Abstract
The motivation to eat, as operationalized by measuring how hard someone will work for food, is cross-sectionally and prospectively related to obesity. Persons high in food reinforcement consume more calories, and energy intake mediates the relationship between food reinforcement and obesity. Research has shown avid sucking for milk in early infancy predicts later adiposity, and the relationship between food reinforcement and excess body weight has been observed in infants as young as 9 months of age. New methodological developments in studying food reinforcement in infants and young children provide the first opportunity to study the origin of food reinforcement. This review seeks to provide background on the measurement of food reinforcement, and to present, for the first time, prenatal and postnatal predictors of infant food reinforcement. Lastly, potential mechanisms for an increasing trajectory of food reinforcement throughout development are proposed.
Keywords: Obesity, Food reinforcement, Infancy, Development
1. Introduction
One of the most important parts of infants’ behavioral repertoire is their ability to seek and consume food; one of the first infant-mother interactions is infant feeding, either through breast or formula feeding. When a mother feeds/nurses her infant, it creates a time to foster bonding. Infants move from bottle feeding to solid food consumption at about 4–6 months (American Academy of Pediatrics, 2009), when they experience new tastes, textures, and smells that can stimulate their appetite. During this transition from a milk-based diet to a wide variety of solid foods, infants develop their food preferences. After mastering the pincer grasp, infants begin to finger-feed, which provides one of the first ways to demonstrate control over their environment. This important developmental trajectory is possible because infants come into the world prepared to eat, and they seek food, will cry when they are hungry, and gain satisfaction and pleasure from food. This is, in part, due to the fact that food is a primary reinforcer (Francis et al., 1999), and infants do not need to learn to want food. The symbiotic relationship between food as a primary reinforcer and the simultaneous infant development of sucking, chewing, gaining motor control to finger-feed themselves, and learning their autonomy in food preferences sets the stage for normal development of eating. The fact that food is a primary reinforcer (Kelley and Berridge, 2002) may provide clues to how food can become too reinforcing, leading to obesity.
Heavier infants (Kong et al., 2015), children (Temple et al., 2008b), and adults (Epstein et al., 2014a; Giesen et al., 2010; Saelens and Epstein, 1996) find food more reinforcing than leaner peers. High levels of food reinforcement also predicts greater weight gain for children (Hill et al., 2009), adolescents (Epstein et al., 2014b), and adults (Carr et al., 2014). In addition, people who find food more reinforcing consume more food in the laboratory and natural environment than those who find food less reinforcing (Epstein et al., 2011). The relative reinforcing value of food is a predictor of short and long-term weight loss, as those who lack access to alternative reinforcers to food have less treatment success (Buscemi et al., 2014).
This review will focus on reviewing evidence of the role of food reinforcement in obesity development starting as young as infancy. There are two aims of this review. First, we seek to provide a brief description of the measurement of food reinforcement, developmental perspective on food reinforcement during infancy, and mechanisms and implications for an increasing trajectory of food reinforcement that may lead to obesity. Second, we use this review to present, for the first time, prenatal and postnatal predictors of infant food reinforcement by combining three sets of data from our laboratory.
2. Measurement of food reinforcement
The basic paradigm to assess food reinforcement is similar to the paradigm for assessing the reinforcing value of drugs of abuse (Epstein et al., 2007a). The paradigm has individuals work for food on progressive ratio schedules of reinforcement. Participants earn a standardized food portion after they meet schedule requirements, and the schedule progressively increases. The maximal amount of work they perform to obtain food determines the reinforcing value of that food. The reinforcing value task is computer based and uses mouse button presses as the instrumental response. In animal laboratories it is common to assess the effect of a schedule of reinforcement on responding for food across multiple sessions, with one schedule per session. It is also common to have animals work for food on the same schedule until their responses are stable before they move on to the next schedule (Richardson and Roberts, 1996). This methodology has also been used to assess reinforcing value of drugs in humans (Bickel et al., 1991; Shahan et al., 1999). However, this paradigm is not feasible for studying individual differences in reinforcing value of food. We have adapted this technology by having subjects advance through progressive schedules within the same session, making it possible to determine reinforcing value of the food(s) studied within one session. The amount of food provided needs to be enough to warrant responding to obtain it, but not so much that subjects will become satiated and not work any longer.
The absolute reinforcing value of food is measured by having the subject only work for food, and the relative reinforcing value by providing access to food and other foods or alternatives to food on concurrent schedules of reinforcement. The relative reinforcing value has been studied in older children (Hill et al., 2009; Temple et al., 2008b) and adults (Epstein et al., 2014a; Giesen et al., 2010; Saelens and Epstein, 1996). In infants, the reinforcing value of food and alternatives to food were studied, but not in a concurrent schedules paradigm. In infant studies (Kong et al., 2016), the choices were presented sequentially in a counterbalanced fashion.
2.1. Measurement of food reinforcement in infants
There is plenty of opportunity to observe how infants use crying as an instrumental response to obtain things they want (i.e. milk), or remove unpleasant things (i.e. wet diaper, needing attention from parents) at a very early age. The reinforcing value of various stimuli has been measured in infants, including the landmark studies in which babies’ level of physical activity was increased by making the motion of a mobile contingent upon movement of the baby’s leg kicks (Rovee and Rovee, 1969). There have been other demonstrations that infants learn an instrumental response to get access to social, auditory/visual, and food reinforcers (Chorna et al., 2014; Lowe et al., 1983; Standley, 2003; Wormith et al., 1975). For example, research has demonstrated that 9 and 10 month-old-infants would press a large bar in front of them to obtain music or food on variable interval schedules of reinforcement (Lowe et al., 1983).
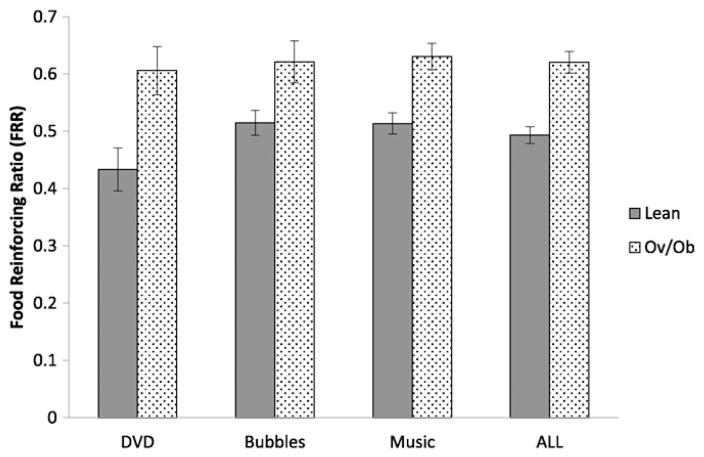
Based on this research, we adapted the reinforcing value task for older infants (≥9 months of age) by using a larger, single button response as the instrumental response in a developmentally appropriate laboratory setting (Kong et al., 2015, 2016). Nine to eighteen month old infants can make purposeful movements to reach for and grab items, most have begun to finger-feed themselves, and they can sit upright. Schedule requirements begin with one response, and progress up to 15 responses to earn a small portion of their favorite food or time accessing an alternative reinforcer. Infants work for the reinforcer until they lose interest, begin to cry, or make it clear they are finished. Our results demonstrate that the reinforcing value of food versus non-food alternatives (food reinforcement ratio, or FRR) is related to infant weight status. We have completed 3 cohorts of infants using the paradigm we developed in our lab by assessing food reinforcement versus three different alternative reinforcers: Baby MacDonald™ video (DVD; n = 27); playing with bubbles (Bubbles; n = 30); and music (Music; n = 49). The food versus non-food reinforcers were offered to the infants in a sequential fashion in each cohort. In each sample we observed a positive relationship between FRR and infant weight status across the three different types of alternatives (DVD, r = 0.60, p < 0.001; Bubbles, r = 0.49, p = 0.006; Music, r = 0.38, p = 0.009) (as shown in Fig. 1). The overall correlation between FRR and weight for length z-score was r = 0.49 (p < 0.0001).
Fig. 1.
Infant obesity status in relation to food/non-food reinforcement. Infants aged 9–18 months old performed the developmentally appropriate food/non-food reinforcement task in three different studies using three different types of non-food reinforcers [Study 1: watching Baby Einstein-Baby MacDonald™ (DVD), lean n = 18, overweight and obese (Ov/Ob) n = 9; Study 2: playing with bubbles (Bubbles), lean n = 17, Ov/Ob n = 13; Study 3: music engagement (Music), lean n = 37, Ov/Ob n = 12]. In the integrated sample, there were 72 lean and 34 Ov/Ob infants. Reinforcing values of food and non-food alternatives were determined using the maximum schedule achieved for food (Food Pmax) and non-food alternative reinforcer (ALT Pmax). Food reinforcing ratio (FRR) was determined by calculating proportion of food responses among all responses [Food Pmax/(Food Pmax + ALT Pmax)]. Linear regression model shows that Ov/Ob infants had significantly higher FRR-DVD (Lean: 0.43 ± 0.04 [mean ± SEM], Ov/Ob: 0.61 ± 0.05; p = 0.009), FRR-Bubbles (Lean: 0.51 ± 0.03, Ov/Ob: 0.62 ± 0.03; p = 0.01) and FRR-Music (Lean: 0.51 ± 0.02, Ov/Ob: 0.63 ± 0.03; p = 0.002). Similarly, when all three studies were combined the pattern of responding between lean vs. Ov/Ob infants remained consistent (Lean: 0.50 ± 0.01, Ov/Ob: 0.62 ± 0.02; p < 0.0001).
There is no research on measurement of food reinforcement in infants younger than 9 months of age. A developmentally appropriate instrumental response for younger infants is sucking. There have been several studies that have used sucking as an operant response to study infant learning. For example, Wormith et al. (1975) showed that infant sucking could be increased by a contingent audio cue. Investigators have shown infant sucking can be used to study infants’ capability of discriminating among pure tones varying in frequency. Premature infants may have an underdeveloped sucking reflex, and making maternal voice (Chorna et al., 2014) or musical stimulation (Standley, 2003) contingent upon sucking can improve their nutritive sucking capability.
There are no studies in which food reinforcement has been assessed through sucking, though there have been several studies in which baby sucking has been measured in relationship to weight status, or growth. These studies suggest that the more intensely the infant sucks for milk or formula, the greater relative weight gain later in childhood (Agras et al., 1990; Agras et al., 1987; Stunkard et al., 2004). The intensity of sucking would suggest that those who suck more avidly for milk would meet higher response requirements to obtain milk. Food reinforcement could be studied in young infants by arranging the schedule such that the infant would need to make increasingly more sucks to obtain milk, or would have to increase the intensity of effort required to derive milk from the bottle. Either approach could be programmed on progressive schedules, so that the breakpoint could be determined. Development of a method to measure food reinforcement in young infants would be a major methodological advancement for studying how early food reinforcement develops, and studying the trajectory of how abnormal food reinforcement can develop.
2.2. Early predictors of food reinforcement in infants
By using the combined data across three studies, we assessed prenatal (maternal pre-pregnancy weight, gestational weight gain (GWG)) and postnatal (duration of breast feeding, introduction of solid foods) factors as predictors of infant food reinforcement. Zero-order correlations indicated that greater maternal pre-pregnancy BMI (r = 0.28, p = 0.003) and greater GWG (r = 0.24, p = 0.01) were associated with higher FRR. For the postnatal factors, shorter breastfeeding duration (r = −0.21, p = 0.03), but not timing of introduction of solid foods (r = −0.007, p > 0.05), was associated with greater FRR. Type of alternative reinforcer did not moderate any of the relationships between prenatal and postnatal factors and food reinforcement or FRR.
We examined the strength of prenatal and postnatal factors on FRR using hierarchical regression analyses. The baseline model included infant age, sex, and birthweight, and maternal parity, age, and education as covariates. We added prenatal factors of pre-pregnancy BMI and maternal GWG into the model as second step and postnatal factors of breastfeeding duration and timing of solid food introduction as third step. The order of variables entered into the model was based on the natural progression of infant growth. To ensure reliability across the three datasets, bootstrapping estimates from 500 samples of n = 35 infants with replacement allowed estimating overall beta, at 99% confidence intervals (CI). Using bootstrapping, we demonstrated positive estimates of the associations of pre-pregnancy BMI (β = 0.006, 99% CI [0.005–0.006]) and GWG (β = 0.064, 99% CI [0.058–0.069]) with FRR, while shorter breastfeeding duration was associated with increased FRR (β = −0.006, 99% CI [−0.006 to −0.005]).
We did not observe an interaction between prenatal and postnatal factors and type of non-food reinforcers for FRR. The baseline model of the hierarchal regression analysis to predict FRR accounted for 9.8% of the variance, with no control variable independently predicting FRR. Adding prenatal factors increased the variance accounted for from 9.8% to 23.9% (FINC(2,98) = 8.26, p = 0.005). FRR was associated with pre-pregnancy BMI (p = 0.002) and GWG (p = 0.02). Adding postnatal factors slightly increased the variance accounted for to 27.5% (FINC(2,98) = 3.70, p = 0.11). When we entered each prenatal and postnatal predictor last in the models, the regression analysis demonstrated that pre-pregnancy BMI (7.2%), maternal GWG (4.1%), and breastfeeding duration (3.7%) each accounted for unique variance in FRR.
2.2.1. Maternal weight at conception
Maternal weight at conception may predict infant FRR because mothers who are overweight/obese at conception may expose the developing fetus to excessive levels of nutrients, such as glucose and free fatty acids (Freinkel, 1980), that could influence brain reward pathways and contribute to future obesity status. Research also suggests that parent and child food reinforcement is similar (Epstein et al., 2008), and this may be influenced by shared genes and environment. There are a variety of genes that have been implicated in high food reinforcement, including dopamine genes and FTO (Epstein et al., 2007b; Scheid et al., 2014). Concordance on the number of Taq1 A1 alleles for the dopamine D2 receptor is associated with strikingly similar changes in parent and child weight change in family-based treatment, which may be due to shared motivation to eat (Epstein et al., 2010). The home environment is important for the development of obesity as easy access to high energy dense foods and sedentary activities, and low access to healthy foods, can be a “toxic” environment (Hill et al., 2003). Infant home environment could influence growth as infants do not make their own choices regarding food or non-food alternative activities.
2.2.2. Gestational weight gain
Elevated GWG may influence child food reinforcement by providing excessive levels of nutrients throughout fetal development. Gestational weight gain is related to birthweight and child obesity, providing a potential pathway for risk factors for high child motivation to eat. Excessive GWG is the result of positive energy balance, which can influence maternal glucose and insulin regulation, which increases risk of large-for-gestational-age babies (Ferraro et al., 2013; Siega-Riz et al., 2009). It may not only be the amount of food that is consumed, but also the types of food and the pattern of intake that is important. Cravings that lead to binges may result in intermittent peaks in glucose metabolism that are as important as the average level for understanding food reward. In drug addiction, research has shown that peaks in blood levels drive drug self-administration, not the average level of a drug (Kimmel et al., 2008; Volkow et al., 2007), and the same process may occur for the developing brain and glucose metabolism.
There is developing literature on how maternal intake can influence infant food reward processes. Both animal and human research has shown that maternal intake can influence infant food preferences. Creative experimental research by Mennella (2014) has shown that this effect may be transmitted in part by infant exposure to flavors of food during gestation, and also during the postnatal period through breast milk. A consistent body of research has studied the effect of maternal food intake on dopaminergic pathways in infant rats and non-human primates, with the demonstration that high fat, high sugar, “junk food” diets reduce expression of dopaminergic activity in offspring (Bayol et al., 2007; Bellinger et al., 2004; Naef et al., 2011; Rivera et al., 2015; Teegarden et al., 2009; Vucetic et al., 2010). Research on dopamine and food reward has shown that food can increase dopaminergic activity (Berridge, 1996), and over time, there is down regulation of dopamine receptors that could lead to the need to increase intake of these foods to derive the same degree of pleasure (Stice et al., 2011). Demonstrating that these effects can be initiated by maternal intake, and can start early, may shape the development of infant food reinforcement.
2.2.3. Breastfeeding duration
We also showed that shorter duration of breastfeeding was associated with greater infant food reinforcement. Feeding delivery method can influence milk intake and appetite regulation. Compared to breastfeeding, bottle feeding allows greater milk consumption and difficulty in controlling appetite (Li et al., 2010, 2012). Bottle feeding enables caregivers to provide set volumes of milk, which could reduce mothers’ and babies’ sensitivity to fullness and satiety cues. It is possible that high food reinforcement, resulting in excessive eating, overshadows babies’ normal satiety response. We have also shown a positive relationship between food reinforcement and general appetite (Kong et al., 2016), suggesting a possible link to development of the satiety response. Another possible mechanism is the difference in nutrient composition and taste between breastmilk and formula (Beauchamp and Mennella, 2009), which can potentially influence the development of food reinforcement at a young age.
2.2.4. Early food exposure
Simple sugar, or glucose, is a major determinant of the reinforcing value of food as it stimulates the release of dopamine in the mesolimbic reward system (Naef et al., 2011; Teegarden et al., 2009; Vucetic et al., 2010). Many of the foods that infants begin to eat are high in carbohydrate and glycemic index (i.e. infant rice cereal, puffs, teething crackers, etc.). Early consumption (<4 months-of-age) of these foods may be associated with early exposure to foods that stimulate brain reward pathways (Ludwig and Currie, 2010). There is an innate preference for sweet foods in infancy (Mennella, 2014; Ventura and Mennella, 2011), and it is easy to understand how parents may learn to provide sweet foods so that their child is eating and growing. The innate preference for sweets is universal across cultures, and tends to decrease as the child gets older (Mennella, 2014), but there are likely to be wide individual differences in preference for sweets. In adults, investigators argue that sugar may be addictive (Avena et al., 2008), and sugar is a major determinant of the reinforcing value of food (Epstein et al., 2011).
An important aspect of drug reinforcement is the rate of uptake of the drug, and the rapid activation of brain reward centers (Kimmel et al., 2008; Volkow et al., 2007). For example, nicotine patches or nicotine gum provide a relatively slow release of nicotine in comparison to smoking, an ideal nicotine delivery system in which nicotine is quickly absorbed, causing a fast release of dopamine in the brain reward systems (Benowitz, 2008). As Schulte et al. (2015) have argued, the same logic may apply to foods high in glycemic index. High glycemic index foods such as fruit juice, candies, white bread, and highly processed grains are comprised of simple sugars, which are readily available to be absorbed, and can rapidly raise blood glucose, which may be then be absorbed by the brain to provide a highly rewarding sensation that can strengthen future consumption.
In addition to innate preference for sweets and the potential for sweet drinks and foods to activate reward centers in the brain, sweet foods also have a calming effect on infants (Blass et al., 1989; Smith and Blass, 1996). Research has shown that providing sweetened water can soothe a baby more than plain water (Barr et al., 1999; Skogsdal et al., 1997), suggesting that sweet foods may have a negatively reinforcing effect by reducing negative affect. Sweet foods may have a positively reinforcing effect, as well, as parents may quickly learn that when fussing, infants can be soothed by sweet liquids such as juices, even if they are not hungry. Fruit juices are not recommended by the American Academy of Pediatrics for children younger than 6 months of age (American Academy of Pediatrics, 2012). Both mother’s milk and formula have moderate glycemic indices, and they may not have the same impact as fruit juice to soothe a crying baby. The relative importance of sweet foods as positive versus negative reinforcers is to be determined. As Bouton (2000) has argued, the initial learning is powerful, and an infant’s developing brain may be particularly susceptible to early experiences, and thus subsequent learning about food does not remove initial learning. It is easy to hypothesize how early experience with food as a calming agent could lead to children and adults using food to regulate their emotion or moods.
3. Food reinforcement and obesity
In addition to the positive relationship between food reinforcement and obesity in infants, the reinforcing value of food is related to adiposity in 3–5 year-old children, in older 8–12 year-old children, and in adults. Research on children, adolescents, and adults have shown that high food reinforcement predicts body fat, zBMI, and weight gain, respectively (Carr et al., 2014; Epstein et al., 2014a, 2014b; Hill et al., 2009). Research has not yet assessed the developmental aspects of food reinforcement. However, it is reasonable to assume that food reinforcement changes over time, based on individual experience and development. It is logical that food reinforcement increases throughout development for those children who become obese and seek food more avidly than leaner peers. Research with adults suggests that food reinforcement can sensitize, or grow, with repeated exposures (Clark et al., 2010; Temple et al., 2009, 2008a; Temple and Epstein, 2012). This effect is dose dependent, and is based on a paradigm in which food was consumed daily, and it is not known whether other patterns of food exposure lead to sensitization. If sensitization of food reinforcement is shown to be a risk factor for increased growth throughout development, then understanding the pattern, timing, and amount of food exposure may be important in modifying a positive trajectory of food reinforcement.
Food also may increase its value if it is used as a reward (Birch et al., 1980). This is a common practice by parents, caregivers, and teachers, and research suggests that using food as a reward can increase the reinforcing value of that food. Of course, the foods that caregivers normally use as an incentive to get children to engage in specific behaviors are generally very palatable and highly energy dense, such as chocolates and candies, which are already very reinforcing to many children. It remains unknown if parents can use healthy foods as reinforcers for child behavior to increase their value. It is important to recognize that healthy food may increase its reinforcing value by being paired with parental attention and parental praise to change child behavior. Parents can differentially emphasize social versus food reinforcers in parenting to strengthen the reinforcing value of healthy foods. Parents can also use social reinforcers to strengthen the value of non-food reinforcers that may shift the choice of children from eating to non-eating behaviors.
It is also possible that regular bouts of food deprivation can act to increase food reinforcement in the short term, and perhaps repeating these bouts over time may sensitize food reinforcement even if food deprivation is not ongoing (Carr, 2002). There are segments of the population, particularly lower income families, who regularly experience some degree of food deprivation (Wilde and Ranney, 2000). While the impression is that infants and young children are often spared from deprivation when older family members do not have enough to eat, there may be widespread lack of food even for younger family members (Alaimo et al., 2001; Casey et al., 2001). One of the basic ways in which food reinforcement is increased is by food deprivation, which has been used for decades in basic animal behavior laboratories as a way to increase food reinforcement (Cuenya et al., 2015). It might be expected that children who live in poverty and experience food deprivation are at greater risk for developing a strong motivation to eat. We have previously theorized that this relationship may be a root cause of the health disparities of obesity in lower income persons (Lin et al., 2012).
There may be waves of food deprivation and food abundance in some families, and these shifts may characterize some families who use SNAP (Supplemental Nutrition Assistance Program) (Wilde and Ranney, 2000). These families get benefits monthly, and they may purchase large quantities of food after they receive their benefits due to recent deprivation. They repeat the cycle of relative deprivation as the month goes on, leading to periods of binges and deprivation. Basic research suggests that these alternating cycles of deprivation and abundance can sensitize the reinforcing value of sugar, and perhaps also the reinforcing value of fat (Corwin et al., 1998; Dimitriou et al., 2000; Wojnicki et al., 2007). Given that the types of foods that people may choose after a period of food deprivation may not be the healthiest options, it is possible that these cycles may lead to increases in the reinforcing value of unhealthy food, leading to excess obesity in these families.
4. Alternative reinforcers/non-food reinforcers and obesity
While the focus of this review is on food reinforcement, for most people eating is a choice, and responding in a choice situation depends in part on the alternatives that are available. This is the case whether more palatable versus less palatable foods are being studied, or food versus other choices. Older overweight children choose food more reliably than non-food alternatives, while leaner children choose alternatives over food (Temple et al., 2008b). In adults, having fewer alternatives to food is related to greater obesity, and lower weight loss in behavioral weight loss programs. This difference may begin early, as Strauss and Knight (1999) showed that children from birth to six years of age who live in environments that have increased access to cognitively stimulating activities are less obese after 6 years of follow-up than those children who have access to fewer alternatives. Our research showed that infant weight status is positively related to the relative reinforcing value of food, and this relationship is primarily due to a stronger motivation to gain access to the alternatives to food instead of food. One implication of this finding is that strengthening alternative reinforcers may reduce the relative reinforcing value of food. To test this hypothesis we randomized 27 infants who were high in FRR to a music enhancement group, designed to provide a new alternative reinforcer to food, or a play date control group. We found a decrease in the relative reinforcing value of food for those in the music engagement group versus control (Kong et al., 2016). These data suggest that differences in access to alternatives that compete for a young child’s attention, and can provide a source of pleasure, may alter the trajectory of food reinforcement. It may not be the food, but the access to non-food alternatives, or the balance of food to non-food alternatives, that is most important for the development of obesity.
5. Conclusions
In summary, the reinforcing value of food can be measured in infancy, and at 9 months of age the relative reinforcing value of food is related to infant weight status. The reinforcing value of food is related to weight across the lifespan from early childhood through adulthood. Both prenatal and postnatal factors independently predict infant food reinforcement, and these factors are modifiable and could change the trajectory of a child’s life. While sucking rate has been related to childhood obesity, methodological advancement in food reinforcement is needed in younger infants to track the early trajectory of food reinforcement. Research suggests that alternatives to food may be important factors in excess energy intake during infancy, as parents can provide access to stimulating non-food activities to foster alternatives to eating. There is a great potential for improving our ability to prevent childhood obesity by using food reinforcement as one important predictor. By developing methods to alter the trajectory of food reinforcement, we may be able to prevent childhood and adult obesity.
Acknowledgments
Funding
The research was funded, in part, by grant R01 DK090106 from the National Institute of Diabetes and Digestive and Kidney Diseases awarded to Dr. Epstein. Study sponsor had no role in study design, collection, analysis, and interpretation of data, writing of the report, or decision to submit the manuscript for publication.
Abbreviations
- GWG
gestational weight gain
- FRR
food reinforcement ratio
Footnotes
Disclosure
Leonard H. Epstein is a consultant for Kurbo Health; Kai Ling Kong has no any potential conflicts of interest to report.
References
- Agras WS, Kraemer HC, Berkowitz RI, Korner AF, Hammer LD. Does a vigorous feeding style influence early development of adiposity? J Pediatr. 1987;110(5):799–804. doi: 10.1016/s0022-3476(87)80029-x. [DOI] [PubMed] [Google Scholar]
- Agras WS, Kraemer HC, Berkowitz RI, Hammer LD. Influence of early feeding style on adiposity at 6 years of age. J Pediatr. 1990;116(5):805–809. doi: 10.1016/s0022-3476(05)82677-0. http://dx.doi.org/10.1016/0891-5245(90)90079-L. [DOI] [PubMed] [Google Scholar]
- Alaimo K, Olson CM, Frongillo EA, Jr, Briefel RR. Food insufficiency, family income, and health in US preschool and school-aged children. Am J Public Health. 2001;91(5):781–786. doi: 10.2105/ajph.91.5.781. http://dx.doi.org/10.1001/archpedi.155.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Caring for your Baby and Young Child: Birth to Age 5. 5. Bantam; New York, NY: 2009. [Google Scholar]
- American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. doi: 10.1542/peds.2011-3552. http://dx.doi.org/10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. http://dx.doi.org/10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RG, Pantel MS, Young SN, Wright JH, Hendricks LA, Gravel R. The response of crying newborns to sucrose: is it a “sweetness” effect? Physiol Behav. 1999;66(3):409–417. doi: 10.1016/s0031-9384(98)00294-7. http://dx.doi.org/10.1016/S0031-9384(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr. 2007;98(4):843–851. doi: 10.1017/S0007114507812037. http://dx.doi.org/10.1017/s0007114507812037. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Mennella JA. Early flavor learning and its impact on later feeding behavior. J Pediatr Gastroenterol Nutr. 2009;48(1):S25–S30. doi: 10.1097/MPG.0b013e31819774a5. http://dx.doi.org/10.1097/MPG.0b013e31819774a5. [DOI] [PubMed] [Google Scholar]
- Bellinger L, Lilley C, Langley-Evans SC. Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br, J Nutr. 2004;92(3):513–520. doi: 10.1079/bjn20041224. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121(4):S3–S10. doi: 10.1016/j.amjmed.2008.01.015. http://dx.doi.org/10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. http://dx.doi.org/10.1016/0149-7634(95)00033-B. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Hughes JR, Higgins ST. Behavioral economics of drug self-administration. II A unit-price analysis of cigarette smoking. J Exp Anal Behav. 1991;55(2):145. doi: 10.1901/jeab.1991.55-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, Zimmerman SI, Hind H. The influence of social-affective context on the formation of children’s food preferences. Child Dev. 1980;5(3):856–861. [Google Scholar]
- Blass EM, Shide DJ, Weller A. Stress-reducing effects of ingesting milk, sugars, and fats — a developmental perspective. Ann N Y Acad Sci. 1989;575:292–305. doi: 10.1111/j.1749-6632.1989.tb53251.x. (discussion 305–306) [DOI] [PubMed] [Google Scholar]
- Bouton ME. A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychol. 2000;19(1):S57–S63. doi: 10.1037/0278-6133.19.suppl1.57. [DOI] [PubMed] [Google Scholar]
- Buscemi J, Murphy JG, Berlin KS, Raynor HA. A behavioral economic analysis of changes in food-related and food-free reinforcement during weight loss treatment. J Consult Clin Psychol. 2014;82(4):659–669. doi: 10.1037/a0036376. http://dx.doi.org/10.1037/a0036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76(3):353–364. doi: 10.1016/s0031-9384(02)00759-x. http://dx.doi.org/10.1016/S0031-9384(02)00759-X. [DOI] [PubMed] [Google Scholar]
- Carr KA, Lin H, Fletcher KD, Epstein LH. Food reinforcement, dietary disinhibition and weight gain in nonobese adults. Obesity (Silver Spring) 2014;22(1):254–259. doi: 10.1002/oby.20392. http://dx.doi.org/10.1002/oby.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey PH, Szeto K, Lensing S, Bogle M, Weber J. Children in food-insufficient, low-income families: prevalence, health, and nutrition status. Arch Pediatr Adolesc Med. 2001;155(4):508–514. doi: 10.1001/archpedi.155.4.508. http://dx.doi.org/10.1001/archpedi.155.4.508. [DOI] [PubMed] [Google Scholar]
- Chorna OD, Slaughter JC, Wang L, Stark AR, Maitre NL. A pacifier-activated music player with mother’s voice improves oral feeding in preterm infants. Pediatrics. 2014;133(3):462–468. doi: 10.1542/peds.2013-2547. http://dx.doi.org/10.1542/peds.2013-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EN, Dewey AM, Temple JL. Effects of daily snack food intake on food reinforcement depend on body mass index and energy density. Am J Clin Nutr. 2010;91(2):300–308. doi: 10.3945/ajcn.2009.28632. http://dx.doi.org/10.3945/ajcn.2009.28632. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65(3):545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- Cuenya L, Annicchiarico I, Serafini M, Glueck AC, Mustaca AE, Papini MR. Effects of shifts in food deprivation on consummatory successive negative contrast. Learn Motiv. 2015;52:11–21. http://dx.doi.org/10.1016/j.lmot.2015.08.002. [Google Scholar]
- Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28(4):436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: a multilevel analysis. Psychol Bull. 2007a;133(5):884–906. doi: 10.1037/0033-2909.133.5.884. http://dx.doi.org/10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci. 2007b;121(5):877–886. doi: 10.1037/0735-7044.121.5.877. http://dx.doi.org/10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Temple JL, Cavanaugh MD. Food reinforcement and impulsivity in overweight children and their parents. Eat Behav. 2008;9(3):319–327. doi: 10.1016/j.eatbeh.2007.10.007. http://dx.doi.org/10.1016/j.eatbeh.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Dearing KK, Erbe RW. Parent-child concordance of Taq1 A1 allele predicts similarity of parent–child weight loss in behavioral family-based treatment programs. Appetite. 2010;55(2):363–366. doi: 10.1016/j.appet.2010.06.006. http://dx.doi.org/10.1016/j.appet.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Carr KA, Lin H, Fletcher KD. Food reinforcement, energy intake, and macronutrient choice. Am J Clin Nutr. 2011;94(1):12–18. doi: 10.3945/ajcn.110.010314. http://dx.doi.org/10.3945/ajcn.110.010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Jankowiak N, Fletcher KD, Carr KA, Nederkoorn C, Raynor HA, Finkelstein E. Women who are motivated to eat and discount the future are more obese. Obesity (Silver Spring) 2014a;22(6):1394–1399. doi: 10.1002/oby.20661. http://dx.doi.org/10.1002/oby.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Yokum S, Feda DM, Stice E. Food reinforcement and parental obesity predict future weight gain in non-obese adolescents. Appetite. 2014b;82:138–142. doi: 10.1016/j.appet.2014.07.018. http://dx.doi.org/10.1016/j.appet.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro ZM, Qiu Q, Gruslin A, Adamo KB. Excessive gestational weight gain and obesity contribute to altered expression of maternal insulin-like growth factor binding protein-3. Int J Womens Health. 2013;5:657–665. doi: 10.2147/IJWH.S49594. http://dx.doi.org/10.2147/IJWH.S49594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O’Doherty J, Browning A, … Smith E. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10(3):453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Freinkel N. Banting lecture 1980. Of pregnancy and progeny Diabetes. 1980;29(12):1023–1035. doi: 10.2337/diab.29.12.1023. (Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7002669) [DOI] [PubMed] [Google Scholar]
- Giesen JC, Havermans RC, Douven A, Tekelenburg M, Jansen A. Will work for snack food: the association of BMI and snack reinforcement. Obesity (Silver Spring) 2010;18(5):966–970. doi: 10.1038/oby.2010.20. http://dx.doi.org/10.1038/oby.2010.20. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Hill C, Saxton J, Webber L, Blundell J, Wardle J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7–10-y-old children. Am J Clin Nutr. 2009;90(2):276–281. doi: 10.3945/ajcn.2009.27479. http://dx.doi.org/10.3945/ajcn.2009.27479. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. 20026361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, … Howell LL. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav. 2008;90(3):453–462. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KL, Eiden RD, Feda DM, Stier CL, Fletcher KD, Woodworth EM, … Epstein LH. Reducing relative food reinforcement in infants by an enriched music experience: a randomized controlled trial. Obesity (Silver Spring) 2016;24:917–923. doi: 10.1002/oby.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KL, Feda DM, Eiden RD, Epstein LH. Origins of food reinforcement in infants. Am J Clin Nutr. 2015;101(3):515–522. doi: 10.3945/ajcn.114.093237. http://dx.doi.org/10.3945/ajcn.114.093237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Fein SB, Grummer-Strawn LM. Do infants fed from bottles lack self-regulation of milk intake compared with directly breastfed infants? Pediatrics. 2010;125(6):e1386–e1393. doi: 10.1542/peds.2009-2549. http://dx.doi.org/10.1542/peds.2009-2549. [DOI] [PubMed] [Google Scholar]
- Li R, Magadia J, Fein SB, Grummer-Strawn LM. Risk of bottle-feeding for rapid weight gain during the first year of life. Arch Pediatr Adolesc Med. 2012;166(5):431–436. doi: 10.1001/archpediatrics.2011.1665. http://dx.doi.org/10.1001/archpediatrics.2011.1665. [DOI] [PubMed] [Google Scholar]
- Lin H, Carr KA, Fletcher KD, Epstein LH. Socioeconomic status, food reinforcement and obestiy. Ann Behav Med. 2012;43:S27. [Google Scholar]
- Lowe CF, Beasty A, Bentall RP. The role of verbal behavior in human learning: infant performance on fixed-interval schedules. J Exp Anal Behav. 1983;39(1):157–164. doi: 10.1901/jeab.1983.39-157. http://dx.doi.org/10.1901/jeab.1983.39-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS, Currie J. The relationship between pregnancy weight gain and birth weight: a within family comparison. Lancet. 2010;376(9745):984–990. doi: 10.1016/S0140-6736(10)60751-9. http://dx.doi.org/10.1016/s0140-6736(10)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA. Ontogeny of taste preferences: basic biology and implications for health. Am J Clin Nutr. 2014;99(3):704S–711S. doi: 10.3945/ajcn.113.067694. http://dx.doi.org/10.3945/ajcn.113.067694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, Walker CD. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience. 2011;176:225–236. doi: 10.1016/j.neuroscience.2010.12.037. http://dx.doi.org/10.1016/j.neuroscience.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66(1):1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rivera HM, Kievit P, Kirigiti MA, Bauman LA, Baquero K, Blundell P, … Sullivan EL. Maternal high-fat diet and obesity impact palatable food intake and dopamine signaling in nonhuman primate offspring. Obesity (Silver Spring) 2015;23(11):2157–2164. doi: 10.1002/oby.21306. http://dx.doi.org/10.1002/oby.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovee CK, Rovee DT. Conjugate reinforcement of infant exploratory behavior. J Exp Child Psychol. 1969;8(1):33–39. doi: 10.1016/0022-0965(69)90025-3. (Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/5804591) [DOI] [PubMed] [Google Scholar]
- Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27(1):41–50. doi: 10.1006/appe.1996.0032. http://dx.doi.org/10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Scheid JL, Carr KA, Lin H, Fletcher KD, Sucheston L, Singh PK, … Epstein LH. FTO polymorphisms moderate the association of food reinforcement with energy intake. Physiol Behav. 2014;132:51–56. doi: 10.1016/j.physbeh.2014.04.029. http://dx.doi.org/10.1016/j.physbeh.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS One. 2015;10(2):e0117959. doi: 10.1371/journal.pone.0117959. http://dx.doi.org/10.1371/journal.pone.0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahan TA, Bickel WK, Madden GJ, Badger GJ. Comparing the reinforcing efficacy of nicotine containing and de-nicotinized cigarettes: a behavioral economic analysis. Psychopharmacology. 1999;147(2):210–216. doi: 10.1007/s002130051162. [DOI] [PubMed] [Google Scholar]
- Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J, … Lohr KN. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol. 2009;201(4):339.e1–339.e14. doi: 10.1016/j.ajog.2009.07.002. http://dx.doi.org/10.1016/j.ajog.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Skogsdal Y, Eriksson M, Schollin J. Analgesia in newborns given oral glucose. Acta Paediatr. 1997;86(2):217–220. doi: 10.1111/j.1651-2227.1997.tb08872.x. [DOI] [PubMed] [Google Scholar]
- Smith BA, Blass EM. Taste-mediated calming in premature, preterm, and full-term human infants. Dev Psychol. 1996;32(6):1084–1089. [Google Scholar]
- Standley JM. The effect of music-reinforced nonnutritive sucking on feeding rate of premature infants. J Pediatr Nurs. 2003;18(3):169–173. doi: 10.1053/jpdn.2003.34. http://dx.doi.org/10.1053/jpdn.2003.34. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011;31(12):4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. http://dx.doi.org/10.1523/jneurosci.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss RS, Knight J. Influence of the home environment on the development of obesity in children. Pediatrics. 1999;103(6):e85. doi: 10.1542/peds.103.6.e85. http://dx.doi.org/10.1542/peds.103.6.e85. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Berkowitz RI, Schoeller D, Maislin G, Stallings VA. Predictors of body size in the first 2 y of life: a high-risk study of human obesity. Int J Obes Relat Metab Disord. 2004;28(4):503–513. doi: 10.1038/sj.ijo.0802517. http://dx.doi.org/10.1038/sj.ijo.0802517. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Scott AN, Bale TL. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience. 2009;162(4):924–932. doi: 10.1016/j.neuroscience.2009.05.029. http://dx.doi.org/10.1016/j.neuroscience.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Epstein LH. Sensitization of food reinforcement is related to weight status and baseline food reinforcement. Int J Obes. 2012;36(8):1102–1107. doi: 10.1038/ijo.2011.210. http://dx.doi.org/10.1038/ijo.2011.210. [DOI] [PubMed] [Google Scholar]
- Temple JL, Chappel A, Shalik J, Volcy S, Epstein LH. Daily consumption of individual snack foods decreases their reinforcing value. Eat Behav. 2008a;9(3):267–276. doi: 10.1016/j.eatbeh.2007.10.001. http://dx.doi.org/10.1016/j.eatbeh.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr. 2008b;87(5):1121–1127. doi: 10.1093/ajcn/87.5.1121. (Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18469229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JL, Bulkley AM, Badawy RL, Krause N, McCann S, Epstein LH. Differential effects of daily snack food intake on the reinforcing value of food in obese and nonobese women. Am J Clin Nutr. 2009;90(2):304–313. doi: 10.3945/ajcn.2008.27283. http://dx.doi.org/10.3945/ajcn.2008.27283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura AK, Mennella JA. Innate and learned preferences for sweet taste during childhood. Curr Opin Clin Nutr Metab Care. 2011;14(4):379–384. doi: 10.1097/MCO.0b013e328346df65. http://dx.doi.org/10.1097/MCO.0b013e328346df65. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64(11):1575–1579. doi: 10.1001/archneur.64.11.1575. http://dx.doi.org/10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151(10):4756–4764. doi: 10.1210/en.2010-0505. http://dx.doi.org/10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde PE, Ranney CK. The monthly food stamp cycle: shopping frequency and food intake decisions in an endogenous switching regression framework. Am J Agric Econ. 2000;82(1):200–213. http://dx.doi.org/10.1111/0002-9092.00016. [Google Scholar]
- Wojnicki FH, Stine JG, Corwin RL. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Physiol Behav. 2007;92(4):566–574. doi: 10.1016/j.physbeh.2007.05.002. http://dx.doi.org/10.1016/j.physbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Wormith SJ, Pankhurst D, Moffitt AR. Frequency discrimination by young infants. Child Dev. 1975;46(1):272–275. [PubMed] [Google Scholar]