Keywords: nerve regeneration, spinal cord injury, tanshinone IIA, spinal pathway, lower urinary tract dysfunction, neurogenic bladder, dorsal root ganglion, detrusor-sphincter dyssynergia, urodynamics, neural regeneration
Abstract
Tanshinone IIA, extracted from Salvia miltiorrhiza Bunge, exerts neuroprotective effects through its anti-inflammatory, anti-oxidative and anti-apoptotic properties. This study intravenously injected tanshinone IIA 20 mg/kg into rat models of spinal cord injury for 7 consecutive days. Results showed that tanshinone IIA could reduce the inflammation, edema as well as compensatory thickening of the bladder tissue, improve urodynamic parameters, attenuate secondary injury, and promote spinal cord regeneration. The number of hypertrophic and apoptotic dorsal root ganglion (L6–S1) cells was less after treatment with tanshinone IIA. The effects of tanshinone IIA were similar to intravenous injection of 30 mg/kg methylprednisolone. These findings suggested that tanshinone IIA improved functional recovery after spinal cord injury-induced lower urinary tract dysfunction by remodeling the spinal pathway involved in lower urinary tract control.
Introduction
Lower urinary tract (LUT) dysfunction after spinal cord injury (SCI) has been recognized as one of the severe complications for SCI patients, even of higher importance than the loss of locomotion (Pikov and Wrathall, 2001; Leung et al., 2007). Urine storage and micturition are complex in the normal condition, requiring the spinal cord to integrate information from the brain, bladder, and urethra (Yu et al., 2003; Birder et al., 2010). SCI impairs LUT by interrupting the communication between the cerebral and spinal circuits that coordinate the bladder detrusor and external urethral sphincter, leading to a severe disorder known as detrusor-sphincter dyssynergia (de Groat and Yoshimura, 2012; Gao et al., 2015). Therefore, the bladder cannot empty efficiently, which inevitably causes more serious consequences, such as urinary tract infections, urinary calculus, hydronephrosis, chronic renal failure and uremia.
Most of the research in LUT dysfunction recovery after SCI focuses on adjusting the bladder or sacral nerves with drug intervention, surgical therapies and functional electrical stimulation. However, these therapeutic methods are less than satisfactory. First, the standard treatment for neurogenic bladder after SCI is usually limited to clean intermittent catheterization, which can lead to repeated urinary tract infection and lower quality of life (Jamison et al., 2013). Second, the antimuscarinic drugs that are currently the first-line choice for the treatment of neurogenic detrusor overactivity cannot be used chronically, because the high dosage required in patients with neurogenic detrusor overactivity often results in more severe side effects, such as dry mouth, constipation, blurred vision, drowsiness, and dry skin and mucosa (Cameron, 2010; Goldmark et al., 2014). Furthermore, surgical options for the neurogenic bladder, such as augmentation cystoplasty, may solve the problems related to bladder capacity but would lead to urinary tract infection, mucus production, urolithiasis and other complications (Kikuno et al., 2009; Lee et al., 2013). Functional electrical stimulation offers another approach to restore LUT function by activating the bladder detrusor and inhibiting the urethral sphincter to produce voiding, alternatively, inhibiting the bladder detrusor to provide urinary continence. Dorsal rhizotomy, before functional electrical stimulation, that transects the dorsal spinal roots to eliminate unwanted bladder and urethral reflexes also eliminates desirable reflexes that affect sexual and bowel functions (Ho et al., 2014).
Tanshinone IIA (TIIA) is an important lipophilic diterpene extracted from Salvia miltiorrhiza Bunge and has been widely used in traditional Chinese medicine for the treatment of many diseases, especially in cardiovascular and cerebrovascular diseases (Xu and Liu, 2013). TIIA also has neuroprotective effects through its anti-inflammatory, anti-oxidative and anti-apoptotic properties (Chen et al., 2012; Gao et al., 2012; Su et al., 2012; Yan et al., 2012). Therefore, we hypothesized that TIIA has great potential in remodeling the spinal pathway. The primary purpose of this study is to evaluate the effect of TIIA in reorganizing the spinal pathway related to LUT control.
Materials and Methods
Animals
A total of 80 specific-pathogen-free female Sprague-Dawley rats, aged 8–10 weeks and weighing 220–250 g, were obtained from Vital River Laboratories in Beijing, China (animal license No. SCXK (Jing) 2012-0001). The rats were housed 3 to 4 per cage, kept on a 12-hour light/dark cycle, and allowed free access to food and water. The experimental protocol was approved by the Animal Care and Use Committee of Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, China (approval No. 2014-14). Adult Sprague-Dawley rats (n = 80) were equally and randomly divided into four groups: sham, SCI, TIIA (SCI + TIIA) and methylprednisolone (MP) (SCI + MP).
Establishment of SCI models
Rats were intraperitoneally anesthetized with 10% chloral hydrate (3.5 mL/kg) and a midline dorsal incision was made over the lower thoracic vertebra to expose the vertebral spines and paravertebral muscles. A laminectomy was made at T9–11 to expose the dura. A standardized mild, incomplete contusive SCI was produced using the multicenter animal spinal cord injury study device (made by New York University, New York, USA) with a 10-g weight dropped from a height of 25 mm onto the exposed dura (Gruner, 1992). The muscle and skin overlying the wound were then sutured in layers, followed by a 3 mL subcutaneous injection of saline solution to replace the blood volume lost during the surgery. After surgery, rats were placed on a heating pad until they awoke. The Basso, Beattie & Bresnahan open field locomotor test and lower abdominal palpation were used to test the success of the SCI model. When the Basso, Beattie & Bresnahan score was 0 (no observable hindlimb movement) and the bladder was excessively filled the rat model was considered successful. Subsequently, the rats were injected with penicillin subcutaneously (200,000 unit/animal/day) for 3 days to prevent incision and urinary tract infections. Bladders of the injured rats were gently manually expressed 2 to 3 times daily by Crede's method, with the volume of expressed urine recorded, until spontaneous micturition was re-established. This provided an estimation of the time required for the re-emergence of spontaneous micturition (urine volume < 2 mL) (Leung et al., 2007). Infection, decubitus, dehydration and autophagia were monitored daily during the process.
Rats were given easy access to food and water and were placed in cages with highly absorbent bedding. Fluid intake was not controlled as it has been shown that this did not affect the time for the recovery of a reflex bladder (Chancellor et al., 1994). Rats failing to establish SCI models or those died were excluded from the experiment and replaced by the same number of rats.
The sham group consisted of rats that were only subjected to laminectomy.
Drug treatment
TIIA injection (batch No. A140133; No. 1 Biochemical Pharmaceutical Co., Ltd., Shanghai, China; 10 mg/2 mL) and MP injection (batch No. A08894; Pfizer Manufacturing Belgium NV, Belgium, 40 mg) were purchased from Dongzhimen Hospital, Beijing University of Chinese Medicine, China, the main component was sodium TIIA sulfonate or methylprednisolone sodium succinate, respectively. The rats in TIIA group were intravenously administered TIIA (20 mg/kg) once a day at the same time from day 1 to day 7 post-surgery. The dose and timing of TIIA administration used were the same as in previous studies (Yin et al., 2012). The rats in the MP group were intravenously administered MP (30 mg/kg) once, immediately post-surgery after confirming the success of the SCI model.
Urodynamic assessment
The urodynamic studies were carried out in awake rats at 2 and 4 weeks after SCI so that the bladder was stabilized after the spinal shock phase (D’Amico and Collins, 2012). To insert a catheter into the bladder, rats were briefly anesthetized with isoflurane. A PE-50 polyethylene catheter was inserted via a midline abdominal incision into the bladder through the bladder dome and secured with cotton thread. The intravesical catheter was connected via a three-way stopcock to a pressure transducer (MP150, BIOPAC Systems, Inc., Goleta, CA, USA) and a micro-infusion pump (WZ-50C6, Smiths Medical Instrument, Co., Zhejiang, China) to record intravesical pressure and infuse saline into the bladder, respectively. Intravesical pressure was recorded continuously using data acquisition software (BIOPAC AcqKnowledge4.2, BIOPAC Systems, Inc.). Anesthesia was stopped after catheter implantation and the rats awakened then left untouched for 30 minutes for bladder stabilization. After this period, rats received a continuous infusion of saline (0.9% NaCl; 37°C) at a rate of 0.1 mL/min (Smith et al., 2008; Artim et al., 2011). It was not necessary to measure abdominal pressure because the abdominal incision was not sutured.
The parameters we assessed included the micturition pressure (maximum bladder pressure during micturition), basal pressure (the lowest bladder pressure between micturitions), threshold pressure (bladder pressure immediately before micturition), and the intercontraction interval. The number of non-voiding contractions was also measured (Andrade et al., 2011).
Saline voided from the urethral meatus was collected and the voided volume was measured. Saline infusion was stopped at the beginning of the voiding contraction to avoid interfering with the measurement of the residual volume. The residual volume was measured by drawing saline through the intravesical catheter and then manually expressing the remaining intravesical contents by exerting pressure on the bladder abdominal wall. The bladder capacity was calculated as the voided volume plus the residual volume. The voiding efficiency (%) is equal to (voided volume/bladder capacity) × 100% (Urakami et al., 2007; Andrade et al., 2011).
Sample drawing
At the end of the urodynamic experiment, the rats were intracardially perfused with saline followed by ice-cold 4% paraformaldehyde. Subsequently, bladders were removed, blotted dry, and weighed. Four dorsal root ganglions (DRGs) on both sides of L6–S1 level, and the 5-mm spinal cord tissues including the central site of injured tissue (T9) were collected. After fixing in 4% paraformaldehyde overnight, tissue blocks were embedded in paraffin. The bladders were cut into 5-μm slices and stained with Masson's trichrome. The spinal cords (T9) were cut transversely into 9-μm slices and stained with Cresyl Violet for Nissl staining. The DRGs were cut transversely into 5-μm slices for terminal deoxyribonucleotidyl transferase (TdT)-mediated biotin-16-dUTP nick-end labeling (TUNEL) assay and 9-μm slices for Nissl staining.
Histological analysis
Hematoxylin-eosin staining
Sections (5-μm thick) of DRGs at 4 weeks after surgery were deparaffinized and washed twice with distilled water and then immersed in hematoxylin solution for 3 minutes, followed by differentiation in 10% acid alcohol for 10 seconds. After washing with tap water for 30 minutes, the sections were dehydrated through 70%, 80%, 90%, 95%, and 100% alcohol, each for 2 minutes, and stained with eosin for 10 seconds. Finally, the sections were dehydrated through increasing concentrations of ethanol, cleared in xylene for 10 minutes and mounted.
Nissl staining
Sections (9-μm thick) of spinal cord and DRGs at 4 weeks after surgery were deparaffinized and washed twice with distilled water. The sections were immersed in 5% Cresyl Violet for 5 minutes. The sections were then dehydrated in increasing concentrations of ethanol and mounted.
Masson's trichrome staining
Sections (5-μm thick) of bladder tissue 4 weeks after surgery were deparaffinized and hydrated through graded alcohols to distilled water. The bladder tissues were stained with Harris Hematoxylin for 2–3 minutes, and then rinsed in distilled water until only the nuclei remained stained. The sections were then stained with Scarlet-Acid Fuchsin for 5 minutes, rinsed twice in distilled water, and immersed in phosphomolybdic acid for 5 minutes. Next, the sections were stained with Brilliant Green for 5 minutes, rinsed, immersed in acetic acid for 30 seconds. Finally, the sections were dehydrated through increasing concentrations of ethanol, air dried, and mounted.
TUNEL assay
TUNEL assay was conducted in accordance with instructions of the TUNEL kit (Roche, Mannheim, Germany). 5-μm thick sections of DRGs at 2 weeks after surgery were washed with phosphate buffered saline (PBS, 0.01 M), incubated with proteinase K at 37°C for 30 minutes, washed with PBS, and then dried. Afterwards, samples were incubated with TUNEL reaction mixture at 37°C for 60 minutes, and washed with PBS.
The slides of Nissl staining, Masson's trichrome staining and TUNEL assay were mounted and analyzed under a bright-field microscope (Nikon, Tokyo, Japan). The injured area in the transverse sections that were located in the lesion epicenter of spinal cord was measured by the Image-Pro plus 6.0 pathological analysis software (Media Cybernetics, Silver Spring, MD, USA). The mean diameter, cross-section areas, cell gaps, and the number of inflammatory cells in L6–S1 DRG were counted and measured. The area of positive cell, mean density and integrated optical density in dorsal root ganglion sections were measured. The integrated optical density was equal to mean density × area of positive cells. The number of motor neurons in the anterior horn of spinal cord and the thickness of muscle layers and vascular congestion in the bladder tissue were all measured by this software. The ratio of injured area was equal to (the injured area/the cross-section of the spinal cord) × 100%.
Statistical analysis
Values were presented as the mean ± SEM. One-way analysis of variance was adopted for comparison between groups. Statistical analyses were performed using SPSS 20.0 statistical software (IBM, Armonk, NY, USA). A value of P < 0.05 was considered statistically significant.
Results
Effect of TIIA on bladder weight after SCI
The weight of a rat's bladder indirectly reflects the extent of compensatory hypertrophy of the detrusor after SCI (Leung et al., 2007). The bladder weights of all the groups were measured at 2 and 4 weeks after surgery (Figure 1). The bladder weights of all experimental groups increased significantly compared with the sham group (P < 0.05), indicating the detrusor hypertrophied after SCI. After drug administration of TIIA or MP, the bladder weights of the TIIA and MP groups were remarkably lower in comparison with the SCI group (P < 0.05). However, there is no significant difference between TIIA and MP groups (P > 0.05).
Figure 1.
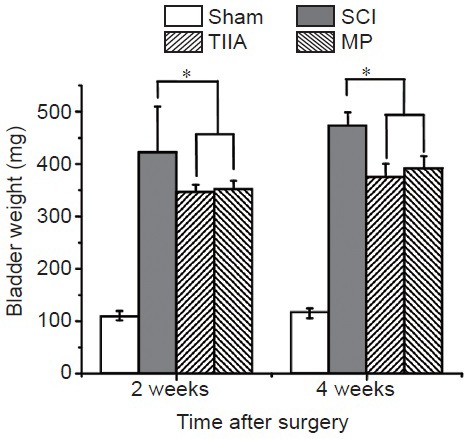
Bladder weights in four groups of rats at 2 and 4 weeks after SCI.
The bladder weights of SCI group, TIIA group and MP group increased significantly compared with sham group at 2 and 4 weeks after SCI. After drug administration of TIIA or MP, the bladder weights of the TIIA and MP groups increased less than those in the SCI group without any treatment (n = 5 in each group, *P < 0.05).
Effect of TIIA on urodynamics after SCI
At 2 weeks after surgery, during the bladder-filling phase, the sham group showed a large number of voiding contractions in this phase. However, the SCI group showed more non-voiding contractions than the sham group (P < 0.05). The SCI group displayed marked alterations, such as reductions in intercontraction interval, voided volume and voiding efficiency. There were also increases in basal pressure, threshold pressure and bladder capacity compared with sham group (P < 0.05; Table 1). On the other hand, the TIIA group exhibited significant improvements compared with the SCI group, such as higher intercontraction interval, voided volume and voiding efficiency (P < 0.05). There were also fewer non-voiding contractions, residual volume and bladder capacity (P < 0.05). The MP group also displayed higher voided volume and voiding efficiency (P < 0.05, vs. SCI group; Table 1). After 4 weeks, similar improvement can also be observed in the TIIA group. Some recovery of bladder function was more obvious after 4 weeks than 2 weeks compared with SCI only (Figure 2).
Table 1.
Changes in urodynamic parameters induced by SCI and effect of treatment with TIIA or MP at 2 and 4 weeks after surgery
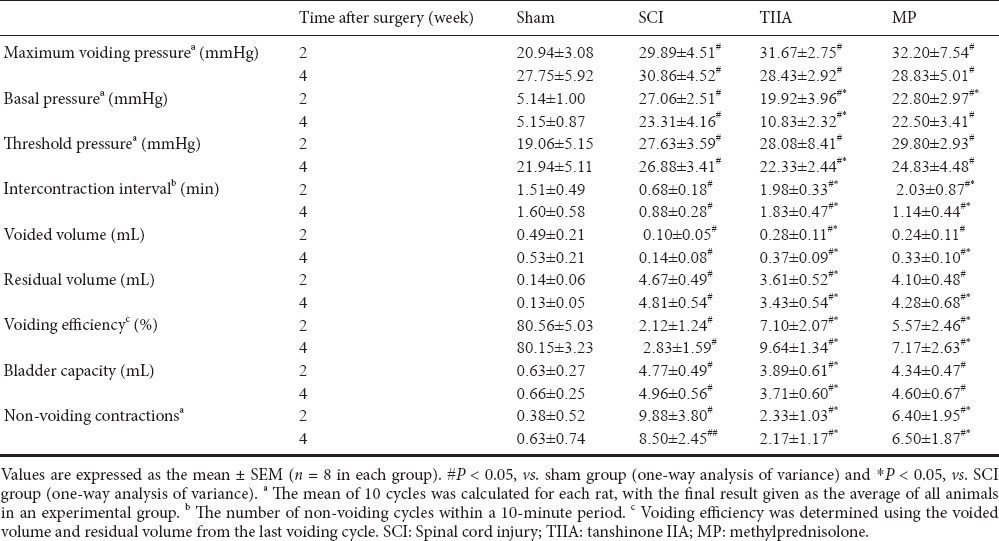
Figure 2.
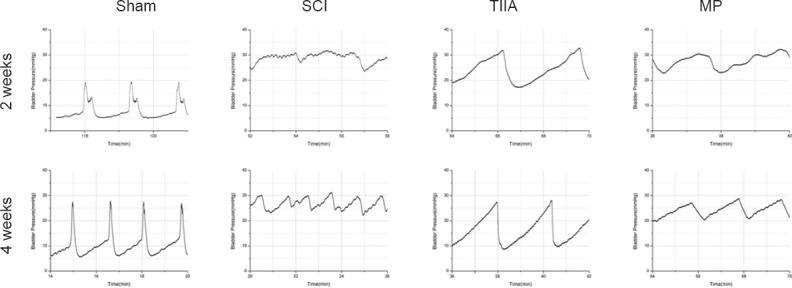
Typical cystometrogram patterns of bladder activity during urodynamics at 2 and 4 weeks after SCI.
Normal bladder in sham group shows stable intravesical pressure without non-voiding contractions. SCI-induced hyperreflexic-bladder shows many large non-voiding contractions (more than 11 mmHg) during saline infusion. At 4 weeks, the micturition interval was more regular than at 2 weeks. After TIIA administration, bladder shows stable intravesical pressure without non-voiding contractions at 2 and 4 weeks after SCI. After MP administration, bladder shows some non-voiding contractions during saline infusion at 2 weeks after SCI, but clearly improved at 4 weeks. SCI: Spinal cord injury; TIIA: tanshinone IIA; MP: methylprednisolone.
Effect of TIIA on secondary injury and spinal cord regeneration after SCI
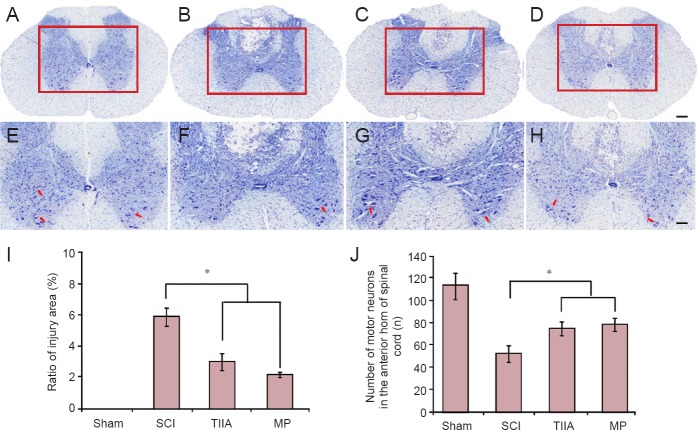
The results of Nissl staining at 4 weeks after surgery are shown in Figure 3. First, compared with the SCI groups, the number of motor neurons in the anterior horn of the sham group was greater and their Nissl bodies and nuclei were clearly visible. The number of motor neurons in the anterior horn in SCI group had decreased dramatically 4 weeks after injury. However, the administration of TIIA or MP lessened this change over the same time. Second, with the development of secondary injury after SCI, the area of injured spinal cord gradually increased. In the TIIA and MP groups the ratio of injured area was significantly less than that of the SCI group (P < 0.05).
Figure 3.
Effect of TIIA on secondary injury and spinal cord regeneration at 4 weeks after SCI (Nissl staining).
(A, E) The motor neurons in the anterior horn of the sham group. Nissl bodies and nuclei were clearly visible. (B, F) The number of motor neurons in the anterior horn in SCI group had decreased dramatically. The administration of TIIA (C, G) or MP (D, H) reduced this change. Red arrows show motor neurons in the anterior horn of spinal cord. Scale bars: 200 μm for A–D; 100 μm for E–H. (I) The ratio of spinal cord injury area (n = 5 in each group). (J) The number of motor neurons in the anterior horn (n = 8 in each group). *P < 0.05. SCI: Spinal cord injury; TIIA: tanshinone IIA; MP: methylprednisolone.
Effect of TIIA on histology of bladder tissue after SCI
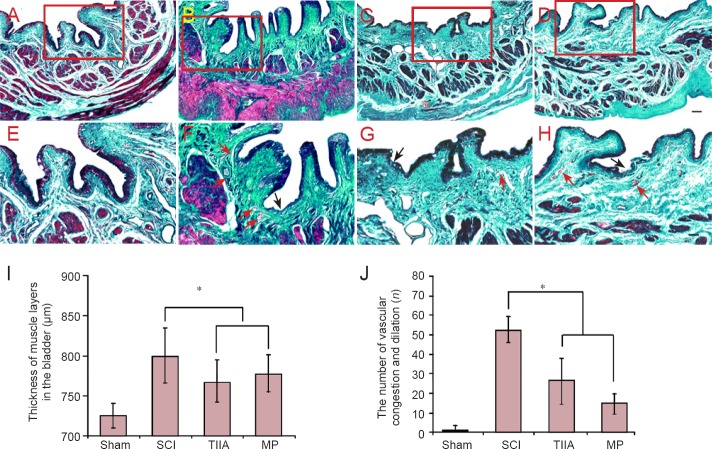
The results of Masson's trichrome staining at 4 weeks after surgery were shown in Figure 4. Bladders from the SCI group revealed the presence of vascular alterations, edema, and proliferation of urothelial layers compared with bladders from the sham group. The continuity of the umbrella cell layer of the urothelium was disrupted in rats at 4 weeks after SCI. A marked neutrophil infiltration to the suburothelial tissue as well as blood vessel congestion and dilation were observed. However, there was less neutrophil infiltration to the suburothelial tissue in the TIIA or MP groups and the muscle layers were not as thick as in the SCI group (P < 0.05).
Figure 4.
Effect of TIIA on histology of bladder tissue at 4 weeks after SCI (Masson's trichrome staining).
(A, E) Normal bladder morphology in sham group: The red color indicates muscle fibers, and the green color indicates collagen. (B, F) Bladders from SCI group reveal the presence of edema (pink) and proliferation of urothelial layers. The continuity of the umbrella cell layer of the urothelium is disrupted. A marked neutrophil infiltration to the suburothelial tissue as well as blood vessel congestion and dilation are observed in the TIIA (C, G) and MP (D, H) groups. The urothelium is disrupted partially and some blood vessel congestion and dilation can be seen. Scale bars: 100 μm for A–D; 50 μm for E–H. The disrupted urothelium is shown by black arrows. Vascular congestion and dilation are shown by red arrows. (I) The thickness of muscle layers. (J) The number of vascular congestion and dilation (n = 8 in each group; *P < 0.05). SCI: Spinal cord injury; TIIA: tanshinone IIA; MP: methylprednisolone.
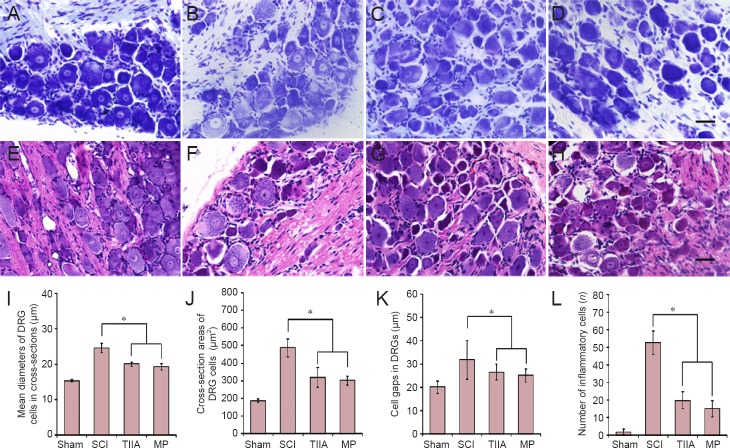
Effect of TIIA on the change of DRG (L6–S1) cells after SCI
The isolated DRGs were examined histologically with Nissl staining and hematoxylin-eosin staining (Figure 5). First, almost no inflammatory cells were observed in the sham group, but there were a large number of inflammatory cells in the SCI group. In the TIIA or MP groups, only some of the ganglion cells were infiltrated by inflammatory cells. Second, the sham group showed typical morphology of normal ganglion cells; SCI group revealed that ganglion cell gaps widened significantly. With the administration of TIIA or MP, the ganglion cell gaps in the TIIA and MP groups were narrowed compared with the SCI group (P < 0.05). Third, the mean diameter and area of DRG cells in cross-section from SCI group was much higher than that of sham group and TIIA and MP groups (P < 0.05). Moreover, the normal cell nuclei in the sham group were round and centered; however, the cell bodies in the SCI group became hypertrophic and elongated with some of the nuclei shrunken or disappeared. Some Nissl bodies also disappeared or were replaced by vacuoles. In the TIIA and MP groups, only a small number of cells were observed with shrunken or absent nuclei and absent or vacuous Nissl bodies.
Figure 5.
Effect of TIIA on the change of dorsal root ganglion (L6–S1) neurons at 4 weeks after SCI (Nissl staining in A–D and hematoxylin-eosin staining in E–H).
(A, E) Typical morphology of normal DRG cells: cell nuclei were round and centered. (B, F) After SCI, cell bodies became hypertrophic and elongated with partial or total loss of their nuclei. Some Nissl bodies also disappeared leaving vacuoles. In the TIIA (C, G) and MP (D, H) treatment groups only a small number of cells was observed with reduced or no nucleus and loss of Nissl bodies. Scale bars: 20 μm for A–D; 10 μm for F–I. (I, J) Mean diameter and area of ganglion cells. (K) Ganglion cell gaps. (L) Number of inflammatory cells (n = 8 in each group; *P < 0.05). SCI: Spinal cord injury; TIIA: tanshinone IIA; MP: methylprednisolone; DRG: dorsal root ganglion.
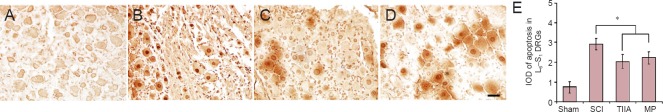
The integrated optical densities of apoptosis in L6–S1 DRGs of TIIA and MP groups were significantly less compared with SCI group at 2 weeks after surgery (P < 0.05). There was no obvious difference in the integrated optical density of apoptosis between the TIIA and MP groups (P > 0.05; Figure 6).
Figure 6.
Apoptosis in L6–S1 DRG cells examined by terminal deoxyribonucleotidyl transferase (TdT)-mediated biotin-16-dUTP nick-end labeling (TUNEL) at 2 weeks after SCI.
(A) Cell nuclei of DRGs were very light brown, not much darker than the cytoplasm, in the sham group. (B) A large number of DRG apoptotic cell nuclei were stained dark brown in the SCI group. (C, D) Apoptotic DRG cell nuclei were stained brown or light brown in the TIIA and MP groups. (E) IOD of apoptotic DRG cells (n = 8 in each group; *P < 0.05). SCI: Spinal cord injury; TIIA: tanshinone IIA; MP: methylprednisolone; DRGs: dorsal root ganglions; IOD: integrated optical density.
Discussion
Urinary complications are very common in patients with SCI, therefore they attract considerable attention. Over the years, most of the therapeutic strategies have concentrated on the upper urinary tract and the improvement of the quality of life by facilitating the storage of urine and bladder emptying (Birder et al., 2010; Cruz and Cruz, 2011). However, the therapeutic effects are far from satisfactory. Recently, many studies have focused on remodeling the spinal pathway related to LUT control (Lee et al., 2013; Kim et al., 2015).
SCI at the cervical or thoracic levels disrupts the voluntary control of micturition as well as the normal reflex pathways that coordinate detrusor and sphincter function. The bladder is initially areflexic, but then becomes hyperreflexic due to the emergence of a spinal micturition reflex pathway. Therefore, the bladder does not empty efficiently because of the loss of coordination between the bladder and urethral outlet, which is called detrusor-sphincter dyssynergia (Leung et al., 2007). Initially detrusor-sphincter dyssynergia after SCI causes bladder outlet obstruction and bladder load increase, leading to chronic inflammation and tissue edema. Then bladder weight increases and bladder wall gradually thickens. Other changes after SCI include the disruption of the continuity of the umbrella cell layer in urothelium and there is a marked neutrophil infiltration to the suburothelial tissue and blood vessel congestion and dilation. Ikeda and Kanai (2008) using optical imaging techniques showed that alterations at the luminal surface of the bladder contribute to the changes in LUT function after SCI. On the other hand, de Groat and Yoshimura (2012) found that detrusor-sphincter dyssynergia after SCI induce somal hypertrophy of bladder afferent neurons in the DRGs (L6–S1). Their studies in cats proved that unmyelinated C-fiber afferents rather than Aδ afferents initiate voiding after SCI. Therefore, SCI not only causes changes in the structure of the bladder itself, but also leads to changes in the neuronal pathways innervating the LUT. These findings suggest that approaches that facilitate the remodeling of the spinal micturition reflex pathway might be useful in treating detrusor-sphincter dyssynergia after SCI.
It has been revealed that the recovery of bladder function after SCI could be mediated by improving the remodeling of the spinal pathway related to LUT control (Zhao et al., 2015). A popular area of research has been the use of cellular implants or the control of the extracellular environment of the injured site. It was found that injection of neural stem cells at the lesion site in the spinal cord of SCI rats enhanced threshold pressure, residual volume and non-voiding contractions (Kim et al., 2015). Olfactory ensheathing cells have also been used in some studies (Fouad et al., 2009). The technique of regulating the extracellular environment of the lesion site and the formation of the glial scar has also been useful in improving bladder function. For example, chondroitinase, which has been used alone or in combination with Schwann cell transplantation, improves recovery of bladder function in rats after SCI (Alluin et al., 2014). Other therapies have combined implants of biomaterials with other approaches mentioned above (Lee et al., 2013).
Our previous study demonstrated that TIIA can facilitate the recovery of neurological function in rats after SCI, and especially promotes the recovery of the sensory function of the hindlimb. Therefore, we hypothesized that the use of drugs for spinal cord repair may also improve the recovery of bladder function after SCI. In this study, detrusor-sphincter dyssynergia following SCI caused bladder outlet obstruction and bladder load increase, which result in gradual thickening of bladder wall and bladder weight increase. The difference of bladder weight between SCI group and sham group suggested that bladder wall thickening is a chronic compensatory action that increases with time (de Groat and Yoshimura, 2012). Tissue edema induced by inflammation contributes to the increase in bladder weight after SCI. After TIIA or MP was administrated to SCI rats, their bladder weight decreased compared with the SCI group. Nevertheless, there is no significant difference between TIIA and MP groups. The urothelial layers were also changed after SCI. The continuity of the umbrella cell layer of the urothelium is disrupted in rats at 4 weeks after SCI. A marked neutrophil infiltration to the suburothelial tissue and vascular congestion and dilation was observed. The changes can be attenuated by treatment with TIIA or MP.
Alterations at the luminal surface of the bladder may contribute to the changes in LUT function after SCI. Optical imaging techniques revealed that phasic contractions in bladders from SCI animals originate from localized sites in the bladder dome and are driven by activity arising in the urothelium (McCarthy et al., 2009). Removal of the mucosa eliminates the phasic contractions in bladders from SCI rats. In addition, gap junction blockers suppress the phasic contractions. It has been proposed that phasic activity in bladders after SCI is due to a signaling pathway that originates in the urothelium and then passes through gap junctions via a network of myofibroblasts in the lamina propria to smooth muscle and afferent nerves (Ikeda and Kanai, 2008). In our study, urodynamic assessment examined at 2 and 4 weeks after SCI supported the positive effect of MP and TIIA on recovery of bladder function. These include increases in voided volume and voiding efficiency and reductions in residual volume and in non-voiding contractions after SCI.
Electrophysiological studies in cats revealed that the recovery of bladder function after SCI is mediated by a change in the afferent limb of the micturition reflex pathway and remodeling of synaptic connections in the spinal cord. In chronic SCI cats, unmyelinated C- fiber afferents rather than Aδ afferents initiate voiding. These findings are supported by pharmacological studies showing that subcutaneous administration of capsaicin, a C-fiber neurotoxin, completely blocks reflex bladder contractions induced by bladder distention in chronic spinal cats; whereas capsaicin has no inhibitory effect on reflex bladder contractions in spinal intact cats (Yoshiyama et al., 1999; de Groat and Yoshimura, 2012). In this study, histological observation showed that bladder afferent neurons in the DRG (L6–S1) undergo somal hypertrophy in SCI rats. However, that could be altered by TIIA or MP intervention, which also prevents bladder hypertrophy. Further research is needed to examine whether TIIA and MP might promote C-fiber bladder afferents to sprout and contribute to the synaptic remodeling of the spinal micturition reflex pathway after SCI.
The ionic mechanisms underlying the hyperexcitability of C-fiber bladder afferents were investigated using whole-cell patch clamp recording in bladder DRG neurons (Yoshimura and de Groat, 1997; Yoshiyama et al., 1999). Dissociated bladder DRG neurons from chronic SCI rats are larger in size and have increased input capacitance. This is consistent with results from histological studies showing that bladder afferent neurons in the DRG (L6–S1) undergo somal hypertrophy in SCI rats (Kruse et al., 1995). The action potentials in bladder afferent neurons are also different after SCI in both rats and cats. In contrast to neurons from spinal intact rats where the majority (approximately 70%) of bladder afferent neurons exhibit high threshold tetrodotoxin-resistant action potentials (Yoshimura et al., 1996), in chronic SCI rats, 60% of bladder afferent neurons exhibit low threshold tetrodotoxin-sensitive action potentials. Therefore, it is likely that following SCI, A-type potassium channels are suppressed in parallel with an increased expression of tetrodotoxin-sensitive Na+ currents, thereby increasing the excitability of C-fiber bladder afferent neurons. Thus, drugs that selectively modulate these types of ion channels might be useful in treating neurogenic detrusor overactivity. Whether TIIA or MP can modulate these types of ion channels would help establish the mechanism of their action.
In summary, TIIA could reduce the inflammation, edema as well as compensatory thickening of the bladder tissue and improve urodynamic parameters in adult rats at 2 and 4 weeks after SCI. Moreover, TIIA inhibited the increase of apoptotic and hypertrophic DRGs (L6–S1) at 4 weeks after SCI. Taken together, TIIA could improve functional recovery in SCI-induced LUT dysfunction in adult rats and its effect is similar to MP. Although there were no dramatic differences between TIIA group and MP group, TIIA might be a better alternative for the treatment of SCI-induced LUT dysfunction. Large doses of MP application has caused many severe complications (Varma et al., 2013) so it is no longer recommended as the first-line drug for acute SCI (Walters et al., 2013). TIIA could be consecutive intravenously injected for 7 days but no side-effect.
Footnotes
Funding: This work was supported by the China Postdoctoral Science Foundation, No. 2015M581120.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Dawes EA, Maxwell R, Yu J, Li CH, Qiu Y, Song LP, Zhao M
References
- Alluin O, Delivet-Mongrain H, Gauthier MK, Fehlings MG, Rossignol S, Karimi-Abdolrezaee S. Examination of the combined effects of chondroitinase ABC, growth factors and locomotor training following compressive spinal cord injury on neuroanatomical plasticity and kinematics. PLoS One. 2014;9:e111072. doi: 10.1371/journal.pone.0111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade EL, Forner S, Bento AF, Leite DF, Dias MA, Leal PC, Koepp J, Calixto JB. TRPA1 receptor modulation attenuates bladder overactivity induced by spinal cord injury. Am J Physiol Renal Physiol. 2011;300:F1223–1234. doi: 10.1152/ajprenal.00535.2010. [DOI] [PubMed] [Google Scholar]
- Artim DE, Kullmann FA, Daugherty SL, Bupp E, Edwards CL, de Groat WC. Developmental and spinal cord injury-induced changes in nitric oxide-mediated inhibition in rat urinary bladder. Neurourol Urodyn. 2011;30:1666–1674. doi: 10.1002/nau.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M. Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn. 2010;29:128–139. doi: 10.1002/nau.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AP. Pharmacologic therapy for the neurogenic bladder. Urol Clin North Am. 2010;37:495–506. doi: 10.1016/j.ucl.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Chancellor MB, Erhard MJ, Hirsch IH, Stass WE., Jr Prospective evaluation of terazosin for the treatment of autonomic dysreflexia. J Urol. 1994;151:111–113. doi: 10.1016/s0022-5347(17)34884-x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wu X, Yu S, Fauzee NJS, Wu J, Li L, Zhao J, Zhao Y. Neuroprotective capabilities of Tanshinone IIA against cerebral ischemia/reperfusion injury via anti-apoptotic pathway in rats. Biol Pharm Bull. 2012;35:164–170. doi: 10.1248/bpb.35.164. [DOI] [PubMed] [Google Scholar]
- Cruz CD, Cruz F. Spinal cord injury and bladder dysfunction: new ideas about an old problem. ScientificWorldJournal. 2011;11:214–234. doi: 10.1100/tsw.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico SC, Collins WF. External urethral sphincter motor unit recruitment patterns during micturition in the spinally intact and transected adult rat. J Neurophysiol. 2012;108:2554–2567. doi: 10.1152/jn.00927.2011. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012;235:123–132. doi: 10.1016/j.expneurol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Pearse DD, Tetzlaff W, Vavrek R. Transplantation and repair: combined cell implantation and chondroitinase delivery prevents deterioration of bladder function in rats with complete spinal cord injury. Spinal Cord. 2009;47:727–732. doi: 10.1038/sc.2009.10. [DOI] [PubMed] [Google Scholar]
- Gao S, Liu Z, Li H, Little PJ, Liu P, Xu S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis. 2012;220:3–10. doi: 10.1016/j.atherosclerosis.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Gao Y, Qu B, Shen Y, Su XJ, Dong XY, Chen XM, Zhou YH, Pi HY. Bibliometric profile of neurogenic bladder in the literature: a 20-year bibliometric analysis. Neural Regen Res. 2015;10:797–803. doi: 10.4103/1673-5374.156985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark E, Niver B, Ginsberg DA. Neurogenic bladder: from diagnosis to management. Curr Urol Rep. 2014;15:448. doi: 10.1007/s11934-014-0448-8. [DOI] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–128. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- Ho CH, Triolo RJ, Elias AL, Kilgore KL, DiMarco AF, Bogie K, Vette AH, Audu M, Kobetic R, Chang SR, Chan KM, Dukelow S, Bourbeau DJ, Brose SW, Gustafson KJ, Kiss Z, Mushahwar VK. Functional electrical stimulation and spinal cord injury. Phys Med Rehabil Clin N Am. 2014;25:631–654. doi: 10.1016/j.pmr.2014.05.001. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Kanai A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: role of mucosal muscarinic receptors. Am J Physiol Renal Physiol. 2008;295:F454–461. doi: 10.1152/ajprenal.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison J, Maguire S, McCann J. Catheter policies for management of long term voiding problems in adults with neurogenic bladder disorders. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD004375.pub2. CD004375. [DOI] [PubMed] [Google Scholar]
- Kikuno N, Kawamoto K, Hirata H, Vejdani K, Kawakami K, Fandel T, Nunes L, Urakami S, Shiina H, Igawa M, Tanagho E, Dahiya R. Nerve growth factor combined with vascular endothelial growth factor enhances regeneration of bladder acellular matrix graft in spinal cord injury-induced neurogenic rat bladder. BJU Int. 2009;103:1424–1428. doi: 10.1111/j.1464-410X.2008.08129.x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Shim SR, Doo SW, Yang WJ, Yoo BW, Kim JM, Ko YM, Song ES, Lim IS, Lee HJ, Song YS. Bladder recovery by stem cell based cell therapy in the bladder dysfunction induced by spinal cord injury: systematic review and meta-analysis. PLoS One. 2015;10:e0113491. doi: 10.1371/journal.pone.0113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse MN, Bray LA, de Groat WC. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Auton Nerv Syst. 1995;54:215–224. doi: 10.1016/0165-1838(95)00011-l. [DOI] [PubMed] [Google Scholar]
- Lee YS, Lin CY, Jiang HH, DePaul M, Lin VW, Silver J. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J Neurosci. 2013;33:10591–10606. doi: 10.1523/JNEUROSCI.1116-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung PY, Johnson CS, Wrathall JR. Comparison of the effects of complete and incomplete spinal cord injury on lower urinary tract function as evaluated in unanesthetized rats. Exp Neurol. 2007;208:80–91. doi: 10.1016/j.expneurol.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy CJ, Zabbarova IV, Brumovsky PR, Roppolo JR, Gebhart GF, Kanai AJ. Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity. J Urol. 2009;181:1459–1466. doi: 10.1016/j.juro.2008.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikov V, Wrathall JR. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. J Neurosci. 2001;21:559–569. doi: 10.1523/JNEUROSCI.21-02-00559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PP, Hurtado E, Smith CP, Boone TB, Somogyi GT. Comparison of cystometric methods in female rats. Neurourol Urodyn. 2008;27:324–329. doi: 10.1002/nau.20512. [DOI] [PubMed] [Google Scholar]
- Su CC, Chien SY, Kuo SJ, Chen YL, Cheng CY, Chen DR. Tanshinone IIA inhibits human breast cancer MDA-MB-231 cells by decreasing LC3-II, Erb-B2 and NF-κBp65. Mol Med Rep. 2012;5:1019–1022. doi: 10.3892/mmr.2012.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakami S, Shiina H, Enokida H, Kawamoto K, Kikuno N, Fandel T, Vejdani K, Nunes L, Igawa M, Tanagho EA, Dahiya R. Functional improvement in spinal cord injury-induced neurogenic bladder by bladder augmentation using bladder acellular matrix graft in the rat. World J Urol. 2007;25:207–213. doi: 10.1007/s00345-006-0142-7. [DOI] [PubMed] [Google Scholar]
- Varma AK, Das A, Wallace G, Barry J, Vertegel AA, Ray SK, Banik NL. Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res. 2013;38:895–905. doi: 10.1007/s11064-013-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BC, Hadley MN, Hurlbert RJ, Aarabi B, Dhall SS, Gelb DE, Harrigan MR, Rozelle CJ, Ryken TC, Theodore N American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60:82–91. doi: 10.1227/01.neu.0000430319.32247.7f. [DOI] [PubMed] [Google Scholar]
- Xu S, Liu P. Tanshinone II-A: new perspectives for old remedies. Expert Opin Ther Pat. 2013;23:149–153. doi: 10.1517/13543776.2013.743995. [DOI] [PubMed] [Google Scholar]
- Yan MY, Chien SY, Kuo SJ, Chen DR, Su CC. Tanshinone IIA inhibits BT-20 human breast cancer cell proliferation through increasing caspase 12, GADD153 and phospho-p38 protein expression. Int J Mol Med. 2012;29:855–863. doi: 10.3892/ijmm.2012.908. [DOI] [PubMed] [Google Scholar]
- Yin X, Yin Y, Cao FL, Chen YF, Peng Y, Hou WG, Sun SK, Luo ZJ. Tanshinone IIA attenuates the inflammatory response and apoptosis after traumatic injury of the spinal cord in adult rats. PLoS One. 2012;7:e38381. doi: 10.1371/journal.pone.0038381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J Physiol. 1997;503:269–276. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, White G, Weight FF, de Groat WC. Different types of Na+ and A-type K+ currents in dorsal root ganglion neurones innervating the rat urinary bladder. J Physiol. 1996;494:1–16. doi: 10.1113/jphysiol.1996.sp021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama M, Nezu FM, Yokoyama O, de Groat WC, Chancellor MB. Changes in micturition after spinal cord injury in conscious rats. Urology. 1999;54:929–933. doi: 10.1016/s0090-4295(99)00234-4. [DOI] [PubMed] [Google Scholar]
- Yu X, Xu L, Zhang XD, Cui FZ. Effect of spinal cord injury on urinary bladder spinal neural pathway: a retrograde transneuronal tracing study with pseudorabies virus. Urology. 2003;62:755–759. doi: 10.1016/s0090-4295(03)00486-2. [DOI] [PubMed] [Google Scholar]
- Zhao WT, Li PP, Zhang HF, Wu NP, Liang JF. Mesenchymal stem cell transplantation for spinal cord injury: a Meta-analysis. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:5865–5871. [Google Scholar]