of single metal ion solution or multi metal ion solution also decides the efficacy of uptake by EPS. Initial metal ions as well as adsorbent (EPS) dosage, duration of interaction etc. are additional parameter determining metal polymer interaction. Thus stringent regulation and control of bacterial growth and EPS production are the deciding factors for production of metal remediation competent polymer. Moreover there must be strict monitoring and regulation of EPS-metal ion sorption experimental conditions in order to yield maximum possible removal. The subsequent notable point is the reusability and selectivity of this polymeric adsorbent. Although several desorbing agents like dilute mineral acids (HCl, HNO3, H2SO4), EDTA, thiosulphates, bases (NaOH, Na2CO3, NaHCO3), organic acids (acetic, citric, lactic) [3], [31], [58], [101] have been dealt for metal desorption, the maximum regeneration limit of EPS as an adsorbent is limited [101]. Also, it has been evident that the sorption through EPS is generally non specific. Overcoming this pitfall requires technological advancements as well as deeper understanding about the polymer and its mechanism of metal uptake. EPS modification and immobilization can be a redeemer here which is still much unexplored field. Several studies have reported different strategies and agents for immobilization [72], [73], [96] yet abundant other unexplored agents can be utilized for immobilization as well as for making hybrid composites of EPS. For example ceramic carrier, polyurethane, porous silica [10], [36], [38], [68], [76], [85], [89] etc. On the other hand, altering and modifying the polymer in desired fashion could presumably enhance its net anionicity. Further it can also improve its selectivity (for metal ions), reusability and regenerability.
The next important subject revolves around materializing the technology out from lab scale to commercial scale for real world applications, which has still been a major shortcoming due to technical as well as nontechnical hurdles. To surmount this many domains need to be worked upon simultaneously. First the source of isolation of such bacterial species must be carefully chosen as there are still many unexplored trenches which can be source of such producers. Use of mixed culture EPS as described in this report can be advantageous but for commercializing and scaling up, mixed culture product cannot be utilized as individual species contribution will be difficult to trace. Rather diverse sources for isolating specific microbial strains which are producer of anionic EPS must be focused. Dairy wastes can stem out pool of GRAS status Lactobacilli offering metal ion adsorbing anionic Exopolysaccharides. These polysaccharides can in turn be employed for metal removal in domestic and consumable water sources, as neither the bacteria nor their polymeric product would be hazardous even if consumed. Similarly industrial wastes (especially those industries utilizing heavy metals) must be naturally abundant in metal tolerant species, and hence can be another hub for desired microorganisms.
Talking about field trials for synthetic and real sample applications, packaging of columns with high anionic polymer yielding strains, either immobilized or attached on suitable carriers, may be utilized as an adsorbent filler material for large scale water purification system. Moreover field experiments go through much adverse environmental conditions where strict regulation and control of temperature, pH, ionic strength, organic and inorganic ligands and other physiochemical parameters are often difficult. While operational conditions in lab scale experiments can be easily controlled. However, this challenge can also be confronted by utilizing either pure EPS or EPS bound with dead biomass which in either case will be unaffected by any environmental distress or harsh operational conditions. It is interesting to note that even under strictly controlled laboratory conditions, attaining the maximum adsorption capacity of EPS is something yet to be achieved. Commercializing a technology has the primary prerequisite of being fast, specific and economical. With EPS as an adsorbent these can be taken care of because as a physical adsorbent it is rapid in adsorption (evident from literature), specificity can be enhanced by bringing about structural modifications as by now discussed and being a bacterial product it is already economical and cheap to produce.
In addition effluent flow rate in industries is another setback as maintaining its contact with adsorbing agent is crucial, without being washed away before the encounter. This can be fixed by the remarkable biotechnological tool ie. immobilization. It can increase the probability, rate and contact duration of EPS and metal ions solving the difficulty of runoff. Now the accountability lies in the efforts required for optimization and establishment of a continuous operational mode for this set up.
In lieu of the discussion made in the article it can be concluded that bacterial EPS requires accurate understanding and appropriate channeling rite from the step of production to the step of application and operation. Consequently it can bring about quantum leap in the direction of developing an extremely sustainable, economic and environmentally restorative method for the removal of heavy metals from terrestrial as well as aquatic systems.
Conflict of interest
All the authors mutually declare that there is no conflict of interest.
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
No funding was received for the present study.
Acknowledgement
Authors acknowledge Mr G.D.Flora from University of Reading for helping in language assessment of the manuscript.
Contributor Information
Pratima Gupta, Email: pguptabiotech@gmail.com, pgupta.bt@nitrr.ac.in.
Batul Diwan, Email: diwan.batul@gmail.com.
References
- 1.Acosta M.P., Valdman E., Leite S., Battaglini F., Ruzal S. Biosorption of copper by Paenibacillus polymyxa cells and their exopolysaccharide. World J. Microbiol. Biotechnol. 2005;21:1157–1163. [Google Scholar]
- 2.Aksu Z. Application of biosorption for the removal of organic pollutants: a review. Process Biochem. 2005;40:997–1026. [Google Scholar]
- 3.Alluri H.K., Ronda S.R., Settalluri V.S., Bondili J.S., Suryanarayana V., Venkateshwar P. Biosorption: an eco-friendly alternative for heavy metal removal. Afr. J. Biotechnol. 2007;6 [Google Scholar]
- 4.Aloni Y., Cohen R., Benziman M., Delmer D. Solubilization of the UDP-glucose: 1, 4-beta-D-glucan 4-beta-D-glucosyltransferase (cellulose synthase) from Acetobacter xylinum. A comparison of regulatory properties with those of the membrane-bound form of the enzyme. J. Biol. Chem. 1983;258:4419–4423. [PubMed] [Google Scholar]
- 5.Barakat M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011;4:361–377. [Google Scholar]
- 6.Bhaskar P., Bhosle N.B. Bacterial extracellular polymeric substance (EPS): a carrier of heavy metals in the marine food-chain. Environ. Int. 2006;32:191–198. doi: 10.1016/j.envint.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Boels I.C., van Kranenburg R., Hugenholtz J., Kleerebezem M., de Vos W.M. Sugar catabolism and its impact on the biosynthesis and engineering of exopolysaccharide production in lactic acid bacteria. Int. Dairy J. 2001;11:723–732. [Google Scholar]
- 8.Chen T., Zhu L., Liu X., Li Y., Zhao C., Xu Z., Yan W., Zhang H. Synthesis and antioxidant activity of phosphorylated polysaccharide from Portulaca oleracea L. with H 3 PW 12 O 40 immobilized on polyamine functionalized polystyrene bead as catalyst. J. Mol. Catal. A Chem. 2011;342:74–82. [Google Scholar]
- 9.A. B Chmurny, E.J. Quintero, RKneer, (1998).Novel heavy metals sorbents produced from hyphomonas and method of use: Google Patents.
- 10.Chu Y.-F., Hsu C.-H., Soma P.K., Lo Y.M. Immobilization of bioluminescent Escherichia coli ells using natural and artificial fibers treated with polyethyleneimine. Bioresour. Technol. 2009;100:3167–3174. doi: 10.1016/j.biortech.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 11.Coleman R.J., Patel Y.N., Harding N.E. Identification and organization of genes for diutan polysaccharide synthesis from Sphingomonas sp. ATCC 53159. J. Ind. Microbiol. Biotechnol. 2008;35:263–274. doi: 10.1007/s10295-008-0303-3. [DOI] [PubMed] [Google Scholar]
- 12.Comte S., Guibaud G., Baudu M. Biosorption properties of extracellular polymeric substances (EPS) towards Cd: Cu and Pb for different pH values. J. Hazard. Mater. 2008;151:185–193. doi: 10.1016/j.jhazmat.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 13.Coplin D., Cook D. Molecular genetics of extracellular polysaccharide biosynthesis in vascular phytopathogenic bacteria. Mol. Plant-Microbe Interact. 1990;3:271–279. doi: 10.1094/mpmi-3-271. [DOI] [PubMed] [Google Scholar]
- 14.Czaczyk K., Myszka K. Biosynthesis of extracellular polymeric substances (EPS) and its role in microbial biofilm formation. Pol. J. Environ. Stud. 2007;16:799–806. [Google Scholar]
- 15.Das N., Vimala R., Karthika P. Biosorption of heavy metals—an overview. Indian J. Biotechnol. 2008;7:159–169. [Google Scholar]
- 16.Davis T.A., Volesky B., Mucci A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003;37:4311–4330. doi: 10.1016/S0043-1354(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 17.De Philippis R., Paperi R., Sili C. Heavy metal sorption by released polysaccharides and whole cultures of two exopolysaccharide-producing cyanobacteria. Biodegradation. 2007;18:181–187. doi: 10.1007/s10532-006-9053-y. [DOI] [PubMed] [Google Scholar]
- 18.De Philippis R., Colica G., Micheletti E. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: molecular basis and practical applicability of the biosorption process. Appl. Microbiol. Biotechnol. 2011;92:697–708. doi: 10.1007/s00253-011-3601-z. [DOI] [PubMed] [Google Scholar]
- 19.De Vuyst L., Degeest B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 1999;23:153–177. doi: 10.1111/j.1574-6976.1999.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 20.D.D. Easson Jr, O.P. Peoples, A.J. Sinskey, (1990).Zoogloea transformation using exopoly saccharide non-capsule producing strains: Google Patents.
- 21.Feng M., Chen X., Li C., Nurgul R., Dong M. Isolation and identification of an Exopolysaccharide‐Producing lactic acid bacterium strain from chinese paocai and biosorption of Pb (II) by its exopolysaccharide. J. Food Sci. 2012;77:T111–T117. doi: 10.1111/j.1750-3841.2012.02734.x. [DOI] [PubMed] [Google Scholar]
- 22.Flora G., Gupta D., Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip. Toxicol. 2012;5:47–58. doi: 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freire-Nordi C.S., Vieira A.A.H., Nascimento O.R. The metal binding capacity of Anabaena spiroides extracellular polysaccharide: an EPR study. Process Biochem. 2005;40:2215–2224. [Google Scholar]
- 24.Freitas F., Alves V.D., Pais J., Costa N., Oliveira C., Mafra L., Hilliou L., Oliveira R., Reis M.A. Characterization of an extracellular polysaccharide produced by a Pseudomonas strain grown on glycerol. Bioresour. Technol. 2009;100:859–865. doi: 10.1016/j.biortech.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Freitas F., Alves V.D., Reis M.A. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 2011;29:388–398. doi: 10.1016/j.tibtech.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Freitas F., Alves V.D., Torres C.A., Cruz M., Sousa I., Melo M.J., Ramos A.M., Reis M.A. Fucose-containing exopolysaccharide produced by the newly isolated Enterobacter strain A47 DSM 23139. Carbohydr. Polym. 2011;83:159–165. [Google Scholar]
- 27.Gavrilescu M. Removal of heavy metals from the environment by biosorption. Chemistry. 2004;28:30. [Google Scholar]
- 28.Gawali Ashruta A., Nanoty V., Bhalekar U. Biosorption of heavy metals from aqueous solution using bacterial EPS. Int. J. Life Sci. 2014;2:373–377. [Google Scholar]
- 29.Guibaud G., Bordas F., Saaid A., D’abzac P., Van Hullebusch E. Effect of pH on cadmium and lead binding by extracellular polymeric substances (EPS) extracted from environmental bacterial strains. Colloids Surf. B Biointerfaces. 2008;63:48–54. doi: 10.1016/j.colsurfb.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez T., Biller D.V., Shimmield T., Green D.H. Metal binding properties of the EPS produced by Halomonas sp: TG39 and its potential in enhancing trace element bioavailability to eukaryotic phytoplankton. Biometals. 2012;25:1185–1194. doi: 10.1007/s10534-012-9581-3. [DOI] [PubMed] [Google Scholar]
- 31.Ha J., Gélabert A., Spormann A.M., Brown G.E. Role of extracellular polymeric substances in metal ion complexation on Shewanella oneidensis: batch uptake thermodynamic modeling, ATR-FTIR, and EXAFS study. Geochim. Cosmochim. Acta. 2010;74:1–15. [Google Scholar]
- 32.Hassiba M., Naima A., Yahia K., Zahra S. Study of lead adsorption from aqueous solutions on agar beads with EPS produced from Paenibacillus polymyxa. Chem. Eng. Trans. 2014;38:31–36. [Google Scholar]
- 33.Iyer A., Mody K., Jha B. Accumulation of hexavalent chromium by an exopolysaccharide producing marine Enterobacter cloaceae. Mar. Pollut. Bull. 2004;49:974–977. doi: 10.1016/j.marpolbul.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 34.Iyer A., Mody K., Jha B. Biosorption of heavy metals by a marine bacterium. Mar. Pollut. Bull. 2005;50:340–343. doi: 10.1016/j.marpolbul.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity: mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang S.H., Min B.G., Jeong Y.G., Lyoo W.S., Lee S.C. Removal of lead ions in aqueous solution by hydroxyapatite/polyurethane composite foams. J. Hazard. Mater. 2008;152:1285–1292. doi: 10.1016/j.jhazmat.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 37.S.C.T.M.T. JESUS, C.N.M.I. Pontes, (2008).Biosorption system produced from biofilms supported on faujasite (fau) zeolite, process obtaining it and its usage for removal of hexavalent chromium (cr (vi)): Google Patents.
- 38.Kariminiaae-Hamedaani H.-R., Kanda K., Kato F. Wastewater treatment with bacteria immobilized onto a ceramic carrier in an aerated system. J. Biosci. Bioeng. 2003;95:128–132. [PubMed] [Google Scholar]
- 39.Kenney J.P. University of Notre Dame; 2010. Metal Adsorption to Bacterial Cells and Their Products. [Google Scholar]
- 40.Kiliç N.K., Kürkçü G., Kumruoğlu D., Dönmez G. EPS production and bioremoval of heavy metals by mixed and pure bacterial cultures isolated from Ankara Stream. Water Sci. Technol. 2015;72:1488–1494. doi: 10.2166/wst.2015.365. [DOI] [PubMed] [Google Scholar]
- 41.Kim S.-Y., Kim J.-H., Kim C.-J., Oh D.-K. Metal adsorption of the polysaccharide produced from Methylobacterium organophilum. Biotechnol. Lett. 1996;18:1161–1164. [Google Scholar]
- 42.Kiran B., Kaushik A. Chromium binding capacity of Lyngbya putealis exopolysaccharides. Biochem. Eng. J. 2008;38:47–54. [Google Scholar]
- 43.Kodali V.P., Sen R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol. J. 2008;3:245–251. doi: 10.1002/biot.200700208. [DOI] [PubMed] [Google Scholar]
- 44.Kralj S., van Geel-Schutten G., Dondorff M., Kirsanovs S., Van Der Maarel M., Dijkhuizen L. Glucan synthesis in the genus Lactobacillus: isolation and characterization of glucansucrase genes, enzymes and glucan products from six different strains. Microbiology. 2004;150:3681–3690. doi: 10.1099/mic.0.27321-0. [DOI] [PubMed] [Google Scholar]
- 45.Kurita K., Sannan T., Iwakura Y. tudies on chitin. VI. Binding of metal cations. J. Appl. Polym. Sci. 1979;23:511–515. [Google Scholar]
- 46.Lakherwal D. Adsorption of heavy metals: a review. Int. J. Environ. Res. Dev. 2014;4:41–48. [Google Scholar]
- 47.Lakzian A. Adsorption capability of lead: nickel and zinc byExopolysaccharide and dried cell of Ensifer meliloti. Asian J. Chem. 2008;20:6075–6080. [Google Scholar]
- 48.Lau T., Wu X., Chua H., Qian P., Wong P. Effect of exopolysaccharides on the adsorption of metal ions by Pseudomonas sp. CU-1. Water Sci. Technol. 2005;52:63–68. [Google Scholar]
- 49.Liu X.-x., Wan Z.-j., Shi L., Lu X.-x. Preparation and antiherpetic activities of chemically modified polysaccharides from Polygonatum cyrtonema Hua. Carbohydr. Polym. 2011;83:737–742. [Google Scholar]
- 50.Liu Y., Lam M., Fang H. Adsorption of heavy metals by EPS of activated sludge. Water Sci. Technol. 2001;43:59–66. [PubMed] [Google Scholar]
- 51.Madhuri K., Prabhakar K.V. Microbial exopolysaccharides: biosynthesis and potential applications. Orient. J. Chem. 2014;30:1401–1410. [Google Scholar]
- 52.Majumder A., Singh A., Goyal A. Application of response surface methodology for glucan production from euconostoc dextranicum and its structural characterization. Carbohydr. Polym. 2009;75:150–156. [Google Scholar]
- 53.Malik A. Metal bioremediation through growing cells. Environ. Int. 2004;30:261–278. doi: 10.1016/j.envint.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Marchal M., Briandet R., Koechler S., Kammerer B., Bertin P.N. Effect of arsenite on swimming motility delays surface colonization in Herminiimonas arsenicoxydans. Microbiology. 2010;156:2336–2342. doi: 10.1099/mic.0.039313-0. [DOI] [PubMed] [Google Scholar]
- 55.Marchal M., Briandet R., Halter D., Koechler S., DuBow M.S., Lett M.-C., Bertin P.N. Subinhibitory arsenite concentrations lead to population dispersal in Thiomonas sp. PLoS One. 2011;6:e23181. doi: 10.1371/journal.pone.0023181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martins P. S. d. O., Almeida N. F. d., Leite S.G.F. Application of a bacterial extracellular polymeric substance in heavy metal adsorption in a co-contaminated aqueous system. Braz. J. Microbiol. 2008;39:780–786. doi: 10.1590/S1517-838220080004000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McIntosh M., Stone B., Stanisich V. Curdlan and other bacterial (1 → 3)-β-D-glucans. Appl. Microbiol. Biotechnol. 2005;68:163–173. doi: 10.1007/s00253-005-1959-5. [DOI] [PubMed] [Google Scholar]
- 58.Mishra S. Adsorption–desorption of heavy metal ions. Curr. Sci. 2014;107:601–612. [Google Scholar]
- 59.Monsan P., Bozonnet S., Albenne C., Joucla G., Willemot R.-M., Remaud-Siméon M. Homopolysaccharides from lactic acid bacteria. Int. Dairy J. 2001;11:675–685. [Google Scholar]
- 60.Morillo J.A., Aguilera M., Ramos-Cormenzana A., Monteoliva-Sánchez M. Production of a metal-binding exopolysaccharide by Paenibacillus jamilae using two-phase olive-mill waste as fermentation substrat. Curr. Microbiol. 2006;53:189–193. doi: 10.1007/s00284-005-0438-7. [DOI] [PubMed] [Google Scholar]
- 61.Mota R., Rossi F., Andrenelli L., Pereira S.B., De Philippis R., Tamagnini P. Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metals bioremediation: interactions between metals and RPS binding sites. Appl. Microbiol. Biotechnol. 2016:1–11. doi: 10.1007/s00253-016-7602-9. [DOI] [PubMed] [Google Scholar]
- 62.Muller D., Simeonova D.D., Riegel P., Mangenot S., Koechler S., Lièvremont D., Bertin P.N., Lett M.-C. Herminiimonas arsenicoxydans sp. nov., a metalloresistant bacterium. Int. J. Syst. Evol. Microbiol. 2006;56:1765–1769. doi: 10.1099/ijs.0.64308-0. [DOI] [PubMed] [Google Scholar]
- 63.Muller D., Médigue C., Koechler S. A tale of two oxidation states: bacterial colonization of arsenic-rich environments. PLoS Genet. 2007;3:e53. doi: 10.1371/journal.pgen.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishimura J. Exopolysaccharides produced from Lactobacillus delbrueckii subsp. bulgaricus. Adv. Microbiol. 2014;4:1017. [Google Scholar]
- 65.Nocelli N., Bogino P.C., Banchio E., Giordano W. Roles of extracellular polysaccharides and biofilm formation in heavy metal resistance of Rhizobia. Materials. 2016;9:418. doi: 10.3390/ma9060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Norberg A., Rydin S. Development of a continuous process for metal accumulation by Zoogloea ramigera. Biotechnol. Bioeng. 1984;26:265–268. doi: 10.1002/bit.260260311. [DOI] [PubMed] [Google Scholar]
- 67.Norberg A.B., Enfors S.-O. Production of extracellular polysaccharide by Zoogloea ramigera. Appl. Environ. Microbiol. 1982;44:1231–1237. doi: 10.1128/aem.44.5.1231-1237.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Obuekwe C., Al-Muttawa E.M. Self-immobilized bacterial cultures with potential for application as ready-to-use seeds for petroleum bioremediation. Biotechnol. Lett. 2001;23:1025–1032. [Google Scholar]
- 69.Öner E.T. Pretreatment Techniques for Biofuels and Biorefineries. Springer; 35–56: 2013. Microbial production of extracellular polysaccharides from biomass. [Google Scholar]
- 70.Oshima T., Kondo K., Ohto K., Inoue K., Baba Y.1. Preparation of phosphorylated bacterial cellulose as an adsorbent for metal ions. React. Funct. Polym. 2008;68:376–383. [Google Scholar]
- 71.Ozdemir G., Ozturk T., Ceyhan N., Isler R., Cosar T. Heavy metal biosorption by biomass of Ochrobactrum anthropi producing exopolysaccharide in activated sludge. Bioresour. Technol. 2003;90:71–74. doi: 10.1016/s0960-8524(03)00088-9. [DOI] [PubMed] [Google Scholar]
- 72.Ozdemir G., Ceyhan N., Manav E. Utilization in alginate beads for Cu (II) and Ni (II) adsorption of an exopolysaccharide produced by Chryseomonas luteola TEM05. World J. Microbiol. Biotechnol. 2005;21:163–167. doi: 10.1016/j.biortech.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 73.Ozdemir G., Ceyhan N., Manav E. Utilization of an exopolysaccharide produced by Chryseomonas luteola TEM05 in alginate beads for adsorption of cadmium and cobalt ions. Bioresour. Technol. 2005;96:1677–1682. doi: 10.1016/j.biortech.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 74.Pal A., Paul A. Microbial extracellular polymeric substances: central elements in heavy metal bioremediation. Indian J. Microbiol. 2008;48:49–64. doi: 10.1007/s12088-008-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pérez J.A.M., García-Ribera R., Quesada T., Aguilera M., Ramos-Cormenzana A., Monteoliva-Sánchez M. Biosorption of heavy metals by the exopolysaccharide produced by Paenibacillus jamilae. World J. Microbiol. Biotechnol. 2008;24:2699–2704. [Google Scholar]
- 76.Quek E., Ting Y.-P., Tan H.M. Rhodococcus sp: F92 immobilized on polyurethane foam shows ability to degrade various petroleum products. Bioresour. Technol. 2006;97:32–38. doi: 10.1016/j.biortech.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 77.Rajendran P., Muthukrishnan J., Gunasekaran P. Microbes in heavy metal remediation. Indian J. Exp. Biol. 2003;41:935–944. [PubMed] [Google Scholar]
- 78.Rani M.J., Hemambika B., Hemapriya J., Kannan V.R. Comparative assessment of heavy metal removal by immobilized and dead bacterial cells: a biosorption approach. Afr. J. Environ. Sci. Technol. 2010:4. [Google Scholar]
- 79.Rasulov B.A., Yili A., Aisa H.A. Biosorption of metal ions by exopolysaccharide produced by Azotobacter chroococcum XU1. J. Environ. Prot. 2013;4:989–993. [Google Scholar]
- 80.Raungsomboon S., Chidthaisong A., Bunnag B., Inthorn D., Harvey N.W. Production: composition and Pb2+ adsorption characteristics of capsular polysaccharides extracted from a cyanobacterium Gloeocapsa gelatinosa. Water Res. 2006;40:3759–3766. doi: 10.1016/j.watres.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 81.Rehm B. Horizon Scientific Press; 2009. Microbial Production of Biopolymers and Polymer Precursors: Applications and Perspectives. [Google Scholar]
- 82.Ruangsomboon S., Chidthaisong A., Bunnag B., Inthorn D., Harvey N.W. Lead (Pb2+) adsorption characteristics and sugar composition of capsular polysaccharides of cyanobacterium Calothrix marchica. Songklanakarin. Songklanakarin J. Sci. Technol. 2007;29:529–541. [Google Scholar]
- 83.Ruas-Madiedo P., Hugenholtz J., Zoon P. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int. Dairy J. 2002;12:163–171. [Google Scholar]
- 84.Salehizadeh H., Shojaosadati S. Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res. 2003;37:4231–4235. doi: 10.1016/S0043-1354(03)00418-4. [DOI] [PubMed] [Google Scholar]
- 85.Samonin V., Elikova E. A study of the adsorption of bacterial cells on porous materials. Microbiology. 2004;73:696–701. [PubMed] [Google Scholar]
- 86.Sarwat F., Qader S.A.U., Aman A., Ahmed N. Production & characterization of a unique dextran from an indigenous Leuconostoc mesenteroides CMG713. Int. J. Biol. Sci. 2008;4:379. doi: 10.7150/ijbs.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharma M., Kaushik A., Bala K., Kamra A. Sequestration of chromium by exopolysaccharides of Nostoc and Gloeocapsa from dilute aqueous solutions. J. Hazard. Mater. 2008;157:315–318. doi: 10.1016/j.jhazmat.2007.12.100. [DOI] [PubMed] [Google Scholar]
- 88.Sheng G.-P., Yu H.-Q., Li X.-Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol. Adv. 2010;28:882–894. doi: 10.1016/j.biotechadv.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 89.Shi W., Tao S., Yu Y., Wang Y., Ma W. High performance adsorbents based on hierarchically porous silica for purifying multicomponent wastewater. J. Mater. Chem. 2011;21:15567–15574. [Google Scholar]
- 90.J. R. Simpson, M. R. Tucker, (1993). Filter device: Google Patents.
- 91.Suflet D.M., Chitanu G.C., Popa V.I. Phosphorylation of polysaccharides: new results on synthesis and characterisation of phosphorylated cellulose. React. Funct. Polym. 2006;66:1240–1249. [Google Scholar]
- 92.Sultan S., Mubashar K., Faisal M. Uptake of toxic Cr (VI) by biomass of exo-polysaccharides producing bacterial strains. Afr. J. Microbiol. Res. 2012;6:3329–3336. [Google Scholar]
- 93.Sutherland I.W. Microbial polysaccharides from Gram-negative bacteria. Int. Dairy J. 2001;11:663–674. [Google Scholar]
- 94.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Springer; 2012. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; pp. 133–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ueshima M., Ginn B.R., Haack E.A., Szymanowski J.E., Fein J.B. Cd adsorption onto Pseudomonas putida in the presence and absence of extracellular polymeric substances. Geochim. Cosmochim. Acta. 2008;72:5885–5895. [Google Scholar]
- 96.Vandevivere P., Kirchman D.L. Attachment stimulates exopolysaccharide synthesis by a bacterium. Appl. Environ. Microbiol. 1993;59:3280–3286. doi: 10.1128/aem.59.10.3280-3286.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vijayaraghavan K., Yun Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008;26:266–291. doi: 10.1016/j.biotechadv.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 98.Volesky B. CRC Press; 1990. Biosorption of Heavy Metals. [Google Scholar]
- 99.Wang J., Chen C. Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol. Adv. 2006;24:427–451. doi: 10.1016/j.biotechadv.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Wang J., Chen C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009;27:195–226. doi: 10.1016/j.biotechadv.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 101.Wang L., Yang J., Chen Z., Liu X., Ma F. Biosorption of Pb (II) and Zn (II) by extracellular polymeric substance (Eps) of Rhizobium Radiobacter: equilibrium, kinetics and reuse studies. Arch.Environ. Prot. 2013;39:129–140. [Google Scholar]
- 102.Wang S., Zhao X. On the potential of biological treatment for arsenic contaminated soils and groundwater. J. Environ. Manage. 2009;90:2367–2376. doi: 10.1016/j.jenvman.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 103.Wang S., Teng S., M Fan. Interaction between heavy metals and aerobic granular sludge. In: Sarkar Santosh Kumar., editor. Environmental Management. Sciyo; Croatia: 2010. pp. 173–188. [Google Scholar]
- 104.Weeger W., Lievremont D., Perret M., Lagarde F., Hubert J.-C., Leroy M., Lett M.-C. Oxidation of arsenite to arsenate by a bacterium isolated from an aquatic environment. Biometals. 1999;12:141–149. doi: 10.1023/a:1009255012328. [DOI] [PubMed] [Google Scholar]
- 105.Whitfield C. Bacterial extracellular polysaccharides. Can. J. Microbiol. 1988;34:415–420. doi: 10.1139/m88-073. [DOI] [PubMed] [Google Scholar]
- 106.Yang J., Wei W., Pi S., Ma F., Li A., Wu D., Xing J. Competitive adsorption of heavy metals by extracellular polymeric substances extracted from Klebsiella sp. J1. Bioresour. Technol. 2015;196:533–539. doi: 10.1016/j.biortech.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 107.Yuan H., Zhang W., Li X., Lü X., Li N., Gao X., Song J. Preparation and in vitro antioxidant activity of κ-carrageenan oligosaccharides and their oversulfated, acetylated, and phosphorylated derivatives. Carbohydr. Res. 2005;340:685–692. doi: 10.1016/j.carres.2004.12.026. [DOI] [PubMed] [Google Scholar]