Abstract
Scleraxis (Scx) is a basic helix-loop-helix transcription factor that is expressed persistently in tendons/ligaments, but transiently in entheseal cartilage. In this study, we generated a novel ScxCre knock-in (KI) allele, by in-frame replacement of most of Scx exon 1 with Cre recombinase (Cre), to drive Cre expression using Scx promoter and to inactivate the endogenous Scx. Reflecting the intensity and duration of endogenous expression, Cre-mediated excision occurs in tendinous and ligamentous tissues persistently expressing Scx. Expression of tenomodulin, a marker of mature tenocytes and ligamentocytes, was almost absent in tendons and ligaments of ScxCre/Cre KI mice lacking Scx to indicate defective maturation. In homozygotes, the transiently Scx-expressing entheseal regions such as the rib cage, patella cartilage, and calcaneus were small and defective and cartilaginous tuberosity was missing. Decreased Sox9 expression and phosphorylation of Smad1/5 and Smad3 were also observed in the developing entheseal cartilage, patella, and deltoid tuberosity of ScxCre/Cre KI mice. These results highlighted the functional importance of both transient and persistent expression domains of Scx for proper integration of the musculoskeletal components.
During early stages of musculoskeletal development, scleraxis (Scx), a basic helix-loop-helix transcription factor, is expressed in the tendon/ligament cell population and the subpopulation of chondrogenic cells that contribute to establishment of chondro-tendinous/ligamentous junctions1,2,3,4. Scx expression occurs only transiently in the chondrogenic lineage because it is rapidly downregulated during the early stages of chondrogenesis3. In contrast, Scx is persistently expressed in the tendon/ligament cell lineage, which also includes the diaphragm and cordae tendinae of the heart, during differentiation and maturation5,6,7,8. Persistent expression of Scx, in the developing tendons and ligaments in vivo, was also visualised in ScxGFP reporter transgenic mice that faithfully recapitulate endogenous Scx-expressing domains under the control of the promoter/enhancer of mouse Scx3,9. In Scx−/− embryos, force-transmitting and intermuscular tendons are reported to be severely affected and are hypoplastic7. However, so far, other Scx-expressing domains have not been explored in detail yet.
Tenomodulin (Tnmd) is an excellent cell surface marker to evaluate differentiation and maturation of dense connective tissues, including tendons and ligaments10,11,12,13. Scx positively regulates the expression of type I collagen (Col1) and Tnmd in tenocytes6,7,14. Transcriptome analysis of mouse embryonic limb tendons also revealed that Tnmd is the second most differentially expressed gene of the top 100 genes differentially expressed between E11.5 and E14.515. Studies on Tnmd null mice demonstrated that Tnmd is necessary for tenocyte proliferation and tendon maturation16. Cellular adhesion in the periodontal ligament is decreased on loss of Tnmd and enhanced on Tnmd overexpression17. In a rat rotator cuff healing model, fibroblast growth factor-2 enhanced tendon-to-bone healing, in which Tnmd expression was associated with formation of aligned collagen fibres in the repair tissue18. Loss of Tnmd results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells19. Furthermore, forced expression of Scx resulted in the conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells that differentiate into Tnmd-expressing cells20. Additionally in tenocytes, Tnmd expression is markedly upregulated by retroviral overexpression of Scx6,21.
In this study, we report a novel ScxCre knock-in (KI) mouse line that was generated by in-frame replacement of most of the exon 1 on mouse Scx locus with Cre recombinase (Cre), to inactivate endogenous Scx and to drive Cre expression using the endogenous Scx promoter. In this established ScxCre KI line, Cre expression was detected using Rosa-CAG-LSL-tdTomato (Rosa-tdTomato) reporter mice expressing tandem dimer Tomato (tdTomato) by Cre-mediated recombination. A number of tdTomato-expressing cells of ScxCre/+; Rosa-tdTomato or ScxCre/Cre; Rosa-tdTomato mice were observed in tendons, ligaments, annulus fibrosus of intervertebral discs, and patella cartilage. Loss of Scx resulted in an almost absent expression of Tnmd in tendons, ligaments, and annulus fibrosus of intervertebral discs. Cartilaginous elements transiently expressing Scx were defective in ScxCre/Cre KI mice lacking Scx. Sox9 expression and phosphorylation of Smad1/5 and Smad 3 were markedly decreased in these Scx+ cartilaginous elements. Our results clearly demonstrated the functional importance of Scx in maturation of tissue domains that express Scx both persistently and transiently during development.
Results
Establishment of Scx Cre KI mice
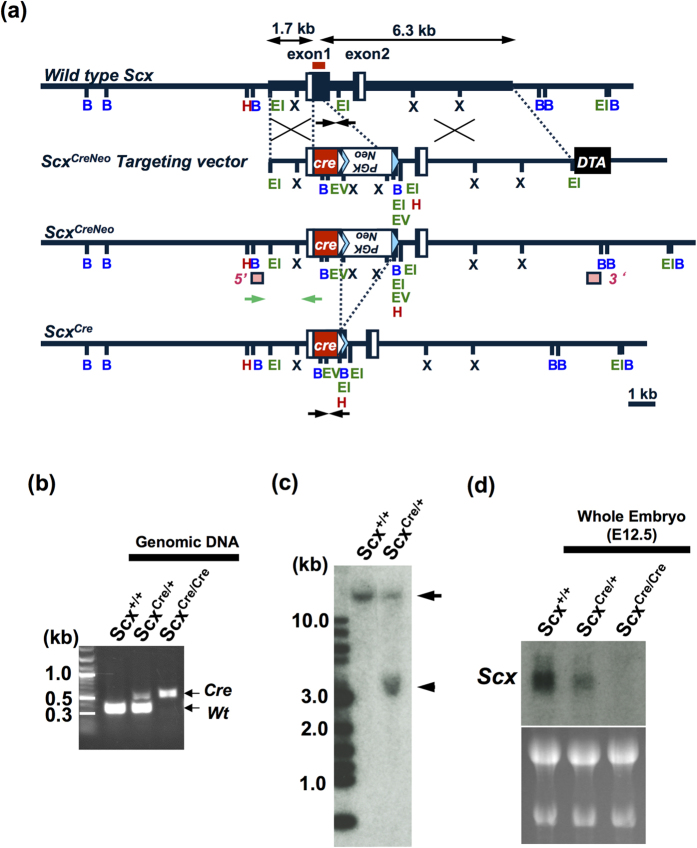
On mouse chromosome 15, Scx gene, consisting of two exons, is located within the third intron of block of proliferation 1 (Bop1), which is transcribed in the opposite orientation9,22. The targeting strategy placed Cre expression under the control of Scx promoter and led to the inactivation of Scx allele by in-frame replacement of most of its exon 1, which encodes for most of the coding region, including a b-HLH region. The linearised ScxCreNeo targeting vector (Fig. 1a) was electroporated into KY1.1 embryonic stem (ES) cell line (C57BL/6J × 129S6/SvEvTac)23. ES cell clone with the correct targeting event was microinjected into blastocysts to obtain chimeric mice. We generated heterozygous mice by crossing these chimeras with C57BL/6 wild type mice and confirmed the successful germ line transmission of ScxCreNeo targeted allele. Heterozygous ScxCreNeo/+ KI mice were viable, whereas homozygous ScxCreNeo/CreNeo KI mice were embryonic lethal. This is consistent with the previous report that conventional Scx knockout mice die around embryonic day (E)10.524. To obtain ScxCre/+ KI mice, we deleted the flippase (FLP) recombinase target (FRT)-flanked neomycin-resistance (Neo) cassette by mating ScxCreNeo/+ KI mice with mice expressing FLP recombinase (FLPe)25. The correctly targeted event of ScxCre/+ KI mice was confirmed by PCR of tail tip DNA and Southern blot analysis (Fig. 1b,c). Expression of Scx was not detected in a ScxCre/Cre KI mouse embryo at E12.5 (Fig. 1d). Thus, we successfully disrupted endogenous Scx by inserting a Cre in-frame into the ATG start site of mouse Scx gene.
Figure 1. Targeting strategy to generate ScxCre KI mice.
(a) The mouse scleraxis (Scx) gene consists of exon 1 and 2. Black boxes represent the coding regions of Scx gene, whereas open boxes represent 5′ and 3′ untranslated regions. The coding region of Cre recombinase (Cre) is shown as red boxes. The neomycin-resistance (Neo) cassette with phosphoglycerate kinase I (PGK) promoter (white open boxes) was used for positive selection of embryonic stem (ES) cells. Diphtheria toxin (DTA) was used for negative selection. White and light blue triangles indicate loxP and FRT sites. Restriction enzyme sites for BamHI (B), HindIII (H), EcoRI (EI), EcoRV (EV), and XbaI (X) are shown. Arrows on wild type allele indicate the 5′ (1.7 kb) and 3′ (6.3 kb) homology arms. To generate ScxCre allele, the Neo cassette was removed by crossing ScxCreNeo mice with flippase-expressing FLPe mice. Black arrows under wild type allele and ScxCre allele are genotyping primers for detection of wild type and ScxCre alleles, respectively. Green arrows under ScxCreNeo allele are genotyping primers for ES screening. The 5′ and 3′ probes for Southern blotting are shown as pink boxes. Red line on wild type allele shows the region used for RNA probes in northern blot analysis. Scale bar represents 1 kb. (b) Tail tip DNA PCR genotyping was performed with primers shown as black arrows in (a). The targeted ScxCre allele (0.5 kb) and wild type (Wt) allele (0.3 kb) are distinguished. (c) Southern blot analysis of mouse genomic DNA isolated from wild type (Scx+/+) and ScxCre/+ KI mice. An arrowhead and an arrow indicate the ScxCre allele (3.5 kb) and wild type allele (19.1 kb), respectively. Hybridised bands on the far left show DNA size markers. (d) Detection of Scx transcripts in whole embryos at E12.5. Scx expression in Scx+/+, ScxCre/+, and ScxCre/Cre KI mice were examined by northern blot analysis. Fifteen micrograms of the total RNA was loaded into each well and equal loading was verified by ethidium bromide staining.
Cre-mediated recombination in Scx Cre KI; Rosa-tdTomato
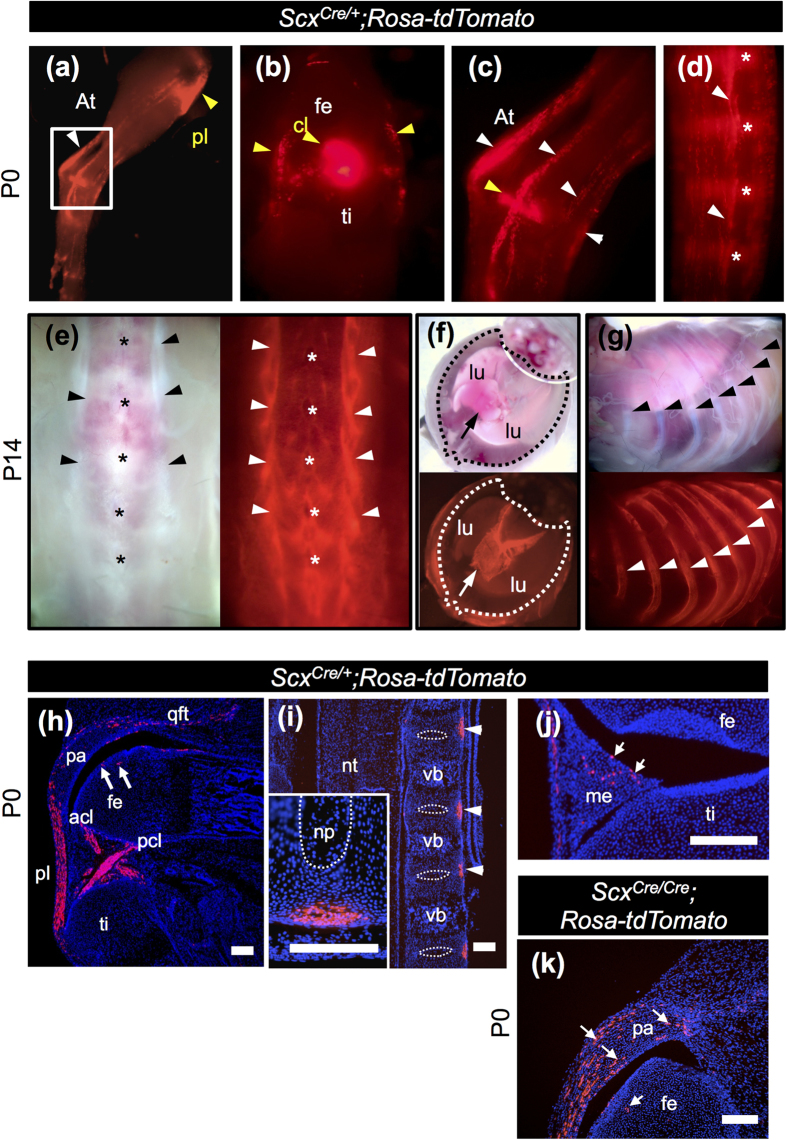
Cre-mediated recombination was examined by crossing ScxCre/+ KI mice with Rosa-tdTomato reporter mice26. Although endogenous expression of Scx is detectable around E9.5, Cre-mediated tdTomato expression in ScxCre/+; Rosa-tdTomato was first detected around E16.5 (data not shown). In the hind limbs of ScxCre/+; Rosa-tdTomato neonates, we detected tdTomato expression in the Achilles tendon and in tendons and ligaments associated with knee joint and heel (Fig. 2a–c). Tail tendons of ScxCre/+; Rosa-tdTomato neonates were also positive for tdTomato (Fig. 2d). Moreover, at postnatal day (P)14, more intense expression of tdTomato was observed in body tendons and ligaments (Fig. 2e,f,g). In the trunk of 2-week-old ScxCre/+; Rosa-tdTomato mice, intense tdTomato expression was observed in tendons of longissimus muscle (Fig. 2e), central tendon of diaphragm (Fig. 2f), and costal tendons (Fig. 2g). In the hindlimb of ScxCre/+; Rosa-tdTomato neonates, tdTomato-positive cells were found in patella ligament, anterior and posterior cruciate ligaments, quadriceps femoris tendon, patella cartilage, femur cartilage, and meniscus (Fig. 2h–j). In the intervertebral region of ScxCre/+; Rosa-tdTomato neonates, tdTomato expression was detected in the outer annulus fibrosus on the ventral side (Fig. 2i). In the hindlimb of ScxCre/Cre; Rosa-tdTomato neonates, the number of tdTomato-positive cells in patella cartilage increased (Fig. 2k). Mosaic Cre-mediated recombination could have occurred because of heterogeneity in the endogenous Scx mRNA levels and time window of Scx expression in the Scx-expressing cells.
Figure 2. Cre-mediated recombination in tendons, ligaments, and diaphragm of ScxCre/+; Rosa-tdTomato mice.
(a–g) Cre-mediated tdTomato expression in P0 (a–d) and P14 (e–g) ScxCre/+; Rosa-tdTomato mice. Bright fluorescence was detected in leg tendons (a–c), tail tendons (d), back tendons (e), diaphragm (f), and costal tendons (g). (a–c) Lateral (a,c) and frontal (b) views of skinned whole mount leg of ScxCre/+; Rosa-tdTomato mice. White and yellow arrowheads in (a–c) indicate tendons and ligaments of the hindlimb, respectively. Arrowheads in (d) indicate tail tendons. Asterisks in (d) indicate intervertebral region. (e–g) Bright and fluorescent images of dorsal view of lumbar tendons (e), ventral view of diaphragm (f), and lateral view of costal tendons (g) are shown. Arrowheads and asterisks in (e) indicate tendons and spinous processes, respectively. Arrows in (f) indicate central tendon of diaphragm. Arrowheads in (g) indicate ribs. Dotted line in (f) encloses the outer boundary of diaphragm. (h–k) Sagittal sections of knee joint (h,j,k) and vertebral column (i) prepared from ScxCre/+; Rosa-tdTomato (h–j) and ScxCre/Cre; Rosa-tdTomato (k) neonates. An inset in (i) shows magnified image of ventral annulus fibrosus. Arrows in (h,j,k) indicate tdTomato-positive chondrocytes in patella, meniscus, and femur, respectively. acl, anterior cruciate ligament; At, Achilles tendon; cl, cruciate ligament; fe, femur; lu, lung; pa, patella; pcl, posterior cruciate ligament; me, meniscus; np, nucleus pulposus; nt, neural tube; pl, patella ligament; qft, quadriceps femoris tendon; ti, tibia; vb, vertebral body. Scale bars, 200 μm (h–k); 100 μm (inset in (i)).
Tendon deficiency and skeletal abnormalities in Scx Cre/Cre KI neonates
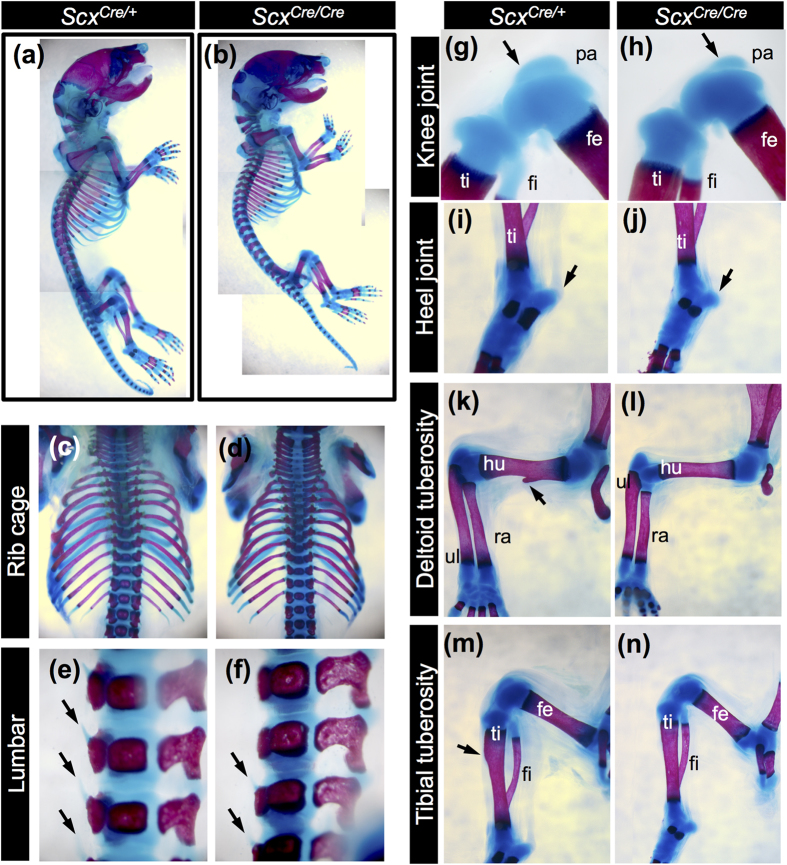
Heterozygous ScxCre/+ KI mice were viable, fertile, and displayed no apparent developmental defects (Fig. 3a,c,e,g). Consistent with previous findings7, morphological defects in force-transmitting and intermuscular tendons were evident at birth, in ScxCre/Cre KI neonates (Fig. 3b,d,f,h). The diaphragm of ScxCre/Cre KI neonates is functional and permits normal breathing. The forelimb autopod of ScxCre/Cre KI neonates was locked in a dorsal flexure (Fig. 4b), compared to that of ScxCre/+ KI neonates (Fig. 4a). The deltoid tuberosity (DT) and tibial tuberosity observed in ScxCre/+ KI neonates (Fig. 4a,k,m) were missing in ScxCre/Cre KI neonates (Fig. 4b,l,n). The rib cage, transverse process of lumbar, patella cartilage, and entheseal cartilage of calcaneus of ScxCre/Cre KI neonates were smaller than that of ScxCre/+ KI neonates (Fig. 4c–j).
Figure 3. Tendon deficiency in ScxCre/Cre KI mice.
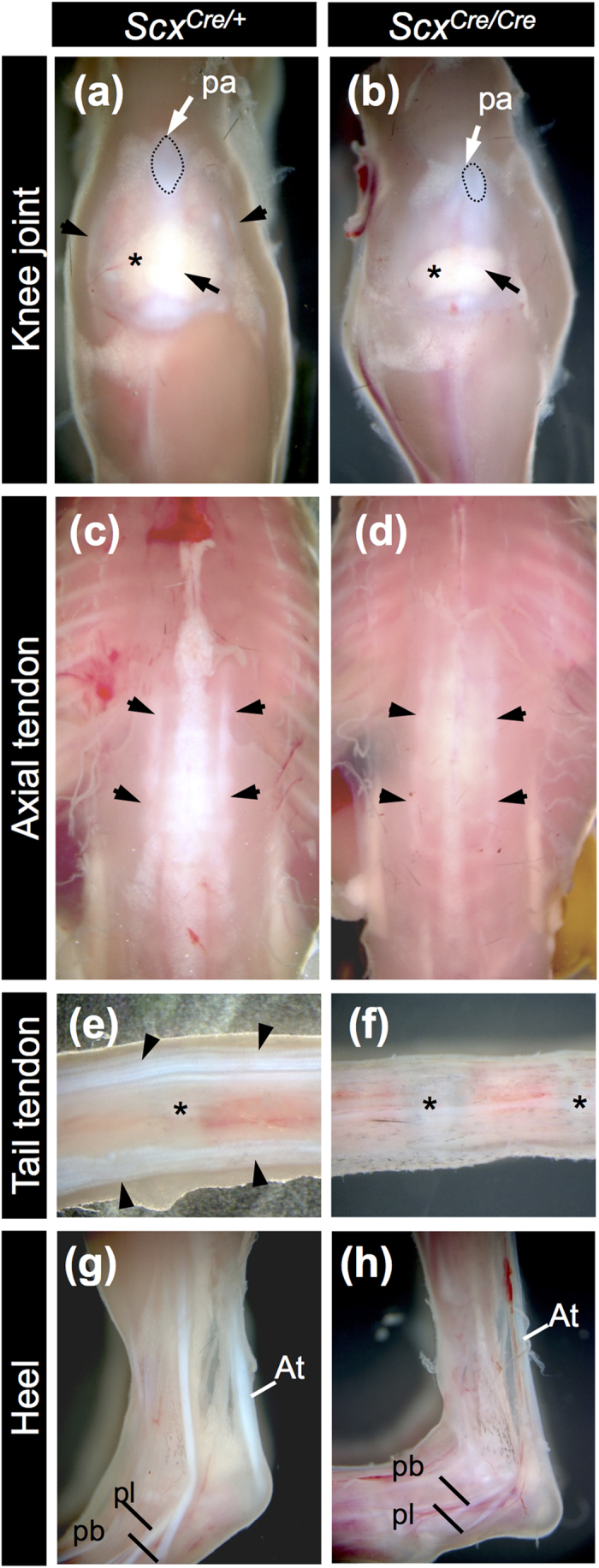
(a–h) Tendons and ligaments of 3-week-old ScxCre/+ (a,c,e,g) and ScxCre/Cre (b,d,f,h) KI mice. Frontal views of knee (a,b), dorsal views of back (c,d), and lateral views of tail (e,f) and heel (g,h) are shown. A black and a white arrow in (a,b) indicate patella ligament and patella, respectively. Arrowheads in (a) indicate collateral ligaments. Asterisk in (a,b) indicates fat pad in the knee joint. Dotted line in (a,b) encloses the patella. Arrowheads in (c,d) indicate back tendons. Arrowheads in (e) indicate tail tendons. Asterisks in (e,f) indicate intervertebral region. At, Achilles tendon; pa, patella; pb, peroneus brevis tendon; pl, peroneus longus tendon.
Figure 4. Skeletal abnormalities in ScxCre/Cre KI mice.
(a–n) Skeletons of ScxCre/+ (a,c,e,g,i,k,m) and ScxCre/Cre (b,d,f,h,j,l,n) KI mice at P0. Lateral (a,b) and dorsal (c,d) views of ribs and lateral views of lumbar (e,f), knee joint (g,h), heel (i,j), humerus (k,l), and lower leg (m,n) are shown. Arrows in (e,f) indicate transverse processes of lumbar. Arrows in (g,h) indicate patella. Arrows in (k,m) indicate deltoid tuberosity and tibial tuberosity, respectively. fe, femur; fi, fibula; hu, humerus; pa, patella; ra, radius; ti, tibia; ul, ulna.
Defective maturation of tendons, ligaments, and annulus fibrosus of intervertebral discs in Scx Cre/Cre KI neonates
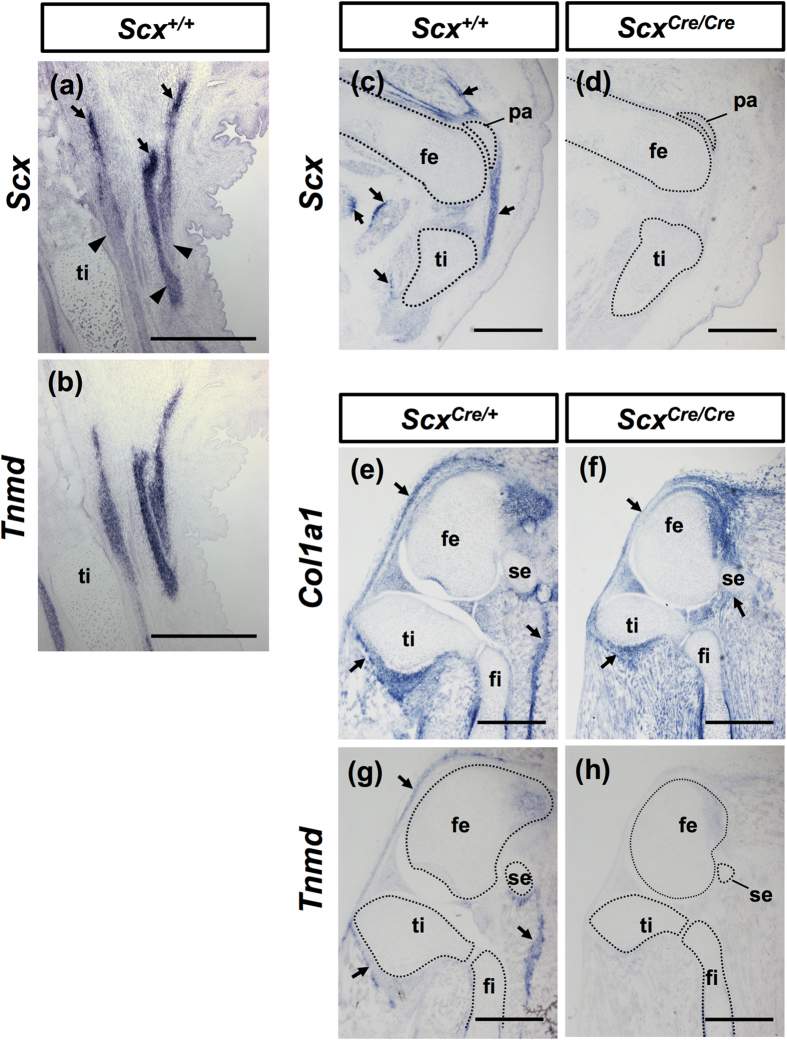
Tnmd is related to a cartilage-derived angiogenesis inhibitor gene product, chondromodulin (Chmd)27, and is a marker of mature tenocytes and ligamentocytes10,11,28. Tnmd expression is positively regulated by Scx6. Scx was heterogeneously expressed in the developing leg tendons (Fig. 5a), whereas uniform expression of Tnmd was observed (Fig. 5b). Higher levels of Scx expression in the tendons were detected near the myotendinous junction (Fig. 5a). We confirmed inactivation of endogenous Scx expression in ScxCre/Cre at E15.5 by in situ hybridisation (Fig. 5c,d). As previously reported7, in Scx null mice, gene expression of Tnmd and type XIV collagen was not detectable in forelimb tendons. However, during postnatal growth, Tnmd expression in other dense connective tissues, such as ligaments and annulus fibrosus of intervertebral discs, has not been evaluated yet. Thus, we investigated localisation of Col1 and Tnmd in tendons and ligaments of ScxCre/+ and ScxCre/Cre KI mice, by in situ hybridisation (Fig. 5e–h) and double immunostaining (Figs 6 and 7). In the hindlimb of a ScxCre/+ neonate, tendons and ligaments were positive for Col1 and Tnmd (Fig. 6a,c,e,g). In the knee joint of a homozygous ScxCre/Cre KI neonate, patella ligament, anterior and posterior cruciate ligaments, and anchoring tendons were positive for Col1, but Tnmd expression was almost absent (Fig. 6b,d). Similarly, in the heel of a homozygous ScxCre/Cre KI neonate, tendons and ligaments, including the Achilles and extensor digitorum longus tendons, were positive for Col1 but negative for Tnmd (Fig. 6f,h). The meniscus was positive for Col1 but negative for Tnmd at E16.5, despite finding tdTomato positive cells in the meniscus of ScxCre/+; Rosa-tdTomato mice (Figs 2j and 6i,j). The outer annulus fibrosus of intervertebral discs in ScxCre/+ KI neonates and the tendinous region of the diaphragm in wild type mice were also positive for Col1 and Tnmd (Fig. 7a,c,e), but Tnmd expression was almost absent in that of ScxCre/Cre KI mice (Fig. 7b,d,f). These results suggest that loss of Scx causes defective maturation of tendons, ligaments, and outer annulus fibrosus of intervertebral discs.
Figure 5. Expression of Tnmd, Scx, and Col1a1 in ScxCre/Cre KI mice.
In situ hybridisation of Scx (a,c,d), Tnmd (b,g,h), and Col1a1 (e,f) on sagittal sections of hindlimbs prepared from Scx+/+ (a–c), ScxCre/+ (e,g), and ScxCre/Cre (d,f,h) mice at E16.5 (a,b), E15.5 (c,d), and P0 (e–h). Arrows and arrowheads in (a) indicate the regions with high and low expression of Scx, respectively. Arrows in (c,e–g) indicate Scx (c), Col1a1 (e,f), and Tnmd (g) positive tendinous region. Dotted lines in (c,d) and (g,h) enclose skeletons. fe, femur; fi, fibula; pa, patella; se, sesamoid bone; ti, tibia. Scal bars, 500 μm.
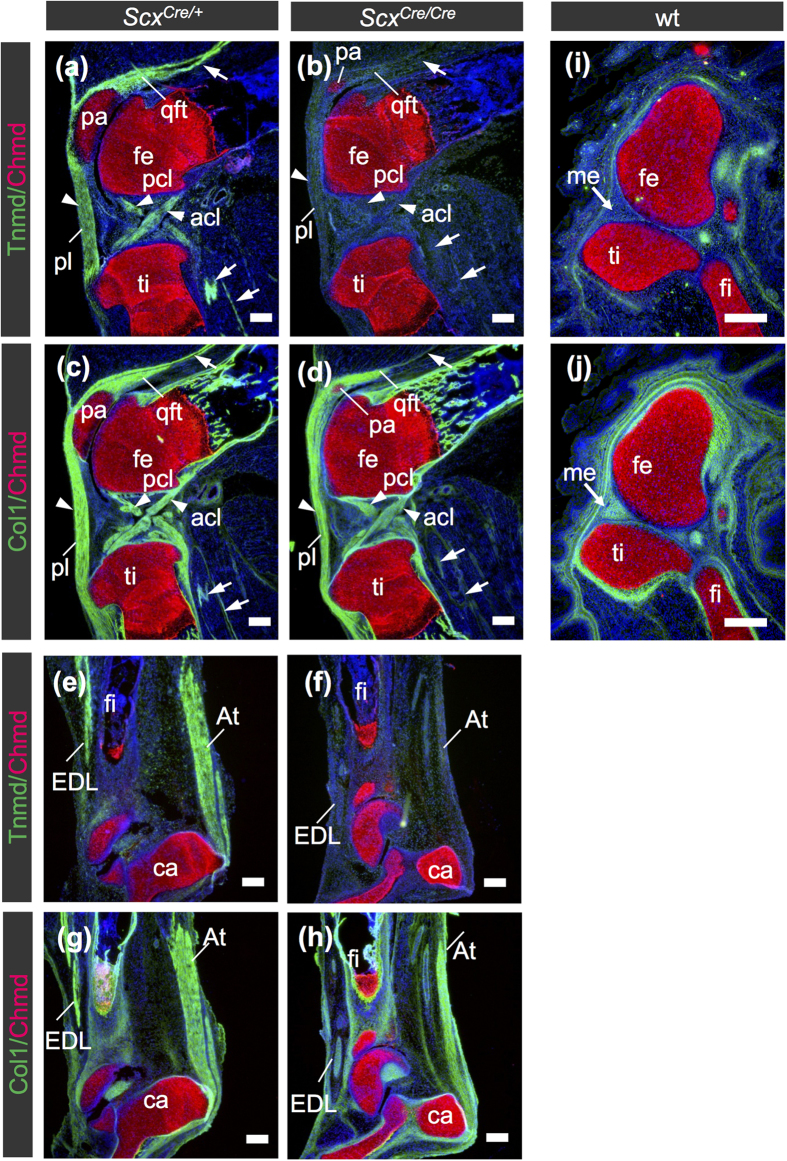
Figure 6. Decreased expression of Tnmd in tendons and ligaments of ScxCre/Cre KI mice.
(a–h) Double immunostaining of Chmd (red) and Tnmd (green) (a,b,e,f,i) or Col1 (green) (c,d,g,h,j) was performed in ScxCre/+ (a,c,e,g) and ScxCre/Cre (b,d,f,h) KI neonates and wild type (wt) embryos at E16.5 (i,j). Frozen sagittal sections of knee (a–d,i,j) and heel (e–h) are shown. Tnmd (a,e) and Col1 (c,g) were detected in tendons and ligaments of ScxCre/+, whereas Col1-positive tendons and ligaments of ScxCre/Cre (d,h) were negative for Tnmd (b,f). Arrows and arrowheads in (a–d) indicate tendons and ligaments respectively, whereas arrows in (i,j) indicate menisci. acl, anterior cruciate ligament; At, Achilles tendon; ca, calcaneus; EDL, extensor digitorum longus tendon; fe, femur; fi, fibula; me, meniscus; pa, patella; pcl, posterior cruciate ligament; pl, patella ligament; qft, quadriceps femoris tendon; ti, tibia. Scale bars, 200 μm.
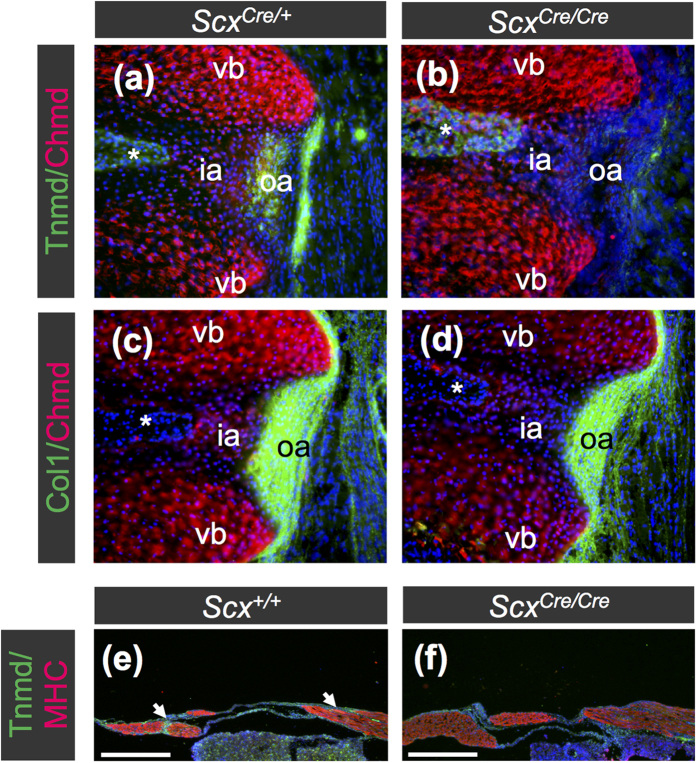
Figure 7. Decreased expression of Tnmd in annulus fibrosus of intervertebral disc and diaphragm of ScxCre/Cre KI mice.
(a–f) Double immunostaining of Chmd (red) and Tnmd (green) (a,b) or Col1 (green) (c,d) was performed in ScxCre/+ (a,c) and ScxCre/Cre (b,d) KI mice and MHC (red) and Tnmd (green) (e,f) was performed in Scx+/+ (e) and ScxCre/Cre (f) KI mice at P0. Frozen frontal sections of vertebral column (a–d) and sagittal sections of diaphragm (e,f) are shown. Tnmd (a) and Col1 (c) were detected in annulus fibrosus of ScxCre/+ KI, whereas Col1-positive annulus fibrosus of ScxCre/Cre (d) were negative for Tnmd (b). Asterisks in (a–d) indicate nucleus pulposus. Arrows in (e) indicate Tnmd-positive tendinous region in diaphragm of Scx+/+ mouse. ia, inner annulus fibrosus; oa, outer annulus fibrosus; vb, vertebral body. Scale bars, 200 μm.
Defective maturation of knee joint of Scx Cre/Cre KI mice
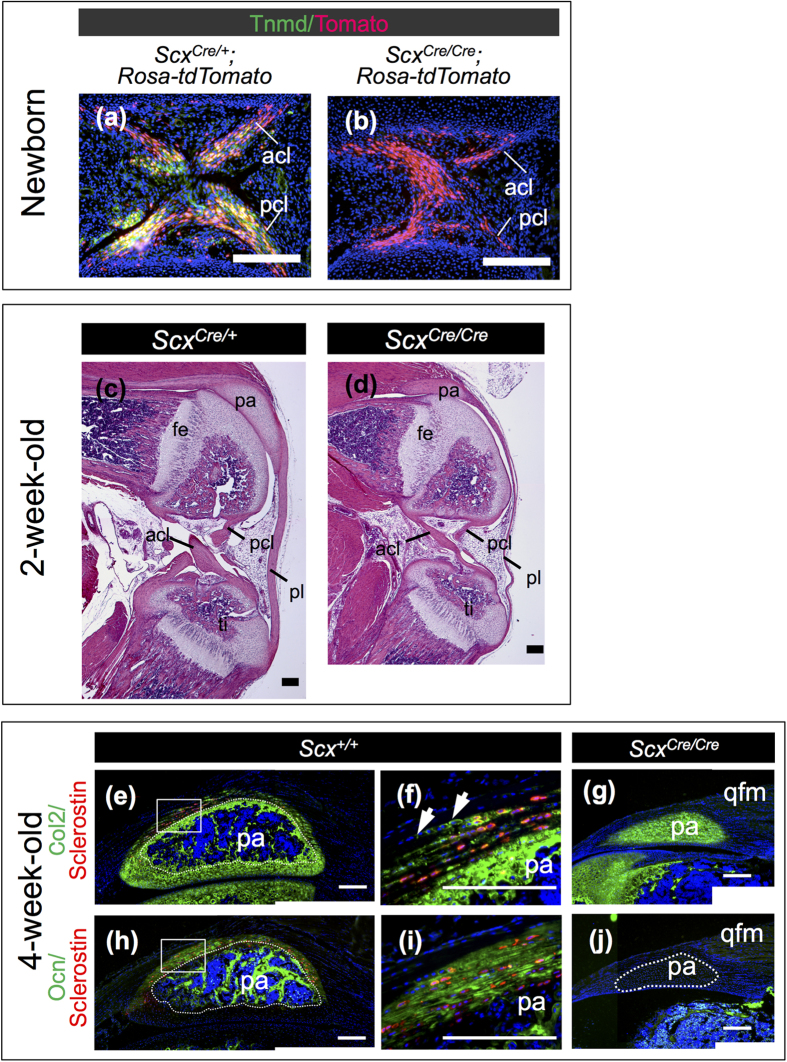
Tnmd expression, observed in cruciate ligaments of ScxCre/+; Rosa-tdTomato mice, was missing in ScxCre/Cre; Rosa-tdTomato mice (Fig. 8a,b). In knee joints of 2-week-old ScxCre/+ KI mice, tendons and ligaments developed well (Fig. 8c), whereas defective maturation was observed in knee joints of homozygous ScxCre/Cre KI mice (Fig. 8d). The size of the patella was also significantly small and entheseal cartilage was defective in a ScxCre/Cre KI mouse (Fig. 8d). To analyse endochondral ossification in the patella, immunostaining using antibodies against type II collagen (Col2), osteocalcin (Ocn), and sclerostin was performed. In a Scx+/+ mouse, a central part of the patella was vascularised to be replaced by bone stained with Ocn, but Col2-positive staining indicates the presence of cartilaginous matrix (Fig. 8e,f,h,i). At this stage, the patella of a ScxCre/Cre KI mouse was Col2-positive without any indication of vascularisation and ossification (Fig. 8g,j), suggesting a delay in the endochondral ossification of the patella. In the entheseal region of patella tendon and ligament of a Scx+/+ mouse, sclerostin-positive cells were observed in Col2- and Ocn-positive calcified fibrocartilage (Fig. 8e,f,h,i). Col2-positive unmineralised fibrocartilage was also present at an adjacent region in patella tendon and ligament of a Scx+/+ mouse (Fig. 8f). Such a structure was not observed in the ScxCre/Cre KI mouse. Taken together, Scx is also required for maturation of tendons, ligaments, and the patella.
Figure 8. Hypoplastic formation of patella and ligaments in knee joint of ScxCre/Cre KI mice.
(a,b) Frozen sagittal sections of anterior and posterior cruciate ligaments of ScxCre/+; Rosa-tdTomato (a) and ScxCre/Cre; Rosa-tdTomato (b) are shown. Cre-mediated tdTomato expression was detected as red fluorescence (a,b). Tnmd-positive cells (green) were visualised by immunostaining using anti-Tnmd antibody. (c,d) Haematoxylin and eosin staining of sagittal sections of knee joint is shown. Paraffin decalcified specimens of 2-week-old ScxCre/+ (c) and ScxCre/Cre (d) KI mice were sliced. (e–j) Double immunostaining of sclerostin (red) and Col2 (green) (e–g) or Ocn (green) (h–j) was performed on frozen sagittal sections of patella prepared from 4-week-old wild type (e,f,h,i) and ScxCre/Cre (g,j) KI mice. Boxed regions in (e,h) are shown at a higher magnification in (f,i), respectively. Arrows in (f) indicate Col2-positive uncalcified fibrochondrocytes. Dotted line in (e,h) encloses a bone region in the patella. Dotted line in (j) encloses patella. acl, anterior cruciate ligament; fe, femur; pa, patella; pcl, posterior cruciate ligament; pl, patella ligament; qfm, quadriceps femoris muscle; ti, tibia. Scale bars, 200 μm.
Decreased Sox9 expression and phosphorylation of Smad1/5 and Smad3 in the developing entheseal cartilage, patella, and deltoid tuberosity of Scx Cre/Cre KI mice
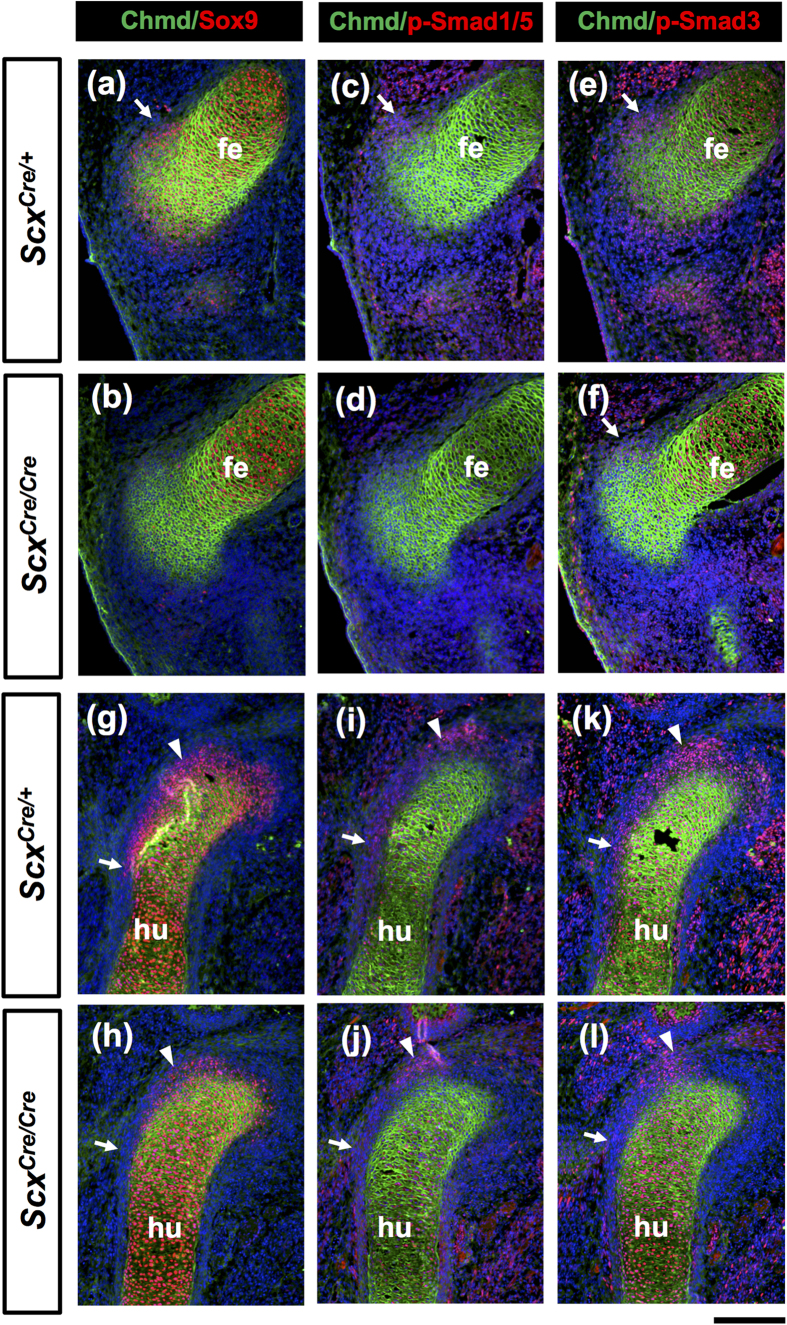
Entheseal cartilage around the tendon/ligament attachment sites and sesamoid bones such as patella are derived from Scx/Sox9 double positive progenitors2,4. Blitz et al. reported that activation of TGF-β and BMP signaling is required for specification and differentiation of these progenitors, respectively4,29. Thus, we examined how loss of Scx affects expression of Sox9 and activation of these signaling pathways in the developing forelimb and hindlimb of heterozygotes and homozygotes at E13.5. Activation of TGF-β and BMP signaling pathways was monitored by phosphorylation of Smad1/5 and Smad3 that are intracellular downstream mediators30. As shown in Fig. 9, mature chondrocytes and progenitor cells of the patella and the deltoid tuberosity were visualized as Chmd positive (green) and Sox9 positive regions (red), respectively. In a ScxCre/Cre KI mouse, Sox9 expression was markedly decreased in the developing patella and entheseal cartilage (Fig. 9b,h), compared with that in a ScxCre/+ KI mouse (Fig. 9a,g). Sox9 positive progenitors in the developing deltoid tuberosity of a ScxCre/+ KI mouse (Fig. 9g) were absent in a ScxCre/Cre KI mouse (Fig. 9h). Similarly, phosphorylation of Smad1/5 and Smad3 observed in a ScxCre/+ KI mouse (Fig. 9c,e,i,k) was markedly decreased especially in the prospective patella and deltoid tuberosity of a ScxCre/Cre KI mouse (Fig. 9d,f,j,l).
Figure 9. Decreased Sox9 expression and phosphorylation of Smad1/5 and Smad3 in the developing patella and deltoid tuberosity of ScxCre/Cre KI mouse.
Frozen sagittal section of hindlimbs (a–f) and forelimbs (g–l) prepared from ScxCre/+ (a,c,e,g,i,k) and ScxCre/Cre (b,d,f,h,j,l) KI embryos at E13.5 are shown. (a–l) Double immunostaining of Chmd (green) and Sox9 (red) (a,b,g,h), p-Smad1/5 (red) (c,d,i,j), or p-Smad3 (red) (e,f,k,l). Arrows in (a,c,e,f) indicate developing patella. Arrows and arrowheads in (g–l) indicate the deltoid tuberosity and the insertion sites of tendon of supraspinous muscle, respectively. fe, femur; hu, humerus. Scale bar, 200 μm.
Gene expression of Col14a1, Decorin, Mohawk, Tnmd and Egr1 in ScxCre/Cre KI mice
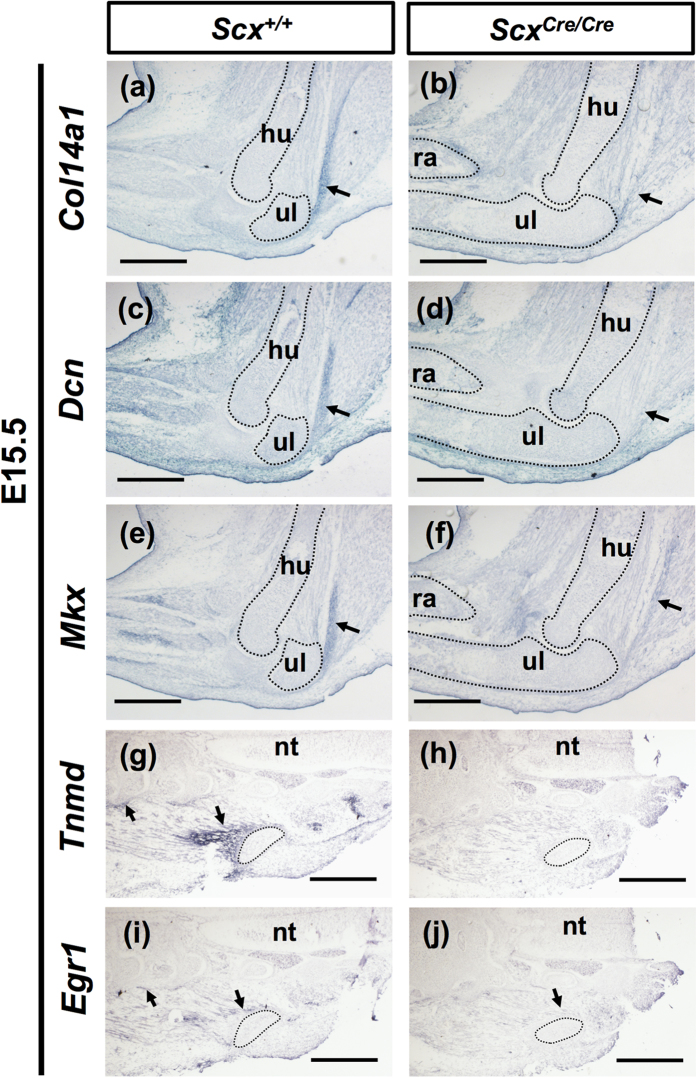
In addition, we investigated the expression of other tendon-associated genes such as Col14a1, Decorin (Dcn), Mohawk (Mkx), Tnmd, and Early growth response 1 (Egr1) in ScxCre/Cre KI embryos at E15.5. Col14a1 is a member of the fibril-associated collagens with interrupted triple helices (FACIT) collagen family31. Dcn is a small leucine-rich proteoglycan involved in regulating collagen fibrillogenesis32 and Mkx is a family member of atypical homeobox genes that are expressed in developing tendons33,34. Egr1 is reported to be a transcription factor regulating the expression of Col1a1 in tendon development35. In a ScxCre/Cre KI mouse, Col14a1 expression was almost absent in the developing triceps brachii tendons (Fig. 10a,b). In a wild type mouse, we found expression of Dcn in the developing tendon, dermis, and soft connective tissues (Fig. 10c), which are consistent with previous findings32. In ScxCre/Cre KI embryos, we found that Dcn expression was almost absent in triceps brachii tendons (Fig. 10d) and also found generally weak expression of Mkx (Fig. 10e,f). Around the pelvic bone and lumbar at E15.5, Tnmd expressing tendons were also positive for Egr1 (Fig. 10g,i). In ScxCre/Cre KI embryos, expression of Tnmd and Egr1 was lost and decreased in theses tendons, respectively (Fig. 10h,j). These findings suggest that loss of Scx affects the expression of genes related to tendon maturation.
Figure 10. Expression of genes related to tendon formation in ScxCre/Cre KI mice.
(a–j) In situ hybridisation of Col14a1 (a,b), Dcn (c,d), Mkx (e,f), Tnmd (g,h), and Egr1 (i,j) on sagittal sections of forelimbs (a–f) and frontal sections of trunks (g–j) prepared from Scx+/+ (a,c,e,g,i) and ScxCre/Cre (b,d,f,h,j) KI embryos at E15.5. Arrows in (a–f) indicate the triceps brachii tendon. Arrows in (g,i,j) indicate Tnmd (g) and Egr1 (i,j) positive tendons. Dotted lines in (a–j) enclose humerus, radius, ulna, and pelvic bone. hu, humerus; nt, neural tube; ra, radius; ul, ulna. Scale bars, 500 μm.
Discussion
We successfully generated ScxCre KI mice that can be used as genetic tools to reveal in vivo function of Scx-expressing tissue domains integrating the musculoskeletal components. In ScxCre KI mice, Cre expression was driven by Scx promoter and endogenous Scx was inactivated. Cre-mediated recombination efficiency of ScxCre KI mice reflected the intensity and duration of endogenous Scx expression. Cre-mediated excision was mainly observed in domains with persistent Scx expression, such as tendons, ligaments, and annulus fibrosus of intervertebral discs. In homozygous ScxCre/Cre KI mice, Tnmd, a marker of mature tenocytes and ligamentocytes, was almost absent. In addition, other tendon markers such as Col14a1, Dcn, Mkx, and Egr1 were downregulated in homozygotes. Moreover, regardless of the short duration of endogenous Scx expression, defective maturation was observed in Scx-expressing domains that are involved in integration of the musculoskeletal components.
Scx is localised in the intron 3 region of Bop1 gene on chromosome 15. Bop1 and Scx are a pair of bidirectional overlapping coding genes related to cellular proliferation and differentiation, respectively7,22. The first reported Scx knockout failed to form mesoderm and died during early stages of embryogenesis24, probably because the neomycin phosphotransferase gene linked to phosphoglycerate kinase promoter (PGK-Neo) cassette affected the expression of Bop1. Later, Murchison et al. reported generation and characterisation of a conditional allele in the Scx locus with FRT-Neo cassette, by flanking the first exon, which includes most of the coding region, with loxP site7. As was the case with homozygotes of Scx knockout with a Neo allele, we too failed to obtain viable ScxCreNeo homozygous pups, but we successfully established a new line of ScxCre KI mice by mating a ScxCreNeo heterozygote with a flippase-expressing FLPe mouse. Homozygous ScxCre/Cre KI mice with tendon defects were viable and similar to Scx knockout mice generated by Murchison et al.7, suggesting that the replacement of most of the exon 1 by Cre does not cause embryonic lethality, unlike as observed on retention of PGK-Neo cassette in this genomic region.
Cre-mediated recombination in a ScxCre/+; Rosa-tdTomato mouse was less efficient than that in a ScxCre-L or a ScxCre-H transgenic mouse mated with a Rosa-tdTomato reporter mouse. Under the control of the endogenous Scx promoter/enhancer in KI mice, Cre expression gradually increased to reach levels sufficient for recombination, thus enabling Cre-mediated tdTomato expression not in the Scx+ progenitor cell population but in the differentiated cell populations of tendons, ligaments, the annulus fibrosus of the intervertebral discs, and patella cartilage. More tdTomato positive cells were observed in ScxCre/Cre; Rosa-tdTomato mice than ScxCre/+; Rosa-tdTomato, suggesting that the number of cells expressing Cre above the threshold increases in homozygotes due to a gene dosage effect. In the Cre-loxP system, recombination occurs only when Cre protein is accumulated above the recombination threshold. Mosaic and later Cre-mediated recombination is considered to be observed due to heterogeneity in the endogenous Scx mRNA levels and a narrow time window of Scx expression.
We previously reported that Scx positively regulates the expression of Tnmd in tenocytes both in vivo and in vitro6. Tnmd expression in Scx-positive cells in the periodontal ligament is also upregulated by lentiviral overexpression of Scx and downregulated by knockdown of endogenous Scx36. In this study, immunostaining of ScxCre/Cre homozygotes with anti-Tnmd antibody revealed that Scx was necessary for the expression of Tnmd in most parts of the developing tendinous and ligamentous tissues. As previously reported, Tnmd acts as a positive regulator of postnatal tendon growth, maturation of collagen fibres, and cellular adhesion in the periodontal ligament17. Loss of Tnmd resulted in abated tenocyte proliferation leading to reduced tenocyte density, greater variation in collagen fibril diameters, and increase in maximal fibril diameters16. Thus, ablation of Tnmd expression in tendons, ligaments, and annulus fibrosus of intervertebral discs of homozygous KI mice suggests defective phenotypes in these tissues, other than the previously reported defects in force-transmitting and intermuscular tendons.
In the spinal column, an intervertebral disc lies between adjacent vertebrae and acts as a shock absorber. Intervertebral discs consist of inner and outer annulus fibrosus of sclerotome origin and notochord-derived nucleus pulposus. The inner annulus fibrosus has cartilaginous matrix associated with Col2 fibres, whereas the outer annulus fibrosus has thick multiple layers of dense connective tissue containing predominantly Col1. We previously reported that Scx+/Sox9+ progenitor population gives rise to both inner and outer annulus fibrosus and that Sox9 in this population is indispensable for the formation of inner annulus fibrosus2. In this study, we found that Tnmd expression in the outer annulus fibrosus was missing in ScxCre/Cre KI neonates. Thus, we demonstrated, for the first time, that Scx is necessary for the maturation of outer annulus fibrosus.
Scx is also transiently expressed in Sox9-expressing entheseal and sesamoid cartilage during early stages of chondrogenic differentiation, as previously reported1,3,37. This is consistent with our previous findings from lineage analysis using ScxCre transgenic mice that entheseal and patella chondroprogenitors were positive for Scx3. In the Scx-expressing domain, mice lacking Sox9 lose the cartilaginous domain that contributes to the establishment of tendon/ligament attachment sites2,4. In this study, we found that expression of Sox9 in Scx+/Sox9+ chondroprogenitors is markedly decreased. Even though Scx expression in Scx+/Sox9+ chondroprogenitors is only transient, loss of Scx caused defective formation of cartilaginous elements arising from the Scx+/Sox9+ progenitor population. Moreover, activation of BMP and TGF-β signalling pathways in these cartilaginous elements was diminished as evidenced by decreased phosphorylation of Smad1/5 and Smad3. These findings indicate that transient expression of Scx in chondroprogenitors is functionally important in the process of entheseal cartilage formation.
Muscle contractions in utero generate mechanical forces that are essential for normal embryonic development, through modulation of cell signalling and gene expression. As reported previously, Scx is the mechanical stress responsive gene that is upregulated in the periodontal ligament in response to tensile force and maintains its fibrogenic state by inhibiting mineralisation36. Conversely, removal of tensile force in the tendon results in a decrease in Scx expression38. The severe defective phenotypes, initially in force-transmitting and intermuscular tendons and later in ligaments of ScxCre/Cre KI mice lacking Scx, raised the possibility that Scx expression in response to mechanical force during embryonic and postnatal growth might be required for the proper development and structural maintenance of these tissues. Further investigation of target genes regulated by tensile force-responsive Scx may reveal how Scx participates in the regulation of formation and maintenance of tendons and ligaments during development and growth, in the presence or absence of mechanical stress. Such studies are now underway.
Materials and Methods
Animals and embryos
Mice were purchased from Japan SLC (Shizuoka, Japan) or from Shimizu Laboratory Supplies (Kyoto, Japan). The generation and establishment of ScxGFP transgenic strains have been reported previously3. For analysis of Cre activity, Rosa-CAG-LSL-tdTomato (Rosa-tdTomato) mice obtained from Jackson laboratory were used26. Cre-mediated recombination was monitored by red fluorescence of tdTomato expression driven by CAG promoter. All animal experimental protocols were approved by the Animal Care Committee of the Institute for Frontier Life and Medical Sciences, Kyoto University and the Committee of Animal Experimentation, Hiroshima University, and conformed to institutional guidelines for the study of vertebrates. All methods were carried out in accordance with relevant guidelines and regulations.
Generation of Scx Cre KI mouse strain
The ScxCreNeo targeting vector was designed to insert the coding region of Cre, along with a translational stop codon followed by FRT-flanked PGK-Neo cassette, in place of the ATG codon of Scx coding region, with simultaneous deletion of most of the Scx exon 1 (Fig. 1a). For construction of the targeting vector, a fused fragment, containing 706 bp Scx genomic region and Cre (706ScxCre)3, was cut with BglII from pBluescript SK (+) (Stratagene). 706ScxCre was inserted into the BamHI site of pPE7neoW-F2LF with PGK-Neo cassette flanked by two FRT sites, so that the PGK promoter was oriented in the opposite direction to the Scx promoter. The 486 bp Scx genomic fragment downstream of BglII site in the first exon and first intron was amplified by PCR using a forward primer with HindIII and BamHI sites (Scx-547F: 5′-AAGCTTGGATCCAGATCTGCACCTTCTGCCTCAG-3′) and a reverse primer with HindIII and NotI sites (Scx-intR2: 5′-AAGCTTGCGGCCGCGGAGGGGTAGTGGCAC-3′). The BamHI site within Scx-547F was introduced for genotyping using Southern blot analysis. The HindIII-digested amplified fragment was inserted into the HindIII site of pPE7neoW-F2LF. PGK-Neo cassette with homology arms was then used for modification of bacterial artificial chromosome clone (RP23-415D19, CHORI) by using Red/ET recombination system (Gene Bridges) to generate ScxBAC-CreNeo, in which Cre was introduced in-frame with ATG of Scx exon 1 and replaced with a 548 bp region. The approximately 0.5 kb homology arms, homologous to extreme 5′ and 3′ ends of the genomic region to be retrieved from ScxBAC-CreNeo, were amplified by PCR. The 5′ homology arm was amplified with forward primer (F1: 5′-AAGCTTCGTTTGCCATCCAGGTCATAACCC-3′) and reverse primer (R1: 5′-ATCGATCCTTGTAAACTACCCACTTGTACCTAAG-3′). The 3′ homology arm was amplified with forward primer (F2: 5′-ATCGATGACAGGCCAGGCCGTGTTCCTGTG-3′) and reverse primer (R2: 5′-CTCGAGCCACATGGCCACTTCACCTCCTTCCTAC-3′). The resultant amplified 5′ and 3′ homology arms were subcloned into HindIII-ClaI and ClaI-XhoI sites of pMCS-DTA, respectively. For generation of the ScxCreNeo targeting vector, the modified genomic region with 1.7 kb short and 6.3 kb long arms was retrieved from ScxBAC-CreNeo and transferred to pMCS-DTA. The PacI-linearised ScxCreNeo targeting vector was electroporated into KY1.1 ES cell line (F1 hybrid of C57BL/6J and 129S6/SvEvTac)23. Total 151 ES cell clones were obtained by positive and negative selection and then screened by PCR using forward primer (F3: 5′-GGATCCTCCTGGGCCCACAGGTCATAG-3′) and reverse primer (Cre-R1: 5′-CTTGCGAACCTCATCACTCGTTGCATCG-3′) (Fig. 1a). We obtained one correctly targeted ES clone that was microinjected into the blastocysts of BDF1 hybrid to generate chimeric mice.
Genotyping
Mice were genotyped using tail tip genomic DNA by PCR or Southern blot analysis. The wild type Scx allele was detected with forward primer (F4: 5′-CTGGTGGGTGAGGCCTGTGGC-3′) and reverse primer (R3: 5′-GAGTCTGTGTCCCAAGGTATG-3′). The ScxCre allele was detected with forward primer (Cre-F1: 5′-TCCAATTTACTGACCGTACACCAA-3′) and reverse primer (Cre-R2: 5′-CCTGATCCTGGCAATTTCGGCTA-3′; Fig. 1b). For Southern blot analysis, 10 μg genomic DNA was digested with HindIII and the resulting DNA fragments were separated via 0.7% agarose gel electrophoresis. After transfer onto Nytran membranes with Turboblotter system (Schleicher and Schuell), hybridisation was performed using probes labelled with [α-32P]- deoxycytidine triphosphate (dCTP) (PerkinElmer).
Northern blot analysis
Total RNA was extracted from wild type and ScxCre/+ KI embryos at E12.5. Fifteen micrograms of the total RNA was denatured with 6% formaldehyde, fractionated using 1% agarose gel electrophoresis, and transferred onto Nytran membranes with Turboblotter system. A specific cDNA probe for Scx was labelled with [α-32P]- dCTP. Hybridisations were performed as previously described6,21.
Histology
ScxCre/+; Rosa-tdTomato and ScxCre/Cre; Rosa-tdTomato neonates were fixed in 4% paraformaldehyde dissolved in phosphate-buffered saline (PFA/PBS) at 4 °C for 3 h, immersed in 20% sucrose/PBS, frozen, and cryosectioned to a thickness of 8 μm. After washing with PBS, nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma). Cre-mediated tdTomato expression was detected with a fluorescent microscope. For histological evaluation of knee joint during postnatal growth, paraffin sections prepared from 2-week-old ScxCre/+ and ScxCre/Cre KI mice were rehydrated and stained with haematoxylin and eosin. The images were captured under the Leica DMRXA microscope equipped with the Leica DC500 camera (Leica Microsystems).
Immunostaining
ScxCre/+KI, ScxCre/Cre KI, ScxCre/+; Rosa-tdTomato, and ScxCre/Cre; Rosa-tdTomato neonates were immersed in 20% sucrose/PBS, frozen, and cryosectioned to a thickness of 8 μm. Sections were fixed in ice-cold acetone prior to double immunostaining of Chmd/Col1, Chmd/Tnmd, and myosin heavy chain (MHC)/Tnmd. For double immunostaining of Chmd and Sox9, p-Smad1/5, or p-Smad3, ScxCre/+ and ScxCre/Cre KI embryos were fixed in 4% PFA/PBS at 4 °C for 3 h, immersed in 20% sucrose/PBS, frozen, and cryosectioned to a thickness of 6 μm. For undecalcified sections, 4-week-old Scx+/+ and ScxCre/Cre KI mice were anesthetised to perfuse with 4% PFA/PBS containing 20% sucrose. The legs were dissected, fixed in 4% PFA/PBS containing 20% sucrose for 3 h, embedded in SCEM (Leica Microsystems), frozen, and cryosectioned to a thickness of 4 μm, according to Kawamoto’s film method39. The sections were decalcified with 0.25 M EDTA/PBS for 60 min and processed for immunostaining. After washing with PBS, the sections were incubated with 2% skim milk in PBS for 20 min and then incubated overnight at 4 °C with primary antibodies diluted with 2% skim milk in PBS. The sections were incubated with appropriate secondary antibodies conjugated with Alexa Fluor 488 or 594 (Life Technologies) and washed again with PBS. The primary antibodies used were anti-Tnmd (diluted 1:1000)2,12, anti-Col1 (1:500; Rockland), anti-Chmd (diluted 1:500; Cosmo Bio), anti-Col2 (diluted 1:500; Rockland), anti-Ocn (1:800; TaKaRa), anti-sclerostin (1:500; R&D), anti-MHC (1:400; sigma), anti-Sox9 (1:800; Millipore), anti-p-Smad1/5 (1:50; Cell Signaling), and anti-p-Smad3 (1:250; Rockland). Nuclei were counterstained with DAPI. Images were captured using either a Leica DMRXA microscope equipped with a Leica DC500 camera (Leica Microsystems) or an OLYMPUS IX70 microscope equipped with an OLYMPUS DP80 camera (OLYMPUS).
In situ hybridisation
Antisense RNA probes for each gene were transcribed from linearised plasmids with a digoxygenin (DIG) RNA labelling kit (Roche) as previously described40. For RNA probes, the cDNAs for Col1a1, Dcn, Mkx, and Egr1 were amplified by RT-PCR using the following primers (mCol1a1_F1: 5′-CCCTCAAGAGCCTGAGTCAGC-3′), (mCol1a1_R1: 5′-GTATTCGATGACTGTCTTGCCC-3′), (mDcn_F1: 5′-TGGTGCAGTGTTCTGATCTGG-3′), (mDcn_R1: 5′-GACTCACAGCCGAGTAGGAAGC-3′), (mfcMkx_F1: 5′-GCGGCCGCCCCCAATCATGAACACCATCG-3′),(mfcMkx_R1: 5′-GCGGCCGCTAGAAGCGCTGCACCAGTGGCA-3′), (mEgr1_F2: 5′-TCTCTTCTTACCCATCCCCAGTGC-3′), and (mEgr1_R2; 5′-TACTGAAATGATTTATCCAATACCATG-3′). RNA probes of Scx, and Tnmd were described previously2,10. Mouse Col14a1 cDNA was also amplified using primers described previously7. For in situ hybridisation on frozen sections, mouse embryos were fixed with 4% PFA/PBS for 3 h, and treated in 20% sucrose before embedding in Tissue-Tek OCT compound (Sakura Finetek). Seven-micron-thick frozen sections of embedded embryos were prepared and postfixed with 4% PFA/PBS for 10 minutes at room temperature and carbethoxylated twice in 0.1% DEPC/PBS. Sections were treated in 5 × SSC, and hybridisation was performed at 58 °C with DIG-labelled antisense RNA probes. To detect DIG-labelled RNA probes, immunological detection was performed using an anti-DIG antibody conjugated with alkaline phosphatase (Anti-DIG-AP Fab fragment, Roche) and BM purple (Roche). Images were captured using an IX70 microscope equipped with a DP80 camera (OLYMPUS).
Skeletal preparation
Mouse neonates were dehydrated with ethanol. After removing skin and soft tissues, the neonates were stained with 0.015% Alcian blue 8GX (Sigma) and cleared with 2% potassium hydroxide. The neonates were then stained with 0.05% Alizarin Red S (Wako) in 1% KOH and cleared with 1% KOH.
Additional Information
How to cite this article: Yoshimoto, Y. et al. Scleraxis is required for maturation of tissue domains for proper integration of the musculoskeletal system. Sci. Rep. 7, 45010; doi: 10.1038/srep45010 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We thank Ms. H. Sugiyama for her valuable secretarial help. This study was partly supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers JP26293395, JP26893164 and the Cooperative Research Program of Institute for Frontier Medical Sciences, Kyoto University, Japan.
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.Y. and C.S. designed the study. Y.Y., A.T., H.W., and G.K. performed the experiments. Y.Y., Y.H., and C.S. analysed the results. Y.Y. and C.S prepared the manuscript.
References
- Cserjesi P. et al. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development 121, 1099–1110 (1995). [DOI] [PubMed] [Google Scholar]
- Sugimoto Y. et al. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 140, 2280–2288, doi: 10.1242/dev.096354 (2013). [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Takimoto A., Hiraki Y. & Shukunami C. Generation and characterization of ScxCre transgenic mice. Genesis 51, 275–283, doi: 10.1002/dvg.22372 (2013). [DOI] [PubMed] [Google Scholar]
- Blitz E., Sharir A., Akiyama H. & Zelzer E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 140, 2680–2690, doi: 10.1242/dev.093906 (2013). [DOI] [PubMed] [Google Scholar]
- Schweitzer R. et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128, 3855–3866 (2001). [DOI] [PubMed] [Google Scholar]
- Shukunami C., Takimoto A., Oro M. & Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Developmental biology 298, 234–247, doi: 10.1016/j.ydbio.2006.06.036 (2006). [DOI] [PubMed] [Google Scholar]
- Murchison N. D. et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134, 2697–2708, doi: 10.1242/dev.001933 (2007). [DOI] [PubMed] [Google Scholar]
- Lincoln J., Alfieri C. M. & Yutzey K. E. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn 230, 239–250, doi: 10.1002/dvdy.20051 (2004). [DOI] [PubMed] [Google Scholar]
- Pryce B. A., Brent A. E., Murchison N. D., Tabin C. J. & Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn 236, 1677–1682, doi: 10.1002/dvdy.21179 (2007). [DOI] [PubMed] [Google Scholar]
- Shukunami C., Oshima Y. & Hiraki Y. Molecular cloning of tenomodulin, a novel chondromodulin-I related gene. Biochemical and biophysical research communications 280, 1323–1327, doi: 10.1006/bbrc.2001.4271 (2001). [DOI] [PubMed] [Google Scholar]
- Brandau O., Meindl A., Fassler R. & Aszodi A. A novel gene, tendin, is strongly expressed in tendons and ligaments and shows high homology with chondromodulin-I. Dev Dyn 221, 72–80, doi: 10.1002/dvdy.1126 (2001). [DOI] [PubMed] [Google Scholar]
- Oshima Y. et al. Anti-angiogenic action of the C-terminal domain of tenomodulin that shares homology with chondromodulin-I. J Cell Sci 117, 2731–2744, doi: 10.1242/jcs.01112 (2004). [DOI] [PubMed] [Google Scholar]
- Kimura N. et al. Local tenomodulin absence, angiogenesis, and matrix metalloproteinase activation are associated with the rupture of the chordae tendineae cordis. Circulation 118, 1737–1747, doi: 10.1161/CIRCULATIONAHA.108.780031 (2008). [DOI] [PubMed] [Google Scholar]
- Dex S., Lin D., Shukunami C. & Docheva D. Tenogenic modulating insider factor: Systematic assessment on the functions of tenomodulin gene. Gene 587, 1–17, doi: 10.1016/j.gene.2016.04.051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havis E. et al. Transcriptomic analysis of mouse limb tendon cells during development. Development 141, 3683–3696, doi: 10.1242/dev.108654 (2014). [DOI] [PubMed] [Google Scholar]
- Docheva D., Hunziker E. B., Fassler R. & Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Molecular and cellular biology 25, 699–705, doi: 10.1128/MCB.25.2.699-705.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y. et al. Tenomodulin expression in the periodontal ligament enhances cellular adhesion. PloS one 8, e60203, doi: 10.1371/journal.pone.0060203 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga T. et al. FGF-2 Stimulates the Growth of Tenogenic Progenitor Cells to Facilitate the Generation of Tenomodulin-Positive Tenocytes in a Rat Rotator Cuff Healing Model. Am J Sports Med 43, 2411–2422, doi: 10.1177/0363546515597488 (2015). [DOI] [PubMed] [Google Scholar]
- Alberton P. et al. Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells Dev 24, 597–609, doi: 10.1089/scd.2014.0314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberton P. et al. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev 21, 846–858, doi: 10.1089/scd.2011.0150 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto A., Oro M., Hiraki Y. & Shukunami C. Direct conversion of tenocytes into chondrocytes by Sox9. Experimental cell research 318, 1492–1507, doi: 10.1016/j.yexcr.2012.04.002 (2012). [DOI] [PubMed] [Google Scholar]
- Arao Y., Carpenter K., Hewitt S. & Korach K. S. Estrogen down-regulation of the Scx gene is mediated by the opposing strand-overlapping gene Bop1. The Journal of biological chemistry 285, 4806–4814, doi: 10.1074/jbc.M109.036681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita K. et al. Development of the circadian oscillator during differentiation of mouse embryonic stem cells in vitro. Proceedings of the National Academy of Sciences of the United States of America 107, 3846–3851, doi: 10.1073/pnas.0913256107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Wagner D., Li X., Richardson J. A. & Olson E. N. Dual role of the basic helix-loop-helix transcription factor scleraxis in mesoderm formation and chondrogenesis during mouse embryogenesis. Development 126, 4317–4329 (1999). [DOI] [PubMed] [Google Scholar]
- Farley F. W., Soriano P., Steffen L. S. & Dymecki S. M. Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28, 106–110 (2000). [PubMed] [Google Scholar]
- Madisen L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140, doi: 10.1038/nn.2467 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraki Y. et al. Identification of chondromodulin I as a novel endothelial cell growth inhibitor. Purification and its localization in the avascular zone of epiphyseal cartilage. The Journal of biological chemistry 272, 32419–32426 (1997). [DOI] [PubMed]
- Shukunami C., Takimoto A., Miura S., Nishizaki Y. & Hiraki Y. Chondromodulin-I and tenomodulin are differentially expressed in the avascular mesenchyme during mouse and chick development. Cell and tissue research 332, 111–122, doi: 10.1007/s00441-007-0570-8 (2008). [DOI] [PubMed] [Google Scholar]
- Blitz E. et al. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Developmental cell 17, 861–873, doi: 10.1016/j.devcel.2009.10.010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. & Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003). [DOI] [PubMed] [Google Scholar]
- Gelse K., Poschl E. & Aigner T. Collagens–structure, function, and biosynthesis. Advanced drug delivery reviews 55, 1531–1546 (2003). [DOI] [PubMed] [Google Scholar]
- Wilda M. et al. A comparison of the expression pattern of five genes of the family of small leucine-rich proteoglycans during mouse development. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 15, 2187–2196, doi: 10.1359/jbmr.2000.15.11.2187 (2000). [DOI] [PubMed] [Google Scholar]
- Ito Y. et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proceedings of the National Academy of Sciences of the United States of America 107, 10538–10542, doi: 10.1073/pnas.1000525107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. et al. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Molecular and cellular biology 30, 4797–4807, doi: 10.1128/MCB.00207-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejard V. et al. EGR1 and EGR2 involvement in vertebrate tendon differentiation. The Journal of biological chemistry 286, 5855–5867, doi: 10.1074/jbc.M110.153106 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto A. et al. Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone. Development 142, 787–796, doi: 10.1242/dev.116228 (2015). [DOI] [PubMed] [Google Scholar]
- Eyal S. et al. On the development of the patella. Development 142, 1831–1839, doi: 10.1242/dev.121970 (2015). [DOI] [PubMed] [Google Scholar]
- Maeda T. et al. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr Biol 21, 933–941, doi: 10.1016/j.cub.2011.04.007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch Histol Cytol 66, 123–143 (2003). [DOI] [PubMed] [Google Scholar]
- Takimoto A., Nishizaki Y., Hiraki Y. & Shukunami C. Differential actions of VEGF-A isoforms on perichondrial angiogenesis during endochondral bone formation. Developmental biology 332, 196–211, doi: 10.1016/j.ydbio.2009.05.552 (2009). [DOI] [PubMed] [Google Scholar]