Abstract
Delivering therapeutics to the central nervous system (CNS) and brain-tumor has been a major challenge. The current standard treatment approaches for the brain-tumor comprise of surgical resection followed by immunotherapy, radiotherapy, and chemotherapy. However, the current treatments are limited in providing significant benefits to the patients and despite recent technological advancements; brain-tumor is still challenging to treat. Brain-tumor therapy is limited by the lack of effective and targeted strategies to deliver chemotherapeutic agents across the blood-brain barrier (BBB). The BBB is the main obstacle that must be overcome to allow compounds to reach their targets in the brain. Recent advances have boosted the nanotherapeutic approaches in providing an attractive strategy in improving the drug delivery across the BBB and into the CNS. Compared to conventional formulations, nanoformulations offer significant advantages in CNS drug delivery approaches. Considering the above facts, in this review, the physiological/anatomical features of the brain-tumor and the BBB are briefly discussed. The drug transport mechanisms at the BBB are outlined. The approaches to deliver chemotherapeutic drugs across the CNS into the brain-tumor using nanocarriers are summarized. In addition, the challenges that need to be addressed in nanotherapeutic approaches for their enhanced clinical application in brain-tumor therapy are discussed.
Keywords: brain-tumor, glioma, CNS, blood-brain barrier, drug transport, nanoformulation, nanocarrier, cell-mediated drug delivery
Introduction
According to the National Brain Tumor Society, in 2016, approximately 688,096 Americans are living with a brain-tumor (http://braintumor.org/brain-tumor-information/brain-tumor-facts/). It is estimated that approximately 78,000 will be diagnosed, and 16,616 people will die from malignant brain-tumors in 2016. Brain-tumor is challenging to cure and therapy is limited by the lack of effective methods to deliver chemotherapeutic drugs across the central nervous system (CNS) barriers. The current treatment consists of surgical resection, followed by immunotherapy, radiotherapy, and chemotherapy (Pourgholi et al., 2016). However, the benefits of current treatment are quite insignificant to patients. Based on the aforementioned facts, in this review, approaches in CNS drug delivery to the brain-tumor using nanocarrier systems are briefly discussed.
Brain-Tumor
Brain-tumor is an abnormal growth of tissue in the CNS that can interrupt the proper brain function. Brain-tumors can be classified according to the types of cells involved or by the tumor location in the brain. Brain-tumors, those originating from glial cells are called gliomas (Gutkin et al., 2016; Meng et al., 2016). The three subtypes of cells from which gliomas arise are astrocytes, oligodendrocytes, and ependymal cells generating astrocytoma, oligodendroglioma, and ependymoma, respectively (Gutkin et al., 2016; Meng et al., 2016).
World Health Organization (WHO) classifies gliomas within four grades (grade I to grade IV) (Louis et al., 2016). Low-grade tumors (grade I/II) are generally treated with careful monitoring and/or surgery. Higher-grade tumors (grade III/IV) (malignant gliomas) are difficult to treat and additional approaches such as radiotherapy, chemotherapy or targeted therapy are required. Malignant gliomas such as glioblastoma multiforme (GBM) are often difficult to treat because of their invasiveness and resistance to surgical procedures as well as chemo-/radiotherapy, poor prognosis, dismal survival rates, increased rate of angiogenesis, resistance to apoptosis, and limited delivery of chemotherapeutics across the CNS (Gutkin et al., 2016; Meng et al., 2016; Pourgholi et al., 2016). Under the standard treatment regimen (resection followed by radiotherapy and/or chemotherapy), the average survival rate for malignant brain-tumor patients is ~34.4% whereas, for GBM, the five-year relative survival rate is ~5.1% (http://braintumor.org/brain-tumor-information/brain-tumor-facts/).
Blood-Brain Barrier (BBB) and Drug Transport Mechanisms
The ineffectiveness of the treatment approaches for the brain-tumor is mainly because of the lack of an effective and targeted drug delivery to the diseased site. The CNS has several barriers to protect itself from invading pathogens, neurotoxic molecules, foreign substances, and circulating blood cells. Among several barriers in the CNS, BBB is the most significant (Gutkin et al., 2016; Meng et al., 2016). Compared with other types of tumors, gliomas are more challenging to treat due to the protection of the BBB. The selective permeability of the BBB is characterized by the absence of fenestrations, presence of tight junctions, and metabolic barriers (enzymes and transport systems) (Gutkin et al., 2016; Meng et al., 2016). The specific and selective molecular permeability of BBB is attributable to special features of the brain cells (presence of a higher number of mitochondria), and the tighter brain capillary endothelial cells compared to normal cells (Meng et al., 2016). These all lower the bioavailability of chemotherapeutic drugs in the diseased tissue. In addition, the expression of several ion channels as well as influx/efflux transporters at the BBB plays a critical role in restricting the transport and permeability of several molecules including the chemotherapeutic drugs (Gutkin et al., 2016; Meng et al., 2016).
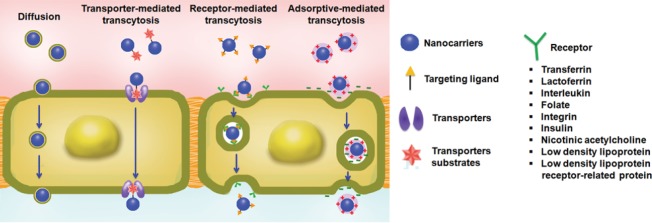
The transport mechanisms at the BBB can be subdivided into three major categories: passive diffusion; transporters mediated transcytosis (TMT); and fluid phase transport by vesicles (Meng et al., 2016; Zhang et al., 2016) (Figure 1). The BBB allows the passage of water, oxygen, carbon dioxide, and lipid-soluble small molecules by passive diffusion, a concentration gradient dependent process. TMT is substrate selective in which carrier facilitates the transport of molecules by the influx and efflux transporters, and carrying substances in and out of the CNS, respectively (Meng et al., 2016). A large number of chemotherapeutic drugs are removed out of the CNS due to the efflux transporters (P-glycoprotein, multidrug resistance-related proteins, and breast cancer resistance protein) expressed at the apical side of the BBB. Fluid phase transport by vesicles allows the transport of large molecule drugs into the CNS and includes two mechanisms; adsorptive mediated transcytosis (AMT) and receptor-mediated endocytosis (RME) (Meng et al., 2016). AMT is mediated by electrostatic interactions between the positively charged delivery systems and the negatively charged brain capillary endothelial cell membrane surface regions. The mechanism of RME is based on the interaction of targeting molecules on drug delivery systems with brain receptors such as transferrin, low-density lipoprotein, and insulin, etc. (Meng et al., 2016). Most macromolecules are delivered to brain cells across the BBB via these specific receptors. In the CNS targeted drug delivery, the RMT pathway has been actively used because of the higher specificity it can provide.
Figure 1.
Nanocarriers-based strategies for crossing the blood-brain barrier (BBB).
The Figure is reproduced with permission from Meng et al., 2016.
Treatment Strategies for the Brain-Tumor
The current standard treatment for brain-tumors usually consists of surgical resection, accompanied by immuno/radio/chemotherapies (Pourgholi et al., 2016). Unfortunately, the benefits resulting from current treatment are limited or insignificant to patients and therefore malignant gliomas remain incurable. In addition, these approaches have several limitations and challenges. The challenges for immunotherapy include the monitoring and assessment of clinical outcomes, and generation of several side-effects. Radiotherapy is the most frequent treatment option for GBM patients. However, response to radiotherapy depends on tumor size and large size tumors poorly respond to radiation compared to smaller size tumors (Pourgholi et al., 2016). In addition, radiotherapy has undesirable side-effects, including damaging of the epithelial surfaces, mouth/throat/gastrointestinal ulcers, swelling, infertility, fibrosis, hair loss, lymphedema, and heart disease (Pourgholi et al., 2016). Chemotherapy approach involves the drugs to destroy tumor cells, generally by stopping the tumor cells ability to mature and divide. However, severe side-effects, including nerve damage, nausea, hair loss, vomiting, infertility, diarrhea, skin rashes, and insomnia are major limitations (Pourgholi et al., 2016).
In order to efficiently deliver chemotherapeutic drugs into the brain, several conventional strategies have been applied, including the disruption of the CNS barriers using biochemical agents, ultrasound and radiation (Azad et al., 2015; Meng et al., 2016). Several invasive methods such as intra-cerebrospinal fluid, intra-thecal, and intra-tumoral injections are also used (Azad et al., 2015; Meng et al., 2016). However, these methods suffer from severe neurotoxicity and neuropathological consequences. The limitation of conventional drug delivery strategies to brain-tumor has led to the development of novel concepts of creating a transient opening or disruption of the BBB integrity by breaking down the tight junctions. Hyperthermia is one of the approaches which is based on generation of heat at the target site (Pourgholi et al., 2016). It activates several intra- and extracellular degradation mechanisms such as protein misfolding, aggregation, and increasing apoptosis. However, the efficiency of hyperthermia treatment significantly depends on the temperature at the targeted site, and the duration of exposure (Pourgholi et al., 2016). Overall, the limitations and challenges of current therapeutic approaches to the brain-tumor highlight the need for effective strategies which can prolong the survival rate of patients while enhancing the quality of life.
Nanotherapeutic Approaches to Brain-Tumor Targeted Drug Delivery
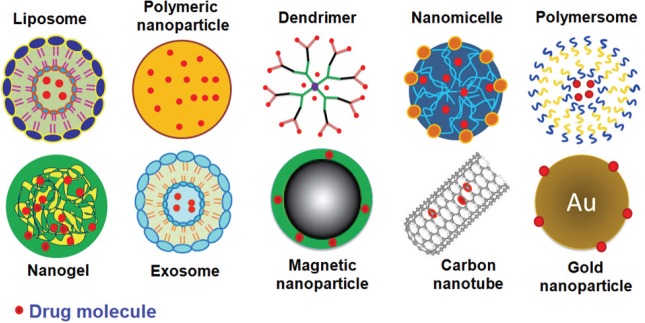
In recent years, the enhanced understanding of mechanisms of drug transport into the brain has boosted the development of nanocarriers with the ability to cross the BBB. Nanocarriers are colloidal systems in the nano size range and have been considered as one of the most promising approaches in brain-tumor targeted therapy due to their unique properties (Gutkin et al., 2016; Mangraviti et al., 2016; Meng et al., 2016). Compared to conventional drug formulations, nanoformulations offer significant advantages such as drug protection from in vivo and in vitro degradation, increase the drug solubility and provide high drug loading, targeted drug delivery by incorporation of ligand molecules, versatile surface modification chemistry, homogenous size distribution, and flexibility in providing controlled or stimuli-responsive drug release behavior (Agrahari et al., 2016a; Meng et al., 2016). Due to these advantages, several nanocarrier systems such as polymeric nanoparticles (NPs), liposomes, dendrimers, nanomicelles, polymerosomes, gold NPs, nanogels, quantum dots, and magnetic NPs, etc., are explored in brain-tumor targeting approaches (Gutkin et al., 2016; Mangraviti et al., 2016; Meng et al., 2016; Pourgholi et al., 2016). A schematic representation of these nanocarriers is shown by Figure 2.
Figure 2.
Nanocarrier systems for brain-tumor therapeutic approaches.
The Figure is reproduced with permission from Meng et al., 2016.
The key attributes those govern the in vivo characteristics of nanocarriers are size, shape, surface charge, and the presence of targeting ligands on their surfaces (Saraiva et al., 2016). Although, nanocarriers provide promising solutions for the treatment of brain-tumors, there are numerous challenges that need to be addressed. These challenges include their low therapeutic efficiency, specificity of targeting ligands to deliver the nanocarriers at the diseased site, and the release of drugs from nanocarriers in a controlled and/or stimuli-responsive behavior. It is important to fabricate nanocarriers that release the drugs only at the targeted brain-tumor site instead of non-targeted sites. There are a number of intrinsic (pH, enzymes, temperature, oxidative stress, etc.) and extrinsic (magnetic field, heat, and light, etc.) signals those can be applied as a stimulus to control the drug release in brain-tumor treatment (Fang et al., 2016; Meng et al., 2016). Stimuli-responsive formulations are attractive since different drug concentrations may be needed at various stages of the treatment and individual's condition. However, toxicity, biodegradation, elimination mechanisms, cost-efficiency, and scalability of nanoformulations must be assessed and needs careful consideration.
Conclusions and Future Directions
The BBB presents a major obstacle in the treatment of CNS diseases, including the brain-tumor. Currently, there are several techniques for transporting drugs across the BBB; however, there are limitations and challenges those needs to be addressed. Current therapy does not provide a long-term solution for brain-tumor patients and failed in improving the quality of patient's life. The nanotherapeutic approaches provide an attractive option for the treatment of brain-tumors and has the potential to address the limitations of current therapeutic approaches. However, the long-term effects of nanotherapeutics are currently unknown and study of biodistribution, pharmacokinetics, and toxicity is warranted to attain regular clinical use. Therefore, the key parameters of nanocarriers must be evaluated to develop an efficient brain-tumor targeted delivery system. In this respect, the design of experiment approach is a valuable tool for screening and optimization of process/formulation parameters in nanocarrier's fabrication as recently applied for various therapeutic applications (Agrahari et al., 2014; Youm et al., 2014).
The application of body's own cells as a drug delivery vehicle represents a novel strategy in targeted drug delivery approaches (Agrahari et al., 2016b). Immune cells (monocytes, macrophages, and neutrophils) and stem cells can cross the intact BBB and potentially allow transportation of therapeutic molecules and nanocarriers (Mariotti et al., 2014; Zhang et al., 2015). These cell-mediated systems represent an exciting strategy to carry therapeutics across the BBB. Despite the exciting clinical potential, there are some limitations such as the encapsulated therapeutic molecule can damage the circulatory cells, and the limited ability of cells to efficiently release the entrapped therapeutics. Further research is needed to optimize the surface characteristics, release profile, and biocompatibility of these novel cell-mediated vehicles to develop a cost-effective and robust system. Recently, the integration of theranostic and imaging techniques with nanotechnology (multi-functional nanocarriers) offers exciting opportunities in brain-tumor targeted therapy (Cheng et al., 2014). Thus, designing multi-functional nanocarrier platform holds great promise and could lead to exciting breakthrough in brain-tumor treatment strategies.
Overall, to achieve the goal of efficient drug delivery to brain-tumor, a better understanding of the physiochemical properties of therapeutic molecules, pharmacokinetics of the delivery systems, molecular mechanisms involved in BBB regulation and drug transportation is required. Also, collaboration among academic and industry scientists is vital to develop a novel system for brain-tumor therapeutics that can extend the patient's survival rate while improving the quality of life.
Footnotes
Conflicts of interest: None declared.
References
- Agrahari V, Agrahari V, Mitra AK. Nanocarrier fabrication and macromolecule drug delivery: challenges and opportunities. Ther Deliv. 2016a;7:257–278. doi: 10.4155/tde-2015-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrahari V, Agrahari V, Mitra AK. Next generation drug delivery: circulatory cells-mediated nanotherapeutic approaches. Expert Opin Drug Deliv. 2016b:1–5. doi: 10.1080/17425247.2017.1254614. [DOI] [PubMed] [Google Scholar]
- Agrahari V, Zhang C, Zhang T, Li W, Gounev TK, Oyler NA, Youan BB. Hyaluronidase-sensitive nanoparticle templates for triggered release of HIV/AIDS microbicide in vitro. AAPS J. 2014;16:181–193. doi: 10.1208/s12248-013-9546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad TD, Pan J, Connolly ID, Remington A, Wilson CM, Grant GA. Therapeutic strategies to improve drug delivery across the blood-brain barrier. Neurosurg Focus. 2015;38:E9. doi: 10.3171/2014.12.FOCUS14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Morshed RA, Auffinger B, Tobias AL, Lesniak MS. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv Drug Deliv Rev. 2014;66:42–57. doi: 10.1016/j.addr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Xiang L, Li-Yuan Z, Qing-Ru S, Min Z, Chen-Xi Z, Jun W, Guo-tao S, Zhong-Hua L. Stimuli-responsive nanocarriers for drug delivery to the central nervous system. Curr Nanosci. 2016;12:4–17. [Google Scholar]
- Gutkin A, Cohen ZR, Peer D. Harnessing nanomedicine for therapeutic intervention in glioblastoma. Expert Opin Drug Deliv. 2016;13:1573–1582. doi: 10.1080/17425247.2016.1200557. [DOI] [PubMed] [Google Scholar]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- Mangraviti A, Gullotti D, Tyler B, Brem H. Nanobiotechnology-based delivery strategies: New frontiers in brain tumor targeted therapies. J Control Release. 2016;240:443–453. doi: 10.1016/j.jconrel.2016.03.031. [DOI] [PubMed] [Google Scholar]
- Mariotti V, Greco SJ, Mohan RD, Nahas GR, Rameshwar P. Stem cell in alternative treatments for brain tumors: potential for gene delivery. Mol Cell Ther. 2014;2:1–10. doi: 10.1186/2052-8426-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Agrahari V, Youm I. Advances in targeted drug delivery approaches for the central nervous system tumors: the inspiration of nanobiotechnology. J Neuroimmune Pharmacol. 2016 doi: 10.1007/s11481-016-9698-1. doi:10.1007/s11481-016-9698-1. [DOI] [PubMed] [Google Scholar]
- Pourgholi F, Hajivalili M, Farhad JN, Kafil HS, Yousefi M. Nanoparticles: Novel vehicles in treatment of Glioblastoma. Biomed Pharmacother. 2016;77:98–107. doi: 10.1016/j.biopha.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Saraiva C, Praca C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release. 2016;235:34–47. doi: 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- Youm I, Agrahari V, Murowchick JB, Youan BB. Uptake and cytotoxicity of docetaxel-loaded hyaluronic acid-grafted oily core nanocapsules in MDA-MB 231 cancer cells. Pharm Res. 2014;31:2439–2452. doi: 10.1007/s11095-014-1339-x. [DOI] [PubMed] [Google Scholar]
- Zhang F, Xu CL, Liu CM. Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma. Drug Des Devel Ther. 2015;9:2089–2100. doi: 10.2147/DDDT.S79592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TT, Li W, Meng G, Wang P, Liao W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater Sci. 2016;4:219–229. doi: 10.1039/c5bm00383k. [DOI] [PubMed] [Google Scholar]