Abstract
Rodents have been widely used in the production of cerebral ischemia models. However, successful therapies have been proven on experimental rodent stroke model, and they have often failed to be effective when tested clinically. Therefore, nonhuman primates were recommended as the ideal alternatives, owing to their similarities with the human cerebrovascular system, brain metabolism, grey to white matter ratio and even their rich behavioral repertoire. The present review is a thorough summary of ten methods that establish nonhuman primate models of focal cerebral ischemia; electrocoagulation, endothelin-1-induced occlusion, microvascular clip occlusion, autologous blood clot embolization, balloon inflation, microcatheter embolization, coil embolization, surgical suture embolization, suture, and photochemical induction methods. This review addresses the advantages and disadvantages of each method, as well as precautions for each model, compared nonhuman primates with rodents, different species of nonhuman primates and different modeling methods. Finally it discusses various factors that need to be considered when modelling and the method of evaluation after modelling. These are critical for understanding their respective strengths and weaknesses and underlie the selection of the optimum model.
Keywords: nerve regeneration, stroke, cerebral ischemia, middle cerebral artery occlusion, nonhuman primates, model selection, neural regeneration
Introduction
Survey data from the World Health Organization indicate that stroke has the second highest mortality rate worldwide and results in high rates of disability (Feigin et al., 2003). The majority of strokes (80%) are ischemic, arising from thromboembolic occlusion of a major cerebral artery or its branches (Zhang, 2010). Approximately 85% of ischemic stroke cases involve the middle cerebral artery (MCA) (Smith et al., 2005).
As a complex disease, stroke is influenced by many factors including genetic profile and the environment (Bacigaluppi et al., 2010). The erratic location, duration and severity of ischemia along with the patients’ other conditions lead to the clinical variability, which poses great challenges for clinical studies. In addition, the response of the brain to stroke and effects from other systems make it impossible to emulate stroke using only in vitro systems (Hainsworth and Markus, 2008). For these reasons, it is necessary to reproduce specific aspects of stroke by using eligible animal models to clarify the pathophysiology of stroke and find efficient preventive and therapeutic solutions. Several animals have been used to establish cerebral ischemic models such as rodents (Fluri et al., 2015; Gao et al., 2015; Feng et al., 2016), canines (Christoforidis et al., 2011), rabbits (Feng et al., 2016), pigs (Platt et al., 2014), sheep (Wells et al., 2015) and nonhuman primates (NHPs).
Rodents are most widely used in ischemia models due to their low cost, abundant source and easy operation (Tian 2015; Yan et al., 2015). While many therapeutics have been proven to be effective on rodent stroke model, they have failed to be successful when tested clinically (Endres et al., 2008). One major reason for the ineffectiveness is that rodents and human brains differ in both their anatomical structure and physiology (Cook and Tymianski, 2011). Accordingly, the Stroke Therapy Academic Industry Roundtable recommends that preclinical trials for stroke treatment use large animals that are more similar to humans, such as NHPs (Saver et al., 2009).
The brains of NHPs are generally composed of multiple gyri. NHPs have a complete cerebral arterial ring similar to humans (Fukuda and del Zoppo, 2003). The distributions of the internal carotid and vertebral arteries in NHPs are also very similar to those in humans. Infarction of the M1 segment (Figure 1) of the MCA of NHPs causes ischemia primarily in the basal ganglia and white matter, similar to the infarct areas observed in human stroke patients (Fukuda and del Zoppo, 2003).
Figure 1.
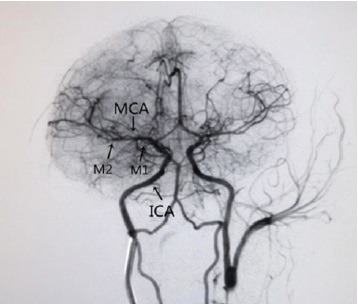
Our model with ensuing mark to show the vessel anatomy of a rhesus macaque via digital subtraction angiography.
MCA: Middle cerebral artery; ICA: internal carotid artery; M1: sphenoidal segment; M2: insular segment.
The pathophysiology of MCA infarction indicates that the components of vascular hemostasis in NHPs mainly include platelets, coagulation proteins in blood plasma, fibrinolytic proteins, inhibitory proteins and polymorphonuclear cells; all similar to the hemostatic agents present in the human cerebrovascular system. Therefore, NHPs stroke models mimic the cerebrovascular reactions to stroke in humans more comprehensively than other animal models of stroke (Fukuda and del Zoppo, 2003).
Studies have demonstrated that the motor dysfunction caused by middle cerebral arterial infarction is more closely correlated with deficits in white matter than with deficits in gray matter (Bihel et al., 2011; Rosso et al., 2011). White matter ischemia may extend the therapeutic time window (Koga et al., 2005). The area ratio of gray matter to white matter in NHPs is similar to that in humans. It has been found that NHP stroke models mimic the changes in gray and white matters and the functional damage after cerebral ischemia more effectively than other animal models of stroke (Bihel et al., 2011). Hence, NHPs may provide the best option for the establishment of cerebral ischemia models (Gao and Cheng, 2002). The middle cerebral artery occlusion (MCAO) model has the greatest clinical similarity to the most common cases of stroke in humans (Feigin et al., 2003).
Over the last few decades, many NHPs ischemic models have been designed to study the pathogenesis and therapeutics. However, different models were constructed for different purposes (Hainsworth and Markus, 2008). Faced with a variety of NHPs models, numerous issues need to be taken into consideration. Therefore, we thoroughly summarize all the NHPs models of focal cerebral ischemia to date. This is critical for understanding their respective strengths and weaknesses so that the optimum model can be selected for the specific conditions and purpose of an experiment.
Methods of Establishing Focal Ischemia Models using NHPs
Surgical methods
The surgical methods of stroke induction can be divided into two categories, an intracranial approach and a transorbital approach, according to different paths into the brain. For the former, MCA was exposed after the parietal bone had been removed and the cerebral dura mater sectioned (Marshall and Ridley, 2003). For the latter, only after the eyeball, orbital medial wall and planum sphenoidale were removed can the MCA be exposed (West et al., 2009).
After MCA exposure, the electrocoagulation and endothelin-1 (ET-1)-induced occlusion methods have been used to establish cerebral ischemia models within the first category (Virley et al., 2004; Yin et al., 2013; Chen et al., 2015); and the microvascular clip occlusion method was employed when utilizing the transorbital approach (Young et al., 1997; Takamatsu et al., 2000; West et al., 2009). These techniques are usually characterized by accurate positioning, good reproducibility and visual inspection of successful MCAO, which made them widely used in the past. However, the limitations of these methods are also evident. (1) Large wounds are inevitable. The craniotomy method requires partial removal of the skull and damages the integrity of the skull, whereas the transorbital method does not. However, the transorbital method requires enucleation of the eyeball, which has a major influence on the physiology and psychology of the animal and thus affects the assessment of an animal's neurological function and motor behavior post operation. There are also ethical concerns regarding animal welfare. (2) The small surgical field and the high precision of the surgery required sometimes results in failure of the operation and death of the animal. (3) Nerves are vulnerable to damage in searching for the MCA. (4) The invasive operation may cause vasospasm, which alters blood flow, affects the progression of cerebral infarction and causes circulation disorders. (5) The scope and size of the infarct are unpredictable.
Electrocoagulation
Using the transcranial electrocoagulation method, Yin et al. (2013) established a rhesus macaque (Macaca mulatta) MCAO model. After the animal was anesthetized, a hole was drilled in the sagittal sinus of the right skull at 2 mm above the center of the right orbit. After the cranium was removed, an incision was made from the cerebral dura mater of the right frontal lobe to the frontal pole, the sagittal sinus and the ventral temporal lobe. The MCA was identified at the juncture of the frontal and temporal lobes, and the M1 segment of the MCA was severed using a bipolar electrocoagulation system to form a permanent cerebral infarct.
ET-1-induced occlusion method
To date, ET-1 is one of the most effective endogenous vasoconstrictors. Regional cerebral blood flow decreases as a result of its potent and long-lasting vasoconstrictive action, which causes focal cerebral infarction (Reid et al., 1995). Studies have indicated that intraductal infusion causes less significant vasospasm than extraductal injection of the blood vessels (Mima et al., 1989; Ogura et al., 1991). Virley et al. (2004) performed a craniotomy to expose the M2 segment of the MCA following the induction of anesthesia in marmosets. In the vicinity of the M2 segment, a stellate incision was performed to open the dura and arachnoid mater. A cannula connecting to an infusion pump was inserted through the incision. ET-1 was injected for 5 minutes at a rate of 5 μL per minute. During the induction of cerebral occlusion, the cerebral arteries constricted resulting in cerebral infarction. The indicator of the successful establishment of the cerebral occlusion model was vasoconstriction and blanching of the cortex. Once the injection was terminated, the arteries recovered gradually, and the infarct area became reperfused. This type of cerebral ischemia model cannot strictly control the duration of cerebral ischemia, and the extent of damage differs significantly according to the magnitude of the individual reactions (Freret et al., 2008).
Microvascular clip occlusion method
Young et al. (1997) used a transorbital method to expose the baboon's (Papio) MCA. When the dura and arachnoid mater were opened, one microvascular clip was placed at the proximal portion of the main trunk of the MCA, with another one placed at the orbitofrontal branch. Clipping the artery shut produced a focal cerebral infarct. Subsequently, removal of the microvascular clip resulted in the reperfusion of the infarct area. Giffard et al. (2005) used this method to mimic mechanical thrombectomy and re-opened the orbit to remove the micro clip after 2 days. Using this method, the duration of cerebral ischemia and reperfusion can be strictly controlled, and the infarct is more specifically defined.
Endovascular intervention method
Endovascular intervention refers to a group of techniques used for the successful establishment of focal cerebral occlusion with the aid of imaging. This method can be divided into two types. In one method, the guiding catheter was inserted into the femoral artery and passed through the thoracic aorta, aortic arch, and common carotid artery to reach the internal carotid artery. Using a microcatheter, an embolus (such as an autologous blood clot, a balloon, a coil or a surgical suture) (Kito et al., 2001; Gao et al., 2006; Guo et al., 2011; Rodriguez-Mercado et al., 2012) was subsequently delivered to a specific position in the MCA to induce focal ischemia. In the other method, the guiding catheter was unnecessary. Instead, a intraluminal thread coated with a thermofusible adhesive at one end was directly delivered via the internal carotid artery (ICA) after an initial incision at the external carotid artery to a specific position of the MCA to form an embolism (Freret et al., 2008).
The advantages of this method are as follows. (1) It is minimally invasive and has little influence on the pain, physiology and psychology of the animals (de Crespigny et al., 2005). (2) It has good repeatability, and most of the methods can achieve reperfusion (Kito et al., 2001; de Crespigny et al., 2005; Gao et al., 2006; Freret et al., 2008; Guo et al., 2011). (3) Its direct effect on intracranial vessels can avoid damaging the endocranium and destroying the intracranial environment. (4) The operation is performed on the lower limb or neck and thus avoids injuring the head, which benefits electrophysiological monitoring of acute and chronic stroke. The disadvantages of this method are as follows: (1) the infarct site is imprecise, and it is easy to form large infarcts (de Crespigny et al., 2005); (2) this technique has substantial technological requirements, as the operation is based on angiography, which requires a skilled operator; and (3) the production cost is high, as are the costs of the catheter and microcatheter.
Autologous blood clot embolization method
The autologous blood clot embolization method uses the blood of experimental animals to produce blood clot emboli, which are then delivered to the MCA to form an occlusion via a microcatheter inserted through a guiding catheter. There is no standard for the composition or size of these emboli. Kito et al. (2001) drew blood from the femoral vein and rested the blood in a catheter for 3 hours to clot. They prepared clots of 0.6–0.7 cm in diameter and 10 cm in length, but in practice, they only used 2-cm-long clots placed in the MCA. They suggested that a length of 2.5–3 cm is sufficient to induce ischemia. Wei et al. (2005) generated a white thrombus using the centrifugal method with autologous blood in vitro. They reasoned that a white thrombus is very similar to a human cerebral thrombus and is not easily autolyzed. Each thrombus was 0.5 cm in diameter and 15 cm in length. The embolization process in this method is the most similar to human cerebral embolization, and it is very valuable for the study of clinical treatments, especially the study of thrombolytic therapy (Wei et al., 2005). A cerebral infarct formed using this method is more stable than those formed in other models (Murphy et al., 2008). The limitation of this method is high variability in stroke location, volume and neurological consequences.
Balloon inflation method
Gao et al. (2006) designed a microcatheter with a balloon attached to one end and a syringe connected to the opposite end. With the propulsion and withdrawal of the syringe piston, the balloon was inflated and deflated. The balloon on the end of the catheter was expanded when air was injected. In vitro and in vivo tests demonstrated that the balloon remained inflated for up to 3 months. The researchers selected a deflated balloon with an outer diameter of 1.1-mm to ensure that the microcatheter with an outer diameter of 0.61 mm could pass through the narrowest region of the blood vessels of the experimental animals. Because the microcatheter is very thin and the balloon is only inflated at the proximal end of the MCA, the damage to the blood vessels is reduced. In addition, the operation is performed only on the intracranial arteries, which avoid damaging the dura mater. The operation for reperfusion is also very simple, only requiring the withdrawal of the air from the balloon. The limitation of the method is that the diameter of the balloon is so large that it can only be used to embolize the proximal MCA. The narrow and circuitous characteristics of remote vessels make this operation unsuitable and prone to failure.
Microcatheter embolization method
Without using emboli, directly inserting a microcatheter into the MCA can also induce focal cerebral ischemia. For instance, de Crespigny et al. (2005) repositioned the microcatheter with a micro-guide wire from the femoral artery to the MCA through the guiding catheter and simultaneously injected contrast medium through the guiding catheter to observe the movement of the microcatheter. The microcatheter was left in the MCA for 3 hours to form a focal embolism, and the microcatheter was subsequently removed to achieve reperfusion. Notably, the final 50 cm of this microcatheter was unreinforced, which enabled steering through the tortuous vasculature. It is also nonmagnetic allowing artifact-free echo planar imaging images. The minimal extraneous damage caused by the model facilitates an improved survival rate, which is advantageous for the study of long-term recovery after stroke.
Coil embolization method
Guo et al. (2011) improved the method developed by de Crespigny et al. (2005) by first inserting the microcatheter into the end of the M1 segment and subsequently placing a coil in this area to cause sustained focal cerebral ischemia. After withdrawing the guiding catheter, magnetic resonance imaging (MRI) of the animal can be done. The vascular sheath and coil can be pulled out to enable blood reperfusion later. This method exhibits increased stability, safety and reproducibility. However, the catheter and coil are more expensive than the tools used for other ischemia induction methods.
Surgical suture embolization method
Surgical sutures can be delivered to a specific position through a microcatheter to stably and permanently induce a focal ischemia. Rodriguez-Mercado et al. (2012) performed a puncture in the femoral artery and inserted a guide catheter; they subsequently delivered the microcatheter to the M1 segment of the MCA. Surgical sutures in saline were injected through a syringe into the microcatheter, depositing 6–8 sutures in the M1 segment to form an occlusion. Angiography was used to confirm the occlusion and to determine the number of sutures released. The embolism formed via this method does not permit reperfusion. The advantages of this method are that it can achieve a continuous expansion of the acute infarct and that the sutures do not interfere with imaging examinations.
Suture method
The suture method was developed by the Japanese researcher Koizumi (Koizumi et al., 1986) for the preparation of a rat model of focal cerebral ischemia. Freret et al. (2008) used this method to establish a monkey model of cerebral ischemia. A nylon thread coated with a thermofusible adhesive at one end, was inserted into the external carotid artery and advanced to the origin of the MCA until the laser-Doppler signal disappeared, indicating the establishment of cerebral ischemia. To achieve reperfusion, the thread must be pulled out. This method causes less damage to the experimental animals and allows for the strict control of the durations of ischemia and reperfusion. However, this method has strict limits regarding animal body weight. Their experimental subjects were 240–330 g marmosets. Freret et al. (2008) tested different diameters of nylon thread with distal cylinders in preliminary studies and found that 3 mm length and 0.54 mm diameter was appropriate. When it comes to different body weights and other species, the experimental conditions will need to be reassessed.
Photochemical induction method
The photochemical induction method takes advantage of the characteristics of a photosensitizer, which initiates chemical reactions upon irradiation by a particular light source. The irradiated area of the cerebral tissue then exhibits edema formation and platelet aggregation, thereby forms a focal infarct. This non-invasive method tends to restrict the infarct within a specific range with low mortality. Ikeda et al. (2013) applied the photochemical induction method to a marmoset (Callithrix) cerebral ischemia model. After anesthesia, Rose Bengal was injected into the tail vein. In the sensorimotor area of the cerebral cortex, a green light of 8 mm in diameter was irradiated on the exposed skull. Under green light irradiation, Rose Bengal induces platelet aggregation, thus resulting in cerebral infarction in the irradiated area. Because the marmoset skull is thicker than the rat skull, researchers had to increase the light intensity applied to marmosets from 150 to 350 W. When it comes to the macaque or higher species with thicker skull, the parameter should be further adjusted on the existing basis. To avoid an increased temperature due to the strong light, the irradiation time was minimized. Cold air and water were also used to maintain the temperature below 38°C.
The advantages of this model are as follow. (1) It is non-invasive and easy to operate. There is no need for craniotomy when inducing infarction, minimizing intracranial infection. (2) This method can be used to produce infarcts of identical size in similar positions between animals (repeatability), and the stability of the ischemic foci is good. (3) The induced embolism is similar to cerebral thrombosis in humans. Based on this evidence, this method is suitable for certain studies using antiplatelet and antithrombotic drugs. However, the infarct is located at the distal end of the vessel, which limits the utility of this method.
Comparison between NHP models and rodent models
Rodents are widely used in the establishment of animal models of stroke and most of the methods described above are modified from rodent models (Fluri et al., 2015). Admittedly, the expense of purchasing and feeding NHPs is greater than that for rodents (Murphy et al., 2008). The ethics and operation requirements for NHPs are stricter than those for rodents (Cook and Tymianski, 2012). Nevertheless, there are irreplaceable advantages in use of the NHPs to establish MCAO: (1) The anatomical, cognitive and behavioral complexities of primates make NHPs more appropriate for modelling human neurological disease than rodents (Bakken et al., 2016). (2) A few features of primates such as prolonged myelination, synapse production and pruning may make some human neurological diseases inadequately modeled in rodents. (3) NHPs and human have a greatly expanded neocortex and functional partitioning, especially of the primary visual cortex, compared with rodents (Bakken et al., 2016). (4) When it comes to MCAO model, rodents differ from primates in having rich collateral anastomoses and most rodents do not have a complete circle of Willis. Subjecting rodents to MCAO produces extensive ischemic infarct volumes rather than injury to specific neuronal circuits (Kwiecien et al., 2014). (5) There is poor correlation between rodents and humans in their responses to inflammation and cell injury (Seok et al., 2013). One hypothesis is that the translational discrepancies between rodent models and clinical trials may be secondary to the interspecies differences in gene expression and genomic responses (Seok et al., 2013).
Comparison of different NHP species
The selection of an experimental animal depends on the aims of the study. Experimental NHPs include animals lacking gyri and animals with multiple gyri. The frequently used species lacking gyri include the marmoset (Callithrix) and the squirrel monkey (Saimiri). Their advantage is that brain function areas are easily identified so these animals are more suitable for the investigation of dysfunction after infarction. The animals with multiple gyri most frequently used include baboons (Papio) and rhesus macaques (Macaca mulatta). Compared with animals lacking gyri, NHPs with multiple gyri possess brains that are structurally more like the human brain, particularly the cerebral cortex and subcortical regions as well as the ratio of gray matter to white matter. Therefore, NHPs with multiple gyri are suitable for multipurpose studies, especially for preclinical trials.
Many NHP species have richer collateral circulation than that of humans, and that compensates for ischemia and helps NHPs to recover after cerebral infarction. The vascular anatomy of NHPs lacking gyri is rat-like, whereas that of NHPs with multiple gyri is more like that of humans. Among the latter, rhesus macaques (Macaca mulatta) have a less extensive collateral circulation than baboons (Papio). In addition, rhesus macaques are relatively docile when domesticated, making these animals more suitable for the assessment of motor, sensory and cognitive disorders (Cook and Tymianski, 2012).
Comparison of different NHP models
The experimental conditions of the surgical methods are more consistent than those of the endovascular intervention and photochemical induction methods. The initial injury site during the surgery and the ensuing infarction are identified visually. Therefore, these methods are frequently used for studies of neuroprotective agents during the acute phase of infarction. However, these methods have several serious drawbacks as mentioned above such as a large wound and unpredictable location. Establishing the cerebral occlusion model using this method exposes the brain to an open environment, which is different from that of human strokes. Moreover, in the transorbital method, enucleating an eyeball impairs the animal's vision affecting cognitive assessments (Mack et al., 2003).
The electrocoagulation and ET-1-induced occlusion methods involve the use of a craniotomy to expose the MCA. The craniotomy can expose the region distal to the M1 branch and the M2 branch of the MCA via the pterional approach (Virley et al., 2004; Chen et al., 2015). Blocking these sites produces a limited infarct area. The microvascular clip occlusion method involves the use of the transorbital approach to expose the main MCA trunk and the orbitofrontal branch (West et al., 2009). Obstructing these sites can generate a larger infarct area than that produced by the craniotomy method. The selection of a specific approach may be determined by the desired infarct area and operation requirements. In the craniotomy, it is more difficult to detach the exposed vessels than in the transorbital method and the former hinders adequately proximally positioning of the occlusion device (Fukuda and del Zoppo, 2003). Considering that the vessels exposed via the transorbital approach are near the main trunk, choosing a method that permits reperfusion would restrict the infarct size and reduce the severity of clinical symptoms.
Compared with the above surgical methods, the endovascular intervention method causes less damage to the experimental animals. Its closed environment is more comparable to the internal microenvironment during the human stroke process, and this method has been widely used in recent studies. Nevertheless, because this method produces a larger infarct area than the surgical methods, its mortality rate is also higher (Cook and Tymianski, 2011).
The suture method that is widely used in rodent animal models has been used less frequently in primates to date. This method has a strict limits regarding animal body weight; therefore, effective modelling parameters are critical for the success of the experiment. The autologous blood clot embolization method produces ischemia via a process that is most comparable to that of human stroke, and is more appropriate for testing thrombolytic therapy. Nevertheless, this model inhibits reperfusion, and the position of the blood clot is difficult to control. This makes the position and size of the damaged area beyond control. These factors limit the application of this method.
In contrast, the balloon inflation, microcatheter embolization and coil embolization methods more effectively control the embolism area and reperfusion time, therefore these methods can be used to study chronic therapies for cerebral infarction. There is a limit to the range of balloon diameter, therefore the brain areas that can be affected by the balloon inflation method are not as deep as those affected by the microcatheter embolization and coil embolization methods. The microcatheter embolization and coil embolization methods can reach deeper brain areas and produce a more focused infarct area, which effectively reduces the mortality rate (de Crespigny et al., 2005). Compared with the microcatheter embolization method, the coil embolization method has the benefit that after placement of the coil, the microcatheter can be removed, and an MRI can be performed.
The photochemical induction method does not require a craniotomy. Its wound is small, and its mortality rate is low. The position, size and degree of the infarct produced by this method can be controlled; thus, this method has good operability. A thrombolytic agent can induce reperfusion but the reperfusion time is difficult to control. This method also exhibits several differences in progression compared with human stroke. The infarct sites reached using this method are frequently at the distal end, which vary in anatomical position (Cook and Tymianski, 2011) (Table 1).
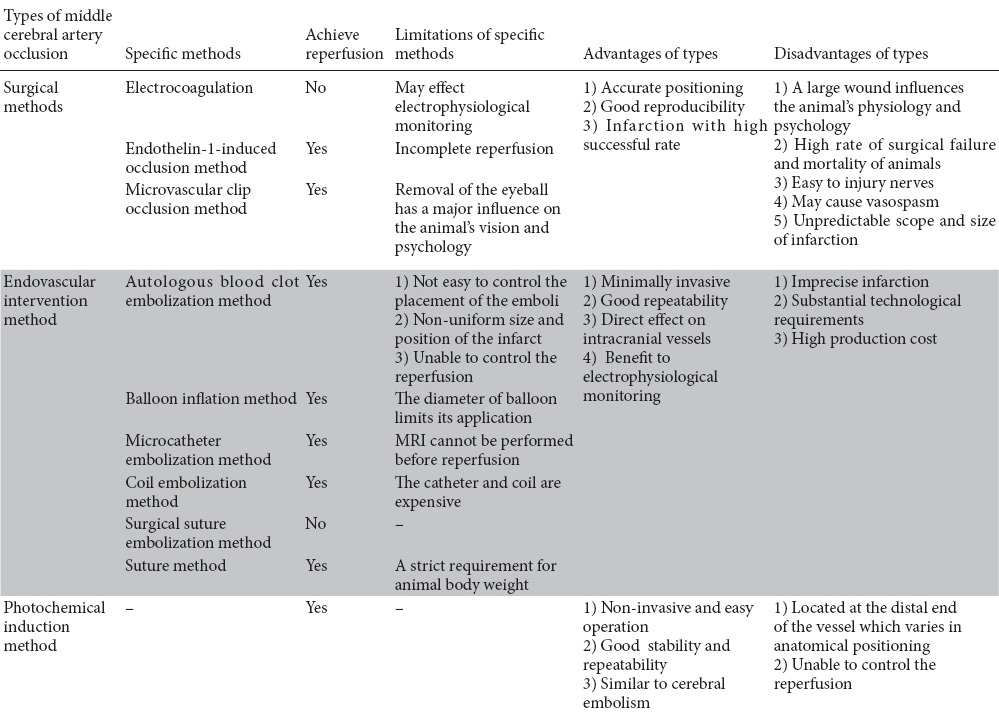
Table 1.
Comparison of the methods of establishing focal ischemia models using nonhuman primates
Other considerations when selecting an animal model of stroke
Several factors must be considered in the selection of a stroke model: permanent infarction versus transient infarction, also the length of time and position of the infarct. Among the above-mentioned methods, all allow reperfusion except for electrocoagulation and surgical suture embolization. In autologous blood clot embolization and photochemical induction, the reperfusion time cannot be controlled. In most studies, the reperfusion time is 1–3 hours (Zhang, 2010). The longer the reperfusion time, the larger the infarct volume is. Unanesthetized animals have larger infarct volumes (Young et al., 1997). One possible reason is that these animals must be immobilized, leading to hypocapnia, which influences cerebral blood flow and metabolic rate. Immobilization for a long period (7–9 hours) may increase the permeability of the blood-brain barrier and awake animals are easier to observe their neurobehavioral status (Fukuda and del Zoppo, 2003). However, to facilitate the operation, most studies choose anesthetization.
The physiological parameters of anesthetized animals should be considered such as heart rate, temperature and blood pressure. In general, the body temperature is maintained within 37–38°C and systolic blood pressure maintained within 100–130 mmHg (Kito et al., 2001; Gao et al., 2006; Rodriguez-Mercado et al., 2012). Some studies controlled the blood pressure at a low level with an average of 60–80 mmHg (West et al., 2009). The use of different species of NHPs complicates the comparisons.
The surgical method can be used to select the infarct site most accurately. When the position is near the distal end, the infarct volumes are smaller. Guo et al. (2011) have reported that the coil embolization method, with a focus of infarction in the distal M1 segment, can produce a larger penumbral area. The penumbra is the critical region for clinical treatment (del Zoppo et al., 2011), but few studies have examined the ischemic penumbra using NHPs.
Gender also has important influences on the development of and recovery from stroke. In general, most trials use only male animals. Murphy et al. (2008) found that the ischemic injury to female rhesus macaques showed greater variability in the infarct area in the entire hemisphere, the putamen, and the caudate nucleus than a similar injury to male rhesus macaques. The investigators speculated that this difference may be related to hormone levels and the menstrual cycle and suggested that subsequent studies should include both male and female animals. The balance of gender between groups should also be noted.
Assessment after establishing a stroke model
After induction of a stroke model, many methods could be used to assess whether the model is successful and to evaluate its stability. The commonly used methods include imaging, motor and sensory function evaluations, neuropathological examination and electroencephalography.
The frequently used imaging methods include interventional angiography, computed tomography (CT), positron emission tomography (PET) and MRI. CT angiography can be used to instantly detect thrombogenesis; it is convenient and direct, and it can accurately locate the infarct site. The disadvantages of CT angiography are that it cannot reflect the functional status, and that introducing the contrast medium causes additional damage. However, when CT angiography is used in combination with the endovascular intervention method, the same catheter can be used to introduce the contrast medium. CT can generate three-dimensional images to accurately locate the infarct site. However, compared with PET and MRI, CT detects only density changes; thus it provides limited data. PET can be used to monitor the metabolic state of oxygen, which can reflect the functional status. However, in terms of accuracy, PET is poorer than the other methods; its resolution is lower, and it cannot accurately locate the infarct. In addition, the cost of PET is higher, which limits its application for research.
For most studies, MRI is more convenient and suitable (Saver et al., 2009). MRI includes multiple-factor imaging; it generates images without the injection of contrast media and can measure a variety of parameters. For example, magnetic resonance angiography can show the signals of blood vessel structure and blood flow. Magnetic resonance spectroscopy can be used to measure molecular components and steric configuration. Diffusion tensor imaging can be used to acquire images of white matter fiber tracts. It can not only detect the infarct site, but also the metabolic status of the infarct area. However, MRI requires a longer time for image generation, and the tested animals must not have metal in their body. Also, life support equipment cannot be used during the imaging. The frequently used MRI techniques include traditional MRI, diffusion-weighted MRI and functional MRI.
Motor and sensory function tests are mainly used for the identification of motor and sensory impairments, after the establishment of a cerebral ischemia model. Commonly used tests include the examination of ipsilateral grip strength, the response to tactile stimulation, other neurological function assessments, and motor function tests (Virley et al., 2004; Freret et al., 2008; Wang et al., 2012). Neuropathological examinations include postoperative histology, immunochemistry, and 2,3,5-triphenyl tetrazolium chloride staining (Wei et al., 2005; Freret et al., 2008). Motor and sensory functional tests and neuropathological examinations can also effectively confirm the establishment of the ischemic model.
Summary
Ischemic stroke is a common clinical disease with high rate of incidence, mortality and morbidity. Establishing an effective ischemic stroke model is vital for the investigation of the mechanisms of stroke, as well as its prevention and treatment. NHP brains are more structurally like human than rodent brains therefore NHP stroke models can better mimic the pathogenesis of human cerebral ischemia compared with rodents. However, their restricted availability and higher associated cost limit their broad application. The establishment of a highly effective ischemia model with a low mortality rate still requires more investigation.
This review will help establish the optimal method, species and strict experimental conditions by analyzing the advantages and disadvantages of each cerebral ischemia model using NHPs. Investigators can choose the appropriate ischemia model according to the aspect of ischemic injury and therapeutic characteristics of their own research.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 81000852 and 81301677; the AHA Award, No. 17POST32530004; the Supporting Project of Science & Technology of Sichuan Province of China, No. 2012SZ0140; the Research Foundation of Zhejiang Province of China, No. 201022896.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Dawes EA, Yajima W, Yu J, Li CH, Qiu Y, Song LP, Zhao M
References
- Bacigaluppi M, Comi G, Hermann DM. Animal models of ischemic stroke. Part one: modeling risk factors. Open Neurol J. 2010;4:26–33. doi: 10.2174/1874205X01004020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken TE, Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Dalley RA, Royall JJ, Lemon T, Shapouri S, Aiona K, Arnold J, Bennett JL, Bertagnolli D, Bickley K, Boe A, Brouner K, Butler S, Byrnes E, et al. A comprehensive transcriptional map of primate brain development. Nature. 2016;535:367–375. doi: 10.1038/nature18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihel E, Roussel S, Toutain J, Bernaudin M, Touzani O. Diffusion tensor MRI reveals chronic alterations in white matter despite the absence of a visible ischemic lesion on conventional MRI: a nonhuman primate study. Stroke. 2011;42:1412–1419. doi: 10.1161/STROKEAHA.110.596650. [DOI] [PubMed] [Google Scholar]
- Chen X, Dang G, Dang C, Liu G, Xing S, Chen Y, Xu Q, Zeng J. An ischemic stroke model of nonhuman primates for remote lesion studies: a behavioral and neuroimaging investigation. Restor Neurol Neurosci. 2015;33:131–142. doi: 10.3233/RNN-140440. [DOI] [PubMed] [Google Scholar]
- Christoforidis GA, Rink C, Kontzialis MS, Mohammad Y, Koch RM, Abduljalil AM, Bergdall VK, Roy S, Khanna S, Slivka AP, Knopp MV, Sen CK. An endovascular canine middle cerebral artery occlusion model for the study of leptomeningeal collateral recruitment. Invest Radiol. 2011;46:34–40. doi: 10.1097/RLI.0b013e3181f0cbc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DJ, Tymianski M. Translating promising preclinical neuroprotective therapies to human stroke trials. Expert Rev Cardiovasc Ther. 2011;9:433–449. doi: 10.1586/erc.11.34. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Tymianski M. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics. 2012;9:371–379. doi: 10.1007/s13311-012-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crespigny AJ, D’Arceuil HE, Maynard KI, He J, McAuliffe D, Norbash A, Sehgal PK, Hamberg L, Hunter G, Budzik RF, Putman CM, Gonzalez RG. Acute studies of a new primate model of reversible middle cerebral artery occlusion. J Stroke Cerebrovasc Dis. 2005;14:80–87. doi: 10.1016/j.jstrokecerebrovasdis.2004.12.005. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Sharp FR, Heiss WD, Albers GW. Heterogeneity in the penumbra. J Cereb Blood Flow Metab. 2011;31:1836–1851. doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Engelhardt B, Koistinaho J, Lindvall O, Meairs S, Mohr JP, Planas A, Rothwell N, Schwaninger M, Schwab ME, Vivien D, Wieloch T, Dirnagl U. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis. 2008;25:268–278. doi: 10.1159/000118039. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- Feng L, Liu J, Liu Y, Chen J, Su C, Lv C, Wei Y. Tirofiban combined with urokinase selective intra-arterial thrombolysis for the treatment of middle cerebral artery occlusion. Exp Ther Med. 2016;11:1011–1016. doi: 10.3892/etm.2016.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YH, Zhu ZH, Wu CX, Zhou GP. Effects of electroacupuncture at points selected by orthogonal experiment on the extracellular signal regulated kinase signal pathway in a rat model of cerebral ischenia-reperfusion injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:5953–5958. [Google Scholar]
- Fluri F, Schuhmann MK, Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther. 2015;9:3445–3454. doi: 10.2147/DDDT.S56071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freret T, Bouet V, Toutain J, Saulnier R, Pro-Sistiaga P, Bihel E, Mackenzie ET, Roussel S, Schumann-Bard P, Touzani O. Intraluminal thread model of focal stroke in the non-human primate. J Cereb Blood Flow Metab. 2008;28:786–796. doi: 10.1038/sj.jcbfm.9600575. [DOI] [PubMed] [Google Scholar]
- Fukuda S, del Zoppo GJ. Models of focal cerebral ischemia in the nonhuman primate. ILAR J. 2003;44:96–104. doi: 10.1093/ilar.44.2.96. [DOI] [PubMed] [Google Scholar]
- Gao H, Liu Y, Lu S, Xiang B, Wang C. A reversible middle cerebral artery occlusion model using intraluminal balloon technique in monkeys. J Stroke Cerebrovasc Dis. 2006;15:202–208. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gao HJ, Liu PF, Li PW, Huang ZY, Yu FB, Lei T, Chen Y, Cheng Y, Mu QC, Huang HY. Ligustrazine monomer against cerebral ischemia/reperfusion injury. Neural Regen Res. 2015;10:832–840. doi: 10.4103/1673-5374.156991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Cheng JS. Progress on the study of monkey cerebral ischemia models. Guowai Yixue: Naoxueguan Jibing Fence. 2002;10:127–128. [Google Scholar]
- Giffard C, Young AR, Mézenge F, Derlon JM, Baron JC. Histopathological effects of delayed reperfusion after middle cerebral artery occlusion in the anesthetized baboon. Brain Res Bull. 2005;67:335–340. doi: 10.1016/j.brainresbull.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Guo J, Zheng HB, Duan JC, He L, Chen N, Gong QY, Tang HH, Li HX, Wang L, Cheng JQ. Diffusion tensor MRI for the assessment of cerebral ischemia/reperfusion injury in the penumbra of non-human primate stroke model. Neurol Res. 2011;33:108–112. doi: 10.1179/016164110X12761752770177. [DOI] [PubMed] [Google Scholar]
- Hainsworth AH, Markus HS. Do in vivo experimental models reflect human cerebral small vessel disease? A systematic review. J Cereb Blood Flow Metab. 2008;28:1877–1891. doi: 10.1038/jcbfm.2008.91. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Harada K, Ohwatashi A, Kamikawa Y, Yoshida A, Kawahira K. A new non-human primate model of photochemically induced cerebral infarction. PLoS One. 2013;8:e60037. doi: 10.1371/journal.pone.0060037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito G, Nishimura A, Susumu T, Nagata R, Kuge Y, Yokota C, Minematsu K. Experimental thromboembolic stroke in cynomolgus monkey. J Neurosci Methods. 2001;105:45–53. doi: 10.1016/s0165-0270(00)00351-4. [DOI] [PubMed] [Google Scholar]
- Koga M, Reutens DC, Wright P, Phan T, Markus R, Pedreira B, Fitt G, Lim I, Donnan GA. The existence and evolution of diffusion-perfusion mismatched tissue in white and gray matter after acute stroke. Stroke. 2005;36:2132–2137. doi: 10.1161/01.STR.0000181066.23213.8f. [DOI] [PubMed] [Google Scholar]
- Koizumi J, Min Z, Imanaka T, Aiba S. Temperature-dependent plasmid integration into and excision from the chromosome of Bacillus stearothermophilus. J Gen Microbiol. 1986;132:1951–1958. doi: 10.1099/00221287-132-7-1951. [DOI] [PubMed] [Google Scholar]
- Kwiecien TD, Sy C, Ding Y. Rodent models of ischemic stroke lack translational relevance… are baboon models the answer? Neurol Res. 2014;36:417–422. doi: 10.1179/1743132814Y.0000000358. [DOI] [PubMed] [Google Scholar]
- Mack WJ, King RG, Hoh DJ, Coon AL, Ducruet AF, Huang J, Mocco J, Winfree CJ, D’Ambrosio AL, Nair MN, Sciacca RR, Connolly ES. An improved functional neurological examination for use in nonhuman primate studies of focal reperfused cerebral ischemia. Neurol Res. 2003;25:280–284. doi: 10.1179/016164103101201346. [DOI] [PubMed] [Google Scholar]
- Marshall JW, Ridley RM. Assessment of cognitive and motor deficits in a marmoset model of stroke. ILAR J. 2003;44:153–160. doi: 10.1093/ilar.44.2.153. [DOI] [PubMed] [Google Scholar]
- Mima T, Yanagisawa M, Shigeno T, Saito A, Goto K, Takakura K, Masaki T. Endothelin acts in feline and canine cerebral arteries from the adventitial side. Stroke. 1989;20:1553–1556. doi: 10.1161/01.str.20.11.1553. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, Kirsch JR, Zhang W, Grafe MR, West GA, del Zoppo GJ, Traystman RJ, Hurn PD. Can gender differences be evaluated in a rhesus macaque (Macaca mulatta) model of focal cerebral ischemia? Comp Med. 2008;58:588–596. [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Takayasu M, Dacey RG., Jr Differential effects of intra- and extraluminal endothelin on cerebral arterioles. Am J Physiol. 1991;261:H531–H537. doi: 10.1152/ajpheart.1991.261.2.H531. [DOI] [PubMed] [Google Scholar]
- Platt SR, Holmes SP, Howerth EW, Duberstein KJJ, Dove CR, Kinder HA, Wyatt EL, Linville AV, Lau VW, Stice SL, Hill WD, Hess DC, West FD. Development and characterization of a Yucatan miniature biomedical pig permanent middle cerebral artery occlusion stroke model. Exp Transl Stroke Med. 2014;6:5. doi: 10.1186/2040-7378-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JL, Dawson D, Macrae IM. Endothelin, cerebral ischaemia and infarction. Clin Exp Hypertens. 1995;17:399–407. doi: 10.3109/10641969509087080. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mercado R, Ford GD, Xu Z, Kraiselburd EN, Martinez MI, Eterović VA, Colon E, Rodriguez IV, Portilla P, Ferchmin PA, Gierbolini L, Rodriguez-Carrasquillo M, Powell MD, Pulliam JV, McCraw CO, Gates A, Ford BD. Acute neuronal injury and blood genomic profiles in a nonhuman primate model for ischemic stroke. Comp Med. 2012;62:427–438. [PMC free article] [PubMed] [Google Scholar]
- Rosso C, Colliot O, Valabrègue R, Crozier S, Dormont D, Lehéricy S, Samson Y. Tissue at risk in the deep middle cerebral artery territory is critical to stroke outcome. Neuroradiology. 2011;53:763–771. doi: 10.1007/s00234-011-0916-5. [DOI] [PubMed] [Google Scholar]
- Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M STAIR VI Consortium. Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations For Extended Window Acute Stroke Therapy Trials. Stroke. 2009;40:2594–2600. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MP. Investigators; MT. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- Takamatsu H, Tsukada H, Kakiuchi T, Nishiyama S, Noda A, Umemura K. Detection of reperfusion injury using PET in a monkey model of cerebral ischemia. J Nucl Med. 2000;41:1409–1416. [PubMed] [Google Scholar]
- Tian X. Variations of brain edema and neurological function of rat models of cerebral infarction after hyperbaric oxygen therapy. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:6423–6428. [Google Scholar]
- Virley D, Hadingham SJ, Roberts JC, Farnfield B, Elliott H, Whelan G, Golder J, David C, Parsons AA, Hunter AJ. A new primate model of focal stroke: endothelin-1-induced middle cerebral artery occlusion and reperfusion in the common marmoset. J Cereb Blood Flow Metab. 2004;24:24–41. doi: 10.1097/01.WCB.0000095801.98378.4A. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang T, Zhao CY, Xu JM, Wen M, Pei ZS. Nonhuman primate chronic stroke model with middle cerebral artery endovascular embolism. Zhongguo Kangfu Lilun yu Shijian. 2012;18:401–405. [Google Scholar]
- Wei Y, Feng FL, Hong ZH, Deng YX, Yu JX, Qiu WJ, He ZK, Zhou ZP, Gan RJ, Wei RM, Rong MZ. Establishment of local cerebral ischemia animal models in monkey by autologous pale thrombus. Zhongguo Xiandai Yixue Zazhi. 2005;15:3715–3717. [Google Scholar]
- Wells AJ, Vink R, Helps SC, Knox SJ, Blumbergs PC, Turner RJ. Elevated Intracranial Pressure and Cerebral Edema following Permanent MCA Occlusion in an Ovine Model. PLoS One. 2015;10:e0130512. doi: 10.1371/journal.pone.0130512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West GA, Golshani KJ, Doyle KP, Lessov NS, Hobbs TR, Kohama SG, Pike MM, Kroenke CD, Grafe MR, Spector MD, Tobar ET, Simon RP, Stenzel-Poore MP. A new model of cortical stroke in the rhesus macaque. J Cereb Blood Flow Metab. 2009;29:1175–1186. doi: 10.1038/jcbfm.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XG, Cheng BH, Wang X, Ding LC, Liu HQ, Chen J, Bai B. Lateral intracerebroventricular injection of Apelin-13 inhibits apoptosis after cerebral ischemia/reperfusion injury. Neural Regen Res. 2015;10:766–771. doi: 10.4103/1673-5374.157243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Gu Z, Pan L, Gan L, Qin DD, Yang B, Guo J, Hu XT, Wang TH, Feng ZT. How does the motor relearning program improve neurological function of brain ischemia monkeys? Neural Regen Res. 2013;8:1445–1454. doi: 10.3969/j.issn.1673-5374.2013.16.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AR, Touzani O, Derlon JM, Sette G, MacKenzie ET, Baron JC. Early reperfusion in the anesthetized baboon reduces brain damage following middle cerebral artery occlusion: a quantitative analysis of infarction volume. Stroke. 1997;28:632–637. doi: 10.1161/01.str.28.3.632. discussion 637-638. [DOI] [PubMed] [Google Scholar]
- Zhang XD. Magnetic resonance imaging of non-human primate ischemic stroke models. Bopu Xue Zazhi. 2010;27:548–561. [Google Scholar]


