Abstract
The impact of the novel basal insulin LY2605541 (LY) on hepatic and nonhepatic glucose uptake (non-HGU) was evaluated. Conscious dogs underwent euglycemic clamps with tracer and hepatic balance measurements. Clamp period infusions were peripheral venous regular insulin (0.1 nmol ⋅ kg−1 ⋅ h−1 [control], n = 6) or LY (bolus [nmol/kg], continuous [nmol ⋅ kg−1 ⋅ h−1]: 0.5, 0.5 [n = 6]; 0.375, 0.375 [n = 5]; 0.25, 0.25 [n = 4]), somatostatin, and glucose, as well as intraportal glucagon (basal). During the clamp, the dogs switched from net hepatic glucose output to uptake (rates reached 2.1 ± 1.2, 0.9 ± 2.1, 8.6 ± 2.3, and 6.0 ± 1.1 µmol ⋅ kg−1 ⋅ min−1 within 5 h in control, LY0.25, LY0.375, and LY0.5, respectively). Non-HGU in LY increased less than in control; the ratio of change from basal in non-HGU to change in net hepatic glucose balance, calculated when glucose infusion rates (GIRs) were ~20 µmol ⋅ kg-1 ⋅ min−1 in all groups, was higher in control (1.17 ± 0.38) versus LY0.25 (0.39 ± 0.33), LY0.375 (−0.01 ± 0.13), and LY0.5 (−0.09 ± 0.07). Likewise, the change from baseline in glucose Rd-to-Ra ratio was greatest in control (1.4 ± 0.3 vs. 0.6 ± 0.4, 0.5 ± 0.2, and 0.6 ± 0.2 in LY0.25, LY0.375, and LY0.5, respectively). In contrast to exogenously administered human insulin, LY demonstrated preferential hepatic effects, similar to endogenously secreted insulin. Therefore, the analog might reduce complications associated with current insulin therapy.
Introduction
Marked improvements have been made in insulin analogs for basal-bolus therapy over the last few years. Insulins detemir and glargine reduce the risk of hypoglycemia compared with the older NPH insulin (1) and come closer to the ideal of creating a peakless basal insulin (2). However, neither detemir nor glargine maintains 24-h basal insulinemia for all individuals on all days (3), and thus improved analogs are needed. Insulin degludec, approved for use in Europe, Japan, and Mexico, is the first of the next-generation analogs to reach the market. The T1/2 of insulin degludec is approximately two times that of glargine (4), with the prolonged activity being achieved by the formation of soluble multihexamers upon subcutaneous injection, followed by gradual release of insulin monomers from the aggregate (5). Compared with glargine in treat-to-target trials, insulin degludec has brought about similar reduction in HbA1c at lower dosages and with lower rates of nocturnal hypoglycemia (6,7). Another new basal insulin, LY2605541 (LY), is currently in phase 3 trials.
LY is insulin lispro with a 20-kDa polyethylene glycol moiety covalently attached to lysine B28 via a urethane bond (8,9). Its large hydrodynamic (i.e., functional) size (10) may contribute to slowing absorption and reducing clearance, resulting in prolonged duration of action. In phase I trials, the half-life of LY was between 1 and 3 days and reached steady-state concentrations within 7–10 days with ~8- to 13-fold increase in exposure at steady-state. After a single dose, the requirement for glucose infusion was at least 36 h, indicating prolonged pharmacodynamic action. At steady-state, nearly peakless glucose infusion profiles were observed due to low peak-to-trough pharmacokinetic fluctuation (11,12). In two phase II trials in patients with type 1 and type 2 diabetes, LY was associated with a lower rate of nocturnal hypoglycemia and glucose variability compared with insulin glargine. At the same time, it resulted in similar glycemic control in the type 2 diabetic patients but better glycemic control in the type 1 diabetic patients. In the type 1 diabetic patients, there was a slightly higher rate of total hypoglycemia but a lower prandial insulin requirement (13,14). In addition, these patients, who were previously treated with other basal insulins, showed modest weight loss when switched to LY compared with modest weight gain with insulin glargine. They also exhibited modestly higher triglycerides compared with those on insulin glargine (13,14). These effects prompted an examination of the effect of LY on specific tissues, including the liver, kidney, and skeletal muscle. The chronically catheterized conscious dog was chosen as the model because it permits cannulation of the vessels required for assessing glucose balance across these organs/tissues and because of the similarity in insulin’s regulation of glucose metabolism in the human and the dog.
Research Design And Methods
Animals and Surgical Procedures
The protocol was approved by the Vanderbilt University Institutional Animal Care and Use Committee, and the animals were housed and cared for according to Association for Assessment and Accreditation of Laboratory Animal Care International guidelines. Approximately 16 days prior to study, each dog underwent surgical preparation under general anesthesia. In the initial studies (part A; n = 12), sampling catheters were placed in a femoral artery, the hepatic portal vein, the left common hepatic vein, a renal vein, and an iliac vein; infusion catheters were inserted in a jejunal and a splenic vein; and ultrasonic flow probes (Transonic Systems, Ithaca, NY) were placed around the hepatic artery, the portal vein, a renal artery, and an external iliac artery as previously described (15–18). In follow-up experiments (part B), the renal and iliac cannulas and flow probes were not placed, but all other aspects of the preparation were as described above. Criteria for use in a study were as previously described (17).
Experimental Protocol
On the morning of study, the proximal ends of the flow probes and catheters were exteriorized under local anesthesia. A primed, continuous infusion of [3-3H]glucose was initiated via a peripheral vein, and the dog was allowed to rest quietly in a Pavlov harness throughout the period of tracer equilibration (90 min). This was followed by 30 min of baseline sampling (90–120 min) and a euglycemic clamp period lasting 5 h (from 120 to 420 min) in part A. At 120 min, infusions of somatostatin (Bachem, Torrance, CA) and insulin were begun via a peripheral vein, while glucagon was administered intraportally at a basal rate (0.5 ng ⋅ kg−1 ⋅ min−1). The dogs in part A were randomly assigned to receive LY initiated with a 0.5 nmol/kg priming dose followed by continuous infusion at 0.5 nmol ⋅ kg−1 ⋅ h−1 (LY0.5 group, n = 6) or regular human insulin (control group, n = 6) at 0.11 nmol ⋅ kg−1 ⋅ h−1 with no priming dose. The analog is designed for subcutaneous injection, but it was infused via peripheral vein in these experiments in order to come to near–steady-state in a reasonable period of time and to mimic the route by which subcutaneously injected insulin arrives at the liver and other insulin-sensitive tissues. There is no known modification of the molecule during the absorption from the subcutaneous tissues, and after absorption it circulates in the same manner as endogenous insulin.
The insulin infusion rates used (determined in pilot studies; data not shown) in the two groups required equivalent glucose infusion rates (GIRs) to maintain euglycemia over the time course of the experiments. Glucose (50%; Hospira, Lake Forest, IL) was infused via peripheral vein as needed to maintain euglycemia. All sampling catheters functioned with the exception of a renal catheter in one LY0.5 animal and a hindlimb catheter in one control dog. At the end of each experiment, the dog was anesthetized, and hepatic biopsies were freeze clamped in situ as previously described (19). Subsequently, the dog was killed and the hypothalamus was removed and freeze clamped (20). Tissues were stored at −80°C to await further analysis.
Part B studies were identical to those in part A, except that the euglycemic clamp lasted 7 h (from 120 to 540 min), and the dogs were randomized to either the LY0.375 (n = 5; 0.375 nmol/kg prime, 0.375 nmol ⋅ kg−1 ⋅ h−1 infusion) or LY0.25 (n = 4; 0.25 nmol/kg prime, 0.25 nmol ⋅ kg−1 ⋅ h−1 infusion) groups. The purpose of part B was to determine whether the hepato-preferential effects of LY, observed in part A, would be evident at different dosages of the analog. Additionally, the longer clamp period was instituted to determine whether lower LY infusion rates sustained the hepato-preferential effects.
Sample Analyses
Plasma glucose, [3H]glucose, glucagon, canine and human insulin, and nonesterified fatty acid (NEFA) levels and blood lactate and glycerol concentrations were measured using standard methods as previously described (19,21). Plasma triglycerides were assayed spectrophotometrically (TR0100; Sigma-Aldrich, St. Louis, MO).
Serum concentrations of LY were assayed using a dual-antibody ELISA specific for LY (Charles River Laboratories, Senneville, QC, Canada). The quantitation range was 20–500 pmol/L, with the standard curve range 15–1,000 pmol/L. Samples with concentrations higher than 500 pmol/L were diluted so that the resulting concentration was within the quantitation range. Standard curve and quality control samples were analyzed with each set of study samples and passed acceptance criteria (within ±20% except at lower limit of quantitation: within ±25%).
Western blotting was carried out on hepatic and hypothalamic tissue from control and LY0.5 dogs; all analytic procedures have previously been described (22). Antibodies against total and phosphorylated Akt (Ser473), signal transducer and activator of transcription 3 (STAT3) (Tyr705), and glycogen synthase kinase-3β (GSK3β) (Ser9) were purchased from Cell Signaling (Danvers, MA). Protein bands were quantified using ImageJ software (http://rsb.info.nih.gov/ij/).
Calculations
Organ balance calculations and estimation of hepatic sinusoidal hormone concentrations and net hepatic carbon retention were carried out as previously described in detail (15–18). Tracer-determined glucose turnover was calculated with the circulatory model of Mari et al. (23). Unidirectional hepatic glucose uptake was calculated endogenous glucose Ra minus net hepatic glucose balance.
Two-way repeated-measures ANOVA (group × time) was used for statistical analysis of time course data, with post hoc analysis by Tukey test (Sigmaplot 11; Systat Software, San Jose, CA). Since part A was 2 h shorter than part B, statistical comparisons do not include the data from the last 2 h of part B. Unpaired t tests were used for comparison of tissue analytes. A P value <0.05 was considered statistically significant.
Results
Hormone Concentrations and Hepatic Blood Flow
Portal vein blood flow declined 15–25% in response to somatostatin infusion (P < 0.05 for Δ from baseline in all groups), while there was a slight (albeit nonsignificant) 0–10% rise in hepatic artery blood flow (Table 1).
TABLE 1.
Hepatic blood flow and plasma glucagon concentrations
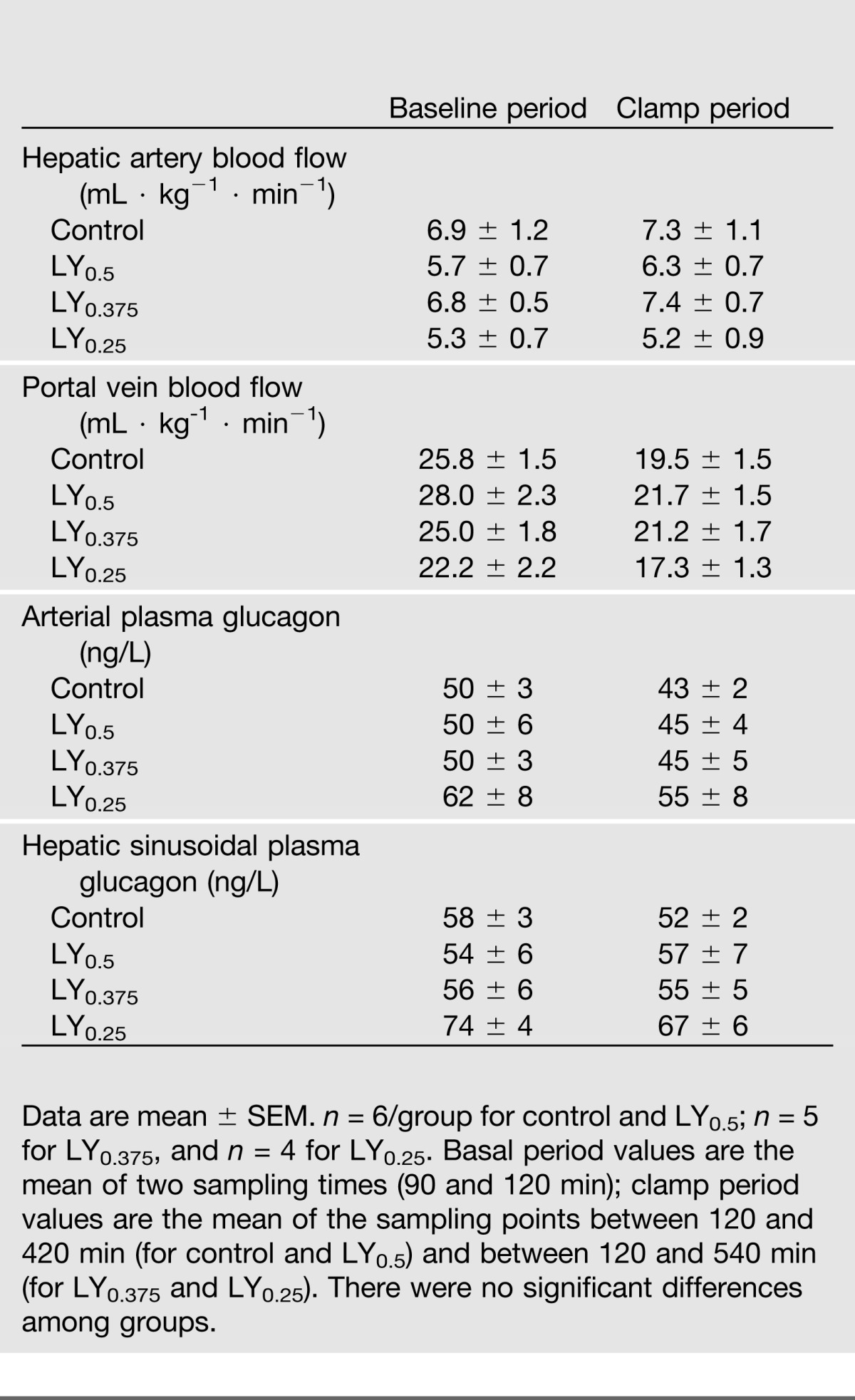
Arterial plasma C-peptide concentrations declined to near the limits of detection in all groups during the clamp period, demonstrating that insulin secretion was fully suppressed (data not shown). Arterial and hepatic sinusoidal glucagon concentrations remained near basal throughout the clamp period and did not differ significantly among groups (P = 0.22) (Table 1).
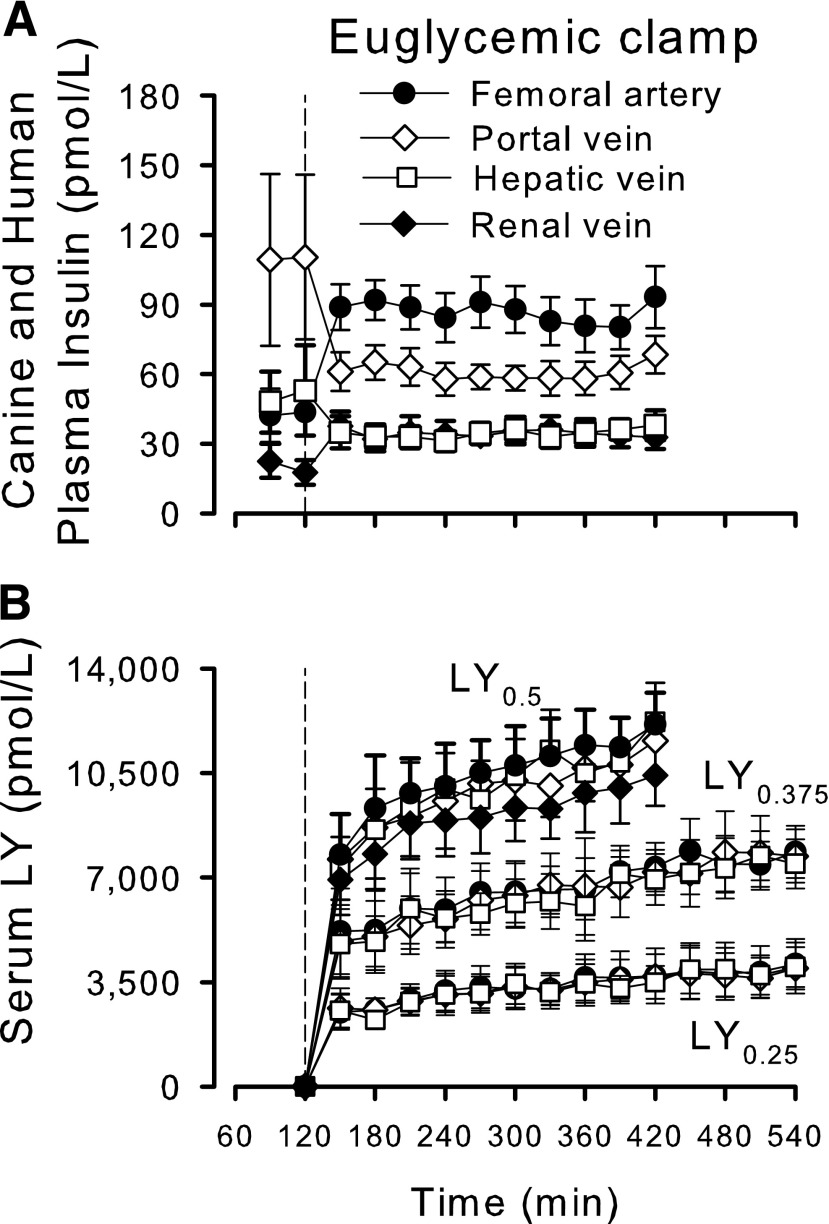
Plasma insulin concentrations during the baseline period were similar in all groups (data shown for control group only [Fig. 1A]). In the control group, the arterial concentrations during the clamp period were twofold basal levels, but peripheral venous insulin delivery resulted in a fall in the portal vein concentrations to ~50% of basal. Consequently, hepatic sinusoidal insulin concentrations in the control group tended to decline (from 96 ± 30 pmol/L [baseline] to 68 ± 7 pmol/L [clamp period]; P = 0.14), while renal vein plasma insulin concentrations increased in parallel with the rise in arterial insulin. The mean arterial LY concentrations during the final hour (near–steady-state) were 11,651 ± 946, 7,589 ± 843, and 3,906 ± 858 pmol/L in LY0.5, LY0.375, and LY0.25, respectively (Fig. 1B).
Figure 1.
Plasma concentrations of endogenous insulin (basal period) and regular human insulin (clamp period) in control dogs (A) and serum concentrations of LY (B). Arterial, portal vein, hepatic vein, and renal vein (available for control and LY0.5 groups only) concentrations of insulin or analog. Data are mean ± SEM; n = 6, 6, 5, and 4 for control, LY0.5, LY0.375, and LY0.25, respectively.
Fractional hepatic, intestinal, and renal extractions of regular insulin during the clamp were 50 ± 3%, 29 ± 2%, and 61 ± 3%, respectively. The extraction of LY by the liver could not be precisely quantified because of the high analog concentrations and relatively low tissue clearance. The extraction by the intestinal tract averaged 5 ± 3% at the highest dose, but at the lower LY infusion rates, the analog concentrations were too similar in the artery and portal vein to detect intestinal extraction. In LY0.5, renal extraction of the LY was 13 ± 4% (not evaluated in LY0.375 and LY0.25).
Glucose Metabolism
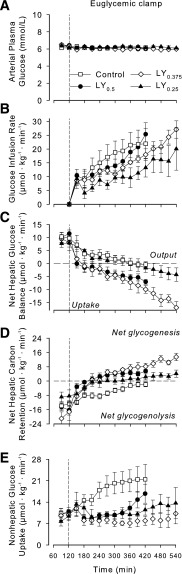
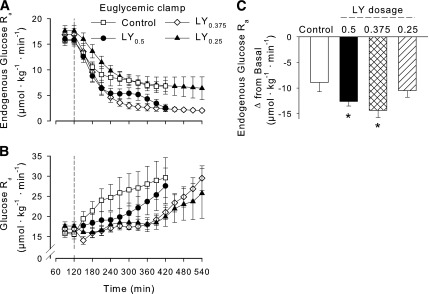
Arterial plasma glucose during the clamp period remained at basal concentrations [~6.1 mmol/L in all groups (Fig. 2A)]. The GIRs were not significantly different between control and LY0.5 or LY0.375 (Fig. 2B) but were lower (P < 0.05) in LY0.25 than in control. All groups exhibited net hepatic glucose output (NHGO) (8–11 µmol ⋅ kg−1 ⋅ min−1) during the baseline period. In the control group, NHGO gradually declined from baseline, reaching approximately zero by 420 min (Δ10.3 ± 1.4 µmol ⋅ kg-1 ⋅ min−1) (Fig. 2C). In LY0.5, on the other hand, there was a rapid and marked impact at the liver, such that there was a switch from NHGO to net hepatic glucose uptake (NHGU) within 30 min of beginning the LY0.5 infusion, and the change from baseline in net hepatic glucose balance reached 16.8 ± 2.2 µmol ⋅ kg−1 ⋅ min−1 by 420 min (P < 0.05 between groups). The change from baseline in LY0.375 and LY0.25 averaged 18.8 ± 2.6 (P < 0.05 vs. control) and 9.5 ± 3.1 (NS vs. control) µmol ⋅ kg−1 ⋅ min−1 by 420 min. All LY dosages were associated with significantly greater net hepatic carbon retention than exhibited by the control group (Fig. 2D). The effect of each treatment on endogenous glucose Ra was very similar to its effect on net hepatic glucose balance (Fig. 3A). In the control group, there was a gradual decline in glucose Ra, with the fall from basal totaling 8.9 ± 1.9 µmol ⋅ kg−1 ⋅ min−1 by the final hour. When the change in endogenous glucose Ra between the basal period and 360–420 min (the final hour during part A) was calculated, it was clear that the change was significantly greater in LY0.5 and LY0.375 than in control (Fig. 3C).
Figure 2.
Arterial plasma glucose concentrations (A), GIRs (B), net hepatic glucose balance (NHGB) (C), net hepatic carbon retention (NHCR) (D), and non-HGU (E). Control (n = 6), LY0.5 (n = 6), LY0.375 (n = 5), and LY0.25 (n = 4). Significant differences (P < 0.05): LY0.5 and LY0.375 vs. control for NHGB, NHCR, and non-HGU; LY0.25 vs. control for GIR, NHCR, and non-HGU; LY0.375 vs. LY0.5 for non-HGU; LY0.25 vs. LY0.5 for NHGB, GIR, and NHCR; LY0.25 vs. LY0.375 for NHGB and NHCR.
Figure 3.
Tracer-determined rate of endogenous glucose Ra (A) and Rd (B). C: Magnitude of the decline in endogenous glucose Ra between the baseline period and 360–420 min (the last hour of study for the control and LY0.5 groups). Control (n = 6), LY0.5 (n = 6), LY0.375 (n = 5), and LY0.25 (n = 4). P < 0.05 for LY0.375 vs. control for both Ra and Rd, P < 0.05 for LY0.25 vs. control for Rd. *P < 0.05 vs. control.
Peripheral insulin infusion in the control group had an immediate impact on nonhepatic glucose uptake (non-HGU), and by the final hour non-HGU had increased 11.1 ± 4.5 µmol ⋅ kg−1 ⋅ min−1 over the basal rate (Fig. 2E). In the LY groups, non-HGU rates initially fell below basal. In the LY0.5 group, it began to increase gradually after 330 min, with the increase from basal in the final hour being 4.1 ± 1.5 µmol ⋅ kg−1 ⋅ min−1 (P = 0.14 versus control). The LY0.25 group also exhibited a small increase in non-HGU during the late part of the clamp, while the rate did not rise above basal at any time in the LY0.375 group. In an effort to compare the control and the different LY groups under equivalent conditions, we calculated the ratio of the change from basal in non-HGU to the change in NHGU at a time when GIRs were equivalent in all groups (~20 µmol ⋅ kg−1 ⋅ min−1). The lower the ratio in Table 2, the more hepato-preferential the action. The ratio was much higher in control than in LY0.5 and LY0.375 (P < 0.01), and it tended to be higher than in LY0.25. No significant differences were seen in either net renal or hindlimb glucose balance between the control and LY0.5 groups (data not shown).
TABLE 2.
Ratios of change from baseline in non-HGU to change in NHGU, as well as change from basal in tracer-determined glucose Rd to the change in endogenous glucose Ra
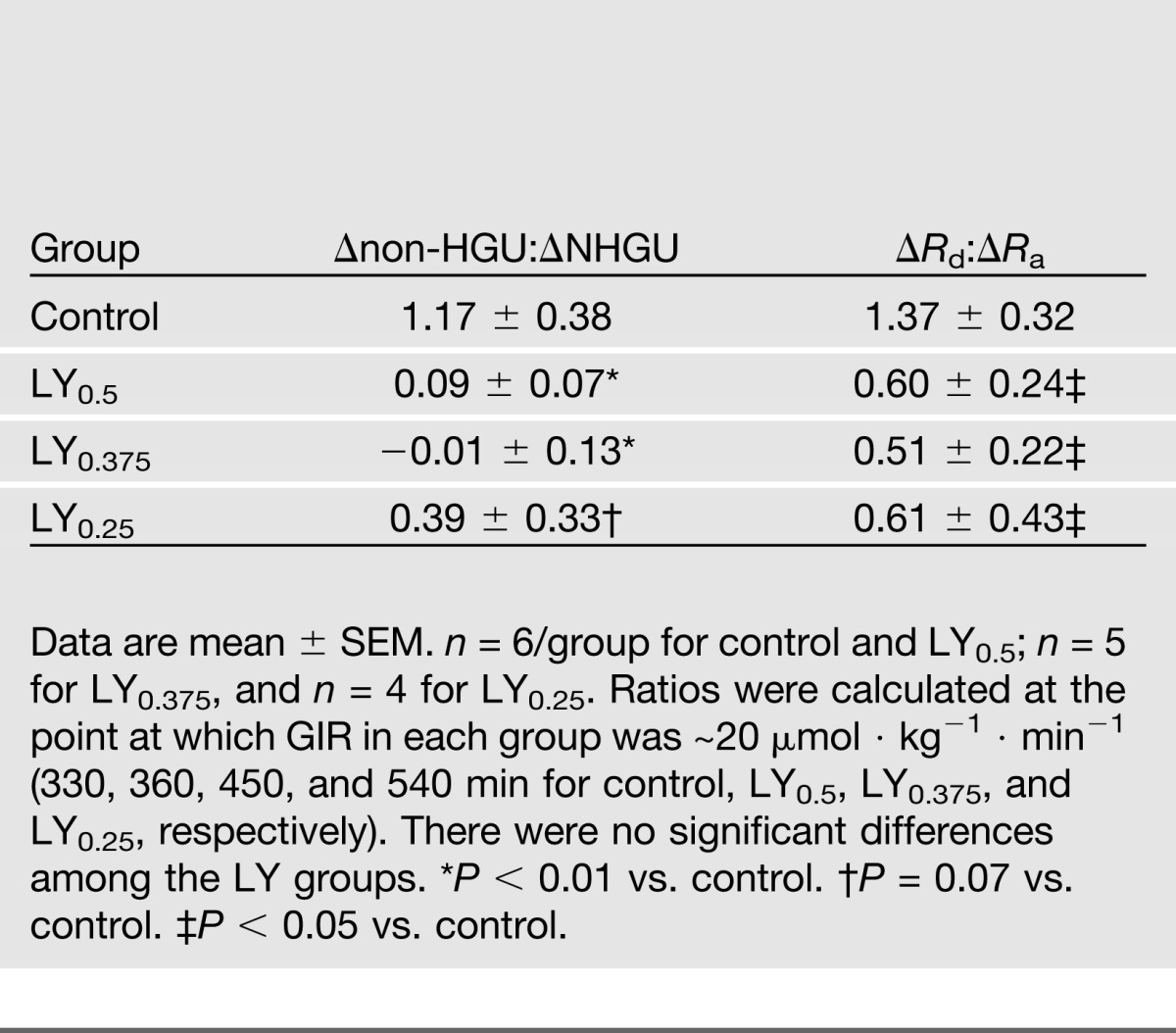
LY0.375 and LY0.25, but not LY0.5, stimulated glucose Rd significantly less than regular insulin through 420 min (Fig. 3B). When the ratio of change from baseline in glucose Rd to change in glucose Ra was calculated at the time when GIR was ~20 µmol ⋅ kg−1 ⋅ min−1 in each group, the control group exhibited a ratio >twofold that in the LY groups (Table 2).
Lactate Metabolism
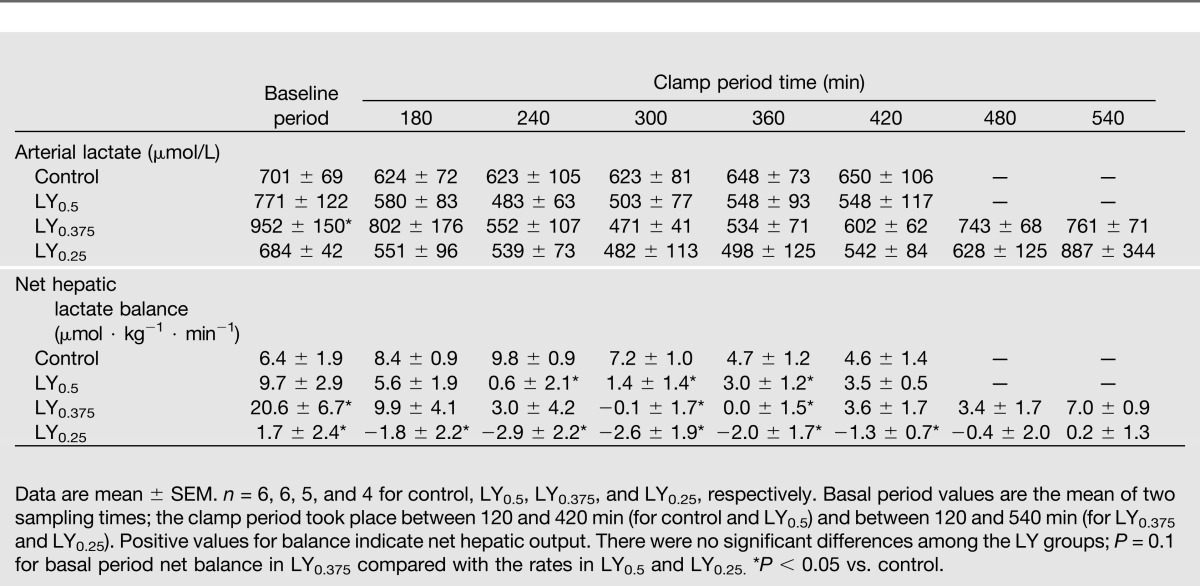
All groups exhibited net hepatic lactate output (NHLO) during the baseline period, with the rates in LY0.375 and LY0.25 being significantly higher and lower, respectively, than in the control group (Table 3). During the clamp period, the control group exhibited a slight increase in NHLO followed by a return to the baseline rate. In all of the LY groups, NHLO during the clamp period declined to a nadir no different from 0 µmol ⋅ kg−1 ⋅ min−1 in LY0.5. The LY0.375 and LY0.25 groups actually switched to net hepatic lactate uptake. NHLO resumed during the latter part of the clamp but did not reach baseline rates by the end of the experiment in any of the LY groups. As a result of these changes, blood lactate concentrations tended to fall initially and then return toward baseline levels in the LY groups.
TABLE 3.
Lactate data
The control group exhibited net hindlimb lactate uptake throughout the clamp, with uptake increasing significantly as NHLO peaked and then declining to near-baseline rates. Conversely, net hindlimb lactate uptake decreased in LY0.5 as NHLO declined, with a subsequent small increase in net limb lactate uptake corresponding to the increase in NHLO by the end of the clamp. Net renal lactate uptake did not change significantly from baseline in either control or LY0.5, and the two groups did not differ at any time point (data not shown).
Glycerol, Fatty Acid, and Triglyceride Metabolism
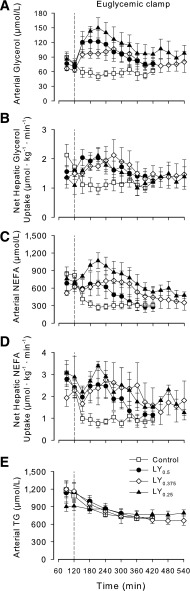
Arterial glycerol and NEFA concentrations in the control group declined promptly under clamp conditions in response to the peripheral hyperinsulinemia. In contrast, the glycerol and NEFA concentrations initially increased in the LY groups and then gradually declined (Fig. 4A and C). Net hepatic and renal (renal not shown) uptakes of glycerol and NEFA decreased during the clamp in the control group and initially increased in the LY groups, corresponding to the changes in the substrate concentrations (Fig. 4B and D). Net hindlimb glycerol output did not differ between the control and LY0.5 groups at any time (data not shown). During the clamp, arterial plasma triglyceride concentrations declined significantly in all groups (Fig. 4E). By 420 min, triglyceride concentrations had fallen ~40% below baseline in the control and LY0.5 and LY0.375 groups and 15% in LY0.25. The concentrations were not significantly different among groups at 420 min.
Figure 4.
Arterial blood glycerol (A), plasma NEFA (C), and plasma triglyceride (TG) (E) concentrations and net hepatic uptakes of glycerol (B) and NEFA (D). Control (n = 6), LY0.5 (n = 6), LY0.375 (n = 5), and LY0.25 (n = 4). P < 0.05 for all LY groups vs. control for arterial glycerol and NEFA and for net hepatic uptake of glycerol and NEFA. There were no significant differences among groups in TG concentrations. There were no significant differences among LY groups for any parameter.
Tissue Analyses
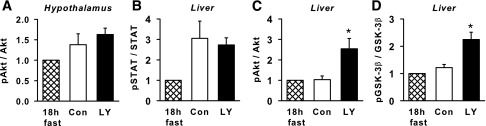
There was no significant difference between regular insulin and LY0.5 in phosphorylation of Akt in the hypothalamus or STAT3 protein in the liver (Fig. 5A and B; Supplementary Fig. 1). On the other hand, phosphorylation of Akt and GSK3β in the liver was significantly enhanced with LY0.5 versus regular insulin (Fig. 5C and D).
Figure 5.
Molecular markers of insulin signaling in the control and LY0.5 groups. Hypothalamic phosphorylated Akt (pAkt), expressed relative to total Akt (A), as well as hepatic pSTAT3/STAT3 (B), pAkt/Akt (C), and pGSK3β/GSK3β (D), in control (con) (white bars) and LY0.5 (black bars) (n = 6/group). The hatched bars are mean data from 2 additional dogs, not included in the experiments, that were fasted for 18 h and then killed to provide reference (non–insulin stimulated) tissue. See Supplementary Fig. 1 for representative blots. *P < 0.05 vs. control.
Discussion
Insulin secretion into the portal vein results in ~threefold greater exposure of the liver, versus other tissues, to insulin. The increased exposure of the liver to the hormone results from its secretion into a limited vascular pool and the fact that the liver extracts 50–60% of the insulin to which it is exposed (24). In contrast, when insulin is administered subcutaneously, the normal portal-peripheral insulin gradient is lost so that the peripheral tissues are comparatively hyperinsulinemic while the liver is comparatively hypoinsulinemic (25,26). The portal vein, hepatic vein, and hepatic sinusoidal insulin concentrations in the control group during the clamp period were ~30–40% below basal, while the arterial concentrations were approximately two times basal. Although the arterial concentrations did not increase as much as would be anticipated after a mixed meal (sixfold basal or more), they were inappropriately high in relation to the other blood vessels sampled, which also exhibit sixfold or more increases in insulin after meal ingestion (27). Partly as a result of the relative overinsulinization of peripheral tissues, even individuals with well-controlled insulin-treated diabetes are apt to experience glycemic fluctuations, hypoglycemia, and dyslipidemia, which have been suggested to contribute to microvascular and macrovascular complications (28). Theoretically, insulin analogs with hepato-preferential actions could reduce the risk of these complications (29,30).
While it may seem counterintuitive that a hepato-preferential insulin could reduce hypoglycemia, since the liver’s response is key to restoring euglycemia, relative overinsulization of peripheral tissues contributes to hypoglycemia and impaired counterregulation. Lipolysis is an important contributor to gluconeogenesis under hypoglycemic conditions, and the relative overinsulinization of the peripheral lipid depots resulting from current peripherally administered insulin therapies would be expected to impede gluconeogenesis (31–33). Moreover, even though skeletal muscle glucose uptake decreases during hypoglycemia associated with conventional insulin therapy, it does not cease. Due to the relatively large mass of skeletal muscle, this results in significant ongoing glucose disposal, a process that would be curtailed by a hepato-preferential insulin. More importantly, a hepato-preferential insulin puts the primary burden of hypoglycemia on the organ best suited to cope because hepatic insulin signaling is markedly reduced in the presence of hypoglycemia; i.e., the insulin receptor is essentially disengaged (34). Even when overinsulinized and deprived of increases in counterregulatory hormones and central neural stimuli, the liver is capable of responding directly to hypoglycemia by increasing its glucose output (35–37).
At the doses examined, LY exhibited a clear hepato-preferential effect compared with human insulin when both products were delivered via peripheral vein. This was demonstrated by two independent measures of glucose metabolism: organ balance and tracer-determined turnover. It should be noted that a single control group was used as a comparator for all three LY dosages. In order for groups to be compared, in terms of glucose disposition, they must be matched for at least one parameter of interest. In that regard, the LY0.5 and LY0.375 groups were not significantly different in GIR from the control group (P = 0.72 and 0.35, respectively). Both LY0.5 and LY0.375 exhibited a faster and greater effect on net hepatic glucose balance than regular insulin, quickly shifting the liver from NHGO to NHGU. Conversely, their effect on non-HGU was delayed and minimal compared with regular insulin. At the lowest LY dose, the analog’s effects on net hepatic glucose balance were comparable to those in the control group throughout 420 min (P = 0.974), making possible a comparison of nonhepatic effects in the presence of similar rates of NHGO. The impact of LY0.25 on non-HGU was markedly lower than that of regular insulin. Of the three LY dosages examined, LY0.375 appeared to have the greatest effect at the liver concomitant with the least effect on nonhepatic tissues.
Tracer methodology yielded results consistent with hepatic glucose balance data. The two higher doses of LY caused a significantly greater fall in endogenous glucose Ra than regular insulin (even though significant differences from control in the rates were not evident as early as they were with organ balance data), while the effect of LY0.25 was very similar to that of regular insulin (P = 0.34). On the other hand, both LY0.375 and LY0.25 stimulated glucose Rd significantly less than regular insulin. Moreover, all doses of LY, compared with regular insulin, were associated with a significantly smaller change in glucose Rd relative to the impact on endogenous Ra.
In contrast to its rapid effects on the liver, as noted above, LY’s effect on nonhepatic tissues was more gradual than that of regular insulin. In particular, LY’s effect on fat metabolism appeared to be slow. Lipolysis is extremely sensitive to an elevation of regular insulin, and thus it was quickly suppressed in the control group, with glycerol concentrations reaching a stable nadir within 30 min of the start of the clamp. In none of the LY groups did the glycerol concentrations fall below the baseline values at any time, suggesting a lack of lipolytic inhibition by the analog at these doses. NEFA levels, on the other hand, fell below baseline levels during the clamp in all LY groups, consistent with an increase in reesterification. Nevertheless, regular insulin lowered NEFA much more promptly than LY. The nadir of NEFA concentrations (271 ± 57 µmol/L) occurred within 90 min after the start of the clamp period in the control group, while the nadir in LY0.5 (278 ± 92 µmol/L) was not reached until 270 min after the start of the clamp, and LY0.375 and LY0.25 did not reach their lowest concentrations (353 ± 130 and 467 ± 96 µmol/L, respectively) until the last hour. An insulin-induced fall in circulating NEFA provides an indirect means for reducing hepatic glucose production (38,39), and thus the sluggish response of glycerol and NEFA concentrations to LY administration suggests that it was the direct effect of the analog that inhibited hepatic glucose production.
A decrease in NEFA levels and hepatic NEFA uptake is associated with an increase in hepatic glycolysis (39). The exact mechanism for this is unclear, but it may well result from a fall in intrahepatic citrate, one of the major inhibitors of phosphofructokinase, the first rate-determining enzyme in the glycolytic pathway. Alternatively, the fall in NEFA could alter the intrahepatic redox state, increasing the NADH-to-NAD ratio and leading to an activation of lactate dehydrogenase. (For further discussion, see Sindelar et al. [39]) Thus, when net hepatic NEFA uptake decreased in the control group, there was an increase in the release of glycolytic carbon (lactate) from the liver. Conversely, there appeared to be a prompt suppression of glycolysis (hepatic lactate release) in response to LY, likely related to the increase in lipolysis early in the experimental period. The two lower rates of LY infusion not only reduced NHLO but also actually prompted the liver to shift to net uptake of lactate, consistent with increased storage of carbon as glycogen in the liver.
The liver is a key organ in the extraction of regular insulin, but in contrast, hepatic extraction of LY appeared to be very low. As with other slowly cleared insulin analogs, such as detemir (40), the plasma LY concentrations were high relative to those of regular insulin. However, it is evident that we achieved three distinct LY levels and near–steady-state concentrations, particularly with the longer studies in Part B. With such high circulating concentrations and low clearance, it is difficult to quantify tissue LY extraction precisely. Even though the kidney was an important site of extraction for LY, renal LY extraction was proportionally much less than with human insulin, as designed (9). When LY was tested in subjects with renal impairment (ranging from mild to end-stage renal disease), the apparent clearance and half-life of the drug did not differ from that in subjects with normal renal function (41). Thus, the pharmacokinetic properties of LY do not appear to be affected by impairment in renal function.
Because of the large hydrodynamic size of LY, it might not be expected to cross the blood-brain barrier (42). Nevertheless, hypothalamic Akt phosphorylation at the end of the experiment was not different in the control and LY-treated animals. The affinity of LY for the insulin receptor, although low (<6% of that of insulin lispro [43]), might permit some passage into the central nervous system (CNS) via saturable mechanisms. Additionally, there is a potential for LY to reach the CNS via extracellular pathways such as are used by some very large molecules, e.g., albumin and certain antibodies (44–46). The current findings are not unique or completely unexpected, since the insulin analog detemir (which is tightly bound to serum albumin [47]) has been reported not to cross the mouse blood-brain barrier (48), and yet it has a more rapid and pronounced effect on hypothalamic insulin receptor phosphorylation than human insulin when both are injected intravenously in mice (49). The similarity of phosphorylated STAT3/STAT3 in the livers of the control and LY0.5 groups is consistent with an effect of LY in the CNS, since hepatic STAT3 phosphorylation has been suggested to be a marker of brain insulin signaling (50,51) that can be blunted with infusion of an ATP-sensitive K+-channel inhibitor or a phosphatidylinositol-3 kinase inhibitor into the 3rd cerebroventricle of the dog (51). Nevertheless, hepatic STAT3 phosphorylation in response to brain insulin signaling occurs slowly also (50–52), affecting gene transcription slowly, and thus could not have been involved in bringing about the rapid response effect on liver glucose metabolism observed with LY infusion.
The significant stimulation of hepatic Akt and GSK3β phosphorylation in the LY-treated versus control animals is in keeping with the hepato-preferential metabolic actions of the analog. Conversely, the lack of apparent stimulation of phosphorylation of these proteins by regular insulin (in view of the data from the 18-h-fasted, non–insulin-treated animals) is in keeping with the lack of increase in hepatic insulin concentrations during the clamp; indeed, the hepatic sinusoidal insulin concentrations were below basal in the control group. GSK3β is inactivated by phosphorylation, thus allowing glycogen synthesis to proceed. Consistent with this, net hepatic carbon retention, a good surrogate measure for hepatic glycogen synthesis (53), was enhanced in the LY-treated groups.
The physiologic basis of the hepato-preferential effect of LY is not fully understood but is likely related to its large hydrodynamic size. While this may impede passage from the capillaries into the interstitium of peripheral target tissues, i.e., muscle and adipose, the hepatic sinusoidal capillaries are fenestrated, with openings of 100–200Å in diameter, allowing entry of larger molecules into the organ (e.g., lipoproteins), with many factors affecting hepatic clearance (54,55).
In conclusion, LY at all dosages examined exhibited a more hepato-preferential effect than regular human insulin when both products were delivered by peripheral vein. The effect on the liver was evident throughout the duration of the clamp period, with the use of both tracer and organ balance techniques. If these effects can be sustained during long-term dosing, peripherally administered LY appears to have the potential to reproduce the hepato-preferential effects of endogenously secreted insulin—an effect that might reduce complications associated with current insulin therapy.
Supplementary Material
Article Information
Funding. The Vanderbilt Diabetes Research and Training Center Metabolic Physiology Shared Resource and Hormone Assay and Analytical Services Cores (SP-60-AM20593) made important contributions to this work. A.D.C. holds the Jacquelyn A. Turner and Dr. Dorothy J. Turner Chair in Diabetes Research.
Duality of Interest. These studies were funded by Eli Lilly and Company. V.P.S. is a shareholder of Eli Lilly and Company and was an employee at the time of this study. J.M.B., M.D.M., and S.J.J. are employees and shareholders of Eli Lilly and Company. A.D.C. is a consultant for Eli Lilly and Company. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.C.M. carried out the studies, interpreted data, and drafted the manuscript. M.S.S. carried out the studies and analyses. V.P.S., S.J.J., and A.D.C. participated in the design of the studies, interpreted data, and reviewed and edited the manuscript. J.M.B. interpreted data and reviewed and edited the manuscript. M.D.M. participated in the design of the studies and reviewed and edited the manuscript. A.D.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, PA, 8–12 June 2012, and at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0826/-/DC1.
V.P.S. is currently affiliated with the U.S. Food and Drug Administration, Silver Spring, MD.
See accompanying commentary, p. 390.
References
- 1.Little S, Shaw J, Home P. Hypoglycemia rates with basal insulin analogs. Diabetes Technol Ther 2011;13(Suppl. 1):S53–S64 [DOI] [PubMed] [Google Scholar]
- 2.Bolli GB, Andreoli AM, Lucidi P. Optimizing the replacement of basal insulin in type 1 diabetes mellitus: no longer an elusive goal in the post-NPH era. Diabetes Technol Ther 2011;13(Suppl. 1):S43–S52 [DOI] [PubMed] [Google Scholar]
- 3.Simon AC, DeVries JH. The future of basal insulin supplementation. Diabetes Technol Ther 2011;13(Suppl. 1):S103–S108 [DOI] [PubMed] [Google Scholar]
- 4.Heise T, Nosek L, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab 2012;14:944–950 [DOI] [PubMed] [Google Scholar]
- 5.Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res 2012;29:2104–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkeland KI, Home PD, Wendisch U, et al. Insulin degludec in type 1 diabetes: a randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care 2011;34:661–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bode BW, Buse JB, Fisher M, et al. BEGIN® Basal-Bolus Type 1 Trial Investigators . Insulin degludec improves glycaemic control with lower nocturnal hypoglycaemia risk than insulin glargine in basal-bolus treatment with mealtime insulin aspart in Type 1 diabetes (BEGIN® Basal-Bolus Type 1): 2-year results of a randomized clinical trial. Diabet Med 2013;30:1293–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandyarajan V, Weiss MA. Design of non-standard insulin analogs for the treatment of diabetes mellitus. Curr Diab Rep 2012;12:697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals JM, Cutler GB, Doyle B, et al. PEGylated insulin lispro compounds. International Patent Application WO 2009/1521128 A1. 17 Dec 2009
- 10.Hansen RJ, Cutler GB, Jr, Vick A, et al. LY2605541: leveraging hydrodynamic size to develop a novel basal insulin (Abstract). Diabetes 2012;61(Suppl. 1):A228 [Google Scholar]
- 11.Sinha VP, Howey DC, Soon DKW, et al. Single-dose pharmacokinetics (PK) and glucodynamics (GD) of the novel, long-acting basal insulin LY2605541 in healthy subjects (Abstract). Diabetes 2012;61(Suppl. 1):A273 [Google Scholar]
- 12.Heise T, Howey DC, Sinha VP, Choi SL, Mace KF. Steady-state pharmacokinetics (PK) and glucodynamics (GD) of the novel, long-acting basal insulin LY2605541 dosed once-daily (QD) in patients with type 2 diabetes mellitus (T2DM). Diabetes 2012;61(Suppl. 1):A256. [DOI] [PubMed] [Google Scholar]
- 13.Bergenstal RM, Rosenstock J, Arakaki RF, et al. A randomized, controlled study of once-daily LY2605541, a novel long-acting basal insulin, versus insulin glargine in basal insulin-treated patients with type 2 diabetes. Diabetes Care 2012;35:2140–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenstock J, Bergenstal RM, Blevins TC, et al. Better glycemic control and weight loss with the novel long-acting basal insulin LY2605541 compared with insulin glargine in type 1 diabetes: a randomized, crossover study. Diabetes Care 2013;36:522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuinness OP, Fugiwara T, Murrell S, et al. Impact of chronic stress hormone infusion on hepatic carbohydrate metabolism in the conscious dog. Am J Physiol 1993;265:E314–E322 [DOI] [PubMed] [Google Scholar]
- 16.Wasserman DH, Lacy DB, Bracy D, Williams PE. Metabolic regulation in peripheral tissues and transition to increased gluconeogenic mode during prolonged exercise. Am J Physiol 1992;263:E345–E354 [DOI] [PubMed] [Google Scholar]
- 17.Winnick JJ, An Z, Moore MC, et al. A physiological increase in the hepatic glycogen level does not affect the response of net hepatic glucose uptake to insulin. Am J Physiol Endocrinol Metab 2009;297:E358–E366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galassetti P, Chu CA, Neal DW, Reed GW, Wasserman DH, Cherrington AD. A negative arterial-portal venous glucose gradient increases net hepatic glucose uptake in euglycemic dogs. Am J Physiol 1999;277:E126–E134 [DOI] [PubMed] [Google Scholar]
- 19.Moore MC, Rossetti L, Pagliassotti MJ, et al. Neural and pancreatic influences on net hepatic glucose uptake and glycogen synthesis. Am J Physiol 1996;271:E215–E222 [DOI] [PubMed] [Google Scholar]
- 20.Ramnanan CJ, Saraswathi V, Smith MS, et al. Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. J Clin Invest 2011;121:3713–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galassetti P, Shiota M, Zinker BA, Wasserman DH, Cherrington AD. A negative arterial-portal venous glucose gradient decreases skeletal muscle glucose uptake. Am J Physiol 1998;275:E101–E111 [DOI] [PubMed] [Google Scholar]
- 22.Edgerton DS, Ramnanan CJ, Grueter CA, et al. Effects of insulin on the metabolic control of hepatic gluconeogenesis in vivo. Diabetes 2009;58:2766–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mari A, Stojanovska L, Proietto J, Thorburn AW. A circulatory model for calculating non-steady-state glucose fluxes. Validation and comparison with compartmental models. Comput Methods Programs Biomed 2003;71:269–281 [DOI] [PubMed] [Google Scholar]
- 24.Chap Z, Ishida T, Chou J, et al. First-pass hepatic extraction and metabolic effects of insulin and insulin analogues. Am J Physiol 1987;252:E209–E217 [DOI] [PubMed] [Google Scholar]
- 25.Sindelar DK, Balcom JH, Chu CA, Neal DW, Cherrington AD. A comparison of the effects of selective increases in peripheral or portal insulin on hepatic glucose production in the conscious dog. Diabetes 1996;45:1594–1604 [DOI] [PubMed] [Google Scholar]
- 26.Sindelar DK, Chu CA, Venson P, Donahue EP, Neal DW, Cherrington AD. Basal hepatic glucose production is regulated by the portal vein insulin concentration. Diabetes 1998;47:523–529 [DOI] [PubMed] [Google Scholar]
- 27.Coate KC, Kraft G, Lautz M, Smith M, Neal DW, Cherrington AD. A high-fat, high-fructose diet accelerates nutrient absorption and impairs net hepatic glucose uptake in response to a mixed meal in partially pancreatectomized dogs. J Nutr 2011;141:1643–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sönksen PH, Russell-Jones D, Jones RH. Growth hormone and diabetes mellitus. A review of sixty-three years of medical research and a glimpse into the future? Horm Res 1993;40:68–79 [DOI] [PubMed] [Google Scholar]
- 29.Hopkins KD, Russell-Jones DL, Lehmann ED, Wheeler MJ, Sönksen PH. Intraperitoneal insulin affects insulin-like growth factor binding protein-1 in a well-controlled type I diabetic patient. Diabetes Care 1993;16:1404–1405 [DOI] [PubMed] [Google Scholar]
- 30.Shojaee-Moradie F, Powrie JK, Sundermann E, et al. Novel hepatoselective insulin analog: studies with a covalently linked thyroxyl-insulin complex in humans. Diabetes Care 2000;23:1124–1129 [DOI] [PubMed] [Google Scholar]
- 31.Avogaro A, Beltramello P, Gnudi L, et al. Alcohol intake impairs glucose counterregulation during acute insulin-induced hypoglycemia in IDDM patients. Evidence for a critical role of free fatty acids. Diabetes 1993;42:1626–1634 [PubMed] [Google Scholar]
- 32.Fanelli C, Calderone S, Epifano L, et al. Demonstration of a critical role for free fatty acids in mediating counterregulatory stimulation of gluconeogenesis and suppression of glucose utilization in humans. J Clin Invest 1993;92:1617–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucidi P, Rossetti P, Porcellati F, et al. Mechanisms of insulin resistance after insulin-induced hypoglycemia in humans: the role of lipolysis. Diabetes 2010;59:1349–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivera N, Ramnanan CJ, An Z, et al. Insulin-induced hypoglycemia increases hepatic sensitivity to glucagon in dogs. J Clin Invest 2010;120:4425–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connolly CC, Myers SR, Neal DW, Hastings JR, Cherrington AD. In the absence of counterregulatory hormones, the increase in hepatic glucose production during insulin-induced hypoglycemia in the dog is initiated in the liver rather than the brain. Diabetes 1996;45:1805–1813 [DOI] [PubMed] [Google Scholar]
- 36.Jackson PA, Cardin S, Coffey CS, et al. Effect of hepatic denervation on the counterregulatory response to insulin-induced hypoglycemia in the dog. Am J Physiol Endocrinol Metab 2000;279:E1249–E1257 [DOI] [PubMed] [Google Scholar]
- 37.Jackson PA, Pagliassotti MJ, Shiota M, Neal DW, Cardin S, Cherrington AD. Effects of vagal blockade on the counterregulatory response to insulin-induced hypoglycemia in the dog. Am J Physiol 1997;273:E1178–E1188 [DOI] [PubMed] [Google Scholar]
- 38.Rebrin K, Steil GM, Mittelman SD, Bergman RN. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J Clin Invest 1996;98:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sindelar DK, Chu CA, Rohlie M, Neal DW, Swift LL, Cherrington AD. The role of fatty acids in mediating the effects of peripheral insulin on hepatic glucose production in the conscious dog. Diabetes 1997;46:187–196 [DOI] [PubMed] [Google Scholar]
- 40.Moore MC, Smith MS, Turney MK, Boysen S, Williams PE. Comparison of insulins detemir and glargine: effects on glucose disposal, hepatic glucose release and the central nervous system. Diabetes Obes Metab 2011;13:832–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linnebjerg H, Choi SL, Lam ECQ, Mace KF, Hodgson TS, Sinha VP. Pharmacokinetics (PK) of the novel, long-acting basal insulin LY2605541 in subjects with varying degrees of renal function (Abstract). Diabetes 2012;61(Suppl. 1):A296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol 2009;9(Suppl. 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owens RA, Lockwood JF, Dunbar JD, et al. In vitro characterisation of novel basal insulin LY2605541: reduced mitogenicity and IGF-IR binding. Diabetologia 2012;55:120 [Google Scholar]
- 44.Banks WA. Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinology 2012;153:4111–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banks WA, Broadwell RD. Blood to brain and brain to blood passage of native horseradish peroxidase, wheat germ agglutinin, and albumin: pharmacokinetic and morphological assessments. J Neurochem 1994;62:2404–2419 [DOI] [PubMed] [Google Scholar]
- 46.Broadwell RD, Sofroniew MV. Serum proteins bypass the blood-brain fluid barriers for extracellular entry to the central nervous system. Exp Neurol 1993;120:245–263 [DOI] [PubMed] [Google Scholar]
- 47.Kurtzhals P, Havelund S, Jonassen I, Kiehr B, Ribel U, Markussen J. Albumin binding and time action of acylated insulins in various species. J Pharm Sci 1996;85:304–308 [DOI] [PubMed] [Google Scholar]
- 48.Banks WA, Morley JE, Lynch JL, Lynch KM, Mooradian AD. Insulin detemir is not transported across the blood-brain barrier. Peptides 2010;31:2284–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hennige AM, Sartorius T, Tschritter O, et al. Tissue selectivity of insulin detemir action in vivo. Diabetologia 2006;49:1274–1282 [DOI] [PubMed] [Google Scholar]
- 50.Inoue H, Ogawa W, Asakawa A, et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab 2006;3:267–275 [DOI] [PubMed] [Google Scholar]
- 51.Ramnanan CJ, Kraft G, Smith MS, et al. Interaction between the central and peripheral effects of insulin in controlling hepatic glucose metabolism in the conscious dog. Diabetes 2013;62:74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramnanan CJ, Edgerton DS, Rivera N, et al. Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes 2010;59:1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satake S, Moore MC, Igawa K, et al. Direct and indirect effects of insulin on glucose uptake and storage by the liver. Diabetes 2002;51:1663–1671 [DOI] [PubMed] [Google Scholar]
- 54.Baker M, Parton T. Kinetic determinants of hepatic clearance: plasma protein binding and hepatic uptake. Xenobiotica 2007;37:1110–1134 [DOI] [PubMed] [Google Scholar]
- 55.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol 2002;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.