Abstract
Objective
Promptly treated erythema migrans (EM) has good prognosis. However, some patients report persistent symptoms. Do patients with EM have more symptoms than the general population? We describe individual symptoms and general function in EM-patients at time of diagnosis and one year after treatment.
Design
Prospective study with 1-year follow up after treatment. Questionnaires included a modified version of the Subjective Health Complaints Inventory, comprising three additional Lyme borreliosis (LB) related symptoms. General function was assessed using a five-point scale modified from the COOP/WONCA charts.
Setting
Norwegian general practice.
Subjects
A total of 188 patients were included in a randomized controlled trial comparing three antibiotic regimens for EM, of whom 139 had complete data for this study.
Main outcome measures
Individual symptoms, symptom load and general function.
Results
Mild symptoms were common, reported by 84.9% at baseline and by 85.6% at follow-up. At baseline, patients reported a mean of 5.4 symptoms, compared with 6.2 after one year. Severely bothersome symptoms and severely impaired general function were rare. Tiredness was the most reported symptom both at baseline and at follow-up. Palsy (other than facial) was the least reported symptom, but the only one with a significant increase. However, this was not associated to the EM.
Conclusion
The symptom load was comparable to that reported in the general population. We found an increase in symptom load at follow-up that did not significantly affect general function.
Implication: Monitoring patients’ symptom loads prior to treatment reduce the probability of attributing follow-up symptoms to LB.
Key points
Erythema migrans has a good prognosis.Patients treated for erythema migrans have a slight increase in symptom load one year after treatment.
This increase does not affect general function.
The levels of subjective health complaints in patients treated for erythema migrans are comparable to the background population.
Keywords: Lyme borreliosis, erythema migrans, subjective health complaints, self-reported symptoms, general practice, Norway
Introduction
In Europe, there is an increasing incidence of Lyme borreliosis (LB) [1–3]. The infection is caused by spirochetes of the Borrelia burgdorferi genospecies complex, transmitted through the bite of an infected tick.
LB is traditionally divided into three stages: early local disease, such as erythema migrans (EM); early disseminated disease, such as Lyme arthritis (LA) or Lyme neuroborreliosis (LNB); and late disseminated disease, such as acrodermatitis chronicum atrophicans, or late LNB [1]. Solitary EM is the most common early manifestation. It is a localised skin lesion around the tick bite that can develop 2–30 days after attachment of the tick [1,4–6]. With prompt treatment, patients with EM have cure rates exceeding 90%. If untreated the Borrelia infection can disseminate and cause more serious disease [1,4,7–10].
In Norway, only cases of disseminated LB are notifiable to the Institute of Public Health [11,12] and the incidence was on average 5.5 cases/100,000 inhabitants/year in our study period 2011–2013, increasing from 2.1 cases in 2000 [11]. EM is estimated to comprise 80–90% of the total incidence of LB [1,13]. A recent Norwegian study found a national estimate of 148 EM/100,000 inhabitants/year, making solitary EMs comprising 96% of the cases of LB in Norway in 2005–2009 [14]. In Sweden, a study from 2006 found an incidence of 464 EM/100,000 inhabitants/year in an endemic county [15] and in Holland, the national incidence of EM was 132/100,000 inhabitants/year in 2010 [16].
Serological tests are not recommended for confirming EM, because less than 50% of the patients have developed antibodies at the time of diagnosis [17]. Hence, a prompt clinical diagnosis is crucial [18]. However, EMs vary in appearance, and reviews have concluded that there are no single factors upon which physicians can rely to make correct diagnoses [5,18–20]. In one study, 72% of the patients with EM were correctly diagnosed in general practice, whereas 23% of patients with other dermatological conditions were given the diagnosis as well. Proper training seemed to improve the clinical diagnosis skills of the GPs [21].
It is common for patients with disseminated LB to have remaining symptoms even after sufficient treatment [22,23]. Despite the good prognosis, some patients treated for early LB also report persistent or new symptoms, such as fatigue, musculoskeletal pain and cognitive impairment, resulting in functional impairment. A new term—post-treatment Lyme borreliosis syndrome (PTLBS)—has been coined, defined as persistent, subjective symptoms without objective manifestations that persist for at least six months after conventional treatment. There is a great deal of controversy regarding this condition. There is a high prevalence of non-specific symptoms in the general population, and no objective evidence of active, persistent Borrelia infection has been found in PTLBS patients [9,24–29]. One recent study reported persistent symptoms ten years after treatment in a few patients with culture-confirmed early LB. However, these symptoms were not associated with functional impairments [30]. Other studies have shown that patients treated for EM and healthy controls report similar numbers of symptoms at controls after six months and 11 years, respectively [7,31]. A recent Norwegian study found that exposure to tick bites and seropositivity to Borrelia did not correlate with subjective health complaints (SHC) in blood donors [32].
This study is part of a randomized controlled trial (RCT) comparing three antibiotic treatment regimens for patients clinically diagnosed with solitary EM in Norwegian general practice. We wanted to assess whether EM patients had a larger symptom burden than the general population. Here, we describe the prevalence of individual symptoms, symptom load and general function among patients with EM at the time of diagnosis and one year after treatment.
Material and methods
Study sample and setting
Forty-four general practitioners (GPs) in Norway included patients 18 years or older clinically diagnosed with EM. Patients were randomized to receive one of three active oral antibiotic treatments – phenoxymethylpenicillin, amoxicillin or doxycycline – for 14 days. Patients were given a neutral carton of study medication. After the consultation they opened the carton and found one of the three treatments in the original packaging and with the manufacturers’ information. This way the study was open for the patients, but blinded for the GPs and the researchers.The GPs attended a 2-day course on tick-borne diseases prior to inclusion of the patients. Data were collected from 10 June 2011 until 18 November 2014.
The case definition for EM was as described by Stanek et al. in 2011 [6]. As EM is a clinical diagnosis, the inclusion criterion was “if the GP would prescribe an antibiotic treatment for EM, the patient should be asked to participate”. There was no screening log registering non-eligible patients or patients declining participation. The patients were asked to complete a questionnaire on the day of the consultation (baseline) and one year after treatment (follow-up). The respondents received the follow-up questionnaires by ordinary mail. Two reminders were sent by text messages to non-responders. In cases of missing or unclear data, responders were contacted by telephone for clarification.
Variables
In the baseline questionnaire, respondents were asked to record demographic information. In baseline and follow-up questionnaires, patients were asked to record whether, during the course of the last 30 days, they had experienced any of 32 symptoms, comprising 29 common, non-specific symptoms from the validated SHC Inventory [33], and three symptoms considered to be associated with LB; “swollen joints”, “facial palsy” and “palsy (other than facial)”. The level of severity of each symptom was recorded on a four-point scale: “not bothered”, “a little bothered”, “moderately bothered” and “severely bothered”. The respondents were asked to record the duration of these symptoms (1–30 days). The response rate for symptom duration was low (14.1%), and we did not use symptom duration data in our analyses.
We used a simple sum score variable for the number of symptoms reported, termed “symptom load”, at baseline and at one year of follow-up (range 0–32). We analysed the numbers of symptoms reported at three levels of severity: Level I (at least a little bothersome), Level II (at least moderately bothersome) and Level III (severely bothersome).
We assessed the patients’ general function by asking the following question at both time points: “At present, how would you describe your ability to perform ordinary daily activities – your general function?” Response options were “as usual”, “hardly reduced at all”, “slightly reduced”, “moderately reduced” and “severely reduced”. The question was adapted from the validated COOP/WONCA chart, which assesses general health [34].
Statistical analyses
Sample size calculation was made for the main outcome of the RCT, the median duration of EM in each treatment group. The unadjusted sample size was 46 in each group, altogether 138 patients. We used descriptive statistics such as frequencies, proportions and means to describe the distribution of demographic characteristics and reports of individual symptoms, symptom load and general function. Two-sample test of proportions was used to compare changes in these reports over time. All p values were two-sided and values below 0.05 were considered statistical significant. After analyses ruling out multicollinearity, multivariate linear regression analyses and analysis of variance (ANOVA) tests were applied to assess the degree of which symptom load and general function at baseline explained variance in these variables at follow-up.
To describe changes in reports of individual symptoms from baseline to follow-up, we used a multi-state model and calculated the transition probabilities for each symptom. At the time points studied, a patient could be in either state 0 (not reported) or state 1 (reported) for each individual symptom. State 1 was defined as report of a symptom at severity level I (at least a little bothersome), whereas state 0 was defined as no report of the symptom. A patient in state 0 at baseline could transit to state 1 after one year or remain in the same state. Similarly, a patient in state 1 at baseline could transit to state 0 after one year or remain in the same state.
To estimate the rate of transition (transition probability), two separate multi-state Markov models were fitted to the data [35]. The first model estimated the rate of progression from healthy to diseased (from not reporting a symptom to reporting a symptom) within one year, whereas the second model estimated the rate of regression (from diseased to healthy).
Analyses were performed using IBM SPSS Statistics for Windows (v. 22; IBM Corp., Armonk, NY). Stata/SE 14.1 (StataCorp LP, College Station, TX) and R for Windows (version 3.0.1; https://cran.r-project.org/bin/windows/base/old/3.0.1/).
Results
Altogether 188 patients were enrolled in the RCT. One hundred and sixty patients returned questionnaires both at baseline and at the 1-year follow-up (response rate 85.1%). One hundred and thirty-nine (73.9%) complete datasets were used in this study. Out of the 139, 91 (65.5%) were women. The median age was 59 years (range 18–85), with distribution: 18–39 years, 10.8%; 40–49 years, 15.8%; 50–59 years, 26.6%; 60–69 years, 32.4%; and 70–85 years, 14.4%.
At least one mildly bothersome symptom (severity level I) was reported by 118/139 (84.9%) patients at baseline and by 119 (85.6%) at follow-up (Figure 1(A)). At these two time points, 25 (18.0%) and 33 (23.7%) reported ten symptoms or more, respectively. The mean number of at least mildly bothersome symptoms was 5.4 at baseline and 6.2 after one year (p = 0.01).
Figure 1.
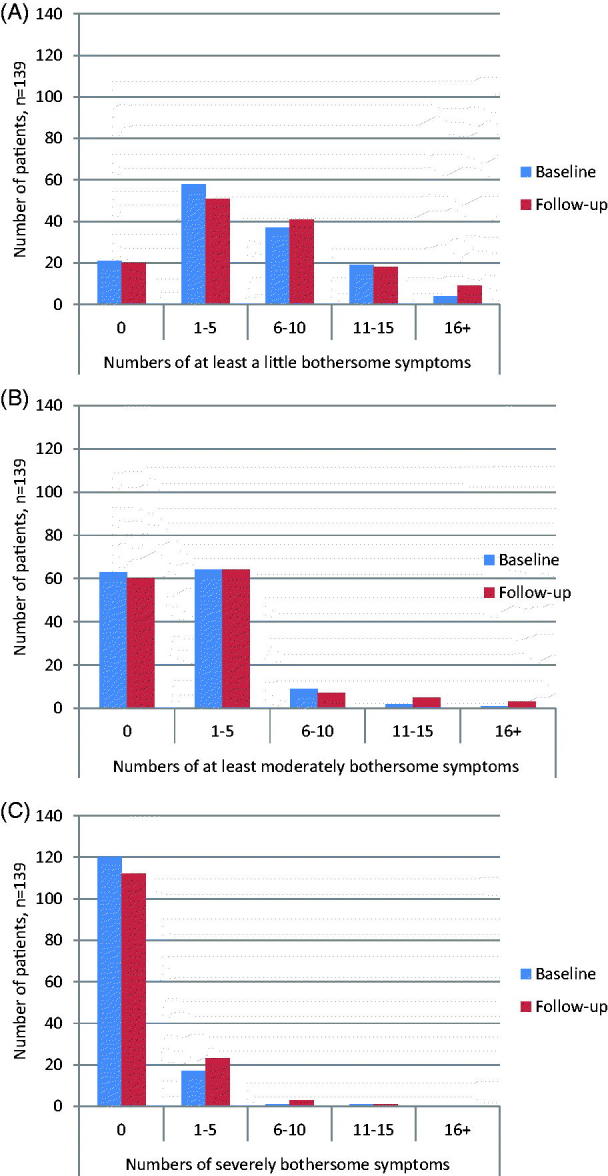
(A) Severity level I. The numbers of symptoms reported to be at least a little bothersome among patients clinically diagnosed with EM at baseline and at the 1-year follow-up (n = 139). (B) Severity level II. The numbers of symptoms reported to be at least a moderately bothersome among patients clinically diagnosed with EM at baseline and at the 1-year follow-up (n = 139). (C) Severity level III. The numbers of symptoms reported to be severely bothersome among patients clinically diagnosed with EM at baseline and at the 1-year follow-up (n = 139).
At least moderately bothersome symptoms (severity level II) were reported by 76/139 (54.7%) at baseline and 79 (56.8%) at follow-up (Figure 1(B)). At these time points, three (2.2%) and eight (5.8%) patients reported ten symptoms or more, respectively. At baseline, patients reported a mean of 1.6 level II symptoms, compared with 2.3 symptoms one year later (p = 0.02).
Severely bothersome symptoms (severity level III) were less frequently reported (Figure 1(C)). At baseline, 19/139 (13.7%) reported at least one symptom, compared to 27 (19.4%) at follow-up. This increase was not statistically significant.
There were no significant differences in symptom load or general function between the three treatment groups neither at baseline or at follow-up, nor at any levels of severity.
The general function was reported “as usual” for 98 patients (70.5%) at baseline, compared with 95 (68.3%) one year later. At baseline, five patients (3.6%) reported severely reduced general function, compared with seven (5.0%) after one year. There were no significant changes in general function at any levels of severity.
Of the individual symptoms at severity level I, tiredness was most frequently reported at baseline by 54/139 (38.8%), followed by headache and low back pain in 53 (38.1%) and 50 (36.0%) patients, respectively. Tiredness was also the most frequently reported symptom at follow-up, reported by 67 patients (48.2%), followed by sleep problems and headache reported by 38.8 and 38.1%, respectively. The only report of an individual symptom that changed significantly from baseline (0.7%) to follow-up (3.6%) was for “palsy (other than facial)” (p = 0.048) (Table 1).The most frequently reported symptoms at severity level II at baseline were headache, shoulder pain and tiredness, reported by 22/139 (15.8%), 21 (15.1%) and 20 (14.4%) of the patients, respectively.
Table 1.
Individual symptoms among patients with EM at baseline and at 1-year follow-up, (n = 139).a
| Prevalence of symptoms | Baseline (%) | 1 year (%) | Absolute change(baseline to 1 year) | Test of proportions(p-value) |
|---|---|---|---|---|
| Tiredness | 38.8 | 48.2 | +9.4 | 0.057 |
| Headache | 38.1 | 38.1 | – | – |
| Low back pain | 36.0 | 35.3 | −0.7 | 0.452 |
| Sleep problems | 29.5 | 38.8 | +9.3 | 0.051 |
| Shoulder pain | 28.8 | 35.3 | +6.5 | 0.123 |
| Neck pain | 28.8 | 28.8 | – | – |
| Diarrhoea | 27.3 | 27.3 | – | – |
| Gas discomfort | 25.9 | 30.2 | +4.3 | 0.212 |
| Cold, flu | 25.9 | 23.7 | −2.2 | 0.336 |
| Hot flushes | 23.0 | 23.7 | +0.7 | 0.445 |
| Arm pain | 20.1 | 24.5 | +4.4 | 0.189 |
| Heartburn | 20.1 | 23.0 | +2.9 | 0.278 |
| Upper back pain | 19.4 | 21.6 | +2.2 | 0.325 |
| Leg pain | 18.7 | 23.7 | +5.0 | 0.154 |
| Stomach pain | 15.1 | 9.4 | −5.7 | 0.074 |
| Dizziness | 18.0 | 23.7 | +5.7 | 0.121 |
| Swollen jointsb | 12.9 | 19.4 | +6.5 | 0.070 |
| Cough | 12.9 | 15.1 | +2.2 | 0.299 |
| Palpitation | 11.5 | 17.3 | +5.8 | 0.084 |
| Allergies | 10.8 | 10.8 | – | – |
| Depression | 10.8 | 14.4 | +3.6 | 0.183 |
| Breathing difficulties | 10.1 | 9.4 | −0.7 | 0.422 |
| Stomach discomfort | 9.4 | 11.5 | +2.1 | 0.284 |
| Migraine | 9.4 | 7.9 | −1.5 | 0.328 |
| Chest pain | 7.9 | 10.8 | +2.9 | 0.203 |
| Asthma | 7.9 | 10.1 | +2.2 | 0.261 |
| Anxiety | 7.2 | 10.1 | +2.9 | 0.195 |
| Eczema | 5.8 | 10.1 | +4.3 | 0.093 |
| Obstipation | 4.3 | 8.6 | +4.3 | 0.072 |
| Facial palsyb | 2.2 | 3.6 | +1.4 | 0.243 |
| Ulcers and non-acid dyspepsia | 2.2 | 2.2 | – | – |
| Palsy (other than facial)b | 0.7 | 3.6 | +2.9 | 0.048 |
Symptoms reported to be at least a little bothersome (severity level I).
Lyme borreliosis-related symptoms, not in the original SHC questionnaire.
Of the severely bothersome symptoms (level III) at baseline, low back pain, sleep problems and tiredness were the most frequently reported, by 5.8, 4.3 and 2.9% of the patients, respectively.
The transition probabilities from baseline to follow-up for each symptom with 95% confidence intervals (CIs) are presented in Table 2. The symptoms with the highest probability of staying unreported (remaining in state 0) were ulcer/dyspepsia (99%), facial palsy (98%), palsy (other than facial) (96%) and stomach pain (96%). The symptoms with the highest probability of still being reported at follow up, if present at baseline, (remaining in state 1) were depression (80%), swollen joints (78%) and sleep problems (73%). For the three LB-related symptoms added to the SHC questionnaire in this study, there were in addition an 89% chance that swollen joints stayed unreported at both time points, and a 67% and four percent chance that facial palsy and palsy (other than facial) if reported at time of diagnosis, were still reported after one year, respectively.
Table 2.
Transition probabilities for individual symptoms among patients with EM from baseline to 1-year follow-up, (n = 139).
| State transition probabilities from baseline to the 1-year follow-up |
||||
|---|---|---|---|---|
| Symptomsa | 0 → 0 | 0 → 1 | 1 → 0 | 1 → 1 |
| Tiredness | 0.70 (0.67, 0.74) | 0.30 (0.26, 0.33) | 0.33 (0.30, 0.37) | 0.67 (0.63, 0.70) |
| Headache | 0.75 (0.72, 0.78) | 0.25 (0.22, 0.28) | 0.40 (0.36, 0.44) | 0.60 (0.56, 0.64) |
| Low back pain | 0.84 (0.75, 0.90) | 0.16 (0.10, 0.25) | 0.30 (0.20, 0.46) | 0.70 (0.54, 0.80) |
| Sleep problems | 0.77 (0.67, 0.83) | 0.23 (0.17, 0.33) | 0.27 (0.16, 0.43) | 0.73 (0.57, 0.84) |
| Shoulder pain | 0.75 (0.66, 0.82) | 0.25 (0.18, 0.34) | 0.40 (0.26, 0.56) | 0.60 (0.44, 0.74) |
| Neck pain | 0.87 (0.79, 0.93) | 0.13 (0.08, 0.21) | 0.38 (0.25, 0.55) | 0.63 (0.45, 0.75) |
| Diarrhoea | 0.83 (0.75, 0.90) | 0.17 (0.10, 0.25) | 0.42 (0.28, 0.58) | 0.58 (0.42, 0.72) |
| Gas discomfort | 0.81 (0.72, 0.87) | 0.19 (0.13, 0.28) | 0.42 (0.27, 0.59) | 0.58 (0.41, 0.73) |
| Cold, flu | 0.84 (0.81, 0.87) | 0.16 (0.13, 0.19) | 0.53 (0.49, 0.60) | 0.47 (0.40, 0.51) |
| Hot flushes | 0.92 (0.85, 0.96) | 0.08 (0.04, 0.15) | 0.31 (0.18, 0.50) | 0.69 (0.50, 0.82) |
| Arm pain | 0.79 (0.72, 0.87) | 0.21 (0.13, 0.28) | 0.54 (0.36, 0.71) | 0.46 (0.29, 0.64) |
| Heartburn | 0.82 (0.74, 0.88) | 0.18 (0.12, 0.26) | 0.55 (0.37, 0.72) | 0.45 (0.28, 0.63) |
| Upper back pain | 0.87 (0.79, 0.92) | 0.13 (0.08, 0.21) | 0.44 (0.27, 0.64) | 0.56 (0.36, 0.73) |
| Leg pain | 0.84 (0.76, 0.90) | 0.16 (0.10, 0.24) | 0.42 (0.27, 0.61) | 0.58 (0.39, 0.73) |
| Stomach pain | 0.96 (0.91, 0.98) | 0.04 (0.02, 0.09) | 0.71 (0.50, 0.88) | 0.29 (0.12, 0.50) |
| Dizziness | 0.82 (0.75, 0.88) | 0.18 (0.12, 0.25) | 0.44 (0.28, 0.63) | 0.56 (0.37, 0.72) |
| Swollen jointsb | 0.89 (0.83, 0.94) | 0.11 (0.06, 0.17) | 0.22 (0.08, 0.48) | 0.78 (0.52, 0.92) |
| Cough | 0.89 (0.86, 0.91) | 0.11 (0.09, 0.14) | 0.65 (0.57, 0.71) | 0.35 (0.29, 0.43) |
| Palpitation | 0.88 (0.82, 0.93) | 0.11 (0.07, 0.18) | 0.38 (0.19, 0.64) | 0.62 (0.36, 0.81) |
| Allergies | 0.93 (0.87, 0.96) | 0.07 (0.04, 0.13) | 0.50 (0.26, 0.76) | 0.50 (0.24, 0.74) |
| Depression | 0.94 (0.88, 0.97) | 0.06 (0.03, 0.12) | 0.20 (0.07, 0.48) | 0.80 (0.52, 0.93) |
| Breathing difficulties | 0.95 (0.90, 0.98) | 0.05 (0.02, 0.10) | 0.50 (0.26, 0.78) | 0.50 (0.22, 0.74) |
| Stomach discomfort | 0.90 (0.84, 0.95) | 0.10 (0.05, 0.16) | 0.69 (0.43, 0.88) | 0.31 (0.12, 0.57) |
| Migraine | 0.93 (0.93, 0.96) | 0.07 (0.04, 0.07) | 0.78 (0.76, 0.89) | 0.22 (0.11, 0.24) |
| Chest pain | 0.91 (0.85, 0.95) | 0.09 (0.05, 0.15) | 0.70 (0.40, 0.89) | 0.30 (0.11, 0.60) |
| Asthma | 0.92 (0.90, 0.95) | 0.08 (0.05, 0.10) | 0.73 (0.68, 0.84) | 0.27 (0.16, 0.32) |
| Anxiety | 0.94 (0.89, 0.97) | 0.06 (0.03, 0.11) | 0.40 (0.16, 0.74) | 0.60 (0.26, 0.84) |
| Eczema | 0.90 (0.84, 0.97) | 0.10 (0.02, 0.15) | 0.88 (0.22, 0.94) | 0.13 (0.06, 0.78) |
| Obstipation | 0.94 (0.88, 0.97) | 0.06 (0.03, 0.12) | 0.33 (0.09, 0.75) | 0.67 (0.25, 0.91) |
| Facial palsyb | 0.98 (0.94, 0.99) | 0.02 (0.01, 0.06) | 0.33 (0.05, 0.92) | 0.67 (0.08, 0.95) |
| Ulcer and non-acid dyspepsia | 0.99 (0.95, 1.00) | 0.01 (0.001, 0.05) | 0.33 (0.05, 0.95) | 0.67 (0.05, 0.95) |
| Palsy (other than facial)b,c | 0.96 | 0.04 | 0.96 | 0.04 |
State 0 means that the patient did not report the symptom. State 1 means that the patient did report the symptom. For example, 0 → 0 means a transition from state 0 (symptom not reported at baseline) to state 0 (symptom not reported after 1 year). 0 → 1 means that the patient did not report the symptom at baseline but did report it at the 1-year follow-up, and so on (95% confidence intervals).
Symptoms reported to be at least a little bothersome (Severity level I).
Lyme borreliosis-related symptoms, not in the original SHC questionnaire.
The confidence interval is not given because of the small numbers reporting this symptom.
Reports of tiredness at severity level I correlated highly with slightly reduced general function both at baseline and one year later (p < 0.05). The correlation was also high between reports of moderately bothersome tiredness (severity level II) and moderately reduced general function at baseline and at follow-up (p < 0.05).
The symptom load of at least mildly bothersome symptoms at baseline (severity level I) explained 53% of the symptom load at follow-up (R2 = 0.53). The numbers of mildly and moderately bothersome symptoms at baseline (severity levels I and II) explained 31% and 23% of the variance in general function, respectively (p < 0.001).
Symptom load at baseline explained less of general function at follow-up. Mildly bothersome symptoms at baseline explained ten percent of the variance in general function one year later (R2 = 0.10, p < 0.001), whereas the moderately bothersome symptoms did not explain any significant variance in general function one year later.
As “palsy (other than face)” stands out as the only symptom with a significant increase to follow-up, we have explored this symptom in detail: The absolute change in reports was from one to five individuals. The one patient reporting mildly bothersome palsy at baseline did not report this symptom at follow-up. Of the five patients reporting palsy at follow-up, one reported this as severely bothersome, and the remaining four as mildly bothersome. Looking at the general function of these six individuals, the one patient at baseline had a decline from “hardly reduced at all” to “moderately reduced”, although palsy was no longer reported. The one patient with severely bothersome palsy at follow-up went from reporting “hardly reduced at all” to “severely reduced” general function. For the four others, one reported worsening from “as usual” to “moderately reduced”, while the other three all reported better or unaltered levels of general function.
Discussion
Strengths and weaknesses of the study
The main strength of this study is its prospective design and that it was performed in general practice. Most other studies assessing symptoms among patients with EM are performed in hospital settings or based on registry data. EM in Norway is primarily diagnosed, handled and treated in primary care [14]. EM is a clinical diagnosis. However, most other studies have made analyses on subgroups with serologically or biopsy-confirmed EM [4,7–10,29,30].
As the data of this study are based on an RCT, a limited number of patients were included. EM is not a highly prevalent diagnosis, and other studies have included a similar number of patients [30]. This might result in a type II statistical bias. However, the responder rate and proportions of completed questionnaires were relatively high, minimizing selection bias.
It is a weakness of the study that we could not compare the results for the cohort with a matched control group without EM [31].
GPs enrolled in the study were given a course on LB prior to inclusion, which is likely to have improved their diagnostic accuracy. This strengthens the study in respect to our intention to include patients with a correct, clinically diagnosed EM.
The selection of symptoms that were included can be questioned. Although the SHC questionnaire is well validated, it was modified in this study. This was essential, as we wanted to include both non-specific symptoms that are highly prevalent in the population and symptoms more specific for LB. However, it can be argued that additional symptoms associated with LB should have been included. There is a difference in time frame between the assessment of general function during the previous two weeks and the assessment of symptom experience during the previous 30 days, which may affect the comparison of these two variables. Besides, the respondents did not comply sufficiently to report the duration of symptoms.
Findings in relation to other studies
Are any of the symptoms reported at baseline caused by the EM itself? Most EMs are accompanied by few general symptoms. In a Swedish prospective EM study, the most common concomitant symptoms at the time of diagnosis were headache in 27% of the cases, muscular or joint pain in 14% and chills in 10% of the cases [5]. In the RCT casing this study there was a median of one concomitant symptom. Reports of symptoms in this study are similar to previous findings in general population studies. We found that 84.9% reported at least one symptom at baseline and 85.6% one year later. In one Norwegian population study and one in the USA, 96% and 80% reported at least one symptom during the last month, respectively [36,37]. In our study, 18.0% and 23.7% reported ten symptoms or more out of 32 at inclusion and one year later, respectively. In comparison, among individuals in one population study, 22% reported more than ten symptoms out of 23 during the last seven days. Only eight percent reported no symptoms at all [38].
The patients in this study reported a mean of 5.4 and 6.2 out of 32 symptoms at baseline and follow-up. In one Norwegian study, unselected patients in general practice reported a mean of 7.6 out of 38 listed symptoms [39]. Furthermore, respondents in another Norwegian population study reported a mean of 6.0 symptoms out of 23 listed [38]. Thus, reports of symptom load in our study, at both baseline and follow-up, seem to be similar to reports both from background population studies and among unselected patients in general practice, although the methodology of the studies differed, especially regarding the numbers of symptoms listed in the questionnaires.
There is growing evidence that the number of symptoms reported per se is associated with health outcomes, regardless of the underlying pathology. Studies on general symptom loads indicate that individuals who report a high symptom load prior to experiencing illness are likely to have worse prognoses at follow-up [40,41]. In this study, we have no records of the patients’ symptom profiles before the EM. Of the few studies assessing symptom load among patients with EM, patients did not report new or increased symptoms at follow-up more often than healthy controls, and symptoms were rarely reported to be functionally disabling [8,31]. Similarly, in our study, we found no significant increase in severely bothersome symptoms or changes in general function over time.
The most commonly reported symptoms in our study were tiredness (38.8 | 48.2%) and headache (38.1|38.1%) . In a study among unselected patients in general practice, headache was reported by 39% and tiredness by 44% (unpublished personal communication, M. Kjeldsberg, University of Oslo; 20 June 2016). A Norwegian study of SHC in the background population, found a prevalence of tiredness of 53% [36]. Fatigue has been documented to be the most common non-specific symptom to persist after early LB. In our study, we only asked about tiredness, which cannot simply be compared with fatigue. Nevertheless, one study found reports of fatigue among almost half of the patients with EM [30], whereas severe fatigue was only found in nine percent of patients with culture-confirmed EM in another prospective study [29]. Symptom reporting seems to be a relatively stable phenomenon. Almost half of patients in a general practice study presenting with physical symptoms had persistence of the symptoms five years later [42]. Thus, it could be expected that many of the symptoms reported at baseline in our study would persist, without necessarily being caused by the Borrelia infection. Current evidence does not indicate the persistence of viable B. burgdorferi bacteria after prompt treatment. Hence, non-specific symptoms should not be attributed to persistent active Borrelia infection [8,24,27].
Palsy (other than facial)
As palsy (other than facial) was both the least-reported symptom and the only individual symptom with a statistically significant increase, using a five percent significance level, we wanted to assess it more rigorously. This was one of the three symptoms added to the SHC questionnaire as an LB-relevant symptom. However, it may be difficult to interpret what any particular patient meant by reporting this symptom. The Norwegian term for palsy was “lammelse”. “Palsy (other than facial)” was “Andre lammelser”. The Norwegian term, as well as the English, is defined as a motoric deficit. The use among the public, however, may cover “numbness” or sensory deficits, as well.
One could easily interpret the decline in general function for the one patient severely bothered with palsy at follow-up to be a possible development of LNB. However, in the RCT casing this study, there were no reports of disseminated LB after one year of follow-up. In addition, this patient had noted other chronic diseases. In August 2016, we acquired supplementary information from all six patients. The one patient with severe palsy, had, as did one of the others, symptoms of ischialgia. One other patient had suffered from apoplexy, one had sequelae from an earlier LNB. One had ulnar numbness in one hand, and the last patient had numbness in one arm due to shoulder arthrosis. None of the symptoms, except for the apoplexy, were new. None of the six patients had reported other LB than their EM during the one year follow-up. The patient with LNB sequelae had also noted facial palsy and joint swelling (both moderately bothersome), at both time points. None of the others had noted other LB-relevant symptoms.
Transition probabilities
Despite being the only symptom with a significant change, the probabilities of reporting palsy (other than facial) after one year, both when reported at baseline (1 to 1) and not reported at baseline (0 to 1), were only four percent (Table 2). In addition, although the second most reported symptom, headache, had equal reported incidences of 38.1% at both time points (Table 1), the probability of being reported at both time points for headache was only 60% (Table 2). The mean probability for a symptom to be reported at both time points in our study was 52%, indicating that the symptoms in this cohort were reported by different individuals at the different time points.
Meaning and implication
We have described the symptom load and general function in a clinically diagnosed cohort of patients with EM in Norwegian general practice.
The results of this study are similar to other outcome studies after treating patients with EM. Severe symptoms and reduced general function are rare: most are mild. There was no significant change in general function at any level of severity.
As the symptom load and functional level of this cohort, at both baseline and follow-up, were similar to other findings from population studies and in general practice settings, and the increase in symptom load did not lead to impaired general function, we cannot conclude that being treated for EM leads to worsening of subjective health or quality of life (QoL). The results of this study correspond quite well with the results of a recent US study on QoL among LB patients, in which EM patients had better QoL-scores than other LB patients, and lower QoL scores were associated with comorbidities [43].
One productive approach could be for the clinician to register patients’ symptom loads prior to treatment, so that unspecific symptoms not unnecessarily are attributed to LB.
Acknowledgements
We would like to thank the GPs including patients for the study, and the patients for participating. We thank Professor Camilla Ihlebæk, Norwegian University for Life Sciences for help regarding SHC and questionnaires. We also thank PhD Ibrahimu Mdala, Department of General Practice, University of Oslo, for essential advice on the statistics.
Funding Statement
Funding for the study was granted by the Institute of Health and Society within the University of Oslo and the Norwegian Research Fund for General Practice (http://legeforeningen.no/Allmennmedisinsk-forskningsfond/). Additional funding was received from the Antibiotic Centre for Primary Care, the NORM surveillance program for antibiotic resistance in human pathogens, the National Centre of Rural Medicine, and the Eckbo Trust. None of those providing economic support were involved in the study design, data collection, analysis and interpretation of data, writing of the report, or in the decision to submit the article for publication.
Ethics approval and consent to participate
The RCT was performed in accordance with the Helsinki Declaration of 1975/83 and Good clinical practice. The study was approved by the Norwegian Regional Committee for Medical and Health Research Ethics (REK South East), approval number 2010/2994. All patients have signed an informed consent form.
Disclosure statement
The authors declare that they have no competing interests in the subjects described in this article.
Trial registration
Part of a randomized control trial comparing three antibiotic regimens for EM in Norwegian general practice, ClinicalTrials.gov identifier: NCT01368341, registered June 6, 2011.
References
- 1.Stanek G, Wormser GP, Gray J, et al. . Lyme borreliosis. Lancet. 2012;379:461–473. [DOI] [PubMed] [Google Scholar]
- 2.Nygard K, Brantsaeter AB, Mehl R. Disseminated and chronic Lyme borreliosis in Norway, 1995–2004. Euro Surveill. 2005;10:235–238. [PubMed] [Google Scholar]
- 3.Lindgren E, Jaenson TGT. Lyme borreliosis in Europe: influences of climate and climate change, epidemiology, ecology and adaptation measures. Copenhagen, Denmark: WHO Regional Office for Europe; 2006. [Google Scholar]
- 4.Smith RP, Schoen RT, Rahn DW, et al. . Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med. 2002;136:421–428. [DOI] [PubMed] [Google Scholar]
- 5.Bennet L, Fraenkel CJ, Garpmo U, et al. . Clinical appearance of erythema migrans caused by Borrelia afzelii and Borrelia garinii – effect of the patient’s sex. Wien Klin Wochenschr. 2006;118:531–537. [DOI] [PubMed] [Google Scholar]
- 6.Stanek G, Fingerle V, Hunfeld KP, et al. . Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect. 2011;17:69–79. [DOI] [PubMed] [Google Scholar]
- 7.Seltzer EG, Gerber MA, Cartter ML, et al. . Long-term outcomes of persons with Lyme disease. JAMA. 2000;283:609–616. [DOI] [PubMed] [Google Scholar]
- 8.Lipsker D, Antoni-Bach N, Hansmann Y, et al. . Long-term prognosis of patients treated for erythema migrans in France. Br J Dermatol. 2002;146:872–876. [DOI] [PubMed] [Google Scholar]
- 9.Hofhuis A, Herremans T, Notermans DW, et al. . A prospective study among patients presenting at the general practitioner with a tick bite or erythema migrans in The Netherlands. PLoS One. 2013;8:e64361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber MA, Shapiro ED, Burke GS, et al. . Lyme disease in children in southeastern Connecticut. Pediatric Lyme Disease Study Group. N Engl J Med. 1996;335:1270–1274. [DOI] [PubMed] [Google Scholar]
- 11.MSIS Statistikk: The Norwegian Surveillance System for Communicable Diseases (MSIS) 2015. [cited 2016 Jul 25]. http://www.msis.no/. [Google Scholar]
- 12.Norwegian Institute of Public Health, Lyme borreliosis 2014. [cited 2016 Jul 25]. https://www.fhi.no/ml/skadedyr/flatt/fakta-om-borreliose/. [Google Scholar]
- 13.Berglund J, Eitrem R, Ornstein K, et al. . An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med. 1995;333:1319–1327. [DOI] [PubMed] [Google Scholar]
- 14.Eliassen KE, Berild D, Reiso H, et al. . Incidence and antibiotic treatment of erythema migrans in Norway 2005–2009. Ticks Tick Borne Dis. 2017;8:1–8. [DOI] [PubMed] [Google Scholar]
- 15.Bennet L, Halling A, Berglund J.. Increased incidence of Lyme borreliosis in southern Sweden following mild winters and during warm, humid summers. Eur J Clin Microbiol Infect Dis. 2006;25:426–432. [DOI] [PubMed] [Google Scholar]
- 16.Hofhuis A, Harms M, Bennema S, et al. . Physician reported incidence of early and late Lyme borreliosis. Parasit Vectors. 2015;8:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen K, Asbrink E.. Serodiagnosis of erythema migrans and acrodermatitis chronica atrophicans by the Borrelia burgdorferi flagellum enzyme-linked immunosorbent assay. J Clin Microbiol. 1989;27:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibbles CD, Edlow JA.. Does this patient have erythema migrans? JAMA. 2007;297:2617–2627. [DOI] [PubMed] [Google Scholar]
- 19.Feder HM Jr, Whitaker DL.. Misdiagnosis of erythema migrans. Am J Med. 1995;99:412–419. [DOI] [PubMed] [Google Scholar]
- 20.Wormser GP. Clinical practice. Early Lyme disease. N Engl J Med. 2006;354:2794–2801. [DOI] [PubMed] [Google Scholar]
- 21.Lipsker D, Lieber-Mbomeyo A, Hedelin G.. How accurate is a clinical diagnosis of erythema chronicum migrans? Prospective study comparing the diagnostic accuracy of general practitioners and dermatologists in an area where Lyme borreliosis is endemic. Arch Dermatol. 2004;140:620–621. [DOI] [PubMed] [Google Scholar]
- 22.Ljøstad U, Mygland A.. Remaining complaints 1 year after treatment for acute Lyme neuroborreliosis; frequency, pattern and risk factors. Eur J Neurol. 2010;17:118–123. [DOI] [PubMed] [Google Scholar]
- 23.Eikeland R, Mygland A, Herlofson K, et al. . European neuroborreliosis: quality of life 30 months after treatment. Acta Neurol Scand. 2011;124:349–354. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira CR, Shapiro ED.. Update on persistent symptoms associated with Lyme disease. Curr Opin Pediatr. 2015;27:100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feder HM Jr, Johnson BJ, O’Connell S, et al. . A critical appraisal of “chronic Lyme disease”. N Engl J Med. 2007;357:1422–1430. [DOI] [PubMed] [Google Scholar]
- 26.Lantos PM. Chronic Lyme disease. Infect Dis Clin North Am. 2015;29:325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halperin JJ. Chronic Lyme disease: misconceptions and challenges for patient management. Infect Drug Resist. 2015;8:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wormser GP, Weitzner E, McKenna D, et al. . Brief Report: Long-term assessment of fibromyalgia in patients with culture-confirmed Lyme disease. Arthritis Rheumatol. 2015;67:837–839. [DOI] [PubMed] [Google Scholar]
- 29.Wormser GP, Weitzner E, McKenna D, et al. . Long-term assessment of fatigue in patients with culture-confirmed Lyme disease. Am J Med. 2015;128:181–184. [DOI] [PubMed] [Google Scholar]
- 30.Weitzner E, McKenna D, Nowakowski J, et al. . Long-term assessment of post-treatment symptoms in patients with culture-confirmed early Lyme disease. Clin Infect Dis. 2015;61:1800–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerar D, Cerar T, Ruzić-Sabljić E, et al. . Subjective symptoms after treatment of early Lyme disease. Am J Med. 2010;123:79–86. [DOI] [PubMed] [Google Scholar]
- 32.Hjetland R, Reiso H, Ihlebaek C, et al. . Subjective health complaints are not associated with tick bites or antibodies to Borrelia burgdorferi sensu lato in blood donors in western Norway: a cross-sectional study. BMC Public Health. 2015;15:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eriksen HR, Ihlebaek C, Ursin H.. A scoring system for subjective health complaints (SHC). Scand J Public Health. 1999;27:63–72. [PubMed] [Google Scholar]
- 34.Bentsen BG, Natvig B, Winnem M. Questions you didn't ask? COOP/WONCA Charts in clinical work and research. World Organization of Colleges, Academies and Academic Associations of General Practitioners/Family Physicists. Fam Pract. 1999;16:190–195. [DOI] [PubMed] [Google Scholar]
- 35.Jackson C. Multi-state models for panel data: the msm package for R. J Stat Soft. 2011;38:28. [Google Scholar]
- 36.Ihlebaek C, Eriksen HR, Ursin H.. Prevalence of subjective health complaints (SHC) in Norway. Scand J Public Health. 2002;30:20–29. [PubMed] [Google Scholar]
- 37.Green LA, Fryer GE Jr., Yawn BP, et al. . The ecology of medical care revisited. N Engl J Med. 2001;344:2021–2025. [DOI] [PubMed] [Google Scholar]
- 38.Tschudi-Madsen H, Kjeldsberg M, Natvig B, et al. . A strong association between non-musculoskeletal symptoms and musculoskeletal pain symptoms: results from a population study. BMC Musculoskelet Disord. 2011;12:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tschudi-Madsen H, Kjeldsberg M, Natvig B, et al. . Multiple symptoms and medically unexplained symptoms–Closely related concepts in general practitioners’ evaluations. A linked doctor–patient study. J Psychosom Res. 2013;74:186–190. [DOI] [PubMed] [Google Scholar]
- 40.olde Hartman TC, Borghuis MS, Lucassen PL, et al. . Medically unexplained symptoms, somatisation disorder and hypochondriasis: course and prognosis. A systematic review. J Psychosom Res. 2009;66:363–377. [DOI] [PubMed] [Google Scholar]
- 41.Kroenke K, Jackson JL.. Outcome in general medical patients presenting with common symptoms: a prospective study with a 2-week and a 3-month follow-up. Fam Pract. 1998;15:398–403. [DOI] [PubMed] [Google Scholar]
- 42.Jackson JL, Passamonti M.. The outcomes among patients presenting in primary care with a physical symptom at 5 years. J Gen Intern Med. 2005;20:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wills AB, Spaulding AB, Adjemian J, et al. . Long-term follow-up of patients with Lyme disease: longitudinal analysis of clinical and quality-of-life measures. Clin Infect Dis. 2016;62:1546–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]


