Abstract
Purpose
We aimed to visualize the relationship of predominant dietary patterns and their associations with AMD.
Methods
A total of 8103 eyes from 4088 participants in the baseline Age-Related Eye Disease Study (AREDS) were classified into three groups: control (n = 2739), early AMD (n = 4599), and advanced AMD (n = 765). Using principle component analysis, two major dietary patterns and eight minor dietary patterns were characterized. Applying logistic regression in our analysis, we related dietary patterns to the prevalence of AMD. Qualitative comparative analysis by operating Boolean algebra and drawing Venn diagrams was used to visualize our findings.
Results
In general, the eight minor patterns were subsets or extensions of either one of the two major dietary patterns (Oriental and Western patterns) and consisted of fewer characteristic foods than the two major dietary patterns. Unlike the two major patterns, which were more strongly associated with both early and advanced AMD, none of the eight minors were associated with early AMD and only four minor patterns, including the Steak pattern (odds ratio comparing the highest to lowest quintile of the pattern score = 1.73 [95% confidence interval: 1.24 to 2.41; Ptrend = 0.02]), the Breakfast pattern (0.60 [0.44 to 0.82]; Ptrend = 0.004]), the Caribbean pattern (0.64 [0.47 to 0.89; Ptrend = 0.009]), and the Peanut pattern (0.64 [0.46 to 0.89; Ptrend = 0.03]), were significantly associated with advanced AMD. Our data also suggested several potential beneficial (peanuts, pizza, coffee, and tea) and harmful (salad dressing) foods for AMD.
Conclusions
Our data indicate that a diet of various healthy foods may be optimal for reducing AMD risk. The effects of some specific foods in the context of overall diet warrant further study.
Keywords: retina, nutrition, diet, aging, dietary pattern, epidemiology, principle component analysis, qualitative comparative analysis, data visualization
Prevention of disease is preferred to treatment and remediation. This is especially true for AMD. Advanced AMD is the major cause of unremediable blindness in persons aged ≥65 years in developed countries. Vision impairment due to advanced AMD significantly reduces quality of life and consumes a large portion of the Medicare budget.1 Most importantly, current treatments for AMD are costly and can only arrest the neovascular type of advanced AMD without preventing progression of visual loss.2 For the most prevalent (>80% of patients) form of advanced AMD, geographic atrophy, there is currently no treatment.
Recent trials from the Age-Related Eye Disease Study (AREDS) and AREDS2 suggest that nutritional intervention is an effective strategy to prevent AMD from progressing to the advanced stages that result in vision loss. The two trials show that use of a supplement containing multiple nutrients, including vitamins C and E, lutein/zeaxanthin, and zinc, delays progression to advanced AMD in persons with intermediate AMD.3,4 This multinutrient strategy has also inspired studies into the possibility of using dietary management, involving multiple foods, to prevent AMD. Consistent with multiple nutrient approach, we recently observed that the benefits of omega-3 fatty acids from foods may depend on the stage of AMD and the status of other nutrients.5 Additionally, our previous studies suggest that the overall quality of carbohydrates in the diet, measured by dietary glycemic index, affect the risk for AMD.5–9 However, it is known that the glycemic index of a specific food may vary depending on the other foods with which it is consumed.10 Together, these observations suggest that intake of one food, with its many ingredients, might affect the bioavailability and nutritional value of another food or nutrient and that the benefits gained through nutrient intake could also vary by health status. Therefore, it is important to study the diet as a whole to understand how it can be optimized to promote health.
Our recent study demonstrated that dietary pattern analysis provides such useful insight regarding optimizing the human diet with regard to AMD.11 Specifically, we identified two major dietary patterns that accounted for less than 20% of the total dietary variance in the AREDS dataset. The data imply that additional minor dietary patterns may account for a substantial amount of dietary variance and that understanding the relationships of these dietary patterns to AMD may further advance our knowledge in diet-related AMD pathogenesis and prevention. To facilitate our analysis, we incorporated qualitative comparative analysis (QCA) into traditional dietary pattern analysis to accomplish finer dietary pattern analysis and to visualize the relationship of patterns and their associations with AMD. Qualitative comparative analysis was developed by Ragin12 and has been applied by various researchers across the social sciences, but remains a novel and underutilized methodology in the field of public health evaluation.13 This method allows for the evaluation of risk of outcome with regard to multiple causation14 and is therefore a useful method for raising new hypotheses. While visualized data relating overall diet to human disease is scanty, our novel approach allowed us to gain additional insight into human dietary patterns and their associations with AMD and helped us discover previously unidentified foods that may be associated with AMD risk in the context of the overall diet.
Methods
Age-Related Eye Disease Study
More details about the AREDS can be found in the AREDS report series.15 This study is an analysis of preexisting data from the AREDS.
The AREDS sponsored by the National Eye Institute of the National Institutes of Health is a long-term multicenter, prospective study dedicated to assessing the clinical course, prognosis, risk factors, and prevention strategies for both AMD and cataracts.15 The protocol adhered to the principles of the Declaration of Helsinki and was approved by a Data and Safety Monitoring Committee as well as by each Institutional Review Board for the 11 participating ophthalmic centers before initiation of the study. Participants were 55 to 80 years of age at enrollment. A total of 4757 participants were enrolled from November 1992 to January 1998. Informed consent was obtained from participants prior to enrollment.16–18
Stereoscopic fundus photographs of the macula and slit lamp and red reflex lens photographs were taken and graded at a central ophthalmic photograph reading center, where the various lesions associated with AMD were assessed using AREDS grading procedures adapted from the Wisconsin age-related maculopathy grading system.17,18 Eyes were classified into one of five groups (see below) according to the size and extent of drusen, presence of geographic atrophy, and neovascular changes of AMD.19 The baseline characteristics for the five study groups have been published previously.7 The five groups, numbered serially and based on increasing severity of drusen or type of AMD, were defined as follows:
Group 1 (Control): Eyes had no drusen or nonextensive, small drusen.
Group 2 (Intermediate Drusen): Eyes had one or more intermediate drusen, extensive small drusen, or pigment abnormalities associated with AMD.
Group 3 (Large Drusen): Eyes had one or more large drusen or extensive intermediate drusen.
Group 4 (Geographic Atrophy): Eyes had geographic atrophy.
Group 5 (Neovascular): Eyes had choroidal neovascularization or RPE detachment.
A 90-item modified Block food frequency questionnaire (FFQ) was administered to AREDS participants at baseline. The FFQ was validated in relation to 24-hour recall using a subset (n = 192) of the AREDS volunteers.20 The FFQ collected information about average frequency and serving sizes of consumption of certain foods over the previous year. For each food item, participants indicated their average frequency of consumption in terms of the specified serving size (small = 0.5 medium, medium, or large = 1.5 medium) by checking one of nine frequency categories ranging from “never or less than once per month” to “two or more times per day.” The medium serving sizes are described by using natural portions (e.g., one banana or two slices of pizza) or by using standard weight and volume measures of the servings commonly consumed by the American population. The selected frequency category and serving sizes for each food item were converted to a daily intake value in proportion to the medium serving sizes. For example, a response of “2 to 4 servings per week” in large servings was converted to 4.5 (3 × 1.5) medium servings per week (4.5 / 7 = 0.643 medium servings per day).
Study Subjects
Of the original 4757 subjects in the AREDS, we first excluded those with diabetes, invalid calorie intake (valid intakes ranged from 400 to 3,000 kcal/d for females and 600 to 3500 kcal/d for males), and missing covariate information. This left 4088 persons contributing 8103 eyes available for analysis. The 8103 eyes consisted of 2739 control eyes (group 1), 4599 early AMD eyes (1801 eyes with intermediate drusen [group 2] plus 2798 eyes with large drusen [group 3]), and 765 advanced AMD eyes (164 eyes with geographic atrophy [group 4] plus 601 eyes with choroidal neovascularization [group 5]).
Statistical Methods
Principle Component Analysis.
We derived dietary patterns by conducting principal component analysis (PCA) on food consumption data from the AREDS FFQ. Principal component analysis is a statistical method that can help reduce the many original data points in a data set into fewer representative data points that summarize the overall information contained in the data. In our case, the individual diet intake information collected by the AREDS FFQ from the AREDS participants are the original data points. In other words, we used PCA to summarize these individual eating styles into several prominent dietary patterns.
As documented previously, we first classified 90 food items in the FFQ into 37 predefined food groups that share similar nutritional properties, biological classifications, or are usually consumed together (Supplementary Table S1).11 The daily intake value of a food group for each subject was calculated by summing the daily intake values of the food items in the food group. The daily intake values of the 37 food groups for each of the 4088 subjects were then entered into our PCA. In our PCA, an orthogonal transformation was used to convert the 37 correlated food group variables into new variables called principal components (factors, i.e., dietary patterns), which are obtained as linear combinations of the original variables (i.e., the 37 food groups).21 A food item or group was defined as a characteristic food for a pattern if its food loading derived from our PCA is higher than or equal to 0.3. The loading factor describes the contribution of the food item or group to the dietary pattern, with a higher loading indicating a greater contribution. The analyses were conducted by using the PROC FACTOR in SAS (version 9.3; SAS Institute, Inc., Cary, NC, USA).
To assess subjects' adherence to a dietary pattern, we did not simply classify our participants as “following” or “not following” a given pattern, but instead ranked them according to how closely their diets line up with a pattern by calculating the pattern score for each subject. The pattern score was constructed by summing observed intakes of the component food items or groups weighted by food loadings in each pattern.
Logistic Analysis.
To evaluate the baseline cross-sectional associations between the eight minor dietary patterns and AMD, we used eyes with AMD lesions (groups 2 through 5) as our cases and those in group 1 as our controls. Odds ratios (ORs) were calculated by dividing the odds of AMD among eyes in the higher quintiles of dietary pattern scores by the odds among eyes in the lowest quintile of dietary pattern scores.
We estimated ORs and 95% confidence intervals (CIs) by logistic regression analysis using SAS PROC GENMOD (version 9.3; SAS Institute, Inc.). The procedure uses the generalized estimating equation (GEE) method to estimate the coefficients and adjust the standard errors (SEs) of the model terms for the correlated data resulting from repeated measurements (both eyes) on the same individual.22
The following baseline characteristics were considered as covariates in our analyses: age, sex, education level (college graduate, and high school or less), race (white and others), body mass index (BMI, computed from weight and height; kg/m2), alcohol intake (g/d), calorie intake, multivitamin use, smoking status (ever and never), sunlight exposure (h/d),23 hypertension history, lens opacity, and refractive error. Nutrient variables were energy adjusted by the residual method.24 We used P < 0.05 to denote statistical significance, and all tests were two sided.
Qualitative Comparative Analysis.
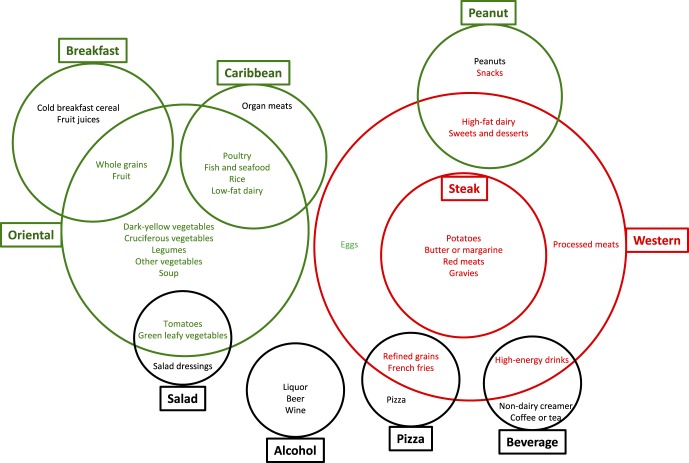
Qualitative comparative analysis is a case-oriented method that allows systematic comparison of cases (i.e., dietary patterns in our example) as configurations of set memberships (i.e., characteristic foods of dietary patterns) based on their attributes and the relationship of these attributes to particular outcomes (i.e., AMD). Qualitative comparative analysis thereby provides an alternative to conventional quantitative approaches, which are generally concerned with isolating the independent effect of one variable while controlling for the influence of others.25 Instead, QCA allows for interactions between multiple attributes and recognizes that the same outcomes may be generated by different configurations of attributes.26 From this aspect, QCA is especially suitable for dietary pattern analysis in which a pattern is composed of several characteristic foods. Qualitative comparative analysis can transform dietary patterns into configurations or combinations of characteristic foods that are referred to as “conditions” that produce an increased or decreased AMD risk. The key question that QCA, therefore, seeks to address is which conditions (or combinations of foods) are “necessary” or “sufficient” to produce the outcome (AMD). To visualize the interrelationships among the prominent dietary patterns and their associations with AMD, we performed QCA by using Boolean algebra and drawing Venn diagrams (Fig.).
Figure.
Visualization of relationship among ten predominant dietary patterns and their associations with advanced AMD in the AREDS. Green indicates healthy, red indicates unhealthy, and black represents neutral/unknown. The size of each circle is proportional to the strength of the association between the corresponding dietary pattern and advanced AMD.
Results
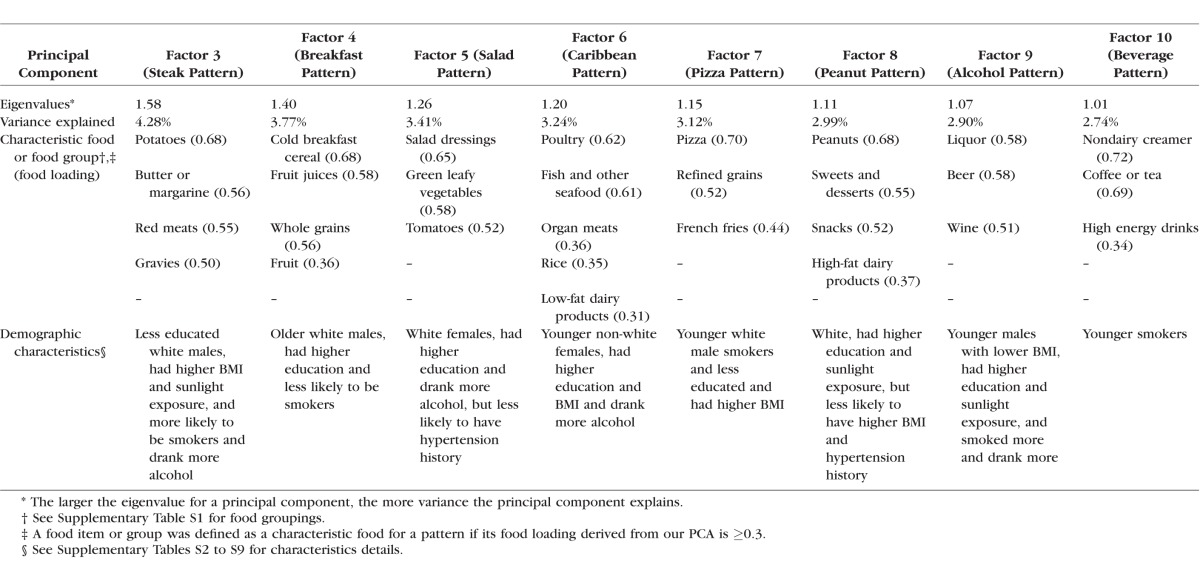
Ten prominent principal components (eigenvalue > 1) were derived from our PCA on the 37 predefined food groups (Supplementary Table S1), including two major factors with an eigenvalue of 4.01 and 3.29, as described previously,11 and eight minor factors with an eigenvalue ranging from 1.58 to 1.01 (Table 1). In this study, we focused on these eight minor factors and characterized them into eight minor dietary patterns. We named each of the eight minor dietary patterns by the major contributing food items or groups in each factor. For example, factor 3 was named “steak pattern” because it was loaded heavily with red meats, potatoes, butter or margarine, and gravies. The eight minor dietary patterns explained 26.5% of the total variance in the diet (Table 1). This is higher than the 19.7% of variance that is explained by the two major dietary patterns. Together, somewhat less than 50% of the dietary variance in the AREDS data set can be characterized into specific patterns.
Table 1.
Eigenvalues, Variances Explained, Characteristic Food Loadings, and Demographic Characteristics for the Eight Minor Dietary Patterns in the AREDS
In terms of the demographic characteristics of each dietary pattern, the AREDS participants who were more closely adherent to the steak pattern tended to be less educated, white males, had higher BMI and sunlight exposure, were more likely to be smokers, and drank more alcohol (Supplementary Table S2). The detailed demographic characteristics of the other seven minor dietary patterns can be found in Supplementary Tables S3 to S9 and are summarized in Table 1. Overall, education level is the most consistently significant demographic factor across these dietary patterns.
In our multivariate logistic analysis relating the eight minor dietary patterns to AMD, none of them were significantly associated with early AMD. However, four patterns were significantly associated with the prevalence of advanced AMD, including the steak, breakfast, Caribbean, and peanut patterns.
Compared with eyes in the first quintile group of the steak pattern score, there was an over 70% (OR = 1.73; 95% CI: 1.24 to 2.41) increased prevalence of advanced AMD for eyes in the fifth quintile group (P for trend = 0.02; Supplementary Fig. S1). Although there was a marginally significantly increased prevalence of early AMD for eyes in the fifth quintile of the steak pattern score (OR = 1.22; 95% CI: 0.99 to 1.50), the trend test was not significant (P = 0.38).
Compared with eyes in the first quintile group of the breakfast pattern score, there was a reduction of at least 26%, and up to 42%, in the prevalence of advanced AMD for eyes in each of the other four quintile groups (P for trend = 0.004; Supplementary Fig. S2).
Compared with eyes in the first quintile group of the Caribbean pattern score, there was an approximately 35% (OR = 0.64; 95% CI: 0.47 to 0.89) decreased prevalence of advanced AMD for eyes in the fifth quintile group (P for trend = 0.009; Supplementary Fig. S3).
The peanut pattern was also associated with reduced prevalence of advanced AMD. Compared with eyes in the first quintile group of the peanut pattern score, there was a decreased prevalence of at least 27%, and up to 36%, for eyes in each of the other four quintile groups (P for trend = 0.03; Supplementary Fig. S4). The other three patterns, including salad pattern, pizza pattern, and alcohol pattern, were not significantly associated with advanced AMD (Supplementary Figs. S5–S7; summary in Table 2). Although there was a significant trend (P = 0.02) in the association between the beverage pattern and advanced AMD, the significance seemed to be due to a random variation (Supplementary Fig. S8).
Table 2.
Associations Between 10 Predominant Dietary Patterns and AMD in the AREDS
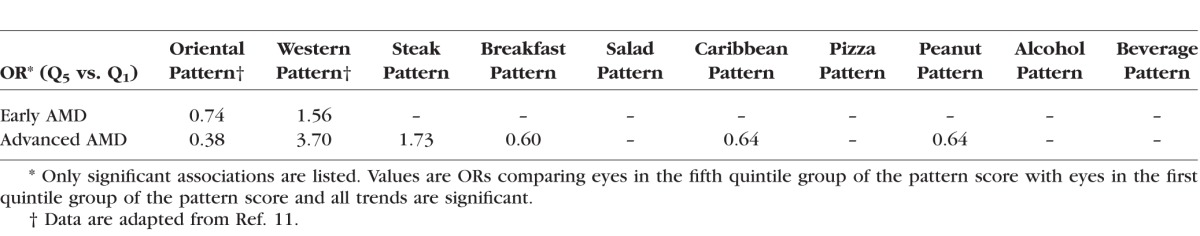
It is interesting to note that the associations with the two major patterns are stronger than those with the eight minor patterns (Table 2). We also noted that in the AREDS cohort, 34% (the two major plus the four minor patterns) of the total dietary variance was significantly associated with risk for AMD (Tables 1 and 2).
Our QCA suggested that, except for the alcohol pattern, each minor pattern is an extension or a subset of one of the two mutually exclusive major patterns (Fig.). Based on the ORs from our logistic analysis (Table 2), each dietary pattern was classified as a healthy (green in Fig.), unhealthy (red), or neutral/unknown (black) dietary pattern for advanced AMD. The size of each circle was made to be proportional to the strength of the association between the corresponding dietary pattern and advanced AMD (Fig.). It seems that harmful patterns are more strongly associated with AMD than beneficial patterns.
Discussion
Our data indicate that, in terms of characteristic foods (Table 1 and Fig.), the compositions of the two major dietary patterns are more diverse than those of the eight minor dietary patterns.11 Unlike the two major dietary patterns, both of which were significantly associated with both early and advanced AMD, four of the eight minor dietary patterns were significantly associated only with advanced AMD, and the ORs are weaker than those for the two major dietary patterns (Table 2). It seems that patterns that include more characteristic food items or groups are more strongly associated with risk for AMD. These observations allow finer interrogation of dietary patterns than prior findings.
Only two studies have used PCA to derive dietary patterns and related the patterns to AMD, including our previous study of the major dietary patterns in the AREDS11 and the Australian Melbourne Collaborative Cohort Study (MCCS).27 The MCCS identified six major factors (dietary patterns) with minimum eigenvalues of 2.7, which together accounted for 33% of the total dietary variance in the MCCS. In our study of the AREDS, there were only two major dietary patterns with eigenvalues greater than 2.7. These findings suggest that there were a larger number of major dietary patterns in the MCCS than in the AREDS.
Few studies have characterized American minor dietary patterns. In a study of participants aged ≥20 years from the third National Health and Nutrition Examination Survey (NHANES III),28 two major dietary patterns (explaining 20% of total dietary variance) and four minor dietary patterns (explaining 17% of total dietary variance) with eigenvalues >1.25 were identified. Despite the difference in age between the NHANES III and the AREDS cohorts, the two NHANES III major dietary patterns, named Western and American-healthy patterns, were similar to the two major dietary patterns that we named Western and Oriental patterns in the AREDS.11 Differences between the studies include higher intakes of tea, potatoes, and salad dressing in the NHANES III American-healthy pattern compared with the AREDS Oriental pattern.
In this study, we used the combination of PCA, logistic analysis, and QCA to visually represent our findings (Fig.) and to discover previously unidentified foods that may be associated with AMD risk in the context of the overall diet. For example, it is somewhat surprising that the peanut pattern guarded against advanced AMD because the pattern was heavily loaded with sweets, snacks, and high-fat dairy products, which are generally considered detrimental to human health. The peanut pattern was, however, most heavily loaded with peanuts, and although it has been suggested that higher peanut intake is beneficial against CVD,29,30 no study has reported the association between peanut intake and AMD risk. Studies to investigate the postulated mechanisms for the beneficial effects of higher peanut intake, such as decreasing oxidative stress,31–33 inhibition of formation of advanced glycation/lipoxidation end products due to their high levels of arginine,31–38 inhibition of inflammation,33–35 and improving endothelial function,33,36–38 are in progress and may be helpful in developing novel therapeutic and prevention strategies against AMD.
Data from our four minor dietary patterns showing no significant associations with AMD are also informative and suggest a need for additional studies into relations between food intake and risk for AMD. For example, the results from the salad pattern implied that a diet higher in green leafy vegetables and tomatoes alone may be insufficient to provide optimal protection against AMD and that salad dressing may be detrimental to the macula. Other foods of interest include pizza and coffee or tea, etc. The results from the alcohol pattern analysis corroborated prior studies that suggested that it may be preferable to evaluate the individual effects of beer, liquor, and wine on disease risk.39
The strengths of this study include use of the well-characterized AREDS cohort, standardized collection of risk factor information and photographic grading of maculopathy, as well as increased power by using eyes as the unit in our analysis. Recall and selection bias in the AREDS were unlikely to explain our findings, because exposure information was collected before outcome evaluation and our retinal classifications were performed in an independent center, by graders masked to our nutrition data. The cross-sectional nature of this study limits its strength in defining causality and our ability to make dietary recommendations. Although in the present study we evaluated diet as a whole and included all known nondietary confounders in our analysis, residual confounding could still be a concern because dietary patterns may be simply a component of lifestyle in general, which is responsible for the underlying relationship.
In summary, our data suggest that a diet consisting of various healthy foods, including previously unidentified beneficial foods, may be optimal for reducing AMD risk and that the effects of combinations of specific foods in the overall diet warrant further study.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health Grant RO1EY021826 (C-JC), US Department of Agriculture Agreements 1950-5100-060-01A, and National Institutes of Health Grants RO1EY013250 and RO1EY021212 (AT). The funding sources had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. The authors declared no conflict of interest. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views or policies of the US Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Disclosure: C.-J. Chiu, None; M.-L. Chang, None; T. Li, None; G. Gensler, None; A. Taylor, None
References
- 1. Klein R. Overview of progress in the epidemiology of age-related macular degeneration. Ophthalmic Epidemiol. 2007; 14: 184–187. [DOI] [PubMed] [Google Scholar]
- 2. Miller JW. Age-related macular degeneration revisited--piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am J Ophthalmol. 2013; 155: 1–35. [DOI] [PubMed] [Google Scholar]
- 3. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age- related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119: 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013; 309: 2005–2015. [DOI] [PubMed] [Google Scholar]
- 5. Chiu CJ,, Klein R,, Milton RC,, Gensler G,, Taylor A. Does eating particular diets alter risk of age-related macular degeneration in users of the Age-Related Eye Disease Study supplements? Br J Ophthalmol. 2009; 93: 1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiu CJ,, Hubbard LD,, Armstrong J,, et al. Dietary glycemic index and carbohydrate in relation to early age-related macular degeneration. Am J Clin Nutr. 2006; 83: 880–886. [DOI] [PubMed] [Google Scholar]
- 7. Chiu CJ,, Milton RC,, Gensler G,, Taylor A. Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the Age-Related Eye Disease Study. Am J Clin Nutr. 2007; 86: 180–188. [DOI] [PubMed] [Google Scholar]
- 8. Chiu CJ,, Milton RC,, Klein R,, Gensler G,, Taylor A. Dietary carbohydrate and progression of age-related macular degeneration, a prospective study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2007; 86: 1210–1218. [DOI] [PubMed] [Google Scholar]
- 9. Chiu CJ,, Milton RC,, Klein R,, Gensler G,, Taylor A. Dietary compound score and risk of age-related macular degeneration in the Age-Related Eye Disease Study. Ophthalmology. 2009; 116: 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moghaddam E,, Vogt JA,, Wolever TM. The effects of fat and protein on glycemic responses in nondiabetic humans vary with waist circumference, fasting plasma insulin, and dietary fiber intake. J Nutr. 2006; 136: 2506–2511. [DOI] [PubMed] [Google Scholar]
- 11. Chiu CJ,, Chang ML,, Zhang FF,, et al. The relationship of major American dietary patterns to age-related macular degeneration. Am J Ophthalmol. 2014; 158: 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ragin CC. The Comparative Method. Berkeley, CA: California University Press; 1987. [Google Scholar]
- 13. Blackman T. Can smoking cessation services be better targeted to tackle health inequalities? Evidence from a cross-sectional study. Health Educ J. 2008; 67: 91–101. [Google Scholar]
- 14. Warren J,, Wistow J,, Bambra C. Applying qualitative comparative analysis (QCA) in public health: a case study of a health improvement service for long-term incapacity benefit recipients. J Public Health (Oxf). 2014; 36: 126–133. [DOI] [PubMed] [Google Scholar]
- 15. Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications AREDS report no. 1. Control Clin Trials. 1999; 20: 573–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: age-related eye disease study report number 3. Ophthalmology. 2000; 107: 2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klein R,, Davis MD,, Magli YL,, Segal P,, Klein BEK,, Hubard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991; 98: 1128–1134. [DOI] [PubMed] [Google Scholar]
- 18. Klein BEK,, Klein R,, Linton KLP,, Magli YL,, Neidler MW. Assessment of cataracts from photographs in the Beaver Dam Eye Study. Ophthalmology. 1990; 97: 1428–1433. [DOI] [PubMed] [Google Scholar]
- 19. Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001; 132: 668–681. [DOI] [PubMed] [Google Scholar]
- 20. Kurinij N, Gensler G, Milton R; for the Age-Related Eye Disease Study (AREDS) Research Group. . Development and valuation of a food frequency questionnaire in a randomized trial of eye diseases. : International Conference on Dietary Assessment Measures. Phoenix, AZ; 1998. [Google Scholar]
- 21. Abdi H,, Williams LJ. Principal Component Analysis. New York: Wiley Interdisciplinary Reviews: Computational Statistics. 2010; 2: 433–459. [Google Scholar]
- 22. Zeger SL,, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986; 42: 121–130. [PubMed] [Google Scholar]
- 23. McCarty CA,, Lee SE,, Livingston PM,, Bissinella M,, Taylor HR. Ocular exposure to UV-B in sunlight: the Melbourne visual impairment project model. Bull World Health Organ. 1996; 74: 353–360. [PMC free article] [PubMed] [Google Scholar]
- 24. Willett W,, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986; 124: 17–27. [DOI] [PubMed] [Google Scholar]
- 25. Blackman T,, Wistow J,, Byrne D. A qualitative comparative analysis of factors associated with trends in narrowing health inequalities in England. Soc Sci Med. 2011; 72: 1965–1974. [DOI] [PubMed] [Google Scholar]
- 26. Rihoux B,, Ragin C. Introduction. : Rihoux B,, Ragin C, Configurational Comparative Method: Qualitative Comparative Analysis (QCA) and Related Techniques. Los Angeles: Sage Publications, Inc.; 2009: xvii–xxi. [Google Scholar]
- 27. Amirul Islam FM,, Chong EW,, Hodge AM,, et al. Dietary patterns and their associations with age-related macular degeneration: the melbourne collaborative cohort study. Ophthalmology. 2014; 121: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 28. Kerver JM,, Yang EJ,, Bianchi L,, Song WO. Dietary patterns associated with risk factors for cardiovascular disease in healthy US adults. Am J Clin Nutr. 2003; 78: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 29. Alper CM,, Mattes RD. Peanut consumption improves indices of cardiovascular disease risk in healthy adults. J Am Coll Nutr. 2003; 22: 133–141. [DOI] [PubMed] [Google Scholar]
- 30. Li TY,, Brennan AM,, Wedick NM,, Mantzoros C,, Rifai N,, Hu FB. Regular consumption of nuts is associated with a lower risk of cardiovascular disease in women with type 2 diabetes. J Nutr. 2009; 139: 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Servetnick DA,, Bryant D,, Wells-Knecht KJ,, Wiesenfeld PL. L-Arginine inhibits in vitro nonenzymatic glycation and advanced glycosylated end product formation of human serum albumin. Amino Acids. 1996; 11: 69–81. [DOI] [PubMed] [Google Scholar]
- 32. Fan X,, Xiaoqin L,, Potts B,, Strauch CM,, Nemet I,, Monnier VM. Topical application of L-arginine blocks advanced glycation by ascorbic acid in the lens of hSVCT2 transgenic mice. Mol Vis. 2011; 17: 2221–2227. [PMC free article] [PubMed] [Google Scholar]
- 33. Chiu CJ,, Taylor A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog Retin Eye Res. 2011; 30: 18–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uchiki T,, Weikel KA,, Jiao W,, et al. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in nondiabetics). Aging Cell. 2012; 11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weikel KA,, Fitzgerald P,, Shang F,, et al. Natural history of age-related retinal lesions that precede AMD in mice fed high or low glycemic index diets. Invest Ophthalmol Vis Sci. 2012; 53: 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang ML,, Chiu CJ,, Shang F,, Taylor A. High glucose activates ChREBP-mediated HIF-1α and VEGF expression in human RPE cells under normoxia. Adv Exp Med Biol. 2014; 801: 609–621. [DOI] [PubMed] [Google Scholar]
- 37. Palmer RM,, Ashton DS,, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988; 333: 664–666. [DOI] [PubMed] [Google Scholar]
- 38. Bhutto IA,, Baba T,, Merges C,, McLeod DS,, Lutty GA. Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD). Exp Eye Res. 2010; 90: 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klatsky AL. Alcohol, cardiovascular diseases and diabetes mellitus. Pharmacol Res. 2007; 55: 237–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.