Abstract
Purpose
EFEMP1 (fibulin-3) is mutated in Malattia Leventinese/Doyne's honeycomb retinal dystrophy (ML/DHRD), an inherited macular dystrophy similar to AMD. Both ML/DHRD and AMD are characterized by the presence of sub-RPE deposits. Efemp1 knockout mice do not develop sub-RPE deposits. This study was to test whether sub-RPE deposits can be induced in Efemp1 knockout mice by experimentally applied stress conditions that cause wild-type mice to develop sub-RPE deposits.
Methods
Efemp1 knockout and control mice at 6, 18, or 24 months old were fed with a synthetic high-fat diet (HFD). Beginning 1 month after starting the HFD, one group of mice was exposed to cigarette smoke daily for 1 month, and another group of mice was subjected to photochemical injury every other day for 2 weeks from a 488-nm argon laser. After the treatments, histologic analysis was performed to assess whether sub-RPE deposits were induced.
Results
Basal laminar deposits (BLamDs), a form of sub-RPE deposits, were observed in the 18- and 24-month-old wild-type mice but not in Efemp1 knockout mice in any age groups after exposure to HFD and cigarette smoke or laser injury.
Conclusions
Mice lacking fibulin-3 do not develop sub-RPE deposits. Environmental oxidative stressors (HFD/cigarette smoke or HFD/laser) known to cause BLamD formation in wild-type mice failed to induce BLamD formation in Efemp1 knockout mice. These results suggest that fibulin-3 is a central player in the development of BLamD, and deletion of fibulin-3 is protective against the development of BLamD.
Keywords: Efemp1, sub-RPE deposits, macular degeneration
The RPE is attached to Bruch's membrane, an acellular pentalaminar structure that is part of the choroid. Bruch's membrane is composed of the RPE basement membrane, an inner collagenous zone, an elastic zone, an outer collagenous zone, and the endothelial basement membrane of the choriocapillaris.1,2 Separating the neurosensory retina from the choroid, the RPE regulates the traffic of nutrients and waste between these compartments. The RPE is a primary site of effect in aging and diseases that cause visual loss.3,4 Deposition of basement membrane-like material and shed membranous debris beneath the basal plasma membrane of the RPE is an early feature of AMD and Malattia Leventinese/Doyne's honeycomb retinal dystrophy (ML/DHRD).5–11 Several forms of sub-RPE deposits are observed in both AMD and ML/DHRD including basal laminar deposits (BLamDs), basal linear deposits (BLinDs), and drusen.5,6,9,11 Basal laminar deposits are internal to BLinDs and lie between the plasma and basement membranes of the RPE.5,6 When confluent, BLamD is often interdigitated with membrane-like debris.5,6 However, this debris is hypothesized to be solid lipoprotein particles that are not well preserved by standard postfixation techniques, resulting in a membranous appearance under an electron microscope.12–16 The BLinD is a layer of lipid rich debris located between the RPE basement membrane and the inner collagenous zone of Bruch's membrane.12–15 These debris build up to form soft drusen.5,6,13,14 The BLinD and soft drusen are two forms of the same material.17,18
The pathogenesis of sub-RPE deposits is poorly understood. Although AMD has a complex etiology with strong environmental and genetic components,19–21 ML/DHRD is a rare monogenic disorder inherited in a dominant manner.7,8 A single mutation (R345W) in the gene EFEMP1 (fibulin-3) is responsible for all the ML/DHRD cases reported to date.22 Fibulin-3 is a basement membrane glycoprotein broadly expressed throughout the body.23–26 It is one of seven highly conserved members of the fibulin family of extracellular matrix (ECM) proteins.26 Fibulin-3 interacts with other basement membrane proteins including tissue inhibitor of metalloproteinase-3 (TIMP-3), collagen XV, and collagen XVIII/endostatin.27–29 It stimulates the expression of TIMP-1 and TIMP-3 but inhibits the expression and activities of matrix metalloprotease-2 (MMP-2), MMP-3, and MMP-9.30–34 The R345W mutation does not appear to impair fibulin-3′s function but rather renders the protein resistant to degradation.23,35,36 Efemp1 knock-in (Efemp1ki/ki) mice carrying the R345W mutation recapitulate the histopathology observed in ML/DHRD patients.35,36 These mice develop sub-RPE deposits and other defects associated with the RPE and Bruch's membrane.35,36 Like ML/DHRD patients, other than retinal abnormalities, Efemp1ki/ki mice appear to be normal.35,36 Mutant fibulin-3 accumulates in the sub-RPE deposits in ML/DHRD patients and Efemp1ki/ki mice.35 Although there is no fibulin-3 mutation found in AMD,22,23 fibulin-3 also accumulates in Bruch's membrane and sub-RPE deposits in AMD.23 Higher amounts of normal or mutant fibulin-3 may alter the basement membrane structural homeostasis through increased enzyme inhibitory activities or other means. This may in turn inhibit the turnover of basement membrane material and entrap shed microvesicle membranous debris to form sub-RPE deposits. In support of this hypothesis, Efemp1 knockout (Efemp1−/−) mice do not express fibulin-3 exhibit premature aging, hernia, and other symptoms associated with heightened ECM enzyme activities and never develop sub-RPE deposits throughout their lifespan.31,37 In contrast, wild-type mice often develop sub-RPE deposits at an advanced age.35 These findings suggest that the presence of fibulin-3 may be required for sub-RPE deposit formation.
Experimentally applied stress conditions have been shown to cause the formation of sub-RPE deposits in wild-type mouse models.38,39 Exposure of mice on a high-fat diet (HFD) to whole cigarette smoke causes BLamD formation.39 A combinational treatment of HFD and laser photochemical injury also induces BLamD-like deposits in mice that are similar to the deposits in AMD.38 Here we report the results of the study to test whether sub-RPE deposits develop in Efemp1−/− mice on a HFD after exposure to whole cigarette smoke or laser photochemical injury.
Methods
Mice
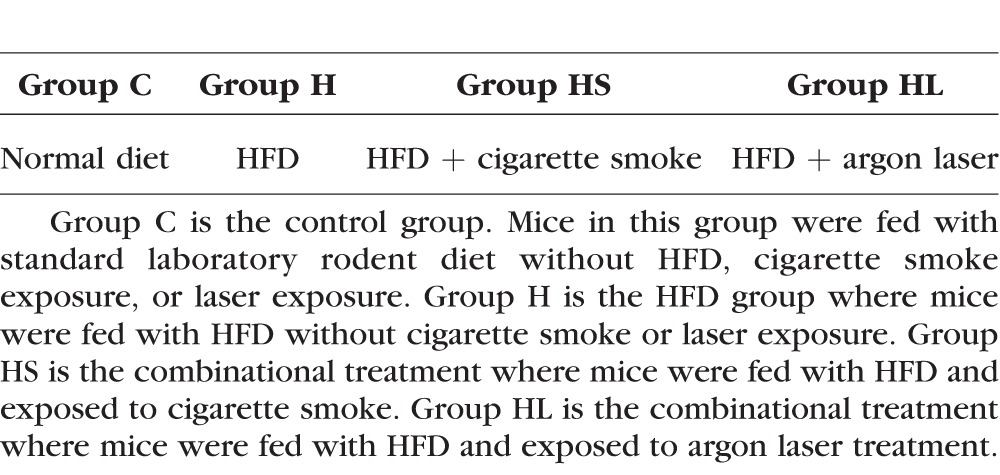
Efemp1−/− and Efemp1ki/ki mice were generated previously.35,37 Efemp1−/− mice and their wild-type littermates at 6, 18, or 24 months of age were used. Efemp1ki/ki mice at the same age groups were used as controls. Both male and female mice were used for all the genotypes, age groups, and treatment conditions. All mice were handled in accordance with the standards of humane animal care described by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, using protocols approved by the Institutional Animal Care and Use Committee of the University of Arizona or Mayo Clinic. Animals were housed under standard conditions and maintained on a 12-hour light/dark cycle with free access to water and food. Mice from three age groups and three genotypes were divided into four groups for treatments (Table 1). Group C is the control group. Mice in this group were fed with standard laboratory rodent diet without an HFD and were not exposed to cigarette smoke or argon laser. Group H mice were fed with an HFD without either cigarette smoke or laser. Group HS mice were fed an HFD and exposed to cigarette smoke. Group HL mice were fed with an HFD and exposed to argon laser treatment.
Table 1.
Treatment Groups of Mice
HFD Treatment
Groups H, HS, and HL mice were fed with a synthetic HFD (TD.88051; Envigo, Indianapolis, IN, USA) containing 15.8% fat, 1.25% cholesterol, and 0.5% sodium cholate. Group C mice were fed with standard laboratory rodent diet. After 1 month on the HFD, groups HS and HL were subjected to smoke or laser exposure while still on the HFD. Group H mice were continuously on the HFD until the experimental endpoints of groups HS and HL.
Cigarette Smoke Exposure
Beginning 1 month after starting the HFD, group HS mice were exposed to cigarette smoke daily for 1 month using a Teague Enterprises mouse smoking system (TE-10; Teague Enterprises, Davis, CA, USA). The cigarette smoke was generated from Kentucky Research 3R4F Reference Cigarettes (University of Kentucky, Lexington, KY, USA). These cigarettes are standardized and have 0.73 mg nicotine/cigarette and total particulate matter of 11.0 mg/cigarette. Forty-eight hours prior to use, the cigarettes were placed in a closed chamber at room temperature along with a solution of glycerin/water mixed in a ratio of 0.76/0.26 to establish a relative humidity of 60%. Total suspended particulate (TSP) in the exposure chamber was calculated using the system calibration. Mice were placed in cages and exposed to smoke in sealed exposure chambers. Smoke dosage was increased gradually over 5 days to allow mice an adaptation period. Mice were exposed daily for 30 days to smoke generated from 10 lit cigarettes for 2 hours each time. The TSP of the chamber was ∼200 mg/m3.
Laser Exposure
After 1 month on the HFD, group HL mice were subjected to photochemical injury every other day for 2 weeks with a 488-nm blue argon laser system Stellar-Pro 488/50 (Modu-Laser, Centerville, UT, USA). Mice were anesthetized with tribromoethanol and placed in a mouse holder in the laser path. The same eye of each mouse was exposed to 30-mJ blue laser for 5 seconds once per day, every other day for 2 weeks. This setting did not cause thermal or phototoxic injury to mouse eyes.38,40
Plasma Cholesterol Measurement
At the end of the experiments, mice were euthanized, and blood samples were taken. Plasma was obtained by centrifugation at 4°C. Total cholesterol was determined using an enzymatic colorimetric method for the quantitative determination of total cholesterol in serum (Wako Dignostics, Richmond, VA, USA).
Ocular Phenotype Analysis
Following the stress treatments, mice were euthanized by CO2 asphyxiation, and eyes were collected for histologic analysis as previously described to assess whether sub-RPE deposits developed in any of the mouse eyes.35,37 For transmission electron microscopy, the eyes were fixed overnight in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.2. After postfixation with 1% osmium tetroxide, the eyes were stained in 2% tannic acid, dehydrated in a graded series of alcohols, and embedded in epoxy resin. One limitation of this postfixation is that it is not optimal for lipid preservation. Thin sections were cut on a Reichert Ultracut microtome (Leica Microsystems, Inc., Buffalo Grove, IL, USA) and stained with uranyl acetate and lead citrate. Samples were examined and photographed using a Philips CM-12 electron microscope equipped with an AMT CCD camera (Advanced Microscopy Techniques Corp., Danvers, MA, USA).
Basal laminar deposit severity and frequency in mice were graded based on a semiquantitative grading system introduced by Cousins et al.40 Mild BLamD referred to the presence of any discrete focal nodule of homogenous deposit between the RPE cell membrane and its basement membrane in at least one micrograph (of at least 10) within a section from an individual specimen. Moderate BLamD was defined as the presence of continuous deposit underlying two or more RPE cells, presence of banded structures, or deposit thickness ≥20% of RPE cell cross-sectional height in at least three micrographs with a section from an individual specimen. Severe BLamD contained continuous sheets of deposits underlying 10 or more RPE cells and deposit thickness >30% of RPE cell height in at least five micrographs within a section from an individual specimen.
Results
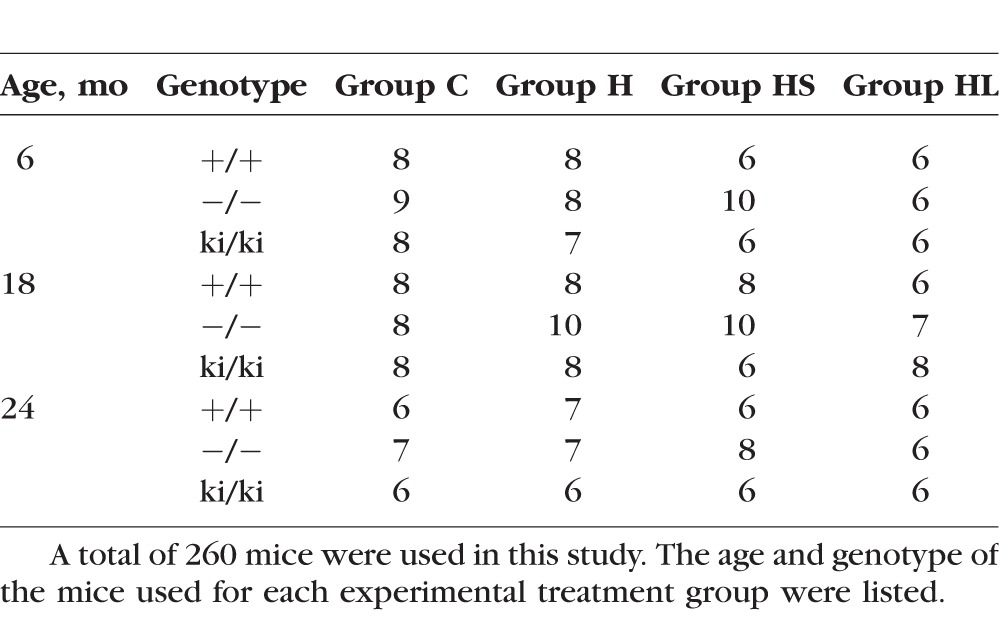
Mice at 6, 18, or 24 months of age from three different genotypes were divided into four groups to be fed with a normal diet or HFD with or without exposure to cigarette smoke or argon laser (Tables 1, 2): group C were fed with standard laboratory rodent diet, group H were fed with an HFD, group HS were fed with an HFD and exposed to cigarette smoke, and group HL were fed with an HFD and exposed to argon laser treatment. Mice fed with an HFD became noticeably heavier at the end of 1 month. The general appearance of the mice was not otherwise affected by the HFD, cigarette smoke, or laser treatment to their eyes. No obvious difference was observed between male and female mice. The morphology of the eyes and the retina appeared to be well preserved. We did not observe obvious differences in choroid and the outer retina between treatment and control groups.
Table 2.
Age and Genotype of the Mice Used in All Experiments
Total Plasma Cholesterol Level
Mice in the three groups (H, HS, HL) fed with an HFD had more than twofold higher total plasma cholesterol levels than the control group mice (C) with corresponding ages fed with a standard rodent diet (Fig. 1). Mice fed with HFD and exposed to cigarette smoke (HS) or argon laser (HL) had similar levels of cholesterol to mice fed with HFD (H) without smoke or laser treatment (Fig. 1). There was no significant difference in total plasma cholesterol levels in the same group of mice with different ages.
Figure 1.
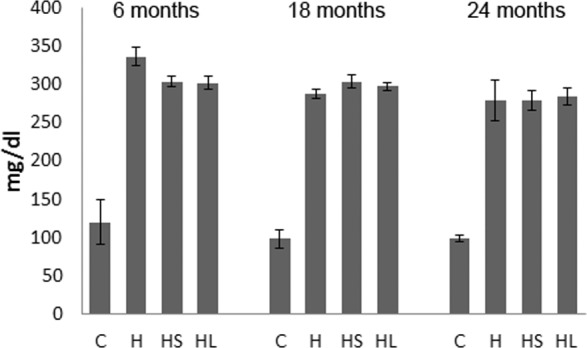
Plasma cholesterol levels in treated mice. Mice fed with HFD (H) in all age groups have more than twofold higher plasma cholesterol levels than the corresponding control group mice fed with a standard rodent diet (C). Mice treated with HFD and cigarette smoke (HS) or HFD and argon laser (HL) had similar levels of cholesterol to mice fed with HFD (H) without smoke or laser treatment.
No Effects on Sub-RPE Deposits by HFD Alone
In the control group C, BLamD was not observed in 6- or 18-month-old wild-type Efemp1+/+ mice (Fig. 2). No BLamD was observed in Efemp1−/− mice at any age (Fig. 2). Consistent with our previous observation, isolated small BLamDs were observed in 24-month-old Efemp1+/+ mice.35 Basal laminar deposits were also observed in Efemp1ki/ki mice at 6, 18, and 24 months of age (Fig. 2).
Figure 2.
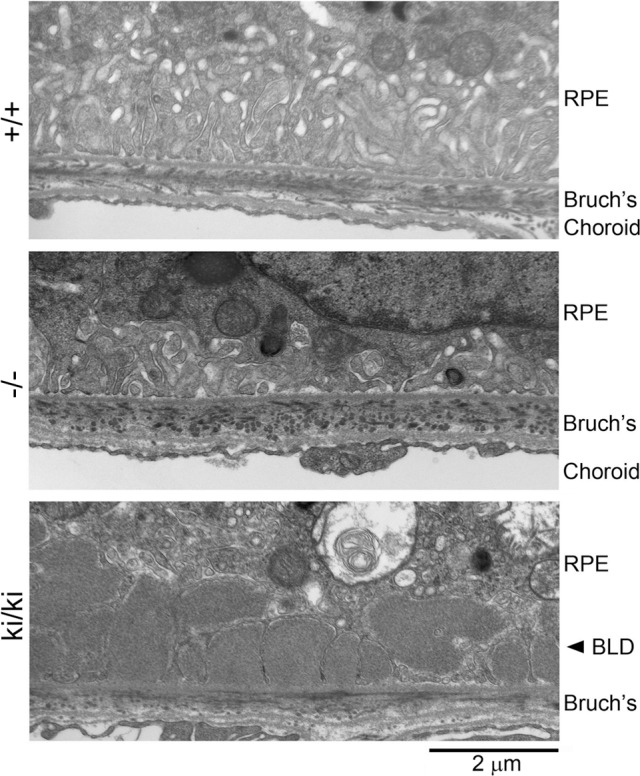
Electron micrographs showing basal RPE, Bruch's membrane, and choroid of 18-month-old wild-type (+/+), Efemp1 knockout (−/−), and Efemp1 knock-in (ki/ki) mice without any treatment. Bruch's, Bruch's membrane.
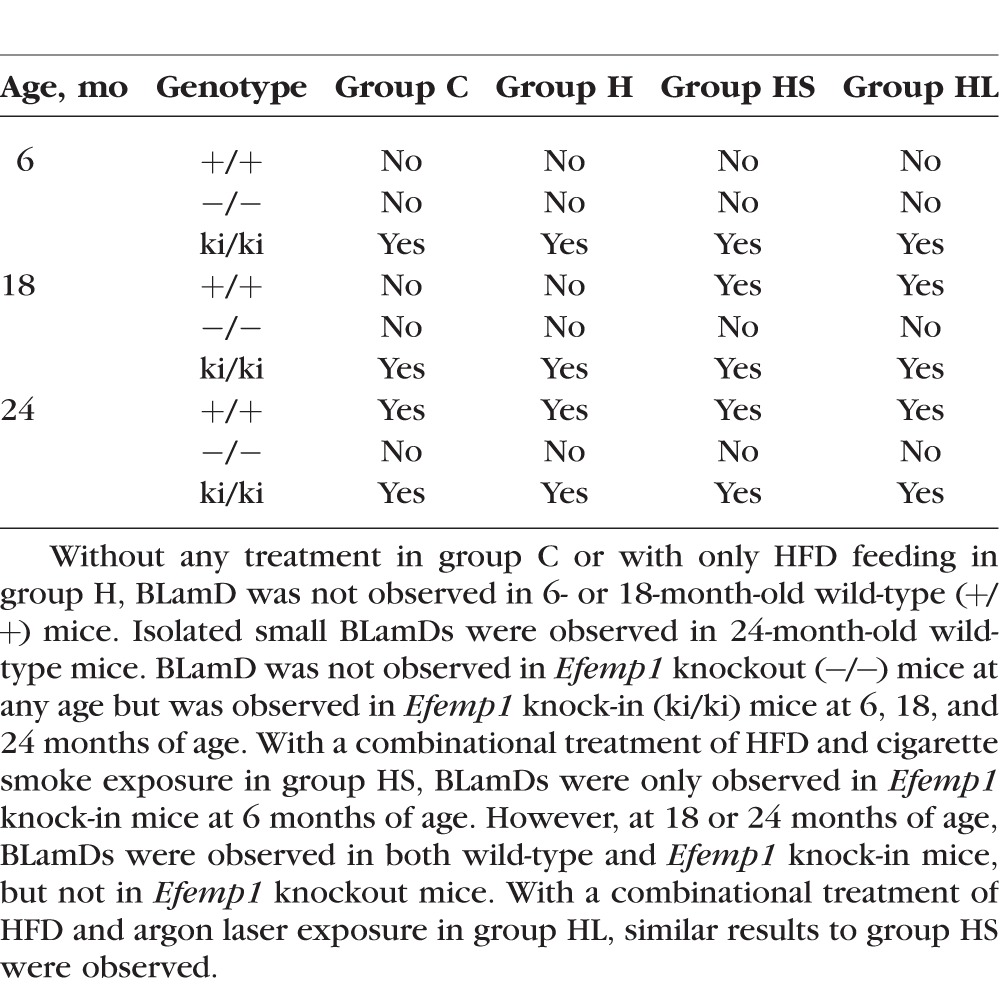
With only an HFD feeding in group H, similar to those in group C, BLamD was not observed in 6- or 18-month-old Efemp1+/+ mice or in Efemp1−/− mice at any age. Basal laminar deposits were observed in 24-month-old Efemp1+/+ mice and in Efemp1ki/ki mice at all three ages (Table 3).
Table 3.
Effects on BLamDs in Mice After HFD, HFD/Smoke, or HFD/Laser Treatment
Effects of HFD in Combination With Cigarette Smoke on Sub-RPE Deposits
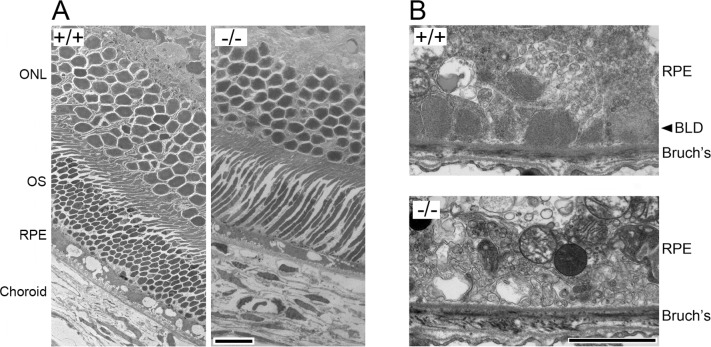
With a combinational treatment of the HFD and cigarette smoke exposure in group HS, BLamDs were observed in Efemp1+/+ mice at 18 and 24 months of age but not at 6 months of age (Table 3). We observed moderate BLamDs in Efemp1+/+ mice at 18 months of age (Fig. 3B) and severe BLamDs at 24 months of age. In Efemp1ki/ki mice, BLamDs were observed at all three ages (6, 18, and 24 months). The severity of BLamDs in treated Efemp1ki/ki mice was similar to that of untreated Efemp1ki/ki mice at the same age.35 The combinational treatment of HFD and smoke exposure did not appear to augment BLamDs in Efemp1ki/ki mice. In contrast, no BLamD was observed in Efemp1−/− mice at any of the three age groups under the same combinational treatment. No other form of sub-RPE deposits was observed in these mice either (Fig. 3B). This finding indicates that Efemp1−/− mice are resistant to these stress conditions in the development of BLamDs.
Figure 3.
Electron micrographs showing the retina (A) and basal RPE and Bruch's membrane area (B) of 18-month-old wild-type (+/+) and Efemp1 knockout (−/−) mice after a combinational treatment of HFD and smoke exposure. ONL, outer nuclear layer, OS, outer segment, Bruch's, Bruch's membrane. Scale bars denote 10 (A) and 2 μm (B).
Effects of HFD in Combination With Argon Laser on Sub-RPE Deposits
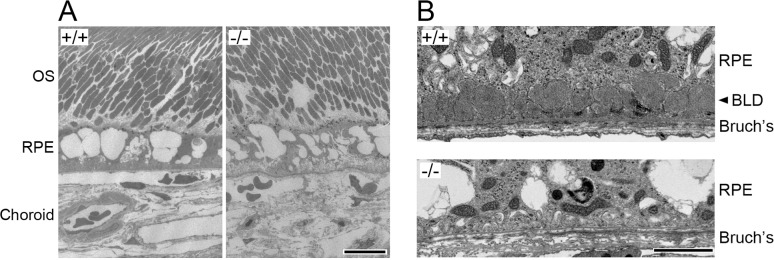
With a combinational treatment of HFD and argon laser exposure of the eyes in group HL, BLamDs were also observed in Efemp1+/+ mice at 18 and 24 months of age, but not at 6 months of age (Table 3). Similar to those treated with HFD and smoke, moderate severe and severe BLamDs were observed in group HL mice at both 18 and 24 months of age (Fig. 4B). In Efemp1ki/ki mice BLamDs were observed at all three ages. The combinational treatment of HFD and laser exposure did not appear to augment BLamDs in Efemp1ki/ki mice. However, no BLamD or any other form of sub-RPE deposits was observed in Efemp1−/− mice at any age under the combinational treatment of HFD and laser (Fig. 4B). This finding indicates that Efemp1−/− mice are also resistant to HFD and argon laser treatment in BLamD formation.
Figure 4.
Electron micrographs showing the retina (A) and basal RPE and Bruch's membrane area of 18-month-old wild-type (+/+) and Efemp1 knockout (−/−) mice after a combinational treatment of HFD and laser exposure. OS, outer segment, Bruch's, Bruch's membrane. Scale bars denote 10 (A) and 2 μm (B).
Effects of HFD in Combination With Smoke or Laser on RPE Morphology
The RPE appeared to be relatively intact in 6-month-old mice regardless of treatment conditions or Efemp1 genotypes. The only difference was the presence of large vacuoles in the RPE cells treated with either HFD/smoke or HFD/laser in mice at this age. However, in both 18- and 24-month-old mice treated with HFD/smoke (Fig. 3A) or HFD/laser (Fig. 4A), regardless of their genotype, the RPE appeared highly stressed, nonuniform, and vacuolated. Although the exact nature of these vacuoles is unknown, they tend to be associated with stressed RPE and may represent an accumulation of phagolysosomes, autophagosomes, and/or autolysosomes caused by stress that inhibits lysosome-mediated turnover of waste materials. Similar to the Efemp1ki/ki mice,35 a loss of RPE basal infoldings near the deposits was observed in 18- and 24-month-old Efemp1+/+ mice treated with HFD/smoke or HFD/laser.
Discussion
In a previous study, we noted that Efemp1−/− mice (which do not express fibulin-3) do not develop BLamDs as they age.37 The results of this study show that no sub-RPE deposit is induced in Efemp1−/− mice even after being fed with a HFD and exposed to cigarette smoke or argon laser photochemical injury. The same stress conditions have induced BLamDs in older wild-type (Efemp1+/+) mice. This demonstrates for the first time that fibulin-3 is essential in the development of sub-RPE deposits, particularly BLamD in mice. Epidemiologic studies have established cigarette smoke is a strong risk factor for AMD.41–43 Photochemical injury also causes BLamD in humans.44 Therefore, it is highly conceivable that fibulin-3 is essential in sub-RPE deposit formation in humans.
How fibulin-3 is involved in sub-RPE deposit formation is not completely understood. Based on its known distribution and function, there are some possible explanations. Fibulin-3 is physically located in basement membrane where BLamD occurs and interacts with TIMP-3 and collagen XVIII, two other known basement membrane components involved in BLamD and other sub-RPE deposit formation.23,26–28 Fibulin-3 stimulates the expression of TIMPs but inhibits the expression of MMPs.30–33 It appears that fibulin-3 helps to maintain basement membrane structural integrity by controlling ECM turnover activities. In Efemp1ki/ki mice and human patients with ML/DHRD or AMD, fibulin-3 accumulates beneath the RPE.23,35 The mutated fibulin-3 protein containing the R345W mutation does not have impaired function and is more resistant to degradation.23,35,36 Higher amounts of fibulin-3 may recruit other components and reduce the signal for turnover of basement membrane and other materials, alter structural homeostasis, activate complement system, and lead to sub-RPE deposit formation. In wild-type mice where a normal amount of fibulin-3 is present, BLamDs do not develop until mice reach an advanced age (>2 years),35 but with extra oxidative stresses caused by the combinational treatment of HFD and cigarette smoke or HFD and argon laser, BLamDs develop in younger animals, presumably when damaged materials exceed the capacity of a fibulin-3–controlled turnover system. In Efemp1−/− mice where fibulin-3 is absent, The RPE basement membrane turnover process is active and damaged materials associated with the basement membrane are promptly removed. Even when the burden increases under extra stress stimulation of HFD/cigarette smoke or HFD/laser, the turnover system can still handle it.
Any of the HFD, cigarette smoke, or argon laser photochemical injury in isolation does not appear to be sufficient to induce BLamD or other forms of sub-RPE deposits in wild-type mice.38,39 It is not known how the interaction between HFD and cigarette smoke or HFD and argon laser causes BLamD. Although our study shows clearly that deletion of fibulin-3 is protective against the development of BLamDs induced by HFD/cigarette smoke or HFD/argon laser in mice, we do not know whether deletion of fibulin-3 is protective against BLamDs caused by other factors. For example, knockout mice lacking collagen XVIII develop BLamDs.45 Transgenic mice expressing an enzymatically inactive form of cathepsin D in RPE cells also develop BLamDs.46 If deleting fibulin-3 prevents these mice from developing sub-RPE deposits, it would add evidence that fibulin-3 is a central player in sub-RPE deposit formation. Sub-RPE deposits are a consistent early finding in ML/DHRD and dry AMD. Currently, there is no treatment for these diseases. Our study suggests that fibulin-3 is a promising target for developing treatments for these diseases.
Acknowledgments
The authors thank Samuel D. Cross for technical assistance, Gina Zhang for assistance with electron microscopy, and Jim Schwiegerling for assistance with the argon laser system.
Supported by National Institutes of Health Grants EY13847 (LYM), EY13160 (ADM), and EY21153 (ADM) and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology at the Mayo Clinic in Rochester, Minnesota.
Disclosure: J.B. Stanton, None; A.D. Marmorstein, None; Y. Zhang, None; L.Y. Marmorstein, None
References
- 1. Booij JC,, Baas DC,, Beisekeeva J,, Gorgels TG,, Bergen AA. The dynamic nature of Bruch's membrane. Progr Retinal Eye Res. 2010; 29: 1–18. [DOI] [PubMed] [Google Scholar]
- 2. Guyer DR,, Schachat, AP,, Green WR. The choroids: structural consideration. : Ryan SJ, Retina. St. Louis, MO: Mosby; 2001: 29. [Google Scholar]
- 3. Fuhrmann S,, Zou C,, Levine EM. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp Eye Res. 2014; 123: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005; 85: 845–881. [DOI] [PubMed] [Google Scholar]
- 5. Sarks S,, Cherepanoff S,, Killingsworth M,, Sarks J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007; 48: 968–977. [DOI] [PubMed] [Google Scholar]
- 6. Sarks SH,, Sarks JP. Age-related maculopathy: nonneovascular age-related macular degeneration and the evolution of geographic atrophy. : Ryan SJ, , Retina. St. Louis, MO: Mosby; 2001: 1064. [Google Scholar]
- 7. Piguet B,, Haimovici R,, Bird AC. Dominantly inherited drusen represent more than one disorder: a historical review. Eye. 1995; 9 Pt 1: 34–41. [DOI] [PubMed] [Google Scholar]
- 8. Marmorstein L. Association of EFEMP1 with malattia leventinese and age-related macular degeneration: a mini-review. Ophthalmic Genet. 2004; 25: 219–226. [DOI] [PubMed] [Google Scholar]
- 9. Dusek J,, Streicher T,, Schmidt K. Hereditary drusen of Bruch's membrane. II: studies of semi-thin sections and electron microscopy results [in German]. Klin Monatsbl Augenheilkd. 1982; 181: 79–83. [PubMed] [Google Scholar]
- 10. Streicher T,, Schmidt K,, Dusek J. Hereditary drusen of Bruch's membrane. I. Clinical and light microscopical study [in German]. Klin Monatsbl Augenheilkd. 1982; 181: 27–31. [DOI] [PubMed] [Google Scholar]
- 11. Collins T. A pathological report on a case of Doyne's choroiditis (‘honeycomb’ or ‘family choroiditis’). Ophthalmoscope. 1913; 11: 537–538. [Google Scholar]
- 12. Milam AH,, Curcio CA,, Cideciyan AV,, et al. Dominant late-onset retinal degeneration with regional variation of sub-retinal pigment epithelium deposits, retinal function, and photoreceptor degeneration. Ophthalmology. 2000; 107: 2256–2266. [DOI] [PubMed] [Google Scholar]
- 13. Curcio CA,, Presley JB,, Millican CL,, Medeiros NE. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp Eye Res. 2005; 80: 761–775. [DOI] [PubMed] [Google Scholar]
- 14. Curcio CA,, Johnson M,, Huang JD,, Rudolf M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Progr Retinal Eye Res. 2009; 28: 393–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curcio CA,, Johnson M,, Rudolf M,, Huang JD. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011; 95: 1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang M,, Esteve-Rudd J,, Lopes VS,, et al. Microtubule motors transport phagosomes in the RPE, and lack of KLC1 leads to AMD-like pathogenesis. J Cell Biol. 2015; 210: 595–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curcio CA,, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999; 117: 329–339. [DOI] [PubMed] [Google Scholar]
- 18. Sarks JP,, Sarks SH,, Killingsworth MC. Evolution of soft drusen in age-related macular degeneration. Eye. 1994; 8 Pt 3: 269–283. [DOI] [PubMed] [Google Scholar]
- 19. Bressler NM,, Bressler SB,, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988; 32: 375–413. [DOI] [PubMed] [Google Scholar]
- 20. de Jong PT. Age-related macular degeneration. New Engl J Med. 2006; 355: 1474–1485. [DOI] [PubMed] [Google Scholar]
- 21. Jager RD,, Mieler WF,, Miller JW. Age-related macular degeneration. New Engl J Med. 2008; 358: 2606–2617. [DOI] [PubMed] [Google Scholar]
- 22. Stone EM,, Lotery AJ,, Munier FL,, et al. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nat Genet. 1999; 22: 199–202. [DOI] [PubMed] [Google Scholar]
- 23. Marmorstein LY,, Munier FL,, Arsenijevic Y,, et al. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002; 99: 13067–13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ehlermann J,, Weber S,, Pfisterer P,, Schorle H. Cloning, expression and characterization of the murine Efemp1, a gene mutated in Doyne-Honeycomb retinal dystrophy. Gene Expr Patterns. 2003; 3: 441–447. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi N,, Kostka G,, Garbe JH,, et al. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J Biol Chem. 2007; 282: 11805–11816. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y,, Marmorstein LY. Focus on molecules: fibulin-3 (EFEMP1). Exp Eye Res. 2010; 90: 374–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klenotic PA,, Munier FL,, Marmorstein LY,, Anand-Apte B. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1). Implications for macular degenerations. J Biol Chem. 2004; 279: 30469–30473. [DOI] [PubMed] [Google Scholar]
- 28. Faye C,, Chautard E,, Olsen BR,, Ricard-Blum S. The first draft of the endostatin interaction network. J Biol Chem. 2009; 284: 22041–22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jorgenson E,, Makki N,, Shen L,, et al. A genome-wide association study identifies four novel susceptibility loci underlying inguinal hernia. Nat Commun. 2015; 6: 10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Albig AR,, Neil JR,, Schiemann WP. Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res. 2006; 66: 2621–2629. [DOI] [PubMed] [Google Scholar]
- 31. Rahn DD,, Acevedo JF,, Roshanravan S,, et al. Failure of pelvic organ support in mice deficient in fibulin-3. Am J Pathol. 2009; 174: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim EJ,, Lee SY,, Woo MK,, et al. Fibulin-3 promoter methylation alters the invasive behavior of non-small cell lung cancer cell lines via MMP-7 and MMP-2 regulation. Int J Oncol. 2012; 40: 402–408. [DOI] [PubMed] [Google Scholar]
- 33. Wang Z,, Cao CJ,, Huang LL,, et al. EFEMP1 promotes the migration and invasion of osteosarcoma via MMP-2 with induction by AEG-1 via NF-kappaB signaling pathway. Oncotarget. 2015; 6: 14191–14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernandez-Godino R,, Garland DL,, Pierce EA. A local complement response by RPE causes early-stage macular degeneration. Hum Mol Genet. 2015; 24: 5555–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marmorstein LY,, McLaughlin PJ,, Peachey NS,, Sasaki T,, Marmorstein AD. Formation and progression of sub-retinal pigment epithelium deposits in Efemp1 mutation knock-in mice: a model for the early pathogenic course of macular degeneration. Hum Mol Genet. 2007; 16: 2423–2432. [DOI] [PubMed] [Google Scholar]
- 36. Fu L,, Garland D,, Yang Z,, et al. The R345W mutation in EFEMP1 is pathogenic and causes AMD-like deposits in mice. Hum Mol Genet. 2007; 16: 2411–2422. [DOI] [PubMed] [Google Scholar]
- 37. McLaughlin PJ,, Bakall B,, Choi J,, et al. Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum Mol Genet. 2007; 16: 3059–3070. [DOI] [PubMed] [Google Scholar]
- 38. Dithmar S,, Sharara NA,, Curcio CA,, et al. Murine high-fat diet and laser photochemical model of basal deposits in Bruch membrane. Arch Ophthalmol. 2001; 119: 1643–1649. [DOI] [PubMed] [Google Scholar]
- 39. Espinosa-Heidmann DG,, Suner IJ,, Catanuto P,, Hernandez EP,, Marin-Castano ME,, Cousins SW. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest Ophthalmol Vis Sci. 2006; 47: 729–737. [DOI] [PubMed] [Google Scholar]
- 40. Cousins SW,, Espinosa-Heidmann DG,, Alexandridou A,, Sall J,, Dubovy S,, Csaky K. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp Eye Res. 2002; 75: 543–553. [DOI] [PubMed] [Google Scholar]
- 41. Smith W,, Assink J,, Klein R,, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001; 108: 697–704. [DOI] [PubMed] [Google Scholar]
- 42. Seddon JM,, George S,, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006; 124: 995–1001. [DOI] [PubMed] [Google Scholar]
- 43. Khan JC,, Thurlby DA,, Shahid H,, et al. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006; 90: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kramer T,, Brown R,, Lynch M,, et al. Molteno implants and operating microscope-induced retinal phototoxicity. A clinicopathologic report. Arch Ophthalmol. 1991; 109: 379–383. [DOI] [PubMed] [Google Scholar]
- 45. Marneros AG,, Keene DR,, Hansen U,, et al. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 2004; 23: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rakoczy PE,, Zhang D,, Robertson T,, et al. Progressive age-related changes similar to age-related macular degeneration in a transgenic mouse model. Am J Pathol. 2002; 161: 1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]