Abstract
Quercetin (QE; 3,5,7,3′,4′-pentahydroxyflavone), a well-known flavonoid, has been shown to prevent against neurodegenerative disorders and ischemic insults. However, few studies are reported regarding the neuroprotective mechanisms of QE after ischemic insults. Therefore, in this study, we investigated the effects of QE on ischemic injury and the expression of antioxidant enzymes in the hippocampal CA1 region of gerbils subjected to 5 minutes of transient cerebral ischemia. QE was pre-treated once daily for 15 days before ischemia. Pretreatment with QE protected hippocampal CA1 pyramidal neurons from ischemic injury, which was confirmed by neuronal nuclear antigen immunohistochemistry and Fluoro-Jade B histofluorescence staining. In addition, pretreatment with QE significantly increased the expression levels of endogenous antioxidant enzymes Cu/Zn superoxide dismutase, Mn superoxide dismutase, catalase and glutathione peroxidase in the hippocampal CA1 pyramidal neurons of animals with ischemic injury. These findings demonstrate that pretreated QE displayed strong neuroprotective effects against transient cerebral ischemia by increasing the expression of antioxidant enzymes.
Keywords: nerve regeneration, flavonoids, transient cerebral ischemia, Cu/Zn superoxide dismutase, catalase, Mn superoxide dismutase, glutathione peroxidase, neural regeneration
Introduction
Flavonoids, polyphenolic compounds from natural biological sources, exist in tea, vegetables, and fruits (Pawlikowska-Pawlega et al., 2003) and have been paid much more attention for their applicability in various brain disorders. For example, quercetin (QE), a 3,5,7,3′,4′-pentahydroxyflavone, has been reported to attenuate motor coordination deficits and anxiety, decrease the proliferation of microglia and increase the number of astrocytes in lesion core in a 3-nitropropionic acid-induced rat model of Huntington's disease (Chakraborty et al., 2014). In addition, various polyphenols including QE have been shown to protect against amyloid-β-induced oxidative damage in animal models of Alzheimer's disease (Pocernich et al., 2011). Furthermore, QE has been reported to display the protective effects against cerebral ischemic injuries (Cherubini et al., 2008; Yao et al., 2012; Chang et al., 2014; Lei et al., 2015).
Patients, who have had an ischemic stroke, can suffer from neuropsychological sequelae (Moulaert et al., 2010; Geri et al., 2014). Transient cerebral ischemia interrupts blood flow in the brain tissue and commonly induces selective neuronal damage/death in specific areas of the brain including the hippocampus (Bae et al., 2015; Lee et al., 2015). In ischemic stroke, oxidative stress activates apoptotic and necrotic signaling pathways (Kahles and Brandes, 2012), hence, antioxidants have undoubtedly become a promising strategy to treat ischemic stroke. In normal physiological system, free radicals are adequately counterbalanced by antioxidants, such as superoxide dismutases (SODs), catalase (CAT) or glutathione peroxidase (GPX) (Kahles and Brandes, 2012), however, this system balance would be broken after ischemic insults. Many studies have demonstrated that antioxidants catalyze superoxide radicals into hydrogen peroxide, which has a protective potential against oxidative stress mediated neuronal damage/death (Kuroda et al., 1999; Crack et al., 2003; O’Collins et al., 2006).
However, few studies are reported on the effects of pretreated QE on the expression of antioxidant enzymes in the gerbil hippocampal CA1 region following transient cerebral ischemia. The hippocampal CA1 region is one of vulnerable and sensitive areas to transient cerebral ischemia, in which selective neuronal damage/death, morphological changes of glial cells and neuroinflammation occur in this region after ischemia (Dirnagl et al., 1999; Neumann et al., 2013; Liu et al., 2014b; Yan et al., 2014b). Therefore, in this study, we investigated the antioxidative effects of QE through examining antioxidant enzymes, such as Cu/Zn superoxide dismutase (SOD1), Mn superoxide dismutase (SOD2), catalase (CAT) and glutathione peroxidase (GPX) as well as the neuroprotective effect of QE using neuronal nuclear antigen (NeuN) immunohistochemistry and Fluoro-Jade B (F-J B) histofluorescence staining in the gerbil hippocampal CA1 region following transient cerebral ischemia.
Materials and Methods
Experimental animals
Eighty-four 6-month-old gerbils, weighing 70–80 g, were used according to the guidelines that are in compliance with the current international laws and policies (Guide for the Care and Use of Laboratory Animals, The National Academies Press, 8th Ed., 2011) and approved by the Institutional Animal Care and Use Committee (IACUC) at Kangwon National University (approval No. KW-12-0018). The animals were divided (n = 14 at each time point in each group) as follows: 1) vehicle-sham group that was pretreated with distilled water and not subjected to ischemia induction, 2) vehicle-ischemia group that was pretreated with distilled water and subjected to ischemia induction, 3) QE-sham group that was pretreated with 20 mg/kg QE and not subjected to ischemia induction, 4) QE-ischemia group that was pretreated with 20 mg/kg QE and subjected to ischemia induction. In the two ischemia groups, rats were observed at 2 and 5 days after ischemia induction.
Pretreatment with QE
QE (Sigma, St. Louis, MO, USA) was dissolved in distilled water according to our previous research (Hwang et al., 2009). Vehicle and QE were administrated orally using a feeding needle once daily for 15 days before ischemia/reperfusion operation (the last treatment was done 30 minutes before ischemia/reperfusion operation) according to our published method (Hwang et al., 2009).
Induction of transient cerebral ischemia
As we previously described (Choi et al., 2016), gerbils were anesthetized via a mask using a gas mixture of 2.5% isoflurane (Baxter, Deerfield, IL, USA) in 33% oxygen and 67% nitrous oxide. Blood flow was completely interrupted by occluding bilateral common carotid arteries for 5 minutes and confirmed by a transient stopping of blood flow in the central retinal artery using an ophthalmoscope (HEINE K180, Heine Optotechnik, Herrsching, Germany). The body (rectal) temperature of normothermic 37 ± 0.5°C conditions was controlled with a rectal temperature probe (TR-100; Fine Science Tools, Foster City, CA, USA), and the temperature was maintained using a thermometric blanket during and after ischemia induction. Sham-operated gerbils were subjected to the same surgical procedure without common carotid artery occlusion.
Tissue processing for histology
For histological examination, sections were prepared from each group (n = 7 at each time point) at designated times (sham, 2 and 5 days after reperfusion). According to our published method (Choi et al., 2016), in brief, the gerbils were anesthetized with pentobarbital sodium (JW Pharm. Co., Ltd., Seoul, Korea, 40 mg/kg, i.p) and transcardially perfused with 4% paraformaldehyde, and their brains were serially cut in a cryostat (Leica, Wetzlar, Germany) into 30-μm thick frontal sections.
NeuN immunohistochemistry
To investigate change in neuronal distribution, NeuN (a marker for neuronal nuclei) immunohistochemistry was performed as we previously described (Choi et al., 2016). In brief, the brain sections were incubated with diluted mouse anti-NeuN (1:1,000, Chemicon International, Temecula, CA, USA) overnight at 4°C and exposed to biotinylated goat anti-mouse IgG (Vector, Burlingame, CA, USA) for 2 hours at room temperature and streptavidin peroxidase complex for 1 hour at room temperature. Finally, they were visualized by staining with 3,3′-diaminobenzidine tetrahydrochloride.
F-J B histofluorescence staining
To examine neuronal death after ischemia, F-J B (a marker for neurodegeneration) histofluorescence staining was used according to our published procedure (Lee et al., 2011). Briefly, the sections were stained with a solution containing sodium hydroxide (1%), a solution of potassium permanganate (0.06%) and a F-J B (Histochem, Jefferson, AR, USA) staining solution (0.0004%). The sections were examined using an epifluorescent microscope (Carl Zeiss, Göttingen, Germany) with blue (450–490 nm) excitation light and a barrier filter.
SOD1, SOD2, CAT and GPX immunohistochemistry
Immunohistochemistry was carried out, according to the above-mentioned method, with sheep anti-SOD1 (1:1,000; Calbiochem, Farmingdale, NY, USA), sheep anti-SOD2 (1:1,000, Calbiochem), rabbit anti-CAT (1:500, AbFrontier, Seoul, Korea), and mouse anti-glutathione peroxidase (GPX, 1:500, Chemicon, Temecula, CA, USA); the primary antisera were exposed to biotinylated donkey anti-sheep IgG, goat anti-rabbit IgG and goat anti-mouse IgG (1:200, Vector, Burlingame, CA, USA) and streptavidin peroxidase complex (1:200, Vector) for 1 hour at room temperature. In order to establish the specificity of the immunostaining, a negative control test was carried out with pre-immune serum instead of primary antibody. The negative control resulted in the absence of immunoreactivity in any structures (data not shown).
Western blot analysis
To examine changes of SOD1, SOD2, CAT and GPX protein expressions in the hippocampal CA1 region, gerbils (n = 7 at each time point in each group) were sacrificed at designated times (sham, 2 and 5 days after ischemia) according to our published method (Lee et al., 2011). In brief, the tissues were homogenized, and protein levels were determined in the supernatants using a micro bicinchoninic acid protein assay kit with bovine serum albumin as a standard (Pierce Chemical, Rockford, IL, USA). Aliquots containing total protein (20 μg) were boiled and loaded onto a polyacryamide gel (12.5%). After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Pall Crop, East Hills, NY, USA). To reduce background staining, the membranes were incubated with non-fat dry milk (5%) and with sheep anti-SOD1 (1:1,000, Calbiochem), sheep anti-SOD2 (1:1,000, Calbiochem), rabbit anti-CAT (1:500, abFrontier), and mouse anti-GPX (1:500, Chemicon) and continuously exposed to peroxidase-conjugated donkey anti-sheep IgG, goat anti-rabbit IgG and goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Antibodies were visualized using an enhanced chemiluminescence kit (Amersham, Buckinghamshire, UK).
Data analysis
All measurements were done to ensure objectivity in blind conditions (three observers for each experiment), and the samples were assayed under the same condition. The studied tissue sections were selected with 90 μm interval, and cell counts were done by averaging cell numbers from 5 sections of each animal according to anatomical landmarks corresponding to anteroposterior –1.4 to –1.9 mm of gerbil brainatlas (Loskota et al., 1974). To evaluate the neuroprotective effect of QE, NeuN-immunoreactive (+) neurons and F-J B-positive (+) cells were counted in the hippocampal CA1 region. In brief, as previously described (Lee et al., 2016), digital images were taken using a light microscope (AxioM1, Carl Zeiss, Germany) equipped with a digital camera (Axiocam, Carl Zeiss, Germany) connected to a PC monitor. Cells were counted in a 250 × 250 μm square including the stratum pyramidale at the center of the hippocampal CA1 region using an image analyzing system (software: Optimas 6.5, CyberMetrics, Scottsdale, AZ, USA). The count ratio was calibrated as %, with vehicle-sham group designated as 100%.
In addition, to quantitatively analyze SOD1, SOD2, CAT and GPX immunoreactivity, five sections of each animal were selected with 90 μm interval. According to our process (Choi et al., 2016), digital images of the hippocampal CA1 region were captured with a light microscope of AxioM1 (Carl Zeiss, Oberkochen, Germany) equipped with a digital camera (Axiocam, Carl Zeiss Microscopy, Thornwood, NY, USA) that was connected to a PC monitor and calibrated into an array of 512 × 512 pixels. The mean immunoreactivity of SOD1, SOD2, CAT and GPX in the CA1 pyramidal neurons of the stratum pyramidale was measured by a 0-255 gray scale system. Background density was subtracted, relative immunoreactivity for SOD1, SOD2, CAT and GPX in the pyramidal neurons of the stratum pyramidale was calibrated as % (relative immunoreactivity) using Adobe Photoshop version 8.0 (Adobe Systems Inc., San Jose, CA, USA) and analyzed using NIH Image 1.59 software (NIH, Bethesda, MD, USA). A ratio of the relative immunoreactivity was calibrated as %, with the vehicle-sham group designated as 100%.
In addition, the protein levels of SOD1, SOD2, CAT and GPX were measured using Scion Image software (Scion Corp., Frederick, MD, USA), which was used for relative optical density (ROD): the ROD ratio was calibrated as % compared with the vehicle-sham group.
Statistical analysis
The data shown in this research represent the means ± SEM. All comparisons were tested for normality and variance homogeneity using SPSS 17.0 software, and all data passed normality test. Differences of the means among the groups were statistically analyzed by one-way analysis of variance (ANOVA) with Duncan's post hoc test to elucidate differences between experimental groups. Statistical significance was considered at P < 0.05.
Results
Neuroprotective effect of QE on hippocampal CA1 pyramidal neurons against ischemia
To investigate the neuroprotective effect of QE in the hippocampal CA1 area after ischemia in gerbils, NeuN immunohistochemistry and F-J B histofluorescence staining were performed (Figure 1).
Figure 1.
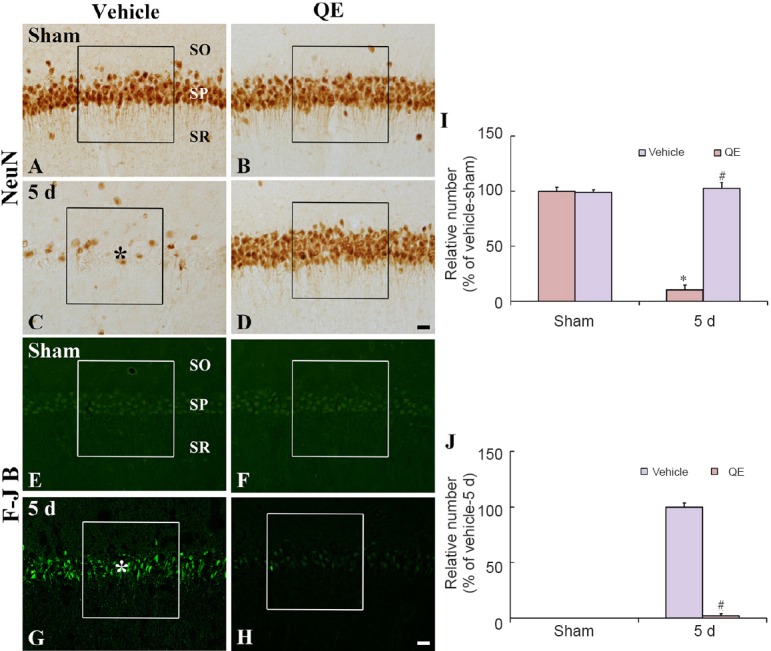
NeuN immunohistochemistry (A–D) and F-J B histofluorescence staining (E–H) in the hippocampal CA1 region of the vehicle-sham (A, E), vehicle-ischemia (C, G), QE-sham (B, F) and QE-ischemia (D, H) groups.
A few NeuN+ neurons and many F-J B+ cells are observed in the SP 5 days after ischemia (asterisks), whereas abundant NeuN+ and few F-J B+ pyramidalneurons are observed in the QE-ischemia group. Scale bars are 20 μm in D and H, valid for A–H. (I, J) Relative analysis as percent in the mean number of NeuN+ and F-J B+ cells in a 250 × 250 μm2 (boxes) (n = 7 at each time point in each group, *P < 0.05, vs. vehicle-sham group; #P < 0.05, vs. corresponding vehicle-ischemia group). Data in I and J are exprossed as the mean ± SEM. QE: Quercetin; NeuN: neuronal nuclear antigen; F-J B: Fluoro-Jade B; SP: stratum pyramidale; SO: stratum oriens; SR: stratum radiatum; d: days.
In the vehicle-sham group, NeuN-immunoreactive (NeuN+) neurons were easily observed in the stratum pyramidale, which are called “pyramidal neurons”, of the hippocampal CA1 region (Figure 1A), and no F-J B-positive (F-J B+) cells, which are damaged or dead cells, were observed in the hippocampal CA1 region (Figure 1E). Whereas, in the vehicle-ischemia group, only a few NeuN+ pyramidal neurons (about 11% of the vehicle-sham group) were observed in the stratum pyramidale at 5 days post-ischemia (Figure 1C and I), and many F-J B+ cells, which represent damaged pyramidal neurons, were detected in the stratum pyramidale (Figure 1G and J).
In the QE-sham group, distribution pattern of NeuN+ pyramidal neurons in the hippocampal CA1 region was similar to that in the vehicle-sham group, and any F–J B+ cells were not found in the hippocampal CA1 region (Figure 1B, F, I and J). In the QE-ischemia group at 5 days after ischemia/reperfusion induction, the distribution pattern and numbers of NeuN+ pyramidal neurons (about 98% of the vehicle-sham group) were, similar to those in the QE-sham group, significantly decreased compared with the vehicle-ischemia group (P < 0.05) and F–J B+ cells (about 2% of the vehicle-ischemia group) were rarely observed in the hippocampal CA1 region (Figure 1D, H, I and J).
Effect of QE on antioxidant enzyme immunoreactivities in the hippocampal CA1 region after transient cerebral ischemia
SOD1 and SOD2 immunoreactivities
Weak SOD1 and SOD2 immunoreactivities were observed in the pyramidal neurons of the CA1region in the vehicle-sham group (Figure 2A and H). In the vehicle-ischemia group, SOD1 and SOD2 immunoreactivities in the CA1 pyramidal neurons were significantly decreased (about 81% and 87% of the vehicle-sham group, respectively) 2 days after transient ischemia, and they were hardly observed in the CA1 pyramidal neurons 5 days after ischemia; however, strong SOD1 and SOD2 immunoreactivities were newly expressed in non-pyramidal cells in the strata oriens and radiatum of the hippocampal CA1 region (Figure 2C, E, G, J, L and N).
Figure 2.
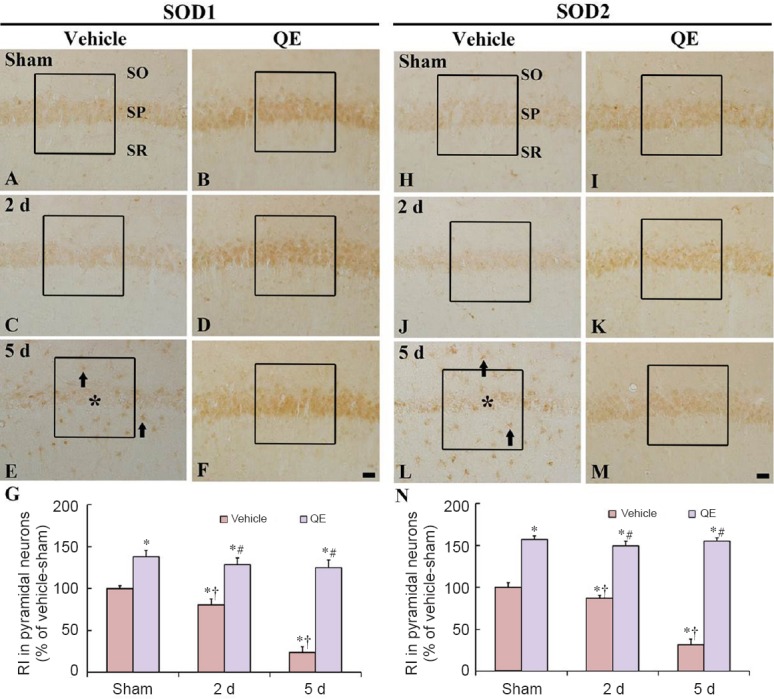
SOD1 and SOD2 immunoreactivities in the hyippocampal CA1 region of the vehicle-sham (A, H), vehicle-ischemia (C, E, J, L), QE-sham (B, I) and QE-ischemia (D, F, K, M) groups.
At 2 days post-ischemia, SOD1 and SOD2 immunoreactivities are decreased in the hippocampal CA1 pyramidal neurons, and at 5 days post-ischemia, SOD1 and SOD2 immunoreactivities in the CA1 pyramidal neurons are very weak (asterisk); however, strong SOD1 and SOD2 immunoreactivities are newly shown in non-pyramidal cells (arrows). In the QE-sham group, SOD1 and SOD2 immunoreactivities are significantly increased in the hippocampal CA1 pyramidal neurons compared with those in the vehicle-sham group. In the QE-ischemia group, SOD1 and SOD2 immunoreactivities are not altered at 2 and 5 days post-ischemia. Scale bars are 20 μm in F and M, valid for A–M. (G, N) Relative immunoreactivity (RI) as percent of SOD1 and SOD2 in the hippocampal CA1 pyramidal neurons in a 140 × 140 μm2 (boxes) (n = 7 at each time point in each group, *P < 0.05, vs. the vehicle-sham group; †P < 0.05, vs. respective pre-time point group; #P < 0.05, vs. corresponding vehicle-ischemia group). Data in G and N are expressed as the mean ± SEM. SOD1: Cu/Zn superoxide dismutase; SOD2: Mn superoxide dismutase; QE: quercetin; SO: stratum oriens; SP: stratum pyramidale; SR: stratum radiatum.
In the QE-sham group, SOD1 and SOD2 immunoreactivities in the hippocampal CA1 pyramidal neurons were significantly higher (about 138% and 157% of the vehicle-sham group, respectively) than that in the vehicle-sham group (P < 0.05) (Figure 2B and I). At 2 and 5 days post-ischemia, SOD1 and SOD2 immunoreactivities in the hippocampal CA1 pyramidal neurons of the QE-ischemia group were significantly higher than those in the vehicle-ischemia group (P < 0.05) (Figure 2D, F, G, K, M and N).
CAT and GPX immunoreactivities
In the vehicle-sham group, immunoreactivities of CAT and GPX were found in the hippocampal CA1 pyramidal neurons (Figure 3A and H). In the vehicle-ischemia group, CAT and GPX immunoreactivities in the hippocampal CA1 pyramidal neurons were slightly decreased (about 96% and 93% of the vehicle-sham group, respectively) 2 days post-ischemia (Figure 3C, G, J and N). CAT and GPX immunoreactivities were rarely observed in the hippocampal CA1 pyramidal neurons 5 days post-ischemia, and at this point time, many non-pyramidal cells newly expressed CAT and GPX immunoreactivities in the CA1 area, especially in the strata oriens and radiatum (Figure 3E and L).
Figure 3.
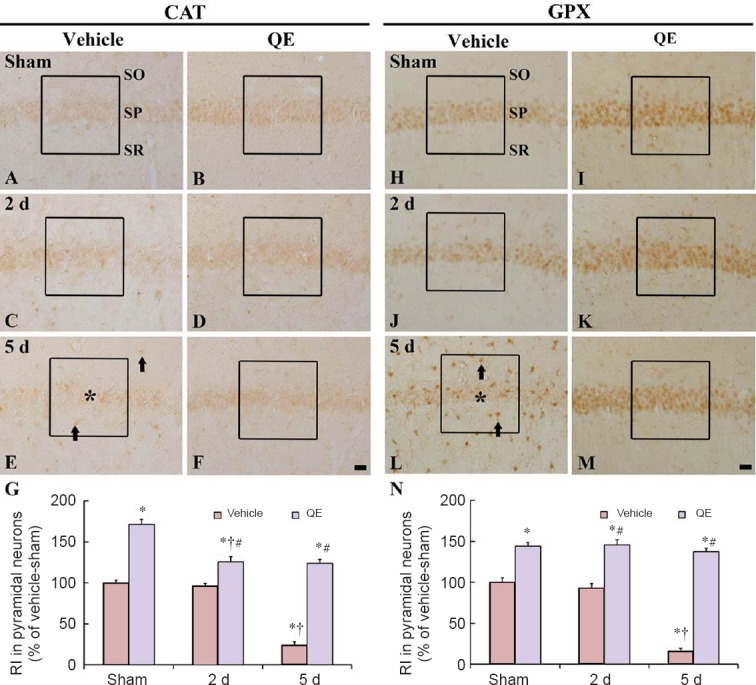
CAT and GPX immunoreactivities in the hippocampal CA1 region of the vehicle-sham (A, H), vehicle-ischemia (C, E, J, L), QE-sham (B, I) and QE-ischemia (D, F, K, M) groups.
At 2 days post-ischemia, CAT and GPX immunoreactivities were gradually decreased in the hippocampal CA1 pyramidal neurons and hardly detected 5 days post-ischemia (asterisks); however, non-pyramidal cells (arrows) show CAT and GPX immunoreactivities. In the QE-sham group, CAT and GPX immunoreactivities were significantly increased in the hippocampal CA1 pyramidal neurons, and in the QE-ischemia group, CAT and GPX immunoreactivities were also strong at 2 and 5 days post-ischemia. Scale bars are 20 μm in F and M, valid for A–M. (G, N) Relative immunoreactivity (RI) as percent of CAT and GPX in hippocampal CA1 pyramidal neurons in a 140 × 140 μm2 (boxes). n = 7 at each time point in each group.*P < 0.05, vs. vehicle-sham group; †P < 0.05, vs. respective pre-time point group; #P < 0.05, vs. corresponding vehicle-ischemia group. Data in F and M are expressed as the mean ± SEM. CAT: Catalase: GPX: glutathione peroxidase; QE: quercetin; SO: stratum oriens; SP: stratum pyramidale; SR: stratum radiatum.
In the QE-sham group, CAT and GPX immunoreactivities were significantly increased (about 171% and 144% of the vehicle-sham group, respectively) in the hippocampal CA1 pyramidal neurons compared with those in the vehicle-sham group (P < 0.05) (Figure 3B, G, I and N). In the QE-ischemia group, CAT and GPX immunoreactivities in the hippocampal CA1 pyramidal neurons were significantly stronger than those in the vehicle-ischemia group at 2 and 5 days post-ischemia (P < 0.05) (Figure 3D, F, G, K, M and N).
Effect of QE on antioxidant enzyme protein expression in the hippocampal CA1 region after transient cerebral ischemia
SOD1 protein level of the vehicle-ischemia group was decreased 2 days post-ischemia; whereas, SOD1 level 5 days post-ischemia was significantly increased compared with the vehicle-sham group because SOD1 was strongly shown in non-pyramidal cells of the ischemic CA1 region (Figure 4A). In the QE-sham group, SOD1 level was significantly increased by about 96% of the vehicle-sham group and maintained in the QE-ischemia group after ischemia (Figure 4A). The change pattern in SOD2 level in both groups was similar to that in SOD1 level in both groups (Figure 4B).
Figure 4.
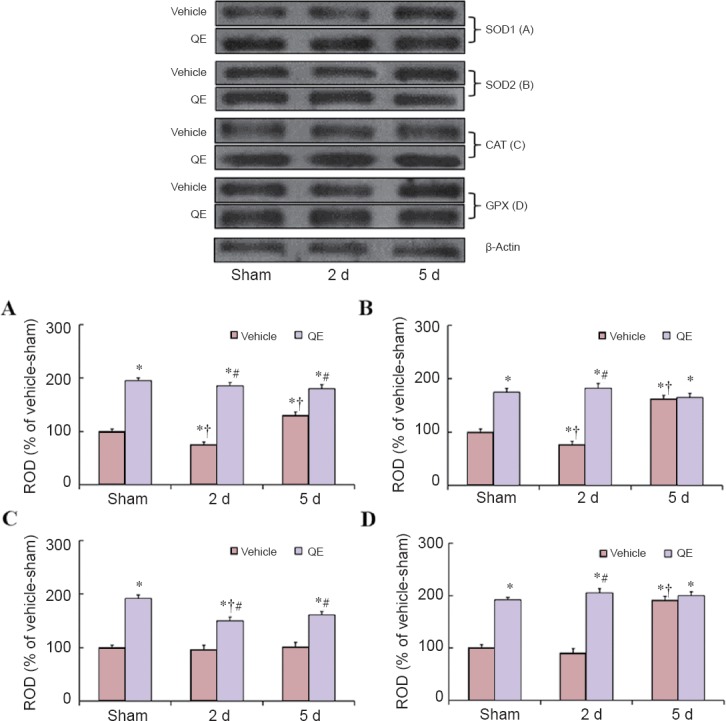
Western blot analyses for SOD1, SOD2, CAT and GPX protein levels in the hippocampal CA1 region of the vehicle-sham, vehicle-ischemia, QE-sham, and QE-ischemia groups.
SOD1, SOD2, CAT and GPX protein levels were significantly increased in the QE-sham groups than in the vehicle-sham groups. Relative optical density (ROD) as % values of the immunoblot band was presented for SOD1 (A), SOD2 (B), CAT (C) and GPX (D) (*P < 0.05, vs. vehicle-sham group; †P < 0.05, vs. respective pre-time point group; #P < 0.05, vs. corresponding vehicle-ischemia group). Data in A–D are expressed as the mean ± SEM. SOD1: Cu/Zn superoxide dismutase; SOD2: Mn superoxide dismutase; CAT: catalase; GPX: glutathione peroxidase; QE: quercetin.
CAT level in the vehicle-ischemia group was not significantly altered after ischemia; however, in the QE-sham group, CAT level was significantly increased by about 92% of the vehicle-sham group and the level was significantly increased in the QE-ischemia group compared with the vehicle-ischemia group (P < 0.05) (Figure 4C).
GPX level in the vehicle-ischemia group was slightly decreased 2 days after transient ischemia and significantly increased 5 days after ischemia by about 89% of the vehicle-sham group, because, at this time, GPX was strongly expressed in non-pyramidal cells (Figure 4D). In the QE-sham group, GPX level was significantly increased by about 94% of the vehicle-sham group and maintained in the QE-ischemia group after ischemia (Figure 4D).
Discussion
Ischemia/reperfusion injury in the central nervous system is characterized by extensive oxidative stress, which is combated by antioxidants. Antioxidants and nutrition have been considered as an approach to slow down ischemia-induced neuronal damage (Crack et al., 2003; Park et al., 2014; Yan et al., 2014a). In this regard, we, in the present study, focused on four antioxidant enzymes that were increased by QE pretreatment and protected hippocampal CA1 pyramidal neurons after 5 minutes of transient cerebral ischemia injury. The neuroprotective effects of QE on the brain after ischemic insults have been reported in some animal models of ischemic insults. Among the previous studies, Hwang et al. (2009) reported that QE displayed a strong possibility of neuroprotection against transient cerebral ischemia using cresyl violet staining alone. However, in this study, to obtain more specific results regarding the neuroprotective effect of QE, we used NeuN immunohistochemistry and F-J B histofluorescence staining, which are recently used as histological methods for identifying neuronal damage/death in neurological diseases including cerebral ischemia (Gascón et al., 2008; Lee et al., 2015) and found that QE strongly protected pyramidal neurons of the gerbil hippocampal CA1 region from ischemia/reperfusion injury. In addition, previous studies have demonstrated that QE significantly reduces ischemia-induced damage in animal models of ischemic insults, such as focal and global cerebral ischemia (Pandey et al., 2011; Yao et al., 2012; Viswanatha et al., 2013). However, the mechanisms underlying the neuroprotective effects of QE on the ischemic brain are disputed. Therefore, in this study, to elucidate the possible neuroprotective effects of QE on transient ischemic damage in gerbil hippocampal CA1 pyramidal neurons, we determined the changes in immunoreactivities and protein levels of endogenous antioxidant enzymes in the gerbil ischemic CA1 region.
It is well known that overproduction of reactive oxygen species (ROS) induces the oxidative modification of cellular constituents that include DNA, proteins and lipids, which can lead to neuronal damage/death (Berlett and Stadtman, 1997; Cui et al., 2004). In this regard, many researchers have demonstrated that antioxidant enzyme system can effectively neutralize and eliminate ROS through catalyzing superoxides into oxygen and hydrogen peroxide (Greenlund et al., 1995; Park et al., 2011b). Hwang et al. (2009) reported that QE attenuated the level of hydroxynonenal (an indicator of lipid peroxidation) in the hippocampus of the gerbil following transient global cerebral ischemia, and, similarly, Ahmad et al. (2011) showed that QE reduced oxidative stress in the rat brain after transient focal cerebral ischemia. Cerebral ischemia was reported to lead to excessive generation of ROS and increased ROS levels, which were regulated by endogenous antioxidant enzymes including SODs, CAT and GPX which were closely associated with neuronal damage/death following cerebral ischemia (Sugawara and Chan, 2003). In addition, we reported that endogenous antioxidant enzymes were increased or maintained by treatments with plant extracts and these enzymes were closely associated with the protective effects of plant extracts against ischemia-induced neuronal death in the gerbil hippocampal CA1 region (Park et al., 2011a, 2015). Therefore, we insist that the upregulation of endogenous antioxidant enzymes by QE prevents or ameliorates ischemia-induced neuronal death by decreasing oxidative stress. On the basis of other reports and our previous reports (Sugawara and Chan, 2003; Park et al., 2015), in this study, to examine how QE protects neurons from ischemia/reperfusion injury, we administered QE before ischemia/reperfusion operation and found that the expression levels of SOD1, SOD2, CAT and GPX were significantly increased in the hippocampal CA1 pyramidal neurons without ischemia/reperfusion injury. This result suggests that pretreatment with QE could increase the activities of antioxidant enzymes in the hippocampal CA1 region. Furthermore, we found that the increased SOD1, SOD2, CAT and GPX expressions in the CA1 pyramidal neurons were maintained by QE after ischemia/reperfusion injury. These findings are supported by a previous study that demonstrated that QE restrained ischemia-induced oxidative stress in the rat heart through increasing SOD, CAT, GPX and glutathione reductase ctivities as well as decreasing malondialdehyde level (Liu et al., 2014a). In addition, some studies showed that some flavonoids, such as pinocembrin (Saad et al., 2015) and luteolin (Fu et al., 2014), reduced ischemic damage and enhanced antioxidant potential. Previous studies demonstrated that SOD1 prevented neuronal damage in vulnerable brain regions following global cerebral ischemia/reperfusion in transgenic rats (Chan et al., 1998) and that SOD2 deficiency resulted in the exacerbation of cerebral infarction induced by middle cerebral artery occlusion in SOD2 knockout mice (Murakami et al., 1998). In addition, Yaidikar and Thakur (2015) reported that CAT and GPX enzymes played a crucial role in protecting neurons from oxidative stress associated damage in a rat model of focal cerebral ischemia. Furthermore, Crack et al. (2003) showed that loss of GPX led infarct volume to threefold increase induced by ischemic insult in transgenic mice compared with wild-type mice. Hence, in the present study, the increases in SOD1, SOD2, CAT and GPX immunoreactivities by QE pretreatment apparently played key roles in protecting hippocampal CA1 pyramidal neurons from ischemia/reperfusion injury.
In conclusion, our findings show that QE pretreatment effectively protected hippocampal CA1 pyramidal neurons from ischemia/reperfusion injury by increasing the activities of SOD1, SOD2, CAT and GPX enzymes.
Acknowledgments
The authors would like to thank Mr. Seung Uk Lee for his technical help in this study.
Footnotes
Funding: This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A01059728), and by Hallym University Research Fund 2014 (HURF-2014-25).
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Edited by Li CH, Song LP, Zhao M
References
- Ahmad A, Khan MM, Hoda MN, Raza SS, Khan MB, Javed H, Ishrat T, Ashafaq M, Ahmad ME, Safhi MM, Islam F. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem Res. 2011;36:1360–1371. doi: 10.1007/s11064-011-0458-6. [DOI] [PubMed] [Google Scholar]
- Bae EJ, Chen BH, Yan BC, Shin BN, Cho JH, Kim IH, Ahn JH, Lee JC, Tae HJ, Hong S, Kim DW, Lee YL, Won MH, Park JH. Delayed hippocampal neuronal death in young gerbil following transient global cerebral ischemia is related to higher and longer-term expression of p63 in the ischemic hippocampus. Neural Regen Res. 2015;10:944–950. doi: 10.4103/1673-5374.158359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Chakraborty J, Singh R, Dutta D, Naskar A, Rajamma U, Mohanakumar KP. Quercetin improves behavioral deficiencies, restores astrocytes and microglia, and reduces serotonin metabolism in 3-nitropropionic acid-induced rat model of Huntington's Disease. CNS Neurosci Ther. 2014;20:10–19. doi: 10.1111/cns.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calagui B, Reola L, Carlson E, Epstein CJ. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Yang YR, Wang PS, Wang RY. Quercetin enhances exercise-mediated neuroprotective effects in brain ischemic rats. Med Sci Sports Exerc. 2014;46:1908–1916. doi: 10.1249/MSS.0000000000000310. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Ruggiero C, Morand C, Lattanzio F, Dell’aquila G, Zuliani G, Di Iorio A, Andres-Lacueva C. Dietary antioxidants as potential pharmacological agents for ischemic stroke. Curr Med Chem. 2008;15:1236–1248. doi: 10.2174/092986708784310431. [DOI] [PubMed] [Google Scholar]
- Choi HY, Park JH, Chen BH, Shin BN, Lee YL, Kim IH, Cho JH, Lee TK, Lee JC, Won MH, Ahn JH, Tae HJ, Yan BC, Hwang IK, Kim YM, Kim SK. Increases of catalase and glutathione peroxidase expressions by lacosamide pretreatment contributes to neuroprotection against experimentally induced transient cerebral ischemia. Neurochem Res. 2016;41:2380–2390. doi: 10.1007/s11064-016-1951-8. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM, de Haan JB, Kola I, Hertzog P, Iannello RC. Glutathione peroxidase-1 contributes to the neuroprotection seen in the superoxide dismutase-1 transgenic mouse in response to ischemia/reperfusion injury. J Cereb Blood Flow Metab. 2003;23:19–22. doi: 10.1097/01.WCB.0000035181.38851.71. [DOI] [PubMed] [Google Scholar]
- Cui K, Luo X, Xu K, Ven Murthy MR. Role of oxidative stress in neurodegeneration: recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:771–799. doi: 10.1016/j.pnpbp.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Fu X, Zhang J, Guo L, Xu Y, Sun L, Wang S, Feng Y, Gou L, Zhang L, Liu Y. Protective role of luteolin against cognitive dysfunction induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav. 2014;126:122–130. doi: 10.1016/j.pbb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Gascón S, Sobrado M, Roda JM, Rodríguez-Peña A, Díaz-Guerra M. Excitotoxicity and focal cerebral ischemia induce truncation of the NR2A and NR2B subunits of the NMDA receptor and cleavage of the scaffolding protein PSD-95. Mol Psychiatry. 2008;13:99–114. doi: 10.1038/sj.mp.4002017. [DOI] [PubMed] [Google Scholar]
- Geri G, Mongardon N, Daviaud F, Empana JP, Dumas F, Cariou A. Neurological consequences of cardiac arrest: where do we stand7#x003F; Ann Fr Anesth Reanim. 2014;33:98–101. doi: 10.1016/j.annfar.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Greenlund LJ, Deckwerth TL, Johnson EM., Jr Superoxide dismutase delays neuronal apoptosis: a role for reactive oxygen species in programmed neuronal death. Neuron. 1995;14:303–315. doi: 10.1016/0896-6273(95)90287-2. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Lee CH, Yoo KY, Choi JH, Park OK, Lim SS, Kang IJ, Kwon DY, Park J, Yi JS, Bae YS, Won MH. Neuroprotective effects of onion extract and quercetin against ischemic neuronal damage in the gerbil hippocampus. J Med Food. 2009;12:990–995. doi: 10.1089/jmf.2008.1400. [DOI] [PubMed] [Google Scholar]
- Kahles T, Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci. 2012;69:2345–2363. doi: 10.1007/s00018-012-1011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S, Tsuchidate R, Smith ML, Maples KR, Siesjo BK. Neuroprotective effects of a novel nitrone, NXY-059, after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1999;19:778–787. doi: 10.1097/00004647-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Lee CH, Park JH, Yoo KY, Choi JH, Hwang IK, Ryu PD, Kim DH, Kwon YG, Kim YM, Won MH. Pre- and post-treatments with escitalopram protect against experimental ischemic neuronal damage via regulation of BDNF expression and oxidative stress. Exp Neurol. 2011;229:450–459. doi: 10.1016/j.expneurol.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Lee JC, Park JH, Ahn JH, Kim IH, Cho JH, Choi JH, Yoo KY, Lee CH, Hwang IK, Cho JH, Kwon YG, Kim YM, Kang IJ, Won MH. New GABAergic neurogenesis in the hippocampal ca1 region of a gerbil model of long-term survival after transient cerebral ischemic injury. Brain Pathol. 2016;26:581–92. doi: 10.1111/bpa.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Chao H, Zhang Z, Lv J, Li S, Wei H, Xue R, Li F, Li Z. Neuroprotective effects of quercetin in a mouse model of brain ischemic/reperfusion injury via anti-apoptotic mechanisms based on the Akt pathway. Mol Med Rep. 2015;12:3688–3696. doi: 10.3892/mmr.2015.3857. [DOI] [PubMed] [Google Scholar]
- Liu H, Guo X, Chu Y, Lu S. Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene. 2014a;545:149–155. doi: 10.1016/j.gene.2014.04.043. [DOI] [PubMed] [Google Scholar]
- Liu YR, Li PW, Suo JJ, Sun Y, Zhang BA, Lu H, Zhu HC, Zhang GB. Catalpol provides protective effects against cerebral ischaemia/reperfusion injury in gerbils. J Pharm Pharmacol. 2014b;66:1265–1270. doi: 10.1111/jphp.12261. [DOI] [PubMed] [Google Scholar]
- Loskota WJ, Lomax P, Rich ST. The gerbil as a model for the study of the epilepsies. Seizure patterns and ontogenesis. Epilepsia. 1974;15:109–119. doi: 10.1111/j.1528-1157.1974.tb04000.x. [DOI] [PubMed] [Google Scholar]
- Moulaert VR, Wachelder EM, Verbunt JA, Wade DT, van Heugten CM. Determinants of quality of life in survivors of cardiac arrest. J Rehabil Med. 2010;42:553–558. doi: 10.2340/16501977-0547. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann JT, Cohan CH, Dave KR, Wright CB, Perez-Pinzon MA. Global cerebral ischemia: synaptic and cognitive dysfunction. Curr Drug Targets. 2013;14:20–35. doi: 10.2174/138945013804806514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Pandey AK, Hazari PP, Patnaik R, Mishra AK. The role of ASIC1a in neuroprotection elicited by quercetin in focal cerebral ischemia. Brain Res. 2011;1383:289–299. doi: 10.1016/j.brainres.2011.01.085. [DOI] [PubMed] [Google Scholar]
- Park JH, Cho JH, Kim IH, Ahn JH, Lee JC, Chen BH, Shin BN, Tae HJ, Yoo KY, Hong S, Kang IJ, Won MH, Kim JD. Oenanthe javanica extract protects against experimentally induced ischemic neuronal damage via its antioxidant effects. Chin Med J (Engl) 2015;128:2932–2937. doi: 10.4103/0366-6999.168063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Joo HS, Yoo KY, Shin BN, Kim IH, Lee CH, Choi JH, Byun K, Lee B, Lim SS, Kim MJ, Won MH. Extract from Terminalia chebula seeds protect against experimental ischemic neuronal damage via maintaining SODs and BDNF levels. Neurochem Res. 2011a;36:2043–2050. doi: 10.1007/s11064-011-0528-9. [DOI] [PubMed] [Google Scholar]
- Park JH, Park OK, Yan B, Ahn JH, Kim IH, Lee JC, Kwon SH, Yoo KY, Lee CH, Hwang IK, Choi JH, Won MH, Kim JD. Neuroprotection via maintenance or increase of antioxidants and neurotrophic factors in ischemic gerbil hippocampus treated with tanshinone I. Chin Med J (Engl) 2014;127:3396–3405. [PubMed] [Google Scholar]
- Park SW, Lee CH, Lee JG, Kim LW, Shin BS, Lee BJ, Kim YH. Protective effects of atypical antipsychotic drugs against MPP(+)-induced oxidative stress in PC12 cells. Neurosci Res. 2011b;69:283–290. doi: 10.1016/j.neures.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Pawlikowska-Pawlega B, Gruszecki WI, Misiak LE, Gawron A. The study of the quercetin action on human erythrocyte membranes. Biochem Pharmacol. 2003;66:605–612. doi: 10.1016/s0006-2952(03)00344-7. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Lange ML, Sultana R, Butterfield DA. Nutritional approaches to modulate oxidative stress in Alzheimer's disease. Curr Alzheimer Res. 2011;8:452–469. doi: 10.2174/156720511796391908. [DOI] [PubMed] [Google Scholar]
- Saad MA, Abdel Salam RM, Kenawy SA, Attia AS. Pinocembrin attenuates hippocampal inflammation, oxidative perturbations and apoptosis in a rat model of global cerebral ischemia reperfusion. Pharmacol Rep. 2015;67:115–122. doi: 10.1016/j.pharep.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal5. 2003:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- Viswanatha GL, Shylaja H, Mohan CG. Alleviation of transient global ischemia/reperfusion-induced brain injury in rats with 1,2,3,4,6-penta-O-galloyl-beta-d-glucopyranose isolated from Mangifera indica. Eur J Pharmacol. 2013;720:286–293. doi: 10.1016/j.ejphar.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Yaidikar L, Thakur S. Arjunolic acid, a pentacyclic triterpenoidal saponin of Terminalia arjuna bark protects neurons from oxidative stress associated damage in focal cerebral ischemia and reperfusion. Pharmacol Rep. 2015;67:890–895. doi: 10.1016/j.pharep.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Yan BC, Park JH, Ahn JH, Kim IH, Park OK, Lee JC, Yoo KY, Choi JH, Lee CH, Hwang IK, Her S, Kim JS, Shin HC, Cho JH, Kim YM, Kwon SH, Won MH. Neuroprotection of posttreatment with risperidone, an atypical antipsychotic drug, in rat and gerbil models of ischemic stroke and the maintenance of antioxidants in a gerbil model of ischemic stroke. J Neurosci Res. 2014a;92:795–807. doi: 10.1002/jnr.23360. [DOI] [PubMed] [Google Scholar]
- Yan BY, Pan CS, Mao XW, Yang L, Liu YY, Yan L, Mu HN, Wang CS, Sun K, Liao FL, Fan JY, Wang XM, Han JY. Icariside II improves cerebral microcirculatory disturbance and alleviates hippocampal injury in gerbils after ischemia-reperfusion. Brain Res. 2014b;1573:63–73. doi: 10.1016/j.brainres.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Yao RQ, Qi DS, Yu HL, Liu J, Yang LH, Wu XX. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem Res. 2012;37:2777–2786. doi: 10.1007/s11064-012-0871-5. [DOI] [PubMed] [Google Scholar]


