Abstract
Survival rates of patients with either early and advanced stage non–small-cell lung cancer (NSCLC) have improved with newer systemic therapy and radiation techniques, including combination regimens, targeted therapies, and immunotherapies. The cancer cooperative groups have historically played a critical role in the advancement of NSCLC therapy. Annually, representatives from cooperative groups worldwide convene at the International Lung Cancer Congress (ILCC). In summer 2015, the ILCC reached its 16th anniversary. This article highlights the NSCLC studies presented by participating groups in 2015.
Keywords: Cooperative group, Mesothelioma, NCTN, NSCLC, SCLC
Introduction
Survival rates of patients with either early and advanced stage non–small-cell lung cancer (NSCLC) have improved with newer systemic therapy and radiation techniques, including combination regimens, targeted therapies, and immunotherapies.1–4 The cancer cooperative groups have historically played a critical role in the advancement of NSCLC therapy. Table 1 lists cooperative group trials currently accruing. Annually, representatives from cooperative groups worldwide convene at the International Lung Cancer Congress (ILCC). In summer 2015, the ILCC reached its 16th anniversary. Here we highlight the NSCLC studies presented by the following participating groups in 2015: the National Clinical Trials Network (NCTN) groups supported by the National Cancer Institute (NCI), including Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN), represented by Suresh Ramalingam, MD; NRG (composed of the legacy groups NSABP [National Surgical Adjuvant Breast and Bowel Project], RTOG [Radiation Therapy Oncology Group], and GOG [Gynecology Oncology Group]), represented by Walter Curran Jr, MD; the Southwest Oncology Group (SWOG), represented by David Gandara, MD; the Alliance for Clinical Trials in Oncology (Alliance), represented by Everett Vokes, MD; the Chinese Thoracic Oncology Group (CTONG), Asia Thoracic Oncology Research Group (ATORG), and Korean Cancer Study Group (KCSG), represented by Tony Mok, MD; the European Organisation for Research and Treatment of Cancer (EORTC) and the European Thoracic Oncology Platform (ETOP), represented by Paul Baas, MD; and the NCIC CTG, represented by Glenwood Goss, MD.
Table 1.
Cooperative Group Trials Currently Accruing
| Study | National Clinical Trial Identifier |
|---|---|
| NSCLC | |
| EA4512 (ALCHEMIST) | NCT02201992 |
| A081105 (ALCHEMIST) | NCT02193282 |
| EA5142 (ANVIL) | NCT02595944 |
| RTOG 1308 | NCT01993810 |
| RTOG 1106 | NCT01507428 |
| RTOG 1306 | NCT01822496 |
| S1400 (Lung-MAP) | NCT02154490 |
| S1403 | NCT02438722 |
| CALGB 140503 | NCT00499330 |
| AFT-09 | NCT02591615 |
| ACCRU RC1126 | NCT01532089 |
| CTONG 1103 (EMERGING) | NCT01407822 |
| CTONG 1104 (ADJUVANT) | NCT01405079 |
| EORTC 22055-08053 (LUNG ART) | NCT00410683 |
| EORTC 08114 (GEM) | NCT01838577 |
| BR31 | NCT02273375 |
| IND219 | NCT02337530 |
| SCLC | |
| CALGB 30610/RTOG 0538 | NCT00632853 |
| ETOP 4-13 (STIMULI) | NCT02046733 |
| Mesothelioma | |
| CALGB 30901 | NCT01085630 |
Abbreviations: NSCLC = non–small-cell lung cancer; SCLC = small-cell lung cancer.
Cooperative group efforts in other thoracic malignancies will also be discussed in brief.
ECOG-ACRIN
ECOG-ACRIN was formed in 2012 by the merger of the Eastern Cooperative Oncology Group and the American College of Radiology Imaging Network. The group’s Thoracic Committee objectives are to individualize treatment options on the basis of specific genotypic aberrations and to integrate targeted therapy into the treatment of lung cancer with a specific emphasis on biologic and imaging biomarker discovery.
ECOG-ACRIN Early Stage Studies
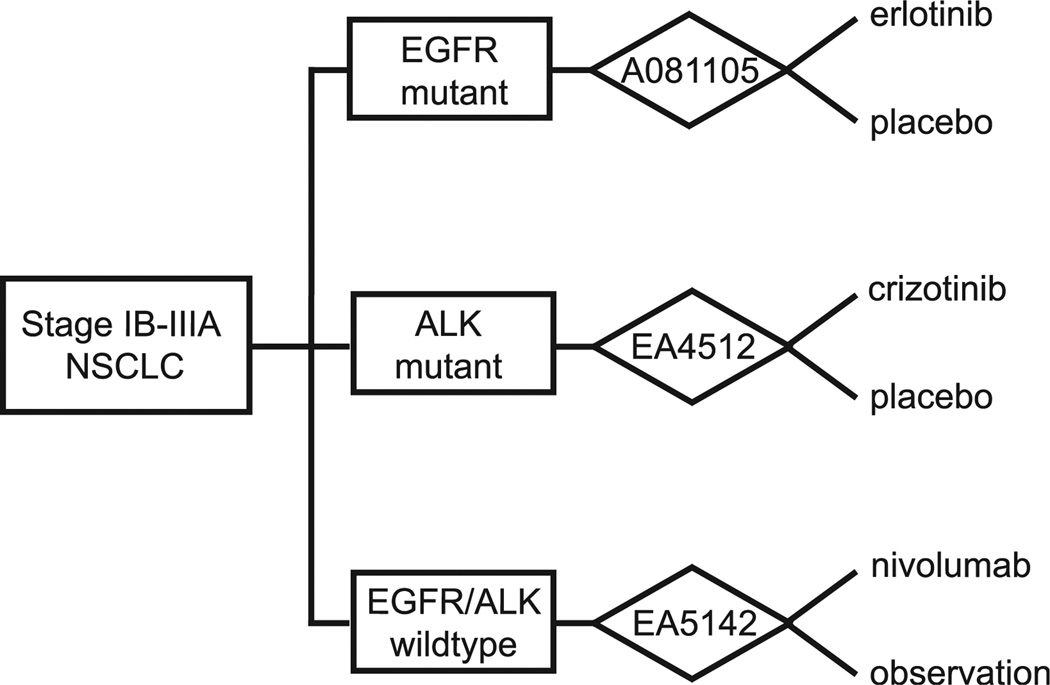
ECOG-ACRIN early stage studies focus on optimal adjuvant therapy for resectable disease. These adjuvant therapies include chemotherapy and bevacizumab in E1505, targeted therapy in E4512, and immunotherapy in EA5142. The primary results of the phase 3 E1505 study, where patients with resected IB-IIIA disease were randomized to receive 4 cycles of a platinum doublet with or without bevacizumab, were presented in fall 2015. The study showed no reported benefit from adjuvant bevacizumab; however, subset analyses and correlative work is ongoing. EA4512, part of the umbrella protocol from the Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial (ALCHEMIST), investigates targeted adjuvant therapy with a primary end point of overall survival (OS) (Figure 1). Specifically, patients with resectable stage I-IIIA ALK-positive nonsquamous NSCLC who have completed surgical and adjuvant treatment are randomized to receive either crizotinib or placebo for 2 years. The soon-to-open EA5142, the Adjuvant Nivolumab in Resected Lung Cancers (ANVIL), expands ALCHEMIST and is a phase 3 randomized controlled trial evaluating the efficacy of nivolumab administered after resection and adjuvant treatment of IB-IIIA NSCLC. Patients without EGFR mutations or ALK translocations will be randomized 1:1 to receive either nivolumab or placebo, and the expected primary end points are median OS and disease-free survival (DFS). Patients with squamous-cell histology will also be eligible to enroll in EA5142 (Figure 1).
Figure 1.
ALCHEMIST Aims to Determine Survival Impact of Additional Targeted Therapy or Immunotherapy in Patients Who Have Undergone Resection and Standard Adjuvant Chemotherapy for Stage IB-IIIA Nonsquamous NSCLC. A081107 Investigates 2 Years of Erlotinib Versus Placebo in EGFR-Mutant NSCLC. EA4512 Compares Crizotinib Versus Placebo in ALK-Mutant NSCLC. EA5142 Randomizes Patients to Receive Either 1 Year of Nivolumab Versus Observation in PD-L1 Positive Patients Defined by >3% Staining by Immunohistochemistry
Abbreviation: NSCLC = non–small-cell lung cancer.
ECOG-ACRIN Advanced Stage Studies
For patients with advanced stage lung cancer, ECOG-ACRIN has 2 studies focusing on the roles of different chemotherapy and targeted therapy regimens. E5508, which has completed accrual but does not yet have results, investigates the impact of differing maintenance therapy regimens on OS. In this study, patients with IIIB-IV stage nonsquamous NSCLC who have at least stable disease after 4 cycles of first-line treatment with carboplatin, paclitaxel, and bevacizumab are randomized to receive maintenance bevacizumab, pemetrexed, or the combination of both. The primary end point to be measured will be OS. E1512, a phase 2 study, demonstrated improved progression-free survival (PFS) with cabozantinib alone (4.2 months, P = .02) or in combination of cabozantinib with erlotinib (4.7 months, P = .02) compared to erlotinib monotherapy (1.9 months) in the setting of second- or third-line treatment of advanced NSCLC lacking actionable EGFR mutations. A follow-up study is in development.
NRG Oncology
In 2015, NRG Oncology reported on lung cancer trials from the legacy RTOG group that emphasized the following questions: Can radiotherapy be improved with better use of functional imaging? What are the benefits of proton therapy? Can targeted therapy improve outcomes over conventional chemotherapy when combined with radiotherapy?
NRG-RTOG Early Stage Studies
RTOG 0813 is a phase 1/2 study that aims to assess the safety and efficacy of stereotactic body radiotherapy for centrally localized tumors. The trial plans to enroll a total of 120 patients in 5 dose-escalation cohorts starting at 10 Gy × 5 fractions with following dose cohorts increasing by 0.5 Gy per fraction to the highest cohort of 12 Gy × 5 fractions totaling 60 Gy. The major primary end point in the phase 1 portion of the trial is the incidence of any treatment-related grade 3 toxicity, while the phase 2 portion has an end point of 2-year primary tumor control rate.
Unlike conventional photon therapy, which delivers radiation throughout the path of the beam, proton therapy has a desirable characteristic of maximal energy delivery at the end of the particle path (eg, the proton beam can be manipulated to deliver the majority of its energy within the volume of a tumor). The objective of the phase 3 randomized NRG-RTOG 1308 trial is to compare OS of patients randomized to photon versus proton radiochemotherapy for stage II-IIIB NSCLC. A total of 560 patients will be randomized between conventional photon radiation therapy totaling 60 Gy at 2 Gy per fraction with weekly platinum doublet chemotherapy versus proton therapy 74 Gy (relative biologic effectiveness) at 2 Gy (relative biologic effectiveness) per fraction in conjunction with weekly platinum doublet chemotherapy.
An update on RTOG 0618, a trial investigating stereotactic body radiotherapy to treat operable early stage lung cancer patients,5 showed that at the 2-year end point, 5 of 26 patients experienced disease recurrence (distant metastases), with a subset who also experienced locoregional tumor failure. An update at 60 months for RTOG 0236, a trial that accrued a total of 55 patients to examine the utility of stereotactic body radiotherapy in early stage inoperable patients, demonstrated that local tumor recurrence at 60 months was 20%, with no treatment-related deaths at 5 years’ follow-up.
NRG-RTOG Advanced Stages Studies
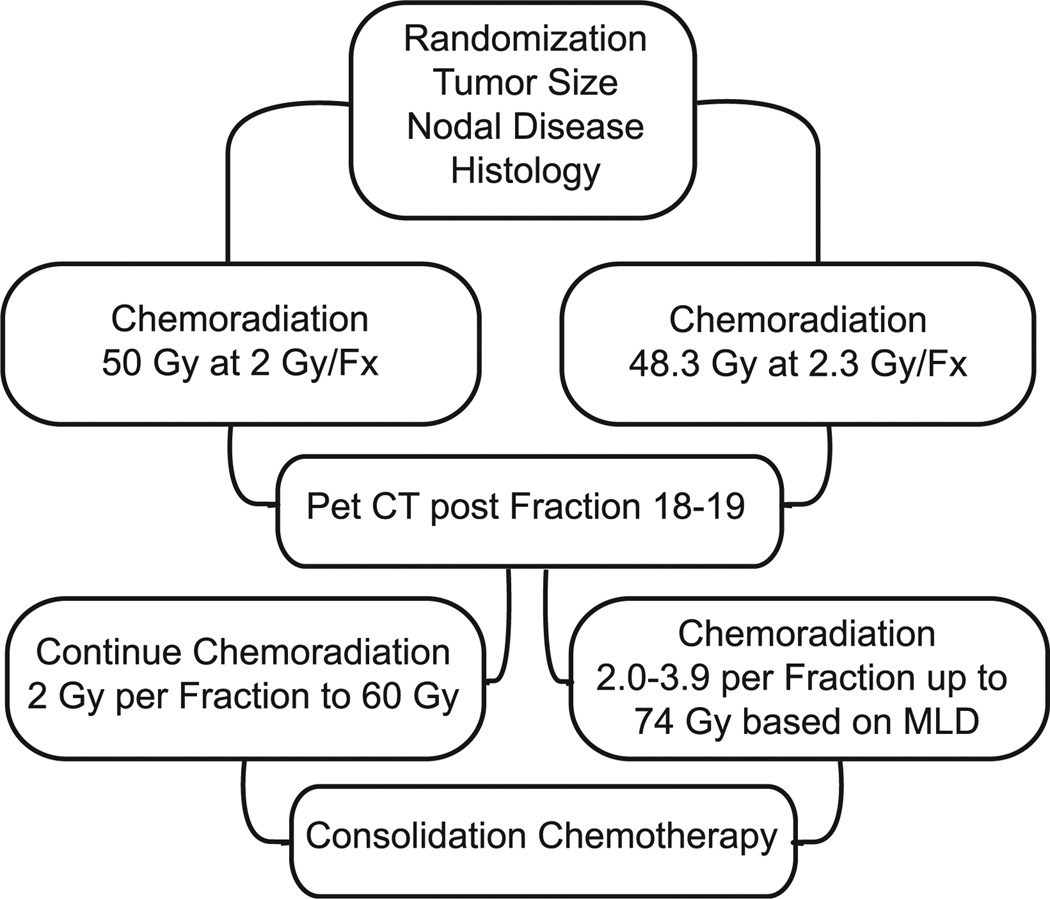
RTOG 1106 examines the use of adaptive radiotherapy versus standard chemoradiation protocol (Figure 2). It is well known that escalating the dose of radiotherapy beyond 60 Gy promotes tumor regression yet can be associated with a significant rise in normal lung tissue toxicity.6 One potential method to circumvent this issue is to adapt the radiotherapy prescription based on positron emission tomography (PET)/computed tomography (CT) tumor assessment midtreatment. For this trial, patients with inoperable or unresectable stage III NSCLC based on 18F-fludeoxyglucose–positron emission tomography/computed tomography (FDG-PET/CT) are randomized to conventional chemoradiotherapy at 50 Gy in 25 fractions (ED2^ = 50 Gy) using the RTOG 0617 uniform dose prescription versus concurrent chemotherapy with radiation with adaptive chemoradiation to mean lung dose 20 Gy in 2.4 to 3.5 Gy per fraction for 9 to 13 fractions to a total of 86 Gy (100 Gy ED2 lung) for 30 fractions. Patients in both arms will receive chemotherapy with radiation up to a defined point of 50 Gy for conventional treatment and 48.3 Gy in the adaptive arm. FDG-PET/CT was then performed during treatment in fraction 18 or 19. Conventional treatment will maintain the original radiotherapy plan to 60 Gy in 30 fractions, whereas the adaptive arm will altered the radiation prescription 2.0 to 3.9 Gy per fraction up to 74 total Gy individualized by the mean lung dose. The primary end point of the trial is locoregional PFS rate at 2 years. The trial has accrued over half of the planned 138 subjects.
Figure 2.
RTOG 1106. RTOG 1106 Compares Standard Chemoradiation (Left) Versus Adaptive Radiation (Right) Protocol in Unresectable NSCLC Patients
Abbreviation: NSCLC = non–small-cell lung cancer.
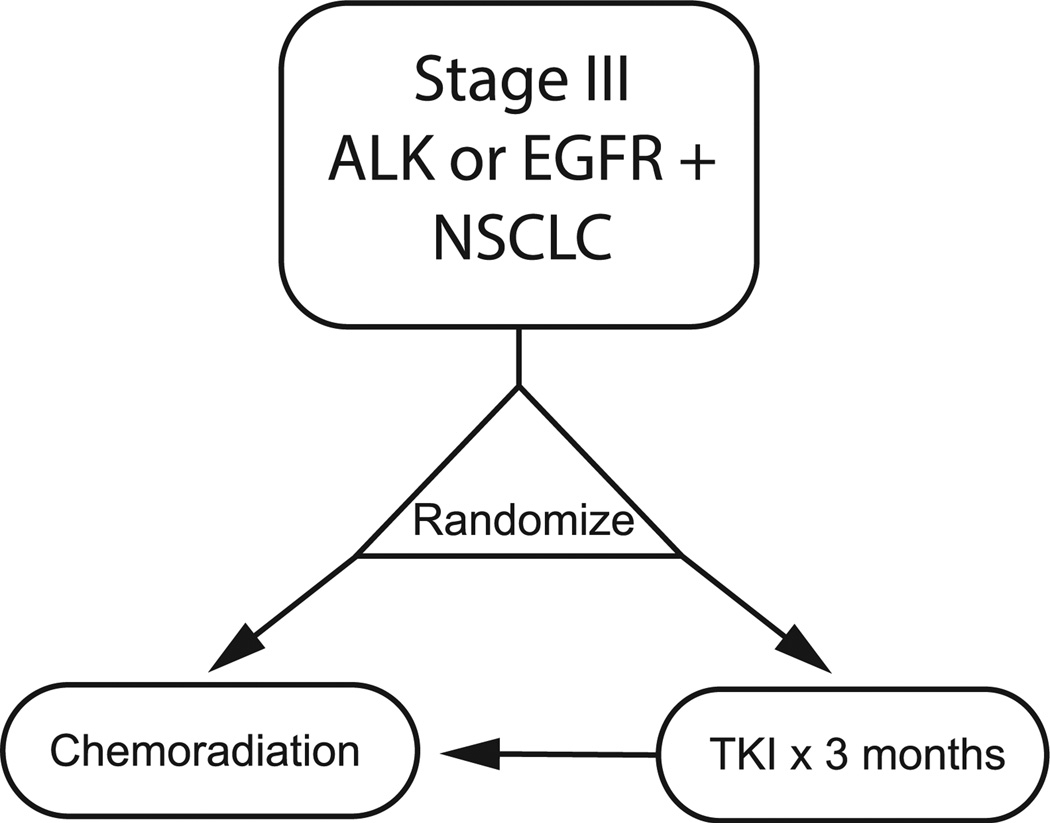
To investigate the activity of targeted therapy administered before definitive concurrent chemotherapy and radiation in patients with unresectable NSCLC, the NRG-RTOG 1306 trial, in partnership with the Alliance NCTN group, will investigate targeted agents against EGFR or ALK mutant stage III NSCLC patients (Figure 3). Patients are randomized to receive 3 months of targeted therapy versus directly proceeding to chemotherapy concurrent with radiation. The study aims to enroll 156 patients in the EGFR cohort and 78 patients in the ALK cohort. The study’s primary end point is PFS.
Figure 3.
NRG/Alliance 1306. NRG/Alliance 1306 Aims to Demonstrate Utility of Targeted Therapy in EGFR- or ALK-Mutant Unresectable NSCLC Before Definitive Concurrent Chemotherapy and Radiation With Either Weekly Carboplatin–Paclitaxel or 2 Cycles of Split-Dose Cisplatin With Etoposide (EP 50/50)
Abbreviation: NSCLC = non–small-cell lung cancer.
The role of immunotherapy in NSCLC patients receiving radiation will be explored in RTOG Foundation Trial 3505, a phase 3 trial of concurrent chemotherapy with radiation with and without adjuvant nivolumab. In this study, stage III patients with any NSCLC histology lacking EGFR or ALK mutations will be randomized to receive nivolumab or placebo after the conclusion of definitive radiotherapy concurrent with chemotherapy. Adjuvant treatment will be administered every 2 weeks for 1 year or until disease progression or unacceptable toxicity is observed. The primary end point of this study is OS and PFS. The study plans to accrue 660 patients.
SWOG
The Southwest Oncology Group (SWOG) strategic priorities in advanced NSCLC are to use genomics, proteomics, and epigenetics to enhance individualize approaches to cancer patients, to devise strategies to circumvent or prevent targeted therapy resistance, to transform checkpoint immunotherapies into targeted therapies, and to integrate novel technologies into trial design to accelerate progress.
SWOG Early Stage Studies
Strategies to Improve Lymph Node Examination in Non–Small Cell Lung Tumors (SILENT), is a prospective randomization study to increase pathologic node collection through 2 corrective interventions in resectable NSCLC. Patients with cT1-3cN0-1 NSCLC will receive either conventional routine surgical lymph node (LN) collection and routine pathologic examination or a special surgical LN collection and pathology examination wherein a checklist of LN stations facilitate collection of LN by surgeons. Furthermore, special collections will be deposited into prelabeled, color-coded International Association for the Study of Lung Cancer LN map and anatomic description. Follow-up data of 51 resections determined that the median number of LNs examined per case was approximately 5 in the control arm and nearly 13 and 20 in the special group with special pathology examination and special specimen collection and special pathology examination, respectively.
SWOG Advanced Stage Studies
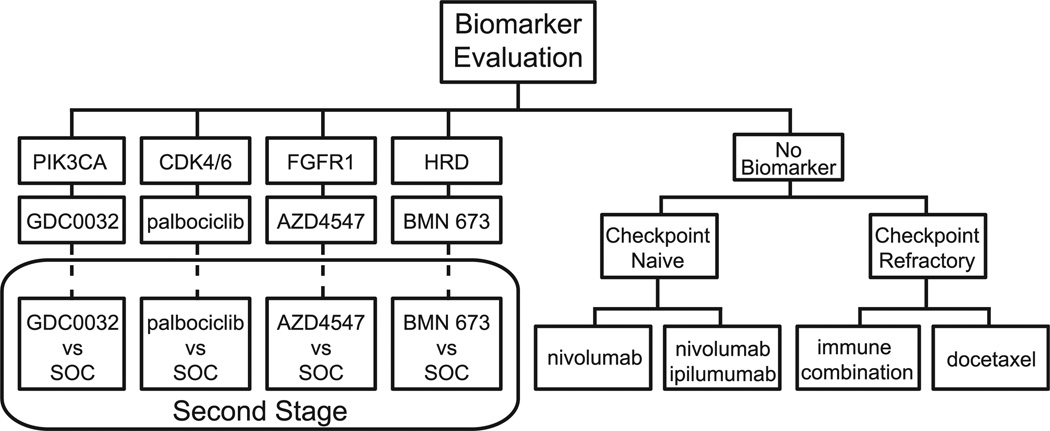
To individualize approaches to cancer patients, S1400, also known as Lung-MAP, is a private–public partnership master protocol that uses an umbrella design to investigate previously treated patients with squamous-cell lung cancer. Here the overarching paradigm is for multiple phase 2/3 substudies with rolling opening and closure to utilize biomarker profiling via Clinical Laboratory Improvement Amendments (CLIA)-certified next-generation sequencing platforms, with each substudy design to be US Food and Drug Administration approval compliant. The design has recently been amended in order to account for the change in therapeutic landscape with the approval of the PD-1–targeted agents nivolumab and pembrolizumab, and also to speed assessment of therapies in the genotypic substudies (Figure 4). Upon identification of a genotypic biomarker match (eg, predefined abnormalities in PIK3CA, CCND1, FGFR, or homologous repair deficiency), patients receive the matched targeted therapy. If a threshold response rate is surpassed, the substudy graduates to a randomized comparison of the targeted drug versus standard-of-care chemotherapy. In the event that the patient does not test for an actionable biomarker (nonmatch), a patient in the second-line setting is randomized to the anti–PDL-1 agent nivolumab or combination immunotherapy with nivolumab–ipilimumab. For those patients whose disease has already failed to respond to second-line PD-1 therapy, a third-line immunotherapy combination is being developed. This study, with participating organizations Alliance, ECOG-ACRIN, NRG, and NCI-Canada, will screen 500 to 1000 patients per year with an expected 65% rate of matching a patient with a drug or biomarker.
Figure 4.
S1400 (Lung-MAP). S1400 Uses An Umbrella Design to Investigate Second-Line Therapy in Treatment of Squamous-Cell Lung Cancer. Multiple Phase 2/3 Substudies With Rolling Opening and Closure Will Utilize Biomarker Profiling via Clinical Laboratory Improvement Amendments (CLIA)-Certified Next-Generation Sequencing Platforms. Patients Will Receive Matched Targeted Therapy Upon Identification of Genotypic Biomarker (eg, PIK3CA, CCND1, FGFR, or Homologous Repair Deficiency). If Threshold Response Rates are Surpassed, Targeted Drugs in Substudies Graduate to Randomized Comparison Versus Standard-of-Care Chemotherapy. Patients Without Actionable Biomarkers and Who Are Checkpoint Naive Will Be Randomized to The Anti–PDL-1 Agent Nivolumab or Combination Immunotherapy With Nivolumab–Ipilimumab. Patients Whose Disease Has Already Failed to Respond to Second-Line PD-1 Therapy (Checkpoint Refractory) Will Be Treated by A Third-Line Immunotherapy Combination Under Development
For circumvention or prevention of targeted therapy resistance, S1403 is a phase 2/3 trial of afatinib with or without cetuximab in patients with stage IIIB-IV EGFR-mutant NSCLC who are tyrosine kinase inhibitor (TKI) naive. The protocol calls for biopsy at progression to study mechanisms of targeted therapy resistance via genomic studies as well as an optional patient-derived xenograft, the latter undertaken by the SWOG translational science center in cooperation with Jackson Laboratories and Cold Spring Harbor. In addition, circulating free DNA analysis will be analyzed by next-generation sequencing every 2 months as well as at the point of disease progression.
Alliance for Clinical Trials in Oncology
The Respiratory Committee of the Alliance oversees NSCLC, small-cell lung cancer (SCLC), and mesothelioma studies to reduce the impact of cancer, with scientists and clinicians committed to discovering, validating, and disseminating strategies for cancer treatment and prevention. The Alliance includes legacy groups Cancer and Leukemia Group B (CALGB) as well as ACOSOG (American College of Surgeons Oncology Group) and NCCTG (North Central Cancer Treatment Group).
Alliance Early Stage Studies
CALGB 140503 is a phase 3 randomized trial of lobectomy versus sublobar resection for ≤ 2 cm peripheral NSCLC. In this study, patients with suspected peripheral T1N0 lung cancers that are < 2 cm in size are randomized after pathologic confirmation of node-negative disease (R level 4, 7, and 10 and L 5 or 6, 7, 10) to undergo either lobectomy or limited resection. Patients are stratified on the basis of tumor size, histology, and smoking status. The primary objective of this study is to determine whether DFS after sublobar resection is noninferior to lobar resection. Imaging objectives are to correlative preoperative CT and PET characteristics with outcome, and to also determine the false-negative rate for PET in hilar and mediastinal nodal metastases. Last, the utility of annual follow-up CT after resection will be determined in this cohort of patients with stage I disease.
Alliance Advanced Stage Studies
A081105, a substudy of ALCHEMIST, investigates 2 years of erlotinib or placebo in patients with resectable stage I-IIIA EGFR-positive NSCLC who have completed surgical and adjuvant treatment. This study occurs in parallel with the EA4512 ALCHEMIST substudy on ALK-positive NSCLC.
AFT-09 is a randomized phase 3 evaluating the optimal sequencing of PD-1 inhibition with MK-3475 (pembrolizumab) and standard platinum based chemotherapy in stage IV NSCLC. Patients with treated central nervous system metastases are included, and adequate tissue is required to evaluate PD-L1 status. One patient cohort will receive a platinum doublet with paclitaxel or pemetrexed every 3 weeks for 4 cycles, followed by MK-3475 every 3 weeks for up to 1 year. The other cohort will receive the same agents with a sequence of MK-3475 for 4 cycles, then platinum doublet, then MK-3475 for up to 1 year. Primary objective is overall response rate, and secondary objectives include PFS and safety and tolerability of MK-3475.
ACCRU RC1126 is a randomized phase 2 trial of erlotinib alone or in combination with bevacizumab in patients with EGFR-mutant NSCLC. The study is currently at over half of 112 total accruals. In this trial, patients with stage IIIB-IV EGFR mutations are randomized 1:1 to either erlotinib or erlotinib in combination with bevacizumab. The primary end point is PFS; secondary end points include OS, tumor response, quality of life, and safety. In a similar trial published by Japanese investigators, at 27 months’ follow-up, median PFS was 16 months for combination therapy as opposed to monotherapy.7 Subgroup analysis identified a median PFS for exon 19–deleted EGFR-mutant patients of 18 months for combination therapy and 10 months for monotherapy compared to exon 21 L858R EGFR-mutant patients, who had 13.9 months and 7.1 months of therapy, respectively.
AFT-07 is a phase 1/2 of veliparib, a poly ADP ribose polymerase (PARP) inhibitor, in combination with carboplatin and paclitaxel and thoracic radiation therapy for unresectable IIIA-IIIB NSCLC. In this 3 + 3 design, patients receive veliparib 3 days before chemoradiation at 1 of 4 dose levels. Veliparib is continued during chemoradiation, then resumed during the 2 additional cycles of carboplatin and paclitaxel starting 3 days before each cycle. The primary end point is PFS, and secondary end points are OS, overall response rate, and chemotherapy-induced peripheral neuropathy.
CTONG
The Chinese Thoracic Oncology Group, established in 2007, comprises 30 member institutions spanning several regions in the country.
CTONG Early Stage Studies
CTONG 1103, the Erlotinib Versus Gemcitabine/Cisplatin as (Neo)Adjuvant Treatment in Non–small Cell Lung Cancer (EMERGING) study, is a randomized phase 2 study examining the effect of neoadjuvant erlotinib versus neoadjuvant chemotherapy in patients with resectable IIIA-N2 NSCLC. In this study, which is currently accruing, treatment-naive patients with IIIA EGFR-mutant NSCLC with N2 disease confirmed by mediastinoscopy, endobronchial ultrasound, or PET are randomized 1:1 to receive either 6 weeks of erlotinib or 2 cycles of gemcitabine with cisplatin. After neoadjuvant treatment, patients without disease progression will proceed to surgery followed by adjuvant treatment with the same agent used in the neoadjuvant setting.
CTONG 1104, the Gefitinib Versus Vinorelbine/Platinum as Adjuvant Treatment in stage II-IIIA (N1-N2) NSCLC With EGFR Mutation (ADJUVANT) study, is a phase 3 randomized trial examining stage II-IIIA (N1-N2) EGFR-mutant (either exon 19 deletion or exon 21 L858R) NSCLC randomized 1:1 to receive either gefitinib daily for 24 months or until disease progression or unacceptable toxicity or cisplatin and vinorelbine every 3 weeks up to 4 cycles. The primary objective is DFS, and secondary end points are OS, 5-year OS rate, 3-year DFS rate, 5-year DFS rate, safety, and exploratory biomarker analysis. The study completed accrual and results are awaited.
CTONG Advanced Stage Studies
CTONG 120 is a phase 3 randomized study examining the efficacy of the first-generation EGFR TKI icotinib on brain metastases in stage IV EGFR-mutant NSCLC. Patients receiving first- or second-line therapy with central nervous system metastases are randomized 1:1 to receive either whole-brain irradiation or icotinib and intracranial PFS is the primary end point.
CTONG 1405 (BENEFIT) is a single-arm trial studying the efficacy and safety of gefitinib in patients with metastatic lung adenocarcinoma with EGFR mutations detected from plasma by digital droplet polymerase chain reaction. The primary end point of this study is overall response rate, and secondary end points are disease control rate, PFS, OS, and safety evaluation.
Previous studies from CTONG highlighted at the meeting included CTONG 0802, the OPTIMAL study that demonstrated first-line erlotinib had a median PFS of 13.1 versus 4.6 months for gemcitabine and carboplatin in EGFR-mutant patients.8 CTONG 0804, the INFORM study, demonstrated no difference in OS between gefitinib and placebo in advanced NSCLC patients not selected for EGFR mutations.9 CTONG 0902 (FASTACT-II) demonstrated a significant increase in median PFS for erlotinib (16.8 months) versus placebo (6.9 months) when intercalated between each cycle of first-line chemotherapy, but no significant difference in OS was noted.10
KCSG
KCSG oversees multicenter trials on thoracic malignancy. One study, KCSG LU05-04, focuses on the benefit of consolidative chemotherapy in patients with locally advanced inoperable stage III NSCLC receiving concurrent chemoradiotherapy with docetaxel and cisplatin. At a median follow-up of 50.7 months, there was no significant difference (P = .438) in median OS between the observation arm (20.63 months) and the consolidative chemotherapy arm (21.78 months).11
LCRG/ATORG
The Lung Cancer Research Group (LCRG), founded in 2001 and including members from Hong Kong, Guangzhou, Shanghai, Seoul, Taipei, Sydney, and Singapore, contributed to the findings of the earlier Iressa Pan-Asia Study (IPASS), which demonstrated efficacy of gefitinib compared to chemotherapy in patients with EGFR mutations.12 The LCRG was dissolved in 2009; however, several member institutions of the LCRG formed the Asian Thoracic Oncology Group in 2015. The first proposed study will be to examine the response rate of the third-generation EGFR TKI osimertinib in patients with EGFR-mutant stage IV adenocarcinoma with EGFR TKI resistance and the presence of EGFR T790M mutation in plasma circulating free DNA. In addition to response rate, the investigators will also monitor the dynamics of T790M mutation signal in patient plasma.
EORTC/ETOP
EORTC, headquartered in Brussels, Belgium, is a multinational and multidisciplinary organization that aims to develop and conduct translational and clinical research in Europe to improve the management of cancer. ETOP, formed in 2009 in Bern, Switzerland, comprises more than 50 member institutions across Europe and is focused on advancing knowledge in thoracic malignancies with the sponsoring and management of translational and clinical studies.
EORTC/ETOP Early Stage Studies
EORTC 22055-08053 (LUNG-ART) is a randomized trial examining the benefit of adjuvant thoracic radiation in patients with completely resected NSCLC with pathology-proven N2 involvement. The primary end point is DFS, with secondary end points being local control, OS, secondary malignancy, and late toxicities. As of 2015, approximately 200 patients have entered this study.
In addition to the above studies, EORTC has currently recruited 236 out of 2000 patients for EORTC 08114 Genetics of EGFR Mutation (GEM), a translational study of the EORTC Lung Cancer Group. The overall objectives of this study are to characterize germ-line DNA variants associated with NSCLC patients that arise in never or former light smokers as well as tumors with EGFR mutations. These germ-line DNA variants will then be correlated with EGFR-mutant NSCLC patient survival.
EORTC/ETOP Advanced Stage Studies
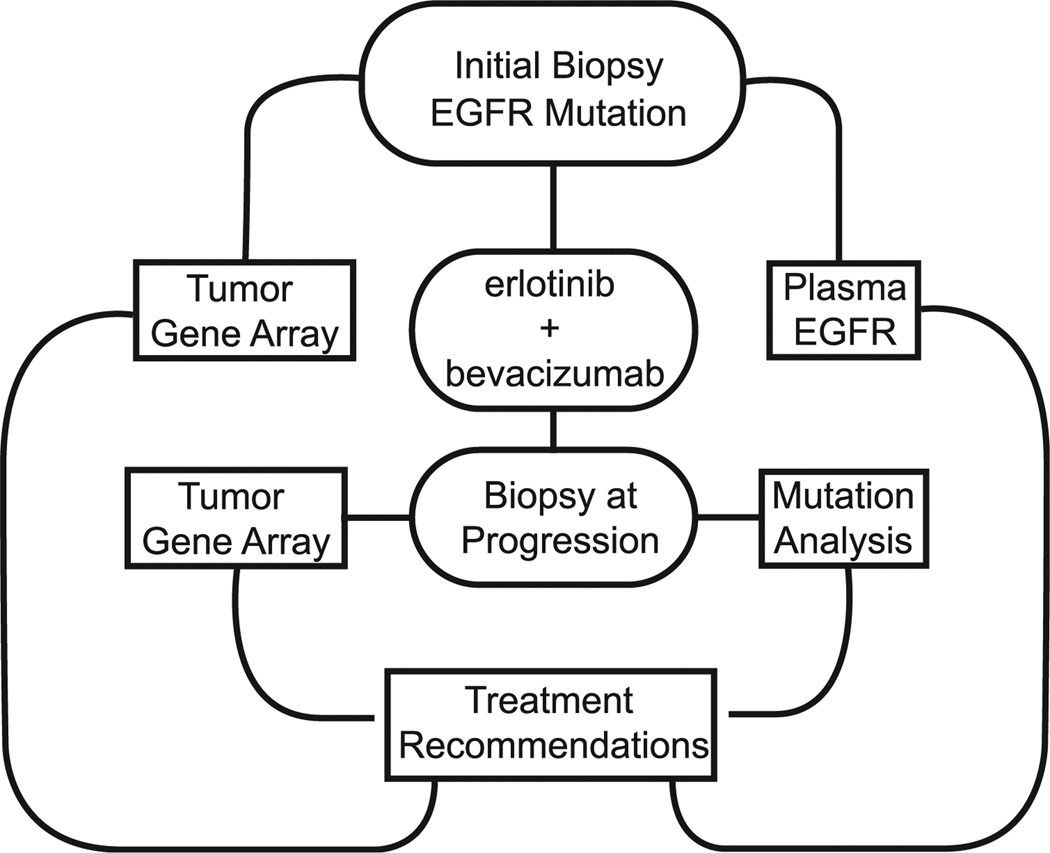
ETOP 2-11 BELIEF is an open-label phase 2 trial of erlotinib and bevacizumab in EGFR-mutant NSCLC (Figure 5). This study, which closed accrual in October 2014 with 102 patients, will treat EGFR-mutant patients with combination erlotinib and bevacizumab until progression or unacceptable toxicity. Data presented at the 2015 European Cancer Congress showed that EGFR T790M-positive patients had a median PFS of 16 months compared to 10.5 months in EGFR-mutant patients without T709M.13 In parallel, translational projects will also examine tumor gene expression array and circulating plasma EGFR mutation monitoring to enhance recommendations for future chemotherapy.
Figure 5.
EORTC BELIEF. The Phase 2 EORTC BELIEF Study Investigates First-Line Erlotinib And Bevacizumab And Utility Of Translational Approaches To Recommend Second-Line Treatments From Tumor Gene Array Of Initial And Postprogression Biopsies. Data Presented At European Cancer Congress Has Shown T790M-Positive Patients Have Increased Progression-Free Survival Compared to T790M-Negative Patients.
ETOP 3-12 EMPHASIS-Lung is a phase 3 randomized trial examining docetaxel versus erlotinib in second-line therapy for advanced squamous-cell NSCLC. In addition to the primary end point, patient outcome will also be examined by stratification before randomization with performance status and the use of a plasma-based assay, VeriStrat, which categorizes patients on the basis of the level of inflammatory proteins in circulation. This study closed early due to slow accrual in December 2014.
EORTC 08092 examined the benefit of pazopanib maintenance therapy versus placebo in patients with IIIB-IV NSCLC who had partial response or > 4 cycles of chemotherapy, without TKI therapy. However, the study was halted after a planned interim analysis of 102 patients showed futility.
One goal of ETOP is a translational project, Lungscape, where participating sites share information from their NSCLC biobanks into a centralized database for the purposes of clinical trial design and generating biologic hypotheses. Recently published studies from the Lungscape project featured at this meeting included correlating clinical outcome by clinical and pathologic parameters, clinical outcomes of resected ALK-positive NSCLC, and quality assessment of EGFR mutation testing.14–16
NCIC CTG
The NCIC CTG focuses on trials in cancer therapy, supportive care, and prevention throughout Canada. NCIC CTG also participates with other cooperative groups in North America.
NCIC CTG Early Stage Studies
BR 31 is a prospective phase 3 randomized trial comparing adjuvant MEDI4736, an anti–PD-L1 monoclonal antibody, to placebo in completely resected NSCLC. Here, patients with IB (< 4 cm) to IIIA NSCLC who have had complete resection are randomized 2:1 to receive MEDI4736 versus placebo, respectively. The first 600 accrued patients will undergo randomization regardless of PD-L1 status, whereas the latter 600 will not undergo randomization and enrollment unless the tumors are PD-L1 positive. The primary end point of this study is DFS in PD-L1–positive patients. Secondary end points are DFS in all patients, OS in all patients, and quality of life.
NCIC CTG is also participating in CALGB 140503 under the name BRC.5.
NCIC CTG Advanced Stage Studies
NCIC CTG IND215 is a multiarm phase 1 trial to evaluate the dose and schedule of standard first-line doublet chemotherapy in combination with the MEK inhibitor selumetinib. The expansion phase of this study will examine the effect of selumetinib in KRAS-mutant patients.
IND219 is a randomized phase 2 trial of selumetinib in conjunction with cisplatin and pemetrexed in patients with wild-type or unknown KRAS nonsquamous NSCLC. Here, patients are randomized to standard induction cisplatin pemetrexed versus the same regimen in combination with varying selumetinib dose schedules. An interim analysis of the response rate for each selumetinib dose schedule will then be undertaken, after which a single selumetinib combination regimen will be used for future enrolled patients compared to standard cisplatin pemetrexed in a 3:1 randomization. The primary end point of this study is response rate.
NCIC CTG IND211 is a randomized phase 2 study of the oncolytic virus Reolysin in combination with second-line therapy in patients with advanced or metastatic NSCLC. The primary end point of this study is PFS, and secondary end points include overall response rate, OS, progression rate at 3 months, and exploratory analysis on circulating tumor cell status with clinical outcome.
Small-Cell Lung Cancer
Cooperative groups presented studies on SCLC and included ECOG-ACRIN, EORTC, ETOP, Alliance, and NRG-RTOG. E2511 is a phase 1/2 randomized double-blind trial examining the PARP inhibitor veliparib versus placebo in combination with cisplatin and etoposide in first-line treatment of extensive stage SCLC. Unconfirmed best response of 7 patients in the phase 1 study was 1 with complete response, 4 with partial response, and 2 with stable disease. ETOP 4-13 STIMULI is a randomized phase 2 trial of consolidation ipilimumab versus placebo in patients who have undergone chemotherapy and radiation for limited stage SCLC. The randomized trial on Chest irRadiation in Extensive disease SCLC (CREST, NTR1527) is a randomized phase 3 trial of extensive stage SCLC patients in the Netherlands examining the 1-year OS between patients who did or did not receive adjunct thoracic radiotherapy after first-line chemotherapy. The EORTC 08072 Concurrent Once-daily Versus twice-daily Radiotherapy (CONVERT) study addresses the question of concurrent cisplatin and etoposide with either 60 Gy of radiotherapy in daily fractions or 45 Gy in twice-daily fractions. The study will be presented in 2016. In a similar vein, CALGB 30610/RTOG 0538 is a phase 2 study comparing 45 Gy radiotherapy in twice-daily fractions versus 70 Gy in daily fractions in patients with limited SCLC who also receive cisplatin and etoposide. The study has currently accrued 446 out of 729 patients. CALGB 30504 is a phase 1/2 trial that examines the role of maintenance sunitinib subsequent to first-line platinum and etoposide in patients with extensive stage SCLC. Intermediate analysis demonstrated a statistically significant increase in median PFS with a 1 sided t test. No difference in OS was observed. However, in patients who underwent prophylactic cranial irradiation, an increase in OS was noted by 3.5 months, whereas no difference was noted in PFS.17
Malignant Pleural Mesothelioma
CALGB 30901 is a randomized phase 2 trial evaluating PFS for maintenance pemetrexed versus observation for patients with malignant pleural mesothelioma without progression after first-line chemotherapy. Nearly half of an expected 137 patients have been accrued.
Conclusion
The treatment of NSCLC and other thoracic malignancies has greatly benefited from the efforts of cooperative groups worldwide. Personalized treatment through the use of biomarkers is an emerging paradigm outlined by many groups during the 16th International Lung Congress across the spectrum of treatment such as chemotherapy, targeted therapy, immunotherapy, and radiation. Translational research and biomarker studies are now routinely included in clinical trials, as highlighted in ALCHEMIST, Lung-MAP, Lungscape, and S1403 in EGFR-mutated lung cancer. Through these cooperative group led studies in the field of lung cancer, more promising individualized and effective treatments are expected that can improve patient survival and enhance clinical outcomes.
Footnotes
Disclosure
The authors have stated that they have no conflict of interest.
References
- 1.Carbone DP, Gandara DR, Antonia SJ, Zielinski C, Paz-Ares L. Non–small-cell lung cancer: role of the immune system and potential for immunotherapy. J Thorac Oncol. 2015;10:974–984. doi: 10.1097/JTO.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen KS, Neal JW, Wakelee H. Review of the current targeted therapies for non–small-cell lung cancer. World J Clin Oncol. 2014;5:576–587. doi: 10.5306/wjco.v5.i4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riess JW, Wakelee HA. Metastatic non–small cell lung cancer management: novel targets and recent clinical advances. Clin Adv Hematol Oncol. 2012;10:226–234. [PubMed] [Google Scholar]
- 4.Wakelee H, Kelly K, Edelman MJ. 50 years of progress in the systemic therapy of non–small cell lung cancer. Am Soc Clin Oncol Educ Book. 2014:177–189. doi: 10.14694/EdBook_AM.2014.34.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timmerman RD, Paulus R, Pass HI, et al. RTOG 0618: stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. J Clin Oncol. 2013;31 abstract 451942. [Google Scholar]
- 6.Belderbos JS, Heemsbergen WD, De Jaeger K, Baas P, Lebesque JV. Final results of a phase I/II dose escalation trial in non–small-cell lung cancer using three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:126–134. doi: 10.1016/j.ijrobp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non–squamous non–small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 8.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Ma F, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non–small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol. 2012;13:466–475. doi: 10.1016/S1470-2045(12)70117-1. [DOI] [PubMed] [Google Scholar]
- 10.Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non–small-cell lung cancer (FAS-TACT-2): a randomised, double-blind trial. Lancet Oncol. 2013;14:777–786. doi: 10.1016/S1470-2045(13)70254-7. [DOI] [PubMed] [Google Scholar]
- 11.Ahn JS, Ahn YC, Kim JH, et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non–small-cell lung cancer: KCSG-LU05-04. J Clin Oncol. 2015;33:2660–2666. doi: 10.1200/JCO.2014.60.0130. [DOI] [PubMed] [Google Scholar]
- 12.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 13.Stahel RA, Dafni U, Gautschi O, et al. A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced non–small-cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations with and without T790M mutation. The Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial. European Cancer Congress, Vienna, Austria, 2015. Eur J Cancer. 2015;51(Suppl 3) abstract S711. [Google Scholar]
- 14.Patton S, Normanno N, Blackhall F, et al. Assessing standardization of molecular testing for non–small-cell lung cancer: results of a worldwide external quality assessment (EQA) scheme for EGFR mutation testing. Br J Cancer. 2014;111:413–420. doi: 10.1038/bjc.2014.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackhall FH, Peters S, Bubendorf L, et al. Prevalence and clinical outcomes for patients with ALK-positive resected stage I to III adenocarcinoma: results from the European Thoracic Oncology Platform Lungscape Project. J Clin Oncol. 2014;32:2780–2787. doi: 10.1200/JCO.2013.54.5921. [DOI] [PubMed] [Google Scholar]
- 16.Peters S, Wader W, Dafni U, et al. Lungscape: resected non–small-cell lung cancer outcome by clinical and pathological parameters. J Thorac Oncol. 2014;9:1675–1684. doi: 10.1097/JTO.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 17.Ready NE, Pang HH, Gu L, et al. Chemotherapy with or without maintenance sunitinib for untreated extensive-stage small-cell lung cancer: a randomized, double-blind, placebo-controlled phase II study—CALGB 30504 (Alliance) J Clin Oncol. 2015;33:1660–1665. doi: 10.1200/JCO.2014.57.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]