Abstract
Phosphatidylinositol-3-kinase (PI3K) signaling plays a crucial role in oncogene-mediated tumor growth and proliferation. Buparlisib (BKM120) is an oral pan-class I PI3K inhibitor. This phase I study was conducted to determine the dose limiting toxicity (DLT) and maximum tolerated dose (MTD) of BKM120 in patients (pts) with relapsed/refractory acute leukemias. Fourteen pts (12 acute myeloid leukemia, 1 acute lymphoblastic leukemia, and 1 mixed phenotype leukemia) were enrolled. Twelve pts received BKM-120 80 mg/day and two 100 mg/day. The MTD was 80 mg/day. Of the 14 patients treated, the best response was stable disease in one patient that lasted 82 days. The median survival for all patients was 75 days (range 10–568). Three patients with a 3q26 chromosome abnormality had a significantly improved median survival of 360 days (range 278–568) as compared to a median survival of 57 days (range, 10–125) among the 11 other patients. The most frequent drug-related toxicities included confusion, mucositis, dysphagia, and fatigue. Western blot profiling revealed a decrease in p-pS6K/total pS6K in 5/7 (71%) available patient samples with a mean quantitative inhibition of 65% (range, 32–100%) and a decrease in p-FOXO3/total FOXO3 in 4/6 (67%) samples with a mean quantitative inhibition of 93% (range, 89–100%). BKM120 administered at 80 mg/day showed modest efficacy and was tolerable in advanced acute leukemias.
Introduction
The phosphatidylinositol-3-kinase (PI3K) signaling pathway plays a critical role in oncogene-mediated tumor growth and proliferation and has regulatory functions in cell survival, apoptosis, protein synthesis, and glucose metabolism.[1–4] The Class-IA PI3K, one of three classes of PI3K, has been shown to be frequently mutated and/or activated in human cancers. Buparlisib (BKM120) is classified as an oral pan-class I PI3K inhibitor belonging to the 2,6-dimorpholino pyrimidine derivative family.[5] Efficacy and tolerance of buparlisib has been shown in advanced solid tumor malignancies.[3] It has shown promise as a combination agent with other therapies such as trastuzumab, letrozole, fulvestrant, and chemotherapy in cases of advanced breast cancer.[6] Phase III trials (BELLE-2 and BELLE-3) are ongoing to evaluate the efficacy of buparlisib in combination with fulvestrant in advanced breast cancer patients.[7]
Deregulated activation of the PI3K/AKT/mTOR pathway has been shown to be a feature of acute myeloid leukemia (AML) patients contributing to an unfavorable outcome.[8,9] It has been demonstrated that the PI3K/AKT pathway is constitutively activated in acute myeloid leukemia (AML), and this activation has been correlated to adverse cytogenetics and inferior overall survival.[9–14] Two separate studies in newly diagnosed AML patients using flow cytometry and reverse phase protein array (RPPA) analysis, respectively, demonstrated that the level of AKT phosphorylation on Thr308, but not on Ser473, significantly correlated with high-risk karyotype in AML.[9,15,16] Furthermore, high levels of p-Thr308 was an adverse prognostic factor for patients with intermediate cytogenetics and was associated with inferior survival compared to patients with low or intermediate pThr308 levels. Increased AKT expression in AML is usually associated with oncogenic mutations of upstream targets, including receptor tyrosine kinases such as BCR-ABL, FLT3, and c-KIT (up to 35–40%), and mutated N-RAS or K-RAS (up to 20%).[4] Buparlisib has been shown to decrease the phosphorylation status of various either direct (GSK3β, FKHRL1/FOXO3a) or indirect downstream AKT effectors (p70S6K, through mTOR) in the PTEN null cell line.[10] These findings and others lent credence to the hypothesis that buparlisib may be an effective agent in patients with advanced leukemias.[17,18]
The clinical trial NCT01396499 was designed to identify an effective dose of BKM120 in patients with relapsed/refractory acute leukemias. To our knowledge, this is the first clinical trial to evaluate a pan PI3K inhibitor in advanced hematologic malignancies. Buparlisib administered at 80 mg/day was tolerable and demonstrated modest single-agent efficacy in pts with previously treated acute leukemias.
Methods
Patients with AML, acute lymphoblastic leukemia (ALL), or mixed phenotype acute leukemia (MPAL) who had relapsed, were refractory to standard chemotherapy, or unsuitable for standard chemotherapy were eligible for this study.
Study design
This was an open label, non-randomized phase I dose escalation study of single agent BKM120 in patients with advanced hematologic malignancies. To estimate the maximum tolerated dose (MTD), up to two dose levels of BKM120 in subsequent cohorts were explored. The starting dose was selected as 80 mg/day which was one dose level lower than the solid tumor MTD of 100 mg/day.[3] Therapy was administered daily continuously by mouth. One cycle was defined as 28 days. Therapy was continued in responders or patients with stable disease for up to 12 cycles or as long as clinical benefit was observed in the absence of clinically significant disease progression or unacceptable toxicity. Patients were evaluated for response by bone marrow aspirate and biopsy at the end of one full cycle of therapy (28 days) and every two cycles thereafter.
Study objectives
The primary objective of this study was to determine the DLT and MTD of BKM120 in patients with relapsed/refractory acute leukemias. Secondary objectives were to determine the safety and tolerability of BKM120, the clinically relevant disease responses to BKM120, and the effect of BKM120 on biomarkers of PI3K/mTOR signaling.
Treatment regimen
Patients received oral BKM120 continuously daily in 28 day cycles. The starting dose was 80 mg/day. For patients unable to tolerate protocol specified dosing schedule, dose adjustments were permitted to keep patients on study and for safety.
Eligibility
Patients were required to be 18 years or older with a diagnosis of AML or ALL, relapsed or refractory to standard chemotherapy. Other inclusion criteria included: ECOG performance status of 0–2, serum creatinine ≤1.5 mg dL−1 or 24-hr clearance ≥50 mL min−1, bilirubin ≤2.0 mg dL−1, SGOT or SGPT ≤3× upper limit of normal (ULN), serum amylase ≤ ULN; serum lipase ≤ ULN, fasting glucose ≤120 mg dL−1, magnesium ≥ the lower limit of normal, normal total calcium and potassium, and an INR ≤ 2.
Biomarker and correlative assessment
Peripheral blood samples were collected before administration and 24 hr after administration of BKM120 during the first cycle of therapy. Bone marrow samples were collected at screening and on Day 28 of the first cycle of therapy. Protein expression in leukemic cells was evaluated using RPPA technology.[16,19] RPPA was used to determine: (1) protein expression of the known upstream regulators of the PI3K/AKT pathway including PP2A, PTEN, and p53, (2) the activation status and modulation by BKM120 of the AKT/mTOR pathway (AKT (Ser473)/(Thr308), p70S6K (Thr389), S6RK, 4EBP1 (Thr70)/(Thr37/46)), total and phospho-mTOR (PRAS40), (3) the downstream apoptotic modulators shown to be inhibited by PI3K/mTOR inhibitors (Bim, Mcl-1, Bcl-2, Bad, HIF-1α), and (4) the effects of BKM120 on activation of other signaling pathways (ERK (p44/42), STAT-3 and STAT-5). Details of RPPA procedure are provided in the Supporting Information.
Results and Discussion
Study population
Baseline characteristics for the study populations are shown in Table I. Fourteen patients received at least one dose of buparlisib (12 AML, 1 MPAL, 1 ALL) and were included in this analysis. The median age was 59 years (range, 29–85). All patients had received prior therapy. The median number of prior therapies was 4 (range, 1–6): hypomethylating agents (n = 13), fludarabine (n = 6), clofarabine (n = 6), cladribine (n = 2), either alone or in combination, and allogeneic stem cell transplant (n = 3). Cytogenetic analysis revealed diploid karyotype in four patients (29%), complex cytogenetics including chromosome 5 and/or 7 abnormalities in three patients (21%), inversion 3 or 3q26 in three patients (21%), and other cytogenetic aberrations in four patients (29%). Molecular analysis showed NRAS mutation in four patients, IDH 1/2 mutations in two patients, and K-RAS, DNMT3A, NPM1, KIT, and FGFR1 mutation in one patient each, respectively.
TABLE I.
Patient Characteristics (N = 14)
| Characteristic | Median (%)/[Range] |
|---|---|
| Age (years) | 59 [29–85] |
| ≥60 years | 7 (50) |
| Diagnosis | |
| AML – de novo | 9 (65) |
| AML – post MDS/CMML | 2 (14) |
| Therapy-related AML | 1 (7) |
| ALL | 1 (7) |
| Mixed phenotype AML | 1 (7) |
| Bone marrow blast percentage | 56 [17–94] |
| Peripheral blast percentage | 32 [0–97] |
| WBC × 109/L | 1.8 [0.5–56] |
| Platelets × 109/L | 23 [2–258] |
| Hemoglobin (g dL−1) | 9.2 [6.9–11.2] |
| Cytogenetics | |
| Diploid | 4 (29) |
| −5/−7 | 3 (21) |
| Other adverse (−17p, −11q, 3q26) | 5 (26) |
| Miscellaneous (+9, +21) | 2 (14) |
| Prior therapies | 4 [1–6] |
| Buparlisib dose | |
| 80 mg | 12 |
| 100 mg | 2 |
AML, acute myeloid leukemia, MDS, myelodysplastic syndrome, CMML, chronic myelomonocytic leukemia, WBC, white blood cells.
Dosing
A 3 + 3 algorithm was used for dose escalation to determine the MTD. Among the first three patients to complete at least one cycle of therapy and be deemed evaluable for DLT at dose level 0 (80 mg/day), one patient experienced a DLT in the form of prolonged CTCAE grade 2 confusion and dysphagia. An additional three patients were enrolled at the 80 mg/day dose and no further DLTs were encountered. Since 1/6 DLTs were experienced the dose level was escalated to 100 mg/day. The first two patients treated at 100 mg/day experienced DLT in the form of grade 3 confusion and grade 3 mucositis with grade 2 confusion, respectively. The 80 mg/day was established as the MTD and three additional patients were enrolled at this level in the expansion phase. One of the patients enrolled at the 80 mg/day dose in the expansion phase but required dose reduction after cycle 1 due to grade 2 hyperglycemia. The patient continued on 60 mg/day for two additional cycles before experiencing disease progression. The median number of cycles received was 1 (range 1–3), and the most common cause of discontinuation was progressive disease (n = 8).
Responses
Of the 14 patients treated, one patient had stable disease at the 80 mg/day dosage and had the longest duration on study at 82 days. The median overall survival for all patients was 75 days (range 10–568). The three patients with cytogenetic findings of 3q26 were found to have the longest overall survival of all study participants with a median survival of 360 days (range 278–568) as compared to a median survival of 57 days (range 10–125) among the 11 patient who did not have 3q26 abnormalities (P value 0.0052). The details of response for each patient are shown in Table II.
TABLE II.
Response to Buparlisib (N = 14)
| Pt | Dx | PreBKM Cyto | PreBKM molecular |
No. prior therapy |
BKM dose (mg) |
Duration on BKM (days) |
Evaluable | Response to therapy |
Post progression therapy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AML | Hyperdiploid, +9 | IDH, RAS | 3 | 80 | 57 | Yes | Progression | Crenolanib |
| 2 | T-AML | del 5, complex | 3 | 80 | 8 | No | Intolerance | None | |
| 3 | CMML-AML | diploid | 5 | 80 | 10 | No | Progression | Hydrea/cytarabine | |
| 4 | MPAL | del 7, +21, complex | 4 | 80 | 28 | Yes | Progression | None | |
| 5 | CMML-AML | Diploid | 6 | 80 | 2 | Yes (toxicity) |
DLT | None | |
| 6 | AML | del 7 | FLT3D835 | 5 | 80 | 7 | No | Progression | None |
| 7 | AML | inv 3 | 5 | 80 | 82 | Yes | Stable | BP100, Erlotinib, hydrea, fludarabine, cytarabine, azacytidine |
|
| 8 | AML | inv 3, (3q26) | NRAS | 1 | 80 | 28 | Yes | Progression | DFP-10917 with fludarabine and cytarabine, azacytadine, trametinib with GSK2141795, PRI-724, decitabine and volasertib |
| 9 | AML | 3q26 | 6 | 80 | 30 | Yes | Progression | Decitabine, AZD-1208, erlotinib, PRI-724, IGN-523, fludarabine and ara-C, AG221, GSK2141795 and trametinib, APTO-253 |
|
| 10 | AML | Hyperdiploid | NPM1, NRAS, IDH1, DNMT3A |
3 | 100 | 16 | Yes (Toxicity) |
DLT | None |
| 11 | AML | 11q23, 13q | KRAS, NRAS | 4 | 100 | 15 | Yes (Toxicity) |
DLT | Decitabine/cytarabine |
| 12 | AML | 17q11, +12 | TP53 | 4 | 80 | 27 | Yes | Progression | None |
| 13 | ALL | 4;11 | 6 | 80 | 27 | Yes | Progression | Decitabine, liposomal vincristine, followed by clofarabine, idarubicin, cytarabine, liposomal vincristine and dexamethasone |
|
| 14 | AML | Diploid | IDH, RAS | 6 | 60 | 79 | Yes | Progression | None |
Pt, patient, Dx, diagnosis, BKM, buparlisib, No., number, AML, acute myeloid leukemia, T-AML, therapy related AML, CMML, chronic myelomonocytic leukemia, MPAL, mixed phenotype acute leukemia, del, deletion, inv, inversion, DLT, dose limiting toxicity.
Safety findings
Neurotoxicity has been seen in prior studies of BKM120. In our study two patients experienced grade 2 confusion, and one patient experienced grade 3 confusion. Confusion was a DLT in all cases in which it was identified. Other drug-related toxicities ≥ grade 2 included grade 3 mucositis (n = 1), grade 2 dysphagia (n = 1), grade 2 fatigue (n = 2), grade 2 elevated serum bilirubin (n = 1) and grade 3 nausea (n = 1). The details of toxicities encountered are shown in Supporting Information Table SI.
Protein profiling
Western blot analysis was performed to evaluate the depth of PI3K/AKT/mTOR inhibition, and is shown in Supporting Information Table SII. A decrease in mTOR target p-pS6K/total pS6K pre- and post- 1 cycle of BKM120 was observed in 5/7 (71%) evaluable samples with a mean quantitative inhibition of 65% (range, 32–100%). Similarly, a decrease in AKT target p-FOXO3/total FOXO3 pre- and post- 1 cycle of BKM120 was observed in 4/6 (67%) evaluable samples with a mean quantitative inhibition of 93% (range, 89–100%). The one patient with stable disease had decreases in both p-pS6K/total PS6K (45%) and p-FOXO3/total FOXO3 (100%).
RPPA analysis
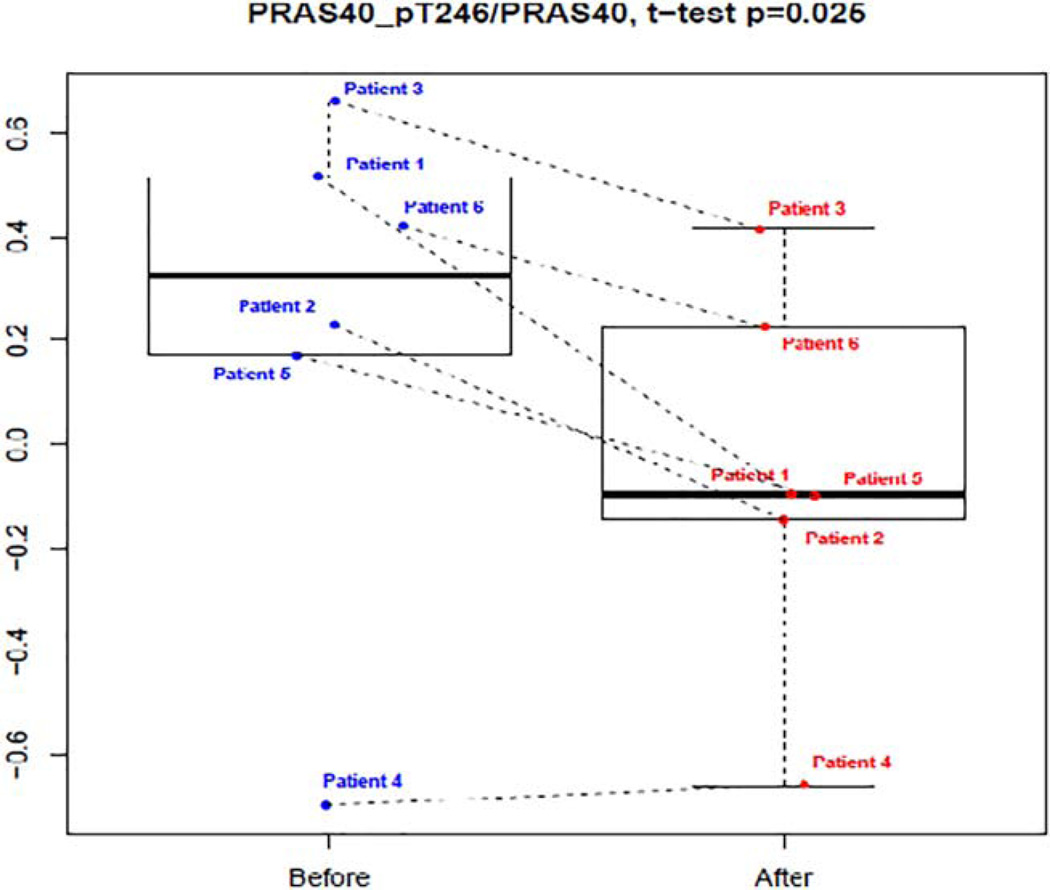
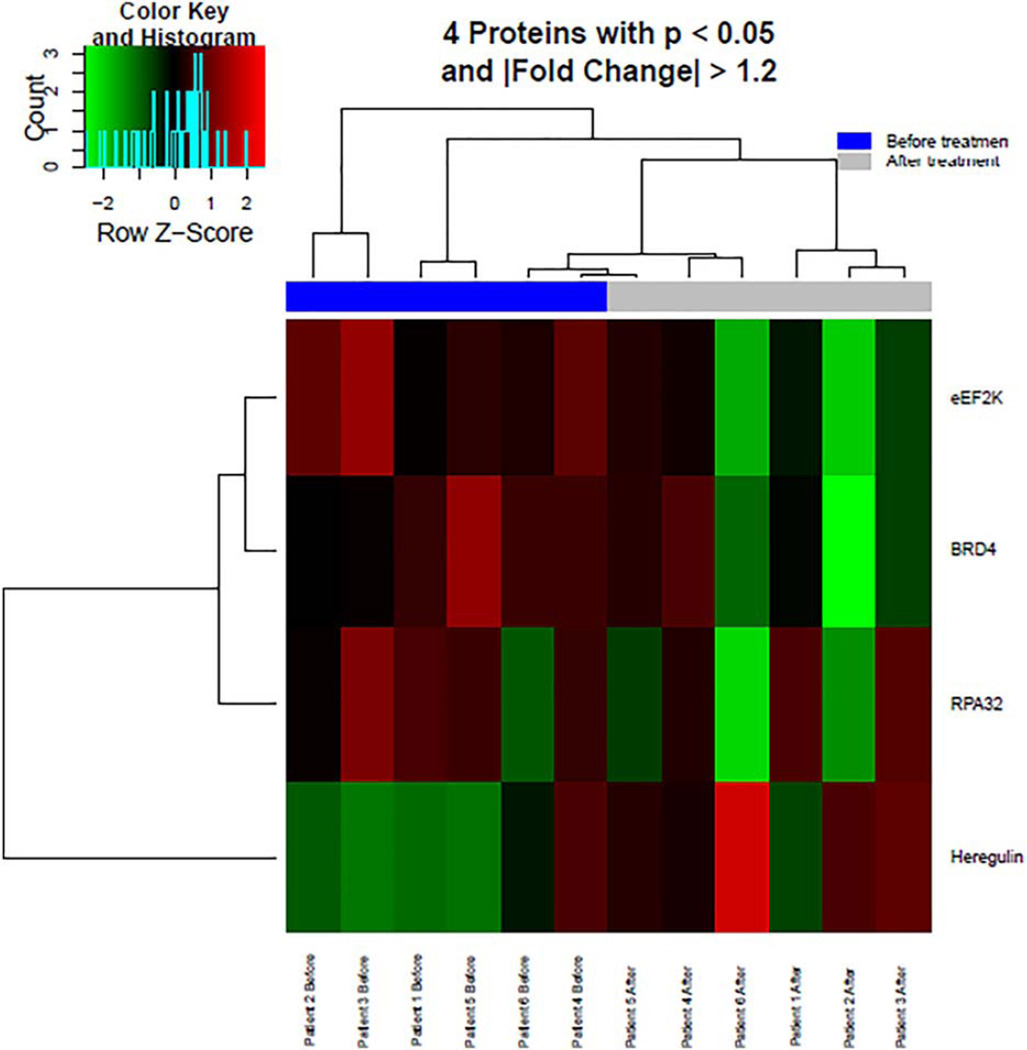
Six patients were evaluated using RPPA. Supporting Information Table SIII demonstrates the RPPA analysis for the proteins of interest in this analysis. Proline-rich AKT substrate of 40 kDa (PRAS40) was the only significantly decreased or down-regulated protein in this analysis; boxplot shown in Fig. 1. A trend toward upregulation by buparlisib was identified in the AKT pathway proteins Akt_pS473 and Akt_pT308. mTOR targets S6_pS240_S2444 and S6_p235_p236 were decreased in four of the six tested but did not reach significance due to the small sample size. Other interesting targets down-regulated by buparlisib included p53 regulator MDM2_pS166 (P = 0.018), known to be phosphorylated by AKT, ribosomal protein RPA32 (P = 0.032), and BRD4 (P = 0.039). A subset of proteins with significant expression change before and after treatment, identified by P < 0.05 and fold change >1.2 or < −1.2, were plotted on a heatmap shown in Fig. 2.
Figure 1.
RPPA box plot for PRAS40_pT246/PRAS40. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
Heatmap of the differentially expressed proteins before and after treatment with buparlisib. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
This trial was the first to evaluate a pan-PI3K inhibitor in advanced leukemias and demonstrates that targeting this pathway elicits modest activity but may have significant toxicity in the leukemic population. The intent of this analysis was to evaluate the tolerability, preliminary efficacy, and biologic activity of BKM120 in advanced leukemias to guide the feasibility and design of future therapeutic investigations using buparlisib and/or alternate PI3K-inhibitors in combination with other targeted inhibitors or cytotoxic therapy. The MTD identified for BKM120 in this trial was 80 mg/day and is lower than the MTD of 100 mg/day established in patients with solid tumors.[3] Furthermore, 3 of 14 patients experienced clinically significant confusion. It is known that BKM120 is effective at crossing the blood-brain barrier, which is supported by neurotoxicity in these patients.[5] Neurotoxicity was observed in early phase trials of BKM120 in patients with metastatic breast cancer and glioblastoma.[5,20] However, unlike in this trial, neurotoxicity was not often a DLT in early phase trials with solid tumors. The differential tolerability and increased CNS toxicity of this agent in patients with relapsed leukemia as opposed to those with solid tumors is unknown.
Correlative studies revealed that BKM120 was effective at suppressing downstream biomarkers p-S6K and p-FOXO3. RPPA analysis also revealed significant down-regulation of PRAS40. This is an important finding as PRAS40 has been shown to relieve inhibitory constraint on the AKT and mTOR signaling pathways of interest.[21] The ability of buparlisib to down-regulate PRAS40 theoretically provides improved inhibition and regulation of the deregulated AKT pathway. Unexpectedly, phosphorylation of direct PI3K target AKT was induced (although this did not reach statistical significance), possibly pointing towards a compensatory feedback secondary to inhibition of other signaling pathways. We found upregulation of MAPK_pT202_Y204 and of Stat3_pY705 in four of the six patients (Supporting Information). These findings confirm the effectiveness of buparlisib at specifically targeting and inhibiting the downstream PI3K/AKT pathway of interest, but such biological suppression did not manifest as improved clinical responses in this population of patients.
A finding of note in the analysis of this trial involves patients with a 3q26 aberration identified on cytogenetic analysis. 3q26.2 gene rearrangement results in overexpression of a protooncogene, transcription factor EVI1.[22,23] High EVI1 expression is one of the common gene expression changes in acute myeloid leukemia and is associated with an extremely poor prognosis.[24] Co-occurrence of RAS/receptor tyrosine kinase mutations are frequently noted in patients with 3q26 aberrations,[25] and these in turn, are known to upregulate PI3K signaling. High EvI1 was also shown to directly inhibit PTEN transcription by recruiting polycomb repressive complexes, and this translated into activation of AKT/mTOR signaling.[24] Thus, patients with 3q26 aberrations frequently have significant upregulated PI3K/AKT/mTOR signaling. Interestingly, the three patients with 3q26 abnormalities detected had a significantly improved overall survival (median 360 days) compared to the 11 non 3q26 patients (median 57 days) with a P value of 0.0052. The three patients of interest all received multiple therapies (median n = 6) following disease progression on BKM120. Though the sample size in this study is too small to make conclusive determinations, these finding suggests that patients with 3q26 gene rearrangements may be more sensitive to the effects of panPI3K inhibition. This would be in consort with the established mechanism of leukemiogenesis associated with 3q26 aberrations. Inversions of the 3q locus (or EVI1 overexpression) may then be candidate biomarkers to select patients with advanced leukemia most likely to benefit from panPI3K inhibitor therapies in subsequent trials.
Based on the results of this investigation, further evaluation of BKM120 either as a single agent or as a combination therapy in advanced hematologic malignancies may be considered, but selection of patients using baseline cytogenetic or molecular biomarkers is warranted to enrich for a population that may derive benefit.
Supplementary Material
Acknowledgments
ND, MK, and JC have received research funding from Novartis.
Contract grant sponsor: MD Anderson Cancer Center Support Grant (CCSG); Contract grant number: CA016672.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures of interest: There are no other relevant conflicts of interest to disclose.
Author Contributions: Brittany Ragon, Naval Daver, Marina Konopleva collected and analyzed the data and wrote the article. All other authors: Hagop Kantarjian, Elias Jabbour, Farhad Ravandi, Jorge Cortes, Gautam Borthakur, LaKiesha DeBose, Zhihong Zeng, Heather Schneider, Naveen Pemmaraju, Guillermo Garcia-Manero, Steven Kornblau, William Wierda, Jan Burger, Courtney DiNardo, Michael Andreeff participated in the discussion, have reviewed and approved the current version of the manuscript.
Naval Daver is responsible for the overall content as guarantor.
References
- 1.Katso R, Okkenhaug K, Ahmadi K, et al. Cellular function of phosphoinositide 3-kinases: Implications for development, immunity, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 2.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 3.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 4.Doepfner KT, Boller D, Arcaro A. Targeting receptor tyrosine kinase signaling in acute myeloid leukemia. Crit Rev Oncol Hematol. 2007;63:215–230. doi: 10.1016/j.critrevonc.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Maira S-M, Pecchi S, Huang A, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Therap. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 6.Ma CX, Luo J, Naughton M, et al. A phase I trial of BKM120 (Buparlisib) in combination with fulvestrant in postmenopausal women with estrogen receptor-positive metastatic breast cancer. Clin Cancer Res. 2016;22:1583–1591. doi: 10.1158/1078-0432.CCR-15-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Leo ACE, Janni W, Eystein LP, O’Regan R, et al. BELLE-3: A phase III study of the panphosphatidylinositol 3-kinase (PI3K) inhibitor buparlisib (BKM120) with fulvestrant in postmenopausal women with HR1/HER2—Locally advanced/metastatic breast cancer (BC) pretreated with aromatase inhibitors (AIs) and refractory to mTOR inhibitor (mTORi)-based treatment. J Clin Oncol. 2015;33(suppl) (abstr TPS626). Available at: http://meetinglibrary.asco.org/content/146016-156. [Google Scholar]
- 8.Allegretti M, Ricciardi MR, Licchetta R, et al. The pan-class I phosphatidyl-inositol-3 kinase inhibitor NVP-BKM120 demonstrates antileukemic activity in acute myeloid leukemia. Sci Rep. 2015;5:18137. doi: 10.1038/srep18137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornblau SM, Womble M, Qiu YH, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–2365. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao S, Konopleva M, Cabreira-Hansen M, et al. Inhibition of phosphatidylinositol 3-kinase dephosphorylates BAD and promotes apoptosis in myeloid leukemias. Leukemia. 2003;18:267–275. doi: 10.1038/sj.leu.2403220. [DOI] [PubMed] [Google Scholar]
- 11.Fransecky L, Mochmann LH, Baldus CD. Outlook on PI3K/AKT/mTOR inhibition in acute leukemia. Mol Cell Ther. 2015;3:2. doi: 10.1186/s40591-015-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinner S, Platanias LC. Targeting the mTOR pathway in leukemia. J Cell Biochem. 2016;117:1745–1752. doi: 10.1002/jcb.25559. [DOI] [PubMed] [Google Scholar]
- 13.Min YH, Eom JI, Cheong JW, et al. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: Its significance as a prognostic variable. Leukemia. 2003;17:995–997. doi: 10.1038/sj.leu.2402874. [DOI] [PubMed] [Google Scholar]
- 14.Xu Q, Simpson SE, Scialla TJ, et al. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 15.Gallay N, Dos Santos C, Cuzin L, et al. The level of AKT phosphorylation on threonine 308 but not on serine 473 is associated with high-risk cytogenetics and predicts poor overall survival in acute myeloid leukaemia. Leukemia. 2009;23:1029–1038. doi: 10.1038/leu.2008.395. [DOI] [PubMed] [Google Scholar]
- 16.Kornblau SM, Tibes R, Qiu YH, et al. Functional proteomic profiling of AML predicts response and survival. Blood. 2009;113:154–164. doi: 10.1182/blood-2007-10-119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martelli AM, Nyakern M, Tabellini G, et al. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–928. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 18.Martelli AM, Evangelisti C, Chiarini F, et al. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert Opin Investig Drugs. 2009;18:1333–1349. doi: 10.1517/14728220903136775. [DOI] [PubMed] [Google Scholar]
- 19.Kornblau SM, Qiu Y, Chen W, et al. Proteomic profiling of 150 proteins in 511 acute myelogenous leukemia (AML) patient samples using reverse phase proteins arrays (RPPA) reveals recurrent proteins expression signatures with prognostic implications. Blood. 2008;112:759. [Google Scholar]
- 20.Shih KCS, Becker K, Baehring J, Liggett W, Burris H, Hainsworth J. A phase II study of the combination of BKM120 (buparlisib) and bevacizumab in patients with relapsed/refractory glioblastoma multiforme (GBM) J Clinical Oncol. 2015;33 (suppl abstr 2065) [Google Scholar]
- 21.Wiza C, Nascimento EBM, Ouwens DM. Role of PRAS40 in Akt and mTOR signaling in health and disease. Am J Physiol Endocrinol Metab. 2012;302:E1453–E1460. doi: 10.1152/ajpendo.00660.2011. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki H, Suzuki M, Otsuki A, et al. A remote GATA2 hematopoietic enhancer drives leukemogenesis in inv(3)(q21;q26) by activating EVI1 expression. Cancer Cell. 2014;25:415–427. doi: 10.1016/j.ccr.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groschel S, Sanders MA, Hoogenboezem R, et al. A single oncogenic enhancer rearrangement causes concomitant EVI1 and GATA2 deregulation in leukemia. Cell. 2014;157:369–381. doi: 10.1016/j.cell.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimi A, Goyama S, Watanabe-Okochi N, et al. Evi1 represses PTEN expression and activates PI3K/AKT/mTOR via interactions with polycomb proteins. Blood. 2011;117:3617–3628. doi: 10.1182/blood-2009-12-261602. [DOI] [PubMed] [Google Scholar]
- 25.Groschel S, Sanders MA, Hoogenboezem R, et al. Mutational spectrum of myeloid malignancies with inv(3)/t(3;3) reveals a predominant involvement of RAS/RTK signaling pathways. Blood. 2015;125:133–139. doi: 10.1182/blood-2014-07-591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.