Abstract
Postoperative cognitive dysfunction is a crucial public health issue that has been increasingly studied in efforts to reduce symptoms or prevent its occurrence. However, effective advances remain lacking. Hyperbaric oxygen preconditioning has proved to protect vital organs, such as the heart, liver, and brain. Recently, it has been introduced and widely studied in the prevention of postoperative cognitive dysfunction, with promising results. However, the neuroprotective mechanisms underlying this phenomenon remain controversial. This review summarizes and highlights the definition and application of hyperbaric oxygen preconditioning, the perniciousness and pathogenetic mechanism underlying postoperative cognitive dysfunction, and the effects that hyperbaric oxygen preconditioning has on postoperative cognitive dysfunction. Finally, we conclude that hyperbaric oxygen preconditioning is an effective and feasible method to prevent, alleviate, and improve postoperative cognitive dysfunction, and that its mechanism of action is very complex, involving the stimulation of endogenous antioxidant and anti-inflammation defense systems.
Keywords: nerve regeneration, brain injury, hyperbaric oxygenation, preconditioning, antioxidants, anti-inflammation, reactive oxygen species, oxidant stress, inflammation, protection, post-operation, cognitive dysfunction, neural regeneration
Introduction
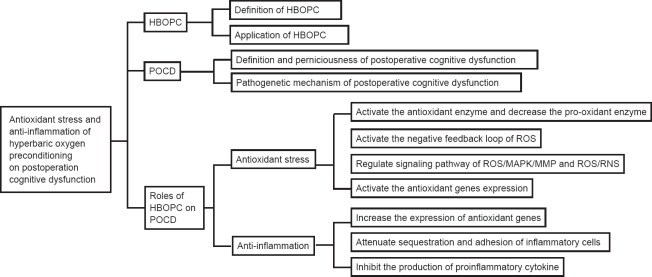
Postoperative cognitive dysfunction (POCD) is a complication of surgery that is widely considered an important clinical problem, particularly in elderly patients (Shoair et al., 2015). However, the pathophysiology underlying POCD is fairly complex, involving numerous mechanisms including oxidant stress, inflammation, and apoptosis (Eckenhoff et al., 2004; Dong et al., 2009; Thom, 2009; Cao et al., 2012; Wilson et al., 2013). Over the past several decades, researchers have explored a wide array of methods for improving POCD, including hyperbaric oxygen preconditioning (HBOPC). HBOPC is one of the most economical, simple, safe, and effective strategies among all the possible choices (Zhu et al. 2016). Indeed, studies have successfully utilized HBOPC to improve cognitive dysfunction (Alex et al., 2005; Peng et al., 2010; Sun et al., 2014). The purpose of this narrative review is to summarize and discuss the literature concerning HBOPC and POCD, with an emphasis on the evidence for a role of HBOPC in treating patients undergoing POCD. The review is organized into the following sections: introduction of HBOPC, mechanisms underlying POCD, and the effect of HBOPC on POCD (Figure 1).
Figure 1.
Summary of article structure.
POCD: Postoperative cognitive dysfunction; HBOPC: hyperbaric oxygen preconditioning; MMP: matrix metalloproteinase; ROS: reactive oxygen species; MAPK: mitogen-activated protein kinase; RNS: reactive nitrogen species.
HBOPC
Definition of HBOPC
During HBO treatment, patients usually inhale pure oxygen (100%) at pressures greater than the atmospheric pressure in a steel vessel (Löndahl, 2012), which increases both the dissolved oxygen and the partial pressure of oxygen in blood plasma (Tibbles and Edelsberg, 1996). Consequently, a large amount of oxygen-dependent reactions and signaling pathways are enhanced (Babchin et al., 2011).
Application of HBOPC
Normobaric oxygen and various levels of HBO have been widely utilized therapeutic agents, and Valenzuela pioneered the application of pure oxygen (as high as 2 MPa) in clinical research (Edwards, 2010). The use of HBO as an adjuvant treatment for a number of medical conditions has been widely supported by the experience of experts in hyperbaric medicine and the scientific literature in areas such as traumatic brain injury (Hu et al., 2016; Zhou et al., 2016a, b) complex refractory wounds (Morykwas and Argenta, 1996), cerebral infarction (Tian, 2015), and radiation-tissue injury (Kindwall and Hunt, 1995; Kindwall and Wheland, 1999). Along with the development of medicines, disease prevention has increasingly become recognized as important. Preconditioning is a type of primary prevention that activates endogenous protective mechanisms, which can reduce the risk of morphologic and functional sequelae. The preconditioning state is typically defined by the response to a sublethal stimulus that extends beyond its presence in the system. This response significantly lessens the level of signal cascades for stress-activated and stress-reactive proteins, which subsequently shows a protective effect for cells. Recently, HBO has become recognized as an effective preconditioning method for reducing mental and cellular stresses, especially in regimented sessions of moderate HBO (Nie et al., 2006; Li et al., 2008). In the clinic, however, HBOPC has had only minimal impact before surgery, and no role in the surgery or post-surgical care of patients (Allen et al., 2014). HBO protocols are performed at 2.0–2.5 atmosphere absolute (ATA) oxygen partial pressures, and usually only applied for one or a few days. The physical adaptations in response to alterations in atmospheric oxygen appear to extend not only to survival, but also a preconditioned state. Similar to ischemic and stress preconditioning, many different paradigms have been used to demonstrate that either rapid or delayed tolerance is affected by the HBO (Stetler et al., 2014). To achieve the best outcome using HBOPC, it requires a certain O2 concentration and high pressure. When air (20% oxygen) rather than 100% O2 was infused into the hyperbaric chamber, the tolerance was negated, demonstrating the need for high O2 concentration in the hyperbaric preconditioned state (Wada et al., 2001). Additionally, Kocaoğullar et al. (2004) compared normobaric oxygen with HBO treatment of rats with cerebral vasospasm after subarachnoid hemorrhage and found that normobaric oxygen was less effective in ameliorating neurological deficits associated with the central nervous system. Many experiments have shown that HBOPC can protect against subsequent multi-organ injury to the brain, heart, or liver (Alex et al., 2005; Yu et al., 2005; Qin et al., 2008). Previous studies suggest that preconditioning with pressures of 2 ATA 3–5 sessions/every other day was effective in inducing tolerance against global ischemia in gerbils (Wad et al., 1996; Wada et al., 2001). Cheng et al. (2011) reported that 2.5 ATA preconditioning, 1 hour daily for 5 days, protected against subsequent global ischemic injury in rats (Cheng et al., 2011). Similarly, pretreatment with HBO has been found both to improve the degree and accelerate the rate of neurologic recovery. Additionally, long-term HBOPC paradigms have been shown to be more effective at establishing tolerance than are acute paradigms (Xiong et al., 2000; Dong et al., 2002; Nie et al., 2006; Liu et al., 2012). Animal studies of ischemia/reperfusion (I/R) injury in the myocardium have indicated that HBO preconditioning can lead to ischemia tolerance, resulting in protection against myocardial ischemia (Kim et al., 2001). In addition to these experimental studies, clinical studies have demonstrated that preconditioning patients who have coronary artery disease with HBO before on-pump cardiopulmonary bypass or coronary artery graft bypass were in a position improved myocardial function, and reduced myocardial injury, the duration of staying in the intensive care unit, blood loss, postoperative complications, and cost (Yogaratnam et al., 2010; Li et al., 2011). Karu et al. (2010) indicated that exposure to hyperoxia for a limited time before ischemia induced a mild oxidative stress and resulted in an (ischemic) preconditioning-like effect in the myocardium, which protected the heart from subsequent injury. Yu et al. (2005) performed an experimental study in rats and reported preconditioning with single-dose HBO (90 minutes) protects the rat liver against subsequent I/R injury. Ren et al. (2008) similarly reported that HBO preconditioning increased the number of new cells and the density of microcirculation in the regenerating liver. Therefore, HBO preconditioning is an encouraging and feasible therapeutic strategy for protecting organs from the subsequent lethal stimulus. The effect and mechanism of HBOPC on POCD will be described below.
POCD
Definition and perniciousness of POCD
Every year, numerous people undergo surgery hoping that the operation will lighten symptoms, heal diseases, and improve quality of life (Berger et al., 2015). Although there is much interest in, and controversy about, the mechanism and treatment of POCD, there is little doubt that cognitive decline after surgery (especially in the elderly population) is a critical clinical issue that shows a high morbidity. POCD is defined as an impairment in mental processes of perception, memory, and information processing that occurs in the postoperative period (Hanning, 2005), and which is diagnosed by specific tests after exclusion of other neurological complications. Both cardiac surgery and non-cardiac surgery are associated with the cognitive dysfunction after hospital discharge from 30% to 50% of patients (Newman et al., 2001; McDonagh et al., 2010; Selnes et al., 2012). One study reported the incidence of cognitive decline to be 53% at discharge, 36% at 6 weeks after discharge, 24% at 6 months after discharge, and 42% at 5 years after coronary-artery bypass grafting (Newman et al., 2001). Shoair et al. (2015) showed that 15.9% of older adult patients developed POCD within 3 months after elective major non-cardiac surgery. Other studies have found that POCD is associated with poor short-term and long-term outcomes, including an increased risk of disability, increased expenditure on hospitalization, inability to cope independently, reduced quality of life, and possible permanent dementia (Hovens et al., 2014; Shoair et al., 2015). Patients with POCD are at an increased risk of death in the first year after surgery and the elderly (aged 60 years or older) are at a significant risk for long-term cognitive problems (Moller et al., 1998; Monk et al., 2008; Avidan et al., 2009; Steinmetz et al., 2009).
Pathogenic mechanism of POCD
There is strong standpoint that cognitive decline experienced by elderly patients is directly mediated by neuro-inflammation and the enhancement of amyloid-beta oligomerization after surgery and general anesthesia (Bedford, 1955; Eckenhoff et al., 2004; Müller et al., 2004; Newman et al., 2007; Dong et al., 2009; Cao et al., 2012). At the same time, some results suggest that surgery results in neuro-inflammation and cognitive impairment, and that anesthesia might not be an essential influential factor for these effects (Zhang et al., 2015; Zhou et al., 2015). Despite these controversies, existing evidence has confirmed that neuro-inflammatory response to operative stress is an independent risk factor associated with the development of POCD (Wan et al., 2007; Barrientos et al., 2012; Hovens et al., 2014; Lu et al., 2015; Ma et al., 2015; Zheng et al., 2015). Amyloid beta, the peptide associated with Alzheimer's disease, was also detected in the serum of POCD patients. Another important player is acetylcholine, which has significant roles in memory, learning, and attention (Hshieh et al., 2008). The most likely mechanism underlying POCD is a central cholinergic deficiency caused by the deregulation of cholinergic anti-inflammatory pathways, which results in increased inflammation (Inouye, 2006; Androsova et al., 2015). Many scholars have highlighted the importance of the cholinergic reflex in resolving the inflammatory pathogenesis of several diseases, including sepsis (Borovikova et al., 2000), rheumatoid arthritis (van Maanen et al., 2009), and colitis (Ghia et al., 2007). Researchers have reported that pro-inflammatory cytokines, including interleukin (IL)-1 and tumor necrosis factor-alpha (TNF-α), play key roles in mediating surgery-induced neuro-inflammation and subsequent cognitive decline (Cibelli et al., 2010; Terrando et al., 2010). Results reveal that surgery, not propofol-based anesthesia, induces neuro-inflammation and the impairment of learning and memory. Pyrrolidine dithiocarbamate attenuates these effects by inhibiting nuclear factor-kappa B activation and downstream matrix metallopeptidase 9 activity (Zhao et al., 2013; Zhang et al., 2014). Other studies have demonstrated that peripheral surgery affects the blood-brain barrier through the release of TNF-α. This promotes macrophage migrating into the hippocampus (Rudolph et al., 2008; Terrando et al., 2011; Vacas et al., 2013). Activation of α7 nicotinic acetylcholine receptors trigger an endogenous inflammation-resolving pathway that has been proven to be useful in blocking TNF-α-induced nuclear factor-kappa B activation and cognitive decline after surgery (Terrando et al., 2011). Jiang et al. (2015) suggested that IL-6 has a crucial role in POCD, and that IL-6R antagonists may serve as novel agents for its prevention or treatment. Chen et al. (2015) also demonstrated that dexmedetomidine reduces the incidence of POCD by suppressing inflammation in aged patients.
Roles of HBOPC on POCD
Cognitive decline after surgery includes deterioration in cognition, disturbance in attention, and reduced awareness of the environment. In light of recent clinical developments, HBO preconditioning has been shown to protect against focal and global cerebral ischemia as well as traumatic brain injury (Cheng et al., 2011; Yan et al., 2011; Lin et al., 2012). Furthermore, HBO preconditioning can promote both cerebral-protective and cardiac-protective effects, as determined by biochemical markers of neuronal and myocardial injury and clinical consequences in patients experiencing on-pump coronary artery bypass-graft surgery (Yogaratnam et al., 2010; Li et al., 2011). Additionally, Alex et al. (2005) indicated that while pretreatment with 2.4-ATA HBO can reduce neuropsychometric dysfunction and modulate the inflammatory response that occurs after cardiopulmonary bypass. In basic studies, Sun et al. (2014) indicated that HBO preconditioning can significantly lessen cognitive impairment, and that it can be considered responsible for decreases in pro-inflammatory (either systemic or central) cytokines and caspase-3 activity. Similarly, Peng et al. (2010) indicated that continuous HBOPC could lead to an apparent improvement in impairments of associative learning and spatial memory. The study also showed that HBOPC has an effective anxiolytic effect and provided experimental evidence that supports the idea that HBOPC is useful for treating some affective disorders, including post-traumatic stress disorder. All these results showed that HBO preconditioning is a safe and feasible procedure that can attenuate cognitive impairments after surgery. Additionally, they show that it is associated with anti-oxidants stress, anti-inflammation, and anti-apoptosis, as well as increased regional cerebral blood flow distribution and improvement of blood-brain barrier integrity (Li et al., 2007; Micarelli et al., 2013; Tian et al., 2013; Sun et al., 2014). Among these phenomena, the anti-oxidative stress and anti-inflammatory action of HBOPC are considered two crucial mechanisms with respect to easing POCD.
Antioxidant stress
(1) Anti-oxidative stress is achieved through activation of antioxidant enzymes and the decrease of pro-oxidant enzymes. HBO can elevate the partial pressure of oxygen and enhance the cellular tolerance against harmful stimuli by inducing the expression of cell protective proteins (Thom, 2009). Several studies have shown that the endogenous antioxidant-defense system becomes active in parallel with the development of HBOPC-induced neuroprotection (Nie et al., 2006; Thom, 2009; Huang et al., 2014). Numerous studies have shown that repeated preconditioning with HBO, but not normal conditions, can protect the spinal cord against I/R damage (Nie et al., 2006; Lu et al., 2012; Huang et al., 2014). These results have been attributed to the protective effect of upregulated HO-1, and the activity of catalase and superoxide dismutase (SOD), which are triggered by HBO preconditioning (Li et al., 2007). Further investigations have shown that when dimethylthiourea, a potent free radical scavenger, was administered before each session of HBO treatment, the HBO-induced catalase and SOD activities were abolished. Similarly, when the catalase inhibitor 3-amino-1,2,4-triazole or dimethylthiourea was administered before spinal cord ischemia, the ischemic tolerance induced by HBOPC was attenuated (Nie et al., 2006; Huang et al., 2014). HBOPC was shown to decrease mortality rate, improve neurological recovery, lessen neuronal injury, reduce the level of malondialdehyde, and increase antioxidant activity of catalase and SOD (Li et al., 2008). Repeated HBO exposure supplies protection against oxygen toxicity in the central nervous system and this may be attributed to the decreased enzymatic activity of the antioxidant system and reduced levels of peroxynitrite, primarily in the hippocampus (Arieli et al., 2014). In related work, Peng et al. (2010) suggested that HBOPC is beneficial for the improvement of anxiety-like behavior and cognitive impairments arising from a single prolonged exposure to stress, and that this effect might be associated with inhibition of neuronal apoptosis via upregulation of thioredoxin reductase in stressed rats. These results confirmed that HBO preconditioning can induce upregulation of antioxidant-enzyme activity, leading to the generation of tolerance against I/R injury in the brain (Li et al., 2008). Expression of antioxidant enzymes, including Cu/Zn-superoxide dismutase, catalase, and glutathione peroxidase, have been shown to be enhanced by HBOPC (Kim et al., 2001; Li et al., 2008). Additionally, levels of pro-oxidant enzymes such as inducible nitric oxide synthase and gp91-phox have been shown to significantly decrease after HBOPC (Zhang and Gould, 2014). However, few animal experiments reported that in the hippocampus of preconditioned rats, the activities of glutathione reductase and glucose-6-phosphate dehydrogenase were substantially decreased, while the activity of glutathione peroxidase was greatly increased (Arieli et al., 2014).
(2) Anti-oxidative stress is also achieved through the reactive oxygen species negative feedback loop. Transiently increased reactive oxygen species (ROS) levels activate a negative feedback loop, which leads to downregulation of oxidant enzymes and upregulation of antioxidant enzymes, thereby limiting subsequent higher levels of reactive species of oxygen and nitrogen production (Zhang and Gould, 2014). Furthermore, these results also indicate that ROS-related enzymes, including inducible nitric oxide synthase and nicotinamide adenine dinucleotide phosphate oxidase, rather than the ROS itself, can be crucial therapeutic targets for inhibiting oxidative stress.
(3) Anti-oxidative stress can also result from regulation of the ROS/mitogen-activated protein kinase (MAPK)/matrix metalloproteinase (MMP) and ROS/reactive nitrogen species (RNS) signaling pathways. HBO repairs ischemic wounds by decreasing the phosphorylation of extracellular signal-regulated kinases 1/2, c-Jun N-terminal kinase, and c-Jun, which suggests that mitogen-activated protein kinase is downregulated. All these results demonstrate that HBO acts via the ROS/MAPK/MMP signaling pathway to decrease neurodegeneration and ameliorate healing of ischemic wounds (Zhang and Gould, 2014). For example, the level of oxidative stress in ischemic wound tissue will be highly enhanced when the effect of HBO is completely blocked (Zhang and Gould, 2014). The oxidized N-linoleoyl tyrosine marker is sufficiently sensitive to detect oxidative stress imposed on cells and cell-free systems and to react selectively with the various ROS/RNS that are induced as a result. Thus, it is very useful for characterizing oxidative stress in general, and possibly also in oxidative stress-associated diseases (Szuchman et al., 2006). In one ingenious and delicate experiment, the oxidized N-linoleoyl tyrosine marker and the protein products of advanced oxidation were analyzed to demonstrate that preconditioning with multiple short HBO exposures followed by a long exposure will lead to a decrease in oxidative adducts, reaching even lower levels than that which initially existed in the control group. Endogenous antioxidant defense mechanisms induced by HBOPC play an important role in the formation of tolerance against long HBO exposure (Palzur et al., 2011).
(4) Antioxidant gene expression is another factor that increases anti-oxidative stress. Ferrer et al. (2007) showed that HBO can also act to activate antioxidant genes in human tissue. Endothelial cells are sensitive to high pressure oxygen exposure, which easily triggers the expression of many Nrf2-regulated antioxidant genes and molecular chaperones (Godman et al., 2010a, b). Additionally, the expression of antioxidant genes also occurs in other cells and tissues activated by HBO (Padgaonkar et al., 1997; Dennog et al., 1999; Rothfuss et al., 2001; Verma et al., 2015).
All these observations serve to illustrate central role that anti-oxidative stress has as a mechanism underlying HBO treatment. The findings strongly suggest that HBO preconditioning is a potentially promising treatment for preventing the development of cognitive impairment after surgery.
Anti-inflammation
Despite advances in surgical techniques, the incidence of neuropsychometric dysfunction after surgery is high. Previous studies have demonstrated that the systemic and central inflammatory response plays a critical role in the development of postoperative cognitive impairment (Cibelli et al., 2010; Fidalgo et al., 2011; Barrientos et al., 2012; He et al., 2012; Hovens et al., 2014; Sun et al., 2014), and HBO treatment can improve POCD by attenuating inflammatory responses (Alex et al., 2005; Daniel et al., 2011; Lin et al., 2012a, b).
(1) Inflammatory responses can be reduced by increased expression of antioxidant genes. ROS plays a significant role in transduction cascades and pathways (Allen and Balin, 1989; Maulik, 2002; Ushio-Fukai and Alexander, 2004; Calabrese et al., 2007). HBO-related anti-inflammatory action can be partially induced through increased expression of antioxidant genes and other cellular defense genes via non-cytotoxic oxidative stimuli (Godman et al., 2010a, b; Matsunami et al., 2010, 2011; He et al., 2011; Simsek et al., 2011).
(2) Attenuation of inflammatory cells sequestration and adhesion can also reduce inflammatory responses. Tissue inflammation can occur when circulating neutrophils adhere to vascular endothelium through interactions with β2-integrins. However, neutrophil β2-integrin function is inhibited by exposure to HBO (Thom et al., 2008; Thom, 2009). In some cases, when animals or humans are exposed to HBO (2.8–3.0 ATA), the ability of circulating neutrophils to adhere to target tissues is temporarily inhibited, and inflammation is subsequently reduced (Thom, 1993; Zamboni et al., 1993; Thom et al., 1997; Labrouche et al., 1999; Kalns et al., 2002). In ameliorating I/R injuries, HBO is notably superior to β2-integrin monoclonal antibodies because it does not compromise the immune system (Mileski et al., 1990; Buras et al., 2006). At the same time, HBO exposure also leads to the impaired synthesis of cyclic guanosine monophosphate (Chen et al., 1996), which consequently reduces the activity of the neutrophil specific adhesion molecule CD18 (Malik and Lo, 1996). In the meantime, intercellular adhesion molecule 1, which is a marker of acute and chronic inflammation, acts as the receptor of leukocyte function associated antigen-1 (CD11a/CDx18). This antigen is expressed on various inflammatory cells, including neutrophils, monocytes, and lymphocytes. For example, some studies have indicated that levels of intercellular adhesion molecule 1 are downregulated by HBO (Buras et al., 2000). By downregulating the accumulation of these cellular adhesion molecules, neutrophil sequestration and adhesion is attenuated, which reduced inflammation (Zamboni et al., 1993).
(3) Inflammation is also reduced through the inhibition of pro-inflammatory cytokine production. The production of pro-inflammatory cytokines by monocyte-macrophages is inhibited after exposure to HBO. Pro-inflammatory cytokine-regulating adhesion molecules and enhancement of heme oxygenase-1 and heat shock proteins (e.g., heat shock protein 70) (Rothfuss et al., 2001) are all mechanisms considered to play important roles in the anti-inflammatory effects of HBO. Compared with cells isolated from HBO-exposed rats (Lahat et al., 1995), those isolated from rats that were not previously exposed to HBO released more TNF-α. Additionally, in endotoxic rats, HBO treatment inhibits the endotoxin lipopolysaccharide-induced pro-inflammatory cytokines in monocytes and macrophages (Benson et al., 2003). Niu et al. (2007) reported that pyrogenic fever is prevented and suppressed by HBO via decreased overproduction of circulating TNF-α and hypothalamic prostaglandin E2. Similarly, several studies have demonstrated that the rise of TNF-α (Huang et al., 2006) and IL-6 (Niu et al., 2009) induced by lipopolysaccharide administration also can be significantly decreased by HBO pretreatment. Further, HBO decreases the release of IL-1β and TNF-a in monocytes and macrophages derived from human blood (Benson et al., 2003). HBO exposure is also indicated to lessen cytokine induction (Yamashita and Yamashita, 2000; Kang et al., 2014). Additionally, HBO pretreatment inhibits activated inflammation and gliosis, and stimulates angiogenesis, neurogenesis, and production of IL-10. This consequently improves outcomes of traumatic brain injury (Lin et al., 2012). Pretreatment with HBO is beneficial for recovery after brain surgery, and can enhance expression of osteopontin, which reduces the expression of IL-1β/nuclear factor-κ-gene binding and expansive protein kinase B (Akt) (Hu et al., 2015).
Discussion
As presented here, there is substantial evidence for a central involvement of oxidant stress and inflammatory response in POCD. In addition, numerous basic and clinical studies have demonstrated that HBOPC has a protective effect on POCD by reducing the detrimental inflammation and balancing the oxygen free radicals. The mechanism underlying preconditioning is not yet fully understood. Many researchers have suggested that HBOPC can alleviate cognitive impairment after surgery (Sun et al., 2014) and subsequently decrease the density of apoptotic cells and further recovery of nerve function (Wang et al., 2009; Lu et al., 2013). The mechanism underlying the protection might involve the reduction of systemic and hippocampal pro-inflammatory cytokines (Cheng et al., 2011) and upregulation of heat-shock protein 32 (Nie et al., 2006). Based on the experimental evidence, the prospect for using HBOPC to reduce cognitive impairment after surgery is bright. However, the number of relevant clinical studies remains low at present. Therefore, further studies are critical for understanding the fundamental mechanisms of this phenomenon and to explore the optimal parameters for pretreatment.
Footnotes
Funding: This study was supported by the Special Research Foundation of Doctoral Course in Colleges and Universities of China in 2013, No. 20133420110009.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Phillips A, Robens J, Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- Alex J, Laden G, Cale AR, Bennett S, Flowers K, Madden L, Gardiner E, McCollum PT, Griffin SC. Pretreatment with hyperbaric oxygen and its effect on neuropsychometric dysfunction and systemic inflammatory response after cardiopulmonary bypass: a prospective randomized double-blind trial. J Thorac Cardiovasc Surg. 2005;130:1623–1630. doi: 10.1016/j.jtcvs.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Allen M, Golembe E, Gorenstein S, Butler G. Protective effects of hyperbaric oxygen therapy (HBO2) in cardiac care-A proposal to conduct a study into the effects of hyperbaric pre-conditioning in elective coronary artery bypass graft surgery (CABG) Undersea Hyperb Med. 2014;42:107–114. [PubMed] [Google Scholar]
- Allen R, Balin AK. Oxidative influence on development and differentiation: an overview of a free radical theory of development. Free Radic Biol Med. 1989;6:631–661. doi: 10.1016/0891-5849(89)90071-3. [DOI] [PubMed] [Google Scholar]
- Androsova G, Krause R, Winterer G, Schneider R. Biomarkers of postoperative delirium and cognitive dysfunction. Front Aging Neurosci. 2015;7:112. doi: 10.3389/fnagi.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieli Y, Kotler D, Eynan M, Hochman A. Hyperbaric oxygen preconditioning protects rats against CNS oxygen toxicity. Respir Physiol Neurobiol. 2014;197:29–35. doi: 10.1016/j.resp.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Avidan MS, Searleman AC, Storandt M, Barnett K, Vannucci A, Saager L, Xiong C, Grant EA, Kaiser D, Morris JC. Long-term cognitive decline in older subjects was not attributable to non-cardiac surgery or major illness. Anesthesiology. 2009;111:964. doi: 10.1097/ALN.0b013e3181bc9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babchin A, Levich E, Sivashinsky G. Osmotic phenomena in application for hyperbaric oxygen treatment. Colloids Surf B Biointerfaces. 2011;83:128–132. doi: 10.1016/j.colsurfb.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012;32:14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford P. Adverse cerebral effects of anaesthesia on old people. Lancet. 1955;266:259–264. doi: 10.1016/s0140-6736(55)92689-1. [DOI] [PubMed] [Google Scholar]
- Benson R, Minter L, Osborne B, Granowitz E. Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophages. Clin Exp Immunol. 2003;134:57–62. doi: 10.1046/j.1365-2249.2003.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Nadler JW, Browndyke J, Terrando N, Ponnusamy V, Cohen HJ, Whitson HE, Mathew JP. Postoperative cognitive dysfunction: minding the gaps in our knowledge of a common postoperative complication in the elderly. Anesthesiol Clin. 2015;33:517–550. doi: 10.1016/j.anclin.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Buras JA, Stahl GL, Svoboda KK, Reenstra WR. Hyperbaric oxygen downregulates ICAM-1 expression induced by hypoxia and hypoglycemia: the role of NOS. Am J Physiol Cell Physiol. 2000;278:C292–C302. doi: 10.1152/ajpcell.2000.278.2.C292. [DOI] [PubMed] [Google Scholar]
- Buras JA, Holt D, Orlow D, Belikoff B, Pavlides S, Reenstra WR. Hyperbaric oxygen protects from sepsis mortality via an interleukin-10–dependent mechanism. Crit Care Med. 2006;34:2624–2629. doi: 10.1097/01.CCM.0000239438.22758.E0. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AMG. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- Cao L, Li L, Lin D, Zuo Z. Isoflurane induces learning impairment that is mediated by interleukin 1β in rodents. PLoS One. 2012;7:e51431. doi: 10.1371/journal.pone.0051431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Banick PD, Thom SR. Functional inhibition of rat polymorphonuclear leukocyte B2 integrins by hyperbaric oxygen is associated with impaired cGMP synthesis. J Pharmacol Exp Ther. 1996;276:929–933. [PubMed] [Google Scholar]
- Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42:484–490. doi: 10.1161/STROKEAHA.110.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS. Role of interleukin-1β in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel RAF, Cardoso VK, Góis E, Jr, Parra RS, Garcia SB, Rocha JJ, Rd, Féres O. Effect of hyperbaric oxygen therapy on the intestinal ischemia reperfusion injury. Acta Cir Bras. 2011;26:463–469. doi: 10.1590/s0102-86502011000600010. [DOI] [PubMed] [Google Scholar]
- Dennog C, Radermacher P, Barnett YA, Speit G. Antioxidant status in humans after exposure to hyperbaric oxygen. Mutat Res. 1999;428:83–89. doi: 10.1016/s1383-5742(99)00034-4. [DOI] [PubMed] [Google Scholar]
- Dong H, Xiong L, Zhu Z, Chen S, Hou L, Sakabe T. Preconditioning with hyperbaric oxygen and hyperoxia induces tolerance against spinal cord ischemia in rabbits. Anesthesiology. 2002;96:907–912. doi: 10.1097/00000542-200204000-00018. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, Culley DJ, Crosby G, Tanzi RE, Xie Z. The common inhalational anesthetic sevoflurane induces apoptosis and increases β-amyloid protein levels. Arch Neurol. 2009;66:620–631. doi: 10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- Edwards ML. Hyperbaric oxygen therapy. Part 1: history and principles. J Vet Emerg Crit Care. 2010;20:284–288. doi: 10.1111/j.1476-4431.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- Fidalgo AR, Cibelli M, White JP, Nagy I, Maze M, Ma D. Systemic inflammation enhances surgery-induced cognitive dysfunction in mice. Neurosci Lett. 2011;498:63–66. doi: 10.1016/j.neulet.2011.04.063. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Blennerhassett P, El-Sharkawy RT, Collins SM. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G711–718. doi: 10.1152/ajpgi.00240.2007. [DOI] [PubMed] [Google Scholar]
- Godman CA, Joshi R, Giardina C, Perdrizet G, Hightower LE. Hyperbaric oxygen treatment induces antioxidant gene expression. Ann N Y Acad Sci. 2010a;1197:178–183. doi: 10.1111/j.1749-6632.2009.05393.x. [DOI] [PubMed] [Google Scholar]
- Godman CA, Chheda KP, Hightower LE, Perdrizet G, Shin D-G, Giardina C. Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress Chaperones. 2010b;15:431–442. doi: 10.1007/s12192-009-0159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanning C. Postoperative cognitive dysfunction. Br J Anaesth. 2005;95:82–87. doi: 10.1093/bja/aei062. [DOI] [PubMed] [Google Scholar]
- He HJ, Wang Y, Le Y, Duan KM, Yan XB, Liao Q, Liao Y, Tong JB, Terrando N, Ouyang W. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci Ther. 2012;18:994–1002. doi: 10.1111/cns.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Xu X, Fan M, Chen X, Sun X, Luo G, Chen L, Mu Q, Feng Y, Mao Q. Preconditioning with hyperbaric oxygen induces tolerance against renal ischemia-reperfusion injury via increased expression of heme oxygenase-1. J Surg Res. 2011;170:e271–277. doi: 10.1016/j.jss.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Hovens IB, Schoemaker RG, van der Zee EA, Absalom AR, Heineman E, van Leeuwen BL. Postoperative cognitive dysfunction: Involvement of neuroinflammation and neuronal functioning. Brain Behav Immun. 2014;38:202–210. doi: 10.1016/j.bbi.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63:764–772. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Manaenko A, Xu T, Guo Z, Tang J, Zhang JH. Hyperbaric oxygen therapy for traumatic brain injury: bench-to-bedside. Med Gas Res. 2016;6:102–110. doi: 10.4103/2045-9912.184720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SL, Huang YX, Hu R, Li F, Feng H. Osteopontin mediates hyperbaric oxygen preconditioning-induced neuroprotection against ischemic stroke. Mol Neurobiol. 2015;52:236–243. doi: 10.1007/s12035-014-8859-6. [DOI] [PubMed] [Google Scholar]
- Huang G, Xu J, Xu L, Wang S, Li R, Liu K, Zheng J, Cai Z, Zhang K, Luo Y. Hyperbaric oxygen preconditioning induces tolerance against oxidative injury and oxygen-glucose deprivation by up-regulating heat shock protein 32 in rat spinal neurons. PLoS One. 2014;9:e85967. doi: 10.1371/journal.pone.0085967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WT, Lin MT, Chang CP. An NMDA receptor-dependent hydroxyl radical pathway in the rabbit hypothalamus may mediate lipopolysaccharide fever. Neuropharmacology. 2006;50:504–511. doi: 10.1016/j.neuropharm.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- Kalns J, Lane J, Delgado A, Scruggs J, Ayala E, Gutierrez E, Warren D, Niemeyer D, Wolf EG, Bowden RA. Hyperbaric oxygen exposure temporarily reduces Mac-1 mediated functions of human neutrophils. Immunol Lett. 2002;83:125–131. doi: 10.1016/s0165-2478(02)00068-8. [DOI] [PubMed] [Google Scholar]
- Kang N, Hai Y, Liang F, Gao CJ, Liu XH. Preconditioned hyperbaric oxygenation protects skin flap grafts in rats against ischemia/reperfusion injury. Mol Med Report. 2014;9:2124–2130. doi: 10.3892/mmr.2014.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Choi H, Chun YS, Kim GT, Park JW, Kim MS. Hyperbaric oxygenation pretreatment induces catalase and reduces infarct size in ischemic rat myocardium. Pflügers Archiv. 2001;442:519–525. doi: 10.1007/s004240100571. [DOI] [PubMed] [Google Scholar]
- Kindwall EP, Hunt TK. Hyperbaric medicine practice. Plast Reconstr Surg. 1995;96:985. [Google Scholar]
- Kindwall EP, Wheland HT. Hyperbaric medicine practice: Best Publishing Company. 1999 [Google Scholar]
- Kocaoğullar Y, Üstün ME, Avci E, Karabacakoglu A, Fossett D. The role of hyperbaric oxygen in the management of subarachnoid hemorrhage. Intensive Care Med. 2004;30:141–146. doi: 10.1007/s00134-003-1916-7. [DOI] [PubMed] [Google Scholar]
- Löndahl M. Hyperbaric oxygen therapy as treatment of diabetic foot ulcers. Diabetes Metab Res Rev. 2012;28:78–84. doi: 10.1002/dmrr.2256. [DOI] [PubMed] [Google Scholar]
- Labrouche S, Javorschi S, Leroy D, Gbikpi-Benissan G, Freyburger G. Influence of hyperbaric oxygen on leukocyte functions and haemostasis in normal volunteer divers. Thromb Res. 1999;96:309–315. doi: 10.1016/s0049-3848(99)00107-3. [DOI] [PubMed] [Google Scholar]
- Lahat N, Bitterman H, Yaniv N, Kinarty A, Bitterman N. Exposure to hyperbaric oxygen induces tumour necrosis factor-alpha (TNF-α) secretion from rat macrophages. Clin Exp Immunol. 1995;102:655–659. doi: 10.1111/j.1365-2249.1995.tb03867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu W, Ding S, Xu W, Guan Y, Zhang JH, Sun X. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia–reperfusion injury by upregulation of antioxidant enzymes in rats. Brain Res. 2008;1210:223–229. doi: 10.1016/j.brainres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Li Q, Li J, Zhang L, Wang B, Xiong L. Preconditioning with hyperbaric oxygen induces tolerance against oxidative injury via increased expression of heme oxygenase-1 in primary cultured spinal cord neurons. Life Sci. 2007;80:1087–1093. doi: 10.1016/j.lfs.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Li Y, Dong H, Chen M, Liu J, Yang L, Chen S, Xiong L. Preconditioning with repeated hyperbaric oxygen induces myocardial and cerebral protection in patients undergoing coronary artery bypass graft surgery: a prospective, randomized, controlled clinical trial. J Cardiothorac Vasc Anesth. 2011;25:908–916. doi: 10.1053/j.jvca.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Lin H, Chang CP, Lin HJ, Lin MT, Tsai CC. Attenuating brain edema, hippocampal oxidative stress, and cognitive dysfunction in rats using hyperbaric oxygen preconditioning during simulated high-altitude exposure. J Trauma Acute Care Surg. 2012a;72:1220–1227. doi: 10.1097/TA.0b013e318246ee70. [DOI] [PubMed] [Google Scholar]
- Lin KC, Niu KC, Tsai KJ, Kuo JR, Wang LC, Chio CC, Chang CP. Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. J Trauma Acute Care Surg. 2012b;72:650–659. doi: 10.1097/TA.0b013e31823c575f. [DOI] [PubMed] [Google Scholar]
- Liu W, Liu K, Tao H, Chen C, Zhang JH, Sun X. Hyperoxia preconditioning: the next frontier in neurology? Neurol Res. 2012;34:415–421. doi: 10.1179/1743132812Y.0000000034. [DOI] [PubMed] [Google Scholar]
- Lu P, Feng H, Yuan S, Zhang R, Li M, Hu R, Liu Z, Yin J. Effect of preconditioning with hyperbaric oxygen on neural cell apoptosis after spinal cord injury in rats. J Neurosurg Sci. 2013;57:253–258. [PubMed] [Google Scholar]
- Lu PG, Hu SL, Hu R, Wu N, Chen Z, Meng H, Lin JK, Feng H. Functional recovery in rat spinal cord injury induced by hyperbaric oxygen preconditioning. Neurol Res. 2012;34:944–951. doi: 10.1179/1743132812Y.0000000096. [DOI] [PubMed] [Google Scholar]
- Lu SM, Gui B, Dong HQ, Zhang X, Zhang SS, Hu LQ, Liu HL, Sun J, Qian YN. Prophylactic lithium alleviates splenectomy-induced cognitive dysfunction possibly by inhibiting hippocampal TLR4 activation in aged rats. Brain Res Bull. 2015;114:31–41. doi: 10.1016/j.brainresbull.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Müller S, Krause N, Schmidt M, Münte T, Münte S. Cognitive dysfunction after abdominal surgery in elderly patients. Z Gerontol Geriatr. 2004;37:475–485. doi: 10.1007/s00391-004-0212-7. [DOI] [PubMed] [Google Scholar]
- Ma Y, Cheng Q, Wang E, Li L, Zhang X. Inhibiting tumor necrosis factor-α signaling attenuates postoperative cognitive dysfunction in aged rats. Mol Med Report. 2015;12:3095–3100. doi: 10.3892/mmr.2015.3744. [DOI] [PubMed] [Google Scholar]
- Malik AB, Lo SK. Vascular endothelial adhesion molecules and tissue inflammation. Pharmacol Rev. 1996;48:213–229. [PubMed] [Google Scholar]
- Matsunami T, Sato Y, Sato T, Ariga S, Shimomura T, Yukawa M. Oxidative stress and gene expression of antioxidant enzymes in the streptozotocin-induced diabetic rats under hyperbaric oxygen exposure. Int J Clin Exp Pathol. 2010;3:177. [PMC free article] [PubMed] [Google Scholar]
- Matsunami T, Sato Y, Hasegawa Y, Ariga S, Kashimura H, Sato T, Yukawa M. Enhancement of reactive oxygen species and induction of apoptosis in streptozotocin-induced diabetic rats under hyperbaric oxygen exposure. Int J Clin Exp Pathol. 2011;4:255. [PMC free article] [PubMed] [Google Scholar]
- Maulik N. Redox signaling of angiogenesis. Antioxid Redox Signal. 2002;4:805–815. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- McDonagh DL, Mathew JP, White WD, Phillips-Bute B, Laskowitz DT, Podgoreanu MV, Newman MF. Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of brain injury. Anesthesiology. 2010;112:852. doi: 10.1097/ALN.0b013e3181d31fd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micarelli A, Jacobsson H, Larsson S, Jonsson C, Pagani M. Neurobiological insight into hyperbaric hyperoxia. Acta Physiologica. 2013;209:69–76. doi: 10.1111/apha.12116. [DOI] [PubMed] [Google Scholar]
- Mileski W, Winn R, Vedder N, Pohlman T, Harlan J, Rice C. Inhibition of CD18-dependent neutrophil adherence reduces organ injury after hemorrhagic shock in primates. Surgery. 1990;108:206–212. [PubMed] [Google Scholar]
- Moller J, Cluitmans P, Rasmussen L, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning C. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, Van Der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiololy. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- Morykwas MJ, Argenta L. Nonsurgical modalities to enhance healing and care of soft tissue wounds. J South Orthop Assoc. 1996;6:279–288. [PubMed] [Google Scholar]
- Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106:572–590. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- Nie H, Xiong L, Lao N, Chen S, Xu N, Zhu Z. Hyperbaric oxygen preconditioning induces tolerance against spinal cord ischemia by upregulation of antioxidant enzymes in rabbits. J Cereb Blood Flow Metab. 2006;26:666–674. doi: 10.1038/sj.jcbfm.9600221. [DOI] [PubMed] [Google Scholar]
- Niu KC, Huang WT, Lin MT, Huang KF. Hyperbaric oxygen causes both antiinflammation and antipyresis in rabbits. Eur J Pharmacol. 2009;606:240–245. doi: 10.1016/j.ejphar.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Padgaonkar VA, Giblin FJ, Fowler K, Leverenz VR, Reddan JR, Dziedzic DC. Heme oxygenase synthesis is induced in cultured lens epithelium by hyperbaric oxygen or puromycin. Exp Eye Res. 1997;65:435–443. doi: 10.1006/exer.1997.0356. [DOI] [PubMed] [Google Scholar]
- Palzur E, Soliman K, Vaya J, Chezar I, Michelis R, Sela S. Effects of hyperbaric oxygen (HBO) preconditioning on plasma constituents. Free Radic Biol Med. 2011;51:S97–S98. [Google Scholar]
- Peng Y, Feng SF, Wang Q, Wang HN, Hou WG, Xiong L, Luo ZJ, Tan QR. Hyperbaric oxygen preconditioning ameliorates anxiety-like behavior and cognitive impairments via upregulation of thioredoxin reductases in stressed rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1018–1025. doi: 10.1016/j.pnpbp.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Rothfuss A, Radermacher P, Speit G. Involvement of heme oxygenase-1 (HO-1) in the adaptive protection of human lymphocytes after hyperbaric oxygen (HBO) treatment. Carcinogenesis. 2001;22:1979–1985. doi: 10.1093/carcin/22.12.1979. [DOI] [PubMed] [Google Scholar]
- Rudolph JL, Ramlawi B, Kuchel GA, McElhaney JE, Xie D, Sellke FW, Khabbaz K, Levkoff SE, Marcantonio ER. Chemokines are associated with delirium after cardiac surgery. J Gerontol A Biol Sci Med Sci. 2008;63:184–189. doi: 10.1093/gerona/63.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selnes OA, Gottesman RF, Grega MA, Baumgartner WA, Zeger SL, McKhann GM. Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med. 2012;366:250–257. doi: 10.1056/NEJMra1100109. [DOI] [PubMed] [Google Scholar]
- Shoair OA, Grasso II MP, Lahaye LA, Daniel R, Biddle CJ, Slattum PW. Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: A prospective study. J Anaesthesiol Clin Pharmacol. 2015;31:30. doi: 10.4103/0970-9185.150530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek K, Ay H, Topal T, Ozler M, Uysal B, Ucar E, Acikel CH, Yesilyurt O, Korkmaz A, Oter S. Long-term exposure to repetitive hyperbaric oxygen results in cumulative oxidative stress in rat lung tissue. Inhal Toxicol. 2011;23:166–172. doi: 10.3109/08958378.2011.558528. [DOI] [PubMed] [Google Scholar]
- Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, Jing Z, Chen J, Zigmond MJ, Gao Y. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol. 2014;114:58–83. doi: 10.1016/j.pneurobio.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Xie K, Zhang C, Song R, Zhang H. Hyperbaric oxygen preconditioning attenuates postoperative cognitive impairment in aged rats. Neuroreport. 2014;25:718–724. doi: 10.1097/WNR.0000000000000181. [DOI] [PubMed] [Google Scholar]
- Szuchman A, Aviram M, Soliman K, Tamir S, Vaya J. Exogenous N-linoleoyl tyrosine marker as a tool for the characterization of cellular oxidative stress in macrophages. Free Radic Res. 2006;40:41–52. doi: 10.1080/10715760500358787. [DOI] [PubMed] [Google Scholar]
- Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-α triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Eriksson LI, Kyu Ryu J, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom S, Mendiguren I, Hardy K, Bolotin T, Fisher D, Nebolon M, Kilpatrick L. Inhibition of human neutrophil beta2-integrin-dependent adherence by hyperbaric O2. Am J Physiol Cell Physiol. 1997;272:C770–777. doi: 10.1152/ajpcell.1997.272.3.C770. [DOI] [PubMed] [Google Scholar]
- Thom SR. Functional inhibition of leukocyte B 2 integrins by hyperbaric oxygen in carbon monoxide-mediated brain injury in rats. Toxicol Appl Pharmacol. 1993;123:248–256. doi: 10.1006/taap.1993.1243. [DOI] [PubMed] [Google Scholar]
- Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol. 2009;106:988–995. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom SR, Bhopale VM, Mancini DJ, Milovanova TN. Actin S-nitrosylation inhibits neutrophil β2 integrin function. J Biol Chem. 2008;283:10822–10834. doi: 10.1074/jbc.M709200200. [DOI] [PubMed] [Google Scholar]
- Tian X. Variations of brain edema and neurological function of rat models of cerebral infarction after hyperbaric oxygen therapy. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:6423–6428. [Google Scholar]
- Tian X, Zhang L, Wang J, Dai J, Shen S, Yang L, Huang P. The possible protective mechanism of hyperbaric oxygen (HBO) in memory impairments induced by Aβ25–35 in rats. Open Med. 2013;8:468–475. [Google Scholar]
- Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642–1648. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling. Role of NAD (P) H oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- Vacas S, Degos V, Feng X, Maze M. The neuroinflammatory response of postoperative cognitive decline. Br Med Bull. 2013;106:161–178. doi: 10.1093/bmb/ldt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maanen MA, Lebre MC, van der Poll T, LaRosa GJ, Elbaum D, Vervoordeldonk MJ, Tak PP. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–122. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- Verma R, Chopra A, Giardina C, Sabbisetti V, Smyth JA, Hightower LE, Perdrizet GA. Hyperbaric oxygen therapy (HBOT) suppresses biomarkers of cell stress and kidney injury in diabetic mice. Cell Stress Chaperones. 2015;20:495–505. doi: 10.1007/s12192-015-0574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wad K, Ito M, Miyazawa T, Katoh H, Nawashiro H, Shima K, Chigasaki H. Repeated hyperbaric oxygen induces ischemic tolerance in gerbil hippocampus. Brain Res. 1996;740:15–20. doi: 10.1016/s0006-8993(96)00831-1. [DOI] [PubMed] [Google Scholar]
- Wada K, Miyazawa T, Nomura N, Tsuzuki N, Nawashiro H, Shima K. Preferential conditions for and possible mechanisms of induction of ischemic tolerance by repeated hyperbaric oxygenation in gerbil hippocampus. Neurosurgery. 2001;49:160–167. doi: 10.1097/00006123-200107000-00025. [DOI] [PubMed] [Google Scholar]
- Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- Wang L, Li W, Kang Z, Liu Y, Deng X, Tao H, Xu W, Li R, Sun X, Zhang JH. Hyperbaric oxygen preconditioning attenuates early apoptosis after spinal cord ischemia in rats. J Neurotrauma. 2009;26:55–66. doi: 10.1089/neu.2008.0538. [DOI] [PubMed] [Google Scholar]
- Wilson B, McLaughlin L, Nair AR, Dange R, Francis J. Inflammation, oxidative stress, and neuroprotective factors in the pathophysiology of PTSD in an animal model. FASEB J. 2013;27:691–695. [Google Scholar]
- Xiong L, Zhu Z, Dong H, Hu W, Hou L, Chen S. Hyperbaric oxygen preconditioning induces neuroprotection against ischemia in transient not permanent middle cerebral artery occlusion rat model. Chin Med J (Engl) 2000;113:836–839. [PubMed] [Google Scholar]
- Yamashita M, Yamashita M. Hyperbaric oxygen treatment attenuates cytokine induction after massive hemorrhage. Am J Physiol Endocrinol Metab. 2000;278:E811–816. doi: 10.1152/ajpendo.2000.278.5.E811. [DOI] [PubMed] [Google Scholar]
- Yan W, Zhang H, Bai X, Lu Y, Dong H, Xiong L. Autophagy activation is involved in neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Brain Res. 2011;1402:109–121. doi: 10.1016/j.brainres.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Yogaratnam JZ, Laden G, Guvendik L, Cowen M, Cale A, Griffin S. Hyperbaric oxygen preconditioning improves myocardial function, reduces length of intensive care stay, and limits complications post coronary artery bypass graft surgery. Cardiovasc Revasc Med. 2010;11:8–19. doi: 10.1016/j.carrev.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Zamboni WA, Roth AC, Russell RC, Graham B, Suchy H, Kucan JO. Morphologic analysis of the microcirculation during reperfusion of ischemic skeletal muscle and the effect of hyperbaric oxygen. Plast Reconstr Surg. 1993;91:1110–1123. doi: 10.1097/00006534-199305000-00022. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jiang W, Zuo Z. Pyrrolidine dithiocarbamate attenuates surgery-induced neuroinflammation and cognitive dysfunction possibly via inhibition of nuclear factor κB. Neuroscience. 2014;261:1–10. doi: 10.1016/j.neuroscience.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tan H, Jiang W, Zuo Z. The choice of general anesthetics may not affect neuroinflammation and impairment of learning and memory after surgery in elderly rats. J Neuroimmune Pharmacol. 2015;10:179–189. doi: 10.1007/s11481-014-9580-y. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Gould LJ. Hyperbaric oxygen reduces matrix metalloproteinases in ischemic wounds through a redox-dependent mechanism. J Invest Dermatol. 2014;134:237–246. doi: 10.1038/jid.2013.301. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Huang G, Wang B, Zhong Y. Inhibition of NF-kappaB activation by Pyrrolidine dithiocarbamate partially attenuates hippocampal MMP-9 activation and improves cognitive deficits in streptozotocin-induced diabetic rats. Behav Brain Res. 2013;238:44–47. doi: 10.1016/j.bbr.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Zheng X, Ma Z, Gu X. Plasma levels of tumor necrosis factor-α in adolescent idiopathic scoliosis patients serve as a predictor for the incidence of early postoperative cognitive dysfunction following orthopedic surgery. Exp Ther Med. 2015;9:1443–1447. doi: 10.3892/etm.2015.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BC, Liu LJ, Liu B. Neuroprotection of hyperbaric oxygen therapy in sub-acute traumatic brain injury: not by immediately improving cerebral oxygen saturation and oxygen partial pressure. Neural Regen Res. 2016a;11:1445–1449. doi: 10.4103/1673-5374.191218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HX, Liu ZG, Liu XJ, Chen QX. Umbilical cord-derived mesenchymal stem cell transplantation combined with hyperbaric oxygen treatment for repair of traumatic brain injury. Neural Regen Res. 2016b;11:107–113. doi: 10.4103/1673-5374.175054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wang Z, Zhou H, Liu T, Lu F, Wang S, Li J, Peng S, Zuo Z. Neonatal exposure to sevoflurane may not cause learning and memory deficits and behavioral abnormality in the childhood of Cynomolgus monkeys. Sci Rep. 2015;5:11145. doi: 10.1038/srep11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JY, Yin GQ, Pang JJ, Chen ZX, Pan XY, Lu SD, Wei QX, Xie ZD. Effect of hyperbaric oxygen pretreatment on the expressions of vascular endothelial growth factor and transforming growth factor beta in over-length dorsal random skin flaps. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:1525–1531. [Google Scholar]