Abstract
Before 1999, leishmaniasis was considered an imported disease in Thailand. Since then, autochthonous leishmaniasis was reported in both immmunocompetent and immmunocompromised patients especially in human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS). A new species was identified and named as Leishmania siamensis consisting of two lineages, that is, lineages TR and PG. Analysis of isoenzymes has clarified the more commonly detected L. siamensis lineage PG as Leishmania martiniquensis (MON-229), a species originally reported from the Martinique Island, whereas the L. siamensis lineage TR has been identified as the true novel species, L. siamensis (MON-324). Both cutaneous leishmaniasis (CL) and visceral leishmaniasis (VL) have been found among Thai patients. Disseminated CL and VL could be presented in some reported patients who had HIV/AIDS coinfection. So far, only sporadic cases have been reported; thus, the true prevalence of leishmaniasis should be determined in Thailand among the high-risk populations such as people with HIV/AIDS. A recent survey among animals identified L. martiniquensis DNA in black rats (Rattus rattus) suggesting a potential animal reservoir. In addition, L. martiniquensis DNA was identified in Sergentomyia gemmea and Sergentomyia barraudi, the predominant sandfly species in the affected areas. However, further studies are needed to prove that these sandflies could serve as the vector of leishmaniasis in Thailand.
Introduction
Leishmania is an intracellular protozoa, a member of the Family Trypanosomatidae, Order Kinetoplastida. The transmission occurs by the bite of the phlebotomine female sandfly. Two subgenera, Leishmania (Leishmania) and Leishmania (Viannia), the members of the subgenus Leishmania develop in the midgut region, whereas those of subgenus Viannia develop in the hindgut region of sandflies.1 Leishmania causes three major clinical forms of infection, that is, cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL), and visceral leishmaniasis (VL). VL or kala-azar, the most severe form, is caused by parasite multiplication in the reticuloendothelial system, mainly the liver, spleen, lymph node, and bone marrow. VL causes a broad range of symptoms from asymptomatic to a fatal outcome.2,3 Symptoms depend on host immunity and species of the parasite.3–5 Concomitant human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) not only increases the risk of active VL but also results in poor responses to treatment.4,5
More than 20 species of Leishmania have been determined as human pathogens, endemic in the Middle East, Central and North America, Indian subcontinent, and Mediterranean basins. Currently, 0.9–1.3 million new cases are annually reported with 20,000–30,000 deaths caused by VL.6,7 Primary causative agents of VL are Leishmania donovani complex species including L. donovani and Leishmania infantum in the Old World and Leishmania chagasi (synonym L. infantum) in the New World. Leishmania tropica and Leishmania amazonensis primarily cause CL, but could be viscerotropic in some cases.8,9 In Thailand, leishmaniasis mainly constituted imported cases before 1999. This review emphasizes the emerging autochthonous leishmaniasis in Thailand. To date, two newly identified species, Leishmania martiniquensis and Leishmania siamensis have caused autochthonous leishmaniasis among Thai patients. The data presented in this review were obtained from published articles of leishmaniasis in Thailand from 1999 to 2016 in PubMed.
Species Identification of Leishmania (Leishmania) Martiniquensis and Leishmania (Leishmania) Siamensis
With regard to species identification, two axenic cultured promastigote isolates from two VL patients10 were sent for isoenzyme analysis at the French Reference Center on Leishmaniasis, Montpellier, France. These isolates were reported as two lineages of L. siamensis: lineage PG and TR.10 The results of 15 isoenzyme analyses revealed two distinct species. The first lineage, L. siamensis lineage PG (MHOM/TH/2011/PG), was identical to the zymodeme MON-229 of L. martiniquensis (MHOM/MQ/92/MAR1). Leishmania martiniquensis is a species originally reported from Martinique Island11 in an HIV-infected patient with diffused nodular CL in 1995.12 Other molecular typings of three target genes of L. martiniquensis (MHOM/MQ/92/MAR1) and the GenBank accession numbers are as follows: RNA polymerase (AF326982), DNA polymerase (AF326983),13 and 18S-rRNA (AF303938).11
Although the L. siamensis lineage TR was confirmed as L. siamensis (MHOM/TH/2010/TR, zymodeme MON-324), a novel species was first reported in Thailand (Figure 1 ).14 Other molecular typings of the three protein coding DNA sequences of L. siamensis (MON-324) are as follows: locus 03.0980, elongation initiation factor 2 a-subunit (JQ586200); locus 04.0580, spermidine synthase 1 (JQ586201); and locus 31.2610 and RNA polymerase II largest subunit (JQ586202).14 Details of isoenzyme analysis using 15 enzymes and molecular markers of the two species as well as other biological characteristics of L. martiniquensis and L. siamensis are summarized in Table 1.15–31
Figure 1.
Locations of the reported autochthonous leishmaniasis cases in Thailand. 1. Surat Thani,32 2. Nan,33 3. Phang-nga,15 4. Chanthaburi,18 5. Songkhla,19,29 6. Trang,14,19 7. Lopburi,34 8.Chiang Rai,20 9. Stun,22 10. Lamphun,27,28 11. Chiang Mai.28
Table 1.
A summary of the biological characteristics of Leishmania martiniquensis and Leishmania siamensis
| L. martiniquensis | L. siamensis | |||
|---|---|---|---|---|
| Characteristic | Reference | Characteristic | Reference | |
| Geographical distribution | Martinique island/French West Indies (human cases) | 11 | Thailand (a human case) | 14 |
| Thailand (human cases) | 15, 18–20, 22, 26–29 | |||
| Germany, Switzerland (horses, cow) | 16, 17 | |||
| United States (a horse) | 21 | |||
| Myanmar (human cases) | 20, 23 | |||
| Clinical type in HIV seronegative | VL | 15, 22 | ||
| CL | 20, 23 | |||
| Clinical type in HIV seropositive | DCL and VL | 19, 20, 26, 28 | DCL and VL | 14 |
| VL | 18, 27, 30 | |||
| CL | 20 | |||
| Clinical type in animals | CL* | 16, 17, 21 | No data | |
| Size of amastigote (light microscopy) | Diameter: 3.99 ± 0.48 μm | 11 | No data | |
| Axenic in vitro cultivation | SDM79 medium supplemented with 15% fetal calf serum, 7 μg/mL hemin, and 2.5 μg/mL 6-biopterin or NNN medium or Schneider's medium supplemented with 20% fetal calf serum | 11 | NNN medium or Schneider's media supplemented with 20% fetal calf serum | Unpublished data |
| Size of promastigote in culture | Body length: 9.44 ± 3.02 μm; body width: 2.20 ± 0.63 μm; flagellum length: 11.59 ± 3.63 μm | 11 | Body length: 7.29 + 1.53 μm; body width: 1.22 + 0.29 μm; flagellum length: 9.80 + 2.52 μm | Unpublished data |
| Enzyme profiles* | MDH150, ME45, ICD95, PGD87, G6PD78, GLUD300, DIA30, NP100, NP285, GOT1170, GOT200, GM104, FH65, MPI,13GPI52 | 11 | MDH112, ME70, ICD100, PGD140, G6PD85, GLUD260, DIA20, NP100, NP2120, GOT1122, GOT2122, PGM109/96, FH60, MPI137, GPI88/76 | Unpublished data |
| WHO Code | MHOM/MQ/92/MAR1 | 11 | MHOM/TH/2010/TR | Unpublished data |
| MHOM/TH/2011/PG (enzyme profiles similar to those of MHOM/MQ/92/MAR1) | Unpublished data | |||
| Zymodeme | MON-229 [MHOM/MQ/92/MAR1] | 11 | MON-324 | Unpublished data |
| MON-229 [MHOM/TH/2011/PG] | Unpublished data | |||
| Molecular typing | RNA polymerase (AF32698) | 13 | Elongation initiation factor 2 a-subunit (JQ586200) | 14 |
| DNA polymerase (AF326983) | 13 | Spermidine synthase 1 (JQ586201) | ||
| 18S rRNA (AF303938) | 11 | RNA polymerase II largest subunit (JQ586202) | ||
| Phylogenetic analysis | Closely related to Leishmania enrietti complex | 27 | Closely related to Leishmania enrietti complex | 14 |
| Experimental vertebrate host | Infective to BALB/c mice and amastigotes were detected in the popliteal and mesenteric lymph nodes, liver, spleen, and brain | 31 | No data | |
| Potential animal reservoir | Black rats (Rattus rattus) (detection by PCR in blood, liver, and spleen) | 25 | No data | |
| Potential sandfly vector | Sergentomyia gemmea, Sergentomyia barraudi (detection by PCR in sandfly) | 24, 25 | No data | |
BALB/c mice = an albino, laboratory-bred strain of the house; CL = cutaneous leishmaniasis; DCL = diffused cutaneous leishmaniasis; HIV = human immunodeficiency virus; NNN = Novy-MacNeal-Nicolle medium; PCR = polymerase chain reaction; VL = visceral leishmaniasis; WHO = World Health Organization.
Isoenzyme analysis was performed at French Reference Center on Leishmaniasis, UMR5290, Montpellier, France.
As shown in Table 2, all L. siamensis published papers from 2008 to 201614–29 were reviewed and the species of Leishmania were confirmed using nucleotide sequences of the SSU-rRNA, ITS1 region of SSU-rRNA, and hsp70 genes from GenBank database and were compared with the nucleotide sequences of each gene using axenic cultures of the reference species, L. siamensis [MON 324] (SSU-rRNA/JQ280883, ITS1/JQ001751, and hsp70/KC202880) and L. martiniquensis [MON-229] (SSU-rRNA/JN885899, ITS1/JX195637, and hsp70/KC202882). The results confirmed that 12 Leishmania infections in Thai and Myanmar patients were L. martiniquensis as well as those reported in horses and cow.16,17,21 Only one published paper by Bualert and others in 2012 identified L. siamensis infection.14
Table 2.
Reported and clarified Leishmania species reported in humans, animals, and sandflies
| Reference | Host | Area | GenBank accession numbers | Reported species | Clarified species§ | ||
|---|---|---|---|---|---|---|---|
| SSU-rRNA* | ITS1† | hsp70‡ | |||||
| 15 | Human | Phang Nga, Thailand | JN885899¶ | EF200012 | N/A | A suspected novel species | L. martiniquensis |
| 16 | Horse | Germany, Switzerland | N/A | GQ281278 | N/A | Closely related to L. siamensis | L. martiniquensis |
| GQ281279 | |||||||
| GQ281280 | |||||||
| GQ281281 | |||||||
| 17 | Cow | Switzerland | N/A | GQ281282 | N/A | A suspected novel species | L. martiniquensis |
| 18 | Human | Chanthaburi, Thailand | GQ226033 | GQ226034 | N/A | A novel species | L. martiniquensis |
| 19, 20 | Human | Songkhla, Thailand | N/A | JQ001751 | N/A | A novel species | L. martiniquensis |
| 19, 20 | Human | Trang, Thailand | N/A | JQ001752∥ | N/A | A novel species | L. martiniquensis |
| 21 | Horse | Florida | N/A | JQ617283 | N/A | L. siamensis | L. martiniquensis |
| 14 | Human | Trang, Thailand | JQ280883 | JX195640 | KC202880 | L. siamensis | L. siamensis |
| 20 | Human | Myanmar | GQ226033 | N/A | N/A | L. siamensis | L. martiniquensis |
| 20 | Human | Chiang Rai, Thailand | N/A | N/A | N/A | L. siamensis | L. martiniquensis |
| 20 | Human | Myanmar | N/A | N/A | N/A | L. siamensis | L. martiniquensis |
| 22 | Human | Stun, Thailand | JN087497 | JX195637 | KC202882 | L. siamensis | L. martiniquensis |
| 20, 23 | Human | Myanmar | N/A | JQ001751 | N/A | L. siamensis | L. martiniquensis |
| 24 | Sandfly (Sergentomyia gemmea) | Trang, Thailand | N/A | N/A | JX852708 | L. siamensis | L. martiniquensis |
| 25 | Sandfly (Sergentomyia barraudi) | Songkhla, Thailand | N/A | JQ866907 | N/A | L. siamensis | L. martiniquensis |
| 25 | Black rats (Rattus rattus) | Trang, Thailand | N/A | JQ866906 | N/A | L. siamensis | L. martiniquensis |
| 26 | Human | Southern Thailand | N/A | JQ001751 | N/A | L. siamensis | L. martiniquensis |
| 27 | Human | Lamphun, Thailand | N/A | JX898938 | N/A | L. martiniquensis | L. martiniquensis |
| 28 | Human | Chiang Mai, Thailand | N/A | KJ 210834 | KP244367 | L. martiniquensis | L. martiniquensis |
| KJ210835 | |||||||
| 28 | Human | Lamphun, Thailand | N/A | KJ 210836 | KP244368 | L. martiniquensis | L. martiniquensis |
| KJ210837 | |||||||
| 29 | Human | Thailand | N/A | KU050863 | N/A | L. martiniquensis | L. martiniquensis |
L. martiniquensis = Leishmania martiniquensis; L. siamensis = Leishmania siamensis; N/A = not available.
Small subunit ribosomal RNA gene.
Internal transcribed spacer 1 of SSU-rRNA gene.
Heat shock protein 70 gene.
Clarified species by sequence analysis using GenBank database of the three genes; L. siamensis (SSU-rRNA/JQ280883, ITS1/JQ001751, hsp70/KC202880) and L. martiniquensis (SSU-rRNA/JN885899, ITS1/JX195637, and hsp70/KC202882).
Unpublished accession number from Sukmee and others (2008), first referred in Bualert and others (2012).
The sequences of accession number JQ001752 and KF227887 to KF227892 were obtained from the specimens collected from the same source but different site of specimen collection. All sequences showed 100% identical.
Phylogenetic Analysis
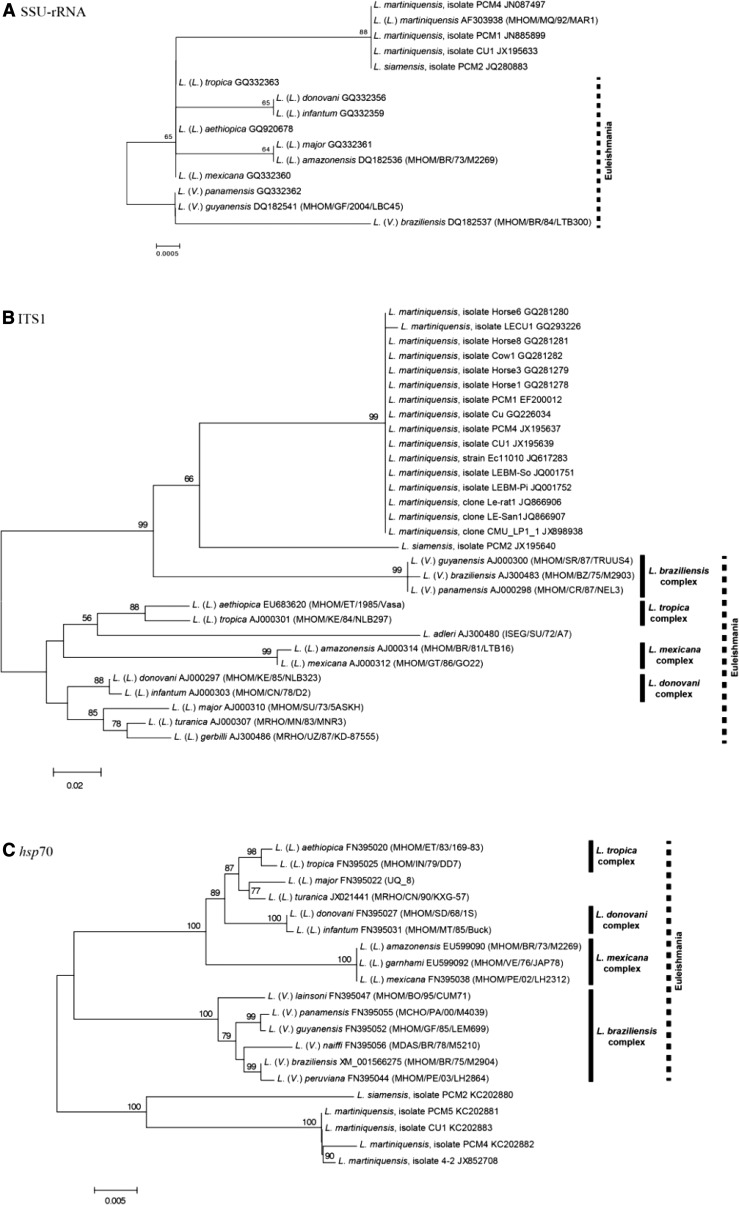
Phylogenetic trees based on three genetic loci, that is, SSU-rRNA, ITS1, and hsp70, including sequencing data of L. siamensis and L. martiniquensis available in GenBank have been reconstructed (Figure 2 ). The SSU-rRNA tree was constructed using three L. martiniquensis isolates, one L. siamensis isolate, and 10 reference sequences of different Leishmania species. The phylogenetic analyses showed that both L. martiniquensis and L. siamensis isolates were grouped together in monophyletic clade implying that their evolutionary processes seemed not to be related to other Leishmania species. The molecular and isoenzymatic techniques of L. martiniquensis clearly show that this species belongs to the subgenus Leishmania. Hence, the grouping of L. siamensis together with L. martiniquensis in the same monophyletic branch primarily suggests that L. siamensis is closely related to members of the same subgenus. However, the SSU-rRNA sequences are unable to discriminate between these two Leishmania species due to the 100% identical sequences (data not shown) indicating that the variation of these gene fragments used to construct phylogenetic tree limits the classification of these parasites at the species level (Figure 2A). The characteristic of this phylogenetic tree was supported by the fact that two pairs of Leishmania major and Leishmania amazonensis; Leishmania guyanensis and Leishmania panamensis sequences were also 100% identical.
Figure 2.
The unrooted phylogenetic tree inferred from DNA sequences of three markers: (A) SSU-rRNA, (B) ITS1, and (C) hsp70 using neighbor-joining method.
The reconstruction of a phylogenetic tree based on ITS1 region including the L. martiniquensis reference sequence together with 13 other Leishmania reference sequences has revised the relationship among the L. siamensis cases previously analyzed. The phylogenetic tree showed that the L. martiniquensis reference sequence was grouped with the same taxa that have been identified as L. siamensis suggesting that most isolates would belong to L. martiniquensis. Figure 2 includes the Leishmania sequences obtained from sandflies and black rats in the study of animal reservoirs and potential vectors of L. siamensis by Chusri and others (JQ866907, JQ866906)25 and sequences belong to L. siamensis lineage PG previously isolated from Thai patients (GQ226034, GQ293226, JQ001751, and JQ001752),10,18,19 horses (JQ617283, GQ281278, GQ281279, GQ281280, and GQ281281),16,21 and cows (GQ281282).17 However, the L. siamensis lineage TR, now termed L. siamensis, still forms a separate branch from L. martiniquensis indicating the close relationship between the two species (Figure 2B).
A few studies have focused on the hsp70 gene compared with the ITS1 for species identification. The information obtained from this gene has been considered one of the most useful data to provide precise relationship of genus Leishmania. Although no reference sequence of L. martiniquensis is available in the database, the hsp70 sequences of Leishmania isolates PCM2, CU1, and PCM4 that have been identified the species by ITS1 region could be logically considered as representative sequences of L. siamensis and L. martiniquensis, respectively. These would implicitly make the case reported by Leelayoova and others (KC202881)10 and the sequence obtained from the potential vector24 to be L. martiniquensis (Figure 2C).
Situation of Leishmaniasis in Thailand
Before 1999, both CL and VL were considered imported diseases. A retrospective review showed 40 CL and 6 VL cases were reported from 1960 to 1997.35 Of 46 cases, 44 comprised Thai workers who returned from endemic areas, that is, Saudi Arabia, Iraq, and Libya. A public health concern of Leishmania infection in Thailand started when the first two autochthonous VL were reported in 1999 and 2007.32,33 However, species identification was not performed. In 2008, a suspected new species of Leishmania causing autochthonous VL in an immunocompetent Thai patient was reported in Phang Nga Province, southern Thailand.15 The map of Thailand shows sporadic cases of CL and VL reported in six southern, one central, one eastern, and four northern provinces. Table 3 shows the characteristics of published leishmaniasis cases in Thailand from 1999 to 2016. Approximately 90% of cases comprised adults with an age range from 30 to 81 years, although the youngest patient was a 3-year-old girl. More than 50% of them were male, lived in the south, and about half of these cases comprised people with HIV/AIDS. All documented VL cases had chronic infection and developed severe symptoms. However, some VL patients who had coinfection with HIV/AIDS could develop disseminated dermal leishmaniasis. In the coinfected patients, most cases had CD4+ counts less than 200 cells/μL. Of these cases, one autochthonous CL was reported in a 5-year-old girl. In 2008, one VL case caused by L. infantum was reported in a Thai male patient living in Bangkok, central Thailand,36 where source of infection, biological vector, and reservoir host could not be identified due to his traveling history. Thus, this case was not considered as autochthonous and was excluded from the map. In addition, a CL was reported in a 3-year-old girl; however, the causative agent was not identified.34
Table 3.
Documented cases of leishmaniasis reported from Thailand during 1999–2016
| Case no. | Age (years) | Sex | Province | Clinical form | HIV status | Diagnostic method | Treatment | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | F | Surat Thani, Thailand | VL | Negative | IFA, PCR | Pentamidine | 31 |
| 2 | 40 | M | Nan, Thailand | VL | Negative | Microscopy, PCR | Amphotericin | 32 |
| 3 | 66 | M | Bangkok, Thailand | VL | Negative | DAT, microscopy, PCR | Amphotericin | 36 |
| 4 | 55 | M | Phang Nga, Thailand | VL | Negative | DAT, microscopy, PCR | Amphotericin | 15 |
| 5 | 37 | M | Chanthaburi, Thailand | VL | Positive | Microscopy, PCR | Amphotericin | 18 |
| 6 | 46 | M | Songkhla, Thailand | CL and VL | Positive | Microscopy, PCR, culture | Amphotericin, itraconazole | 19 |
| 7 | 30 | M | Trang, Thailand | DCL and VL | Positive | Microscopy, PCR | Amphotericin, itraconazole | 19 |
| 8 | 32 | F | Trang, Thailand | DCL and VL | Positive | Microscopy, PCR, culture | Amphotericin, itraconazole | 14 |
| 9 | 3 | F | Lopburi, Thailand | CL | Negative | Microscopy | Itraconazole | 34 |
| 10 | 45 | F | Chiang Rai, Thailand | CL, VL | Positive | PCR | No treatment | 20 |
| 11 | 34 | M | Yangon, Myanmar | CL | Positive | PCR | Amphotericin | 20 |
| 12 | 22 | F | Yangon, Myanmar | Asymptomatic | Negative | PCR | No treatment | 20 |
| 13 | 60 | M | Yangon, Myanmar | DCL | Negative | Microscopy, PCR | Amphotericin | 23 |
| 14 | 5 | F | Satun, Thailand | VL | Negative | DAT, microscopy, PCR | Amphotericin | 22 |
| 15 | 52 | M | Lamphun, Thailand | VL | Negative | Microscopy, PCR | Amphotericin | 27 |
| 16 | 48 | M | Chiang Mai, Thailand | DCL | Positive | Microscopy, PCR | Amphotericin, itraconazole | 28 |
| 17 | 38 | M | Lamphun, Thailand | DCL | Positive | Microscopy, PCR | Amphotericin, itraconazole | 28 |
| 18 | 28 | F | Songkhla, Thailand | Asymptomatic | Positive | PCR | No treatment | 29 |
CL = cutaneous leishmaniasis; DAT = direct agglutination test; DCL = diffused cutaneous leishmaniasis; F = female; HIV = human immunodeficiency virus; IFA = indirect immunofluorescence assay; M = male; PCR = polymerase chain reaction; VL = visceral leishmaniasis.
Interestingly, one case involved a Burmese male patient living in Yangon, Myanmar, who came to Thailand to seek medical care. He was an immunocompetent host who developed multiple erythematous and nodules on his face, trunk, and extremities after receiving steroid therapy, and afterward received a diagnosis of diffused cutaneous leishmaniasis (DCL) caused by L. martiniquensis.20,23 His daughter, who lived in the same house, also had asymptomatic L. martiniquensis infection.20 Until now, L. martiniquensis infection has been reported in different geographical areas, that is, Martinique Island/French West Indies, Thailand, and Myanmar (Table 1).
With regard to L. siamensis, only one DCL and VL was recorded in a person with AIDS14; however, our preliminary survey revealed a number of asymptomatic VL caused by L. siamensis in immunocompetent and immunocompromised individuals (J. Manomat and others, unpublished data). Public health personnel should be aware of L. siamensis transmission and be ready for a substantial increase in the numbers of symptomatic cases in the future.
Clinical Spectrum of L. martiniquensis and L. siamensis Infections
CL in immunocompetent patients, caused by L. martiniquensis, shows lesions present only at the site of inoculation on the skin and is called localized CL (LCL) (S. Natesuwan and others, unpublished data). However, the majority of lesions on skin in HIV/AIDS cases could be single, multiple nodular, or generalized papular forms. The lesion could be disseminated as multiple nonulcerative nodules, papular with ulcerative lesions or chronic generalized fibrotic lesions to other parts of the body besides a primary lesion on the face, ears (along the helix and antihelix of the pinna), the dorsum of hands, knuckles of fingers, elbows, extensor surface of the forearm, or on the trunk and lower extremities,19,20,23,28 called DCL. For L. siamensis infection, only one DCL was reported in a person with AIDS. Thick and hard skin nodules covering the body especially on the face, trunk, and extremities were observed. Histological examination revealed diffused irregular hard subcutaneous nodules varying in size.14
Clinical characteristics of VL caused by L. martiniquensis are similar to typical VL reported in L. donovani and L. infantum for which main clinical symptoms include prolonged fever, anemia, hepatosplenomegaly, and cachexia. Due to thrombocytopenia, some patients could have bleeding gums and epistaxis. Gastrointestinal symptoms were also observed in some cases. Laboratory findings showed pancytopenia and hyperglobulinemia. Similar clinical pictures were also found in a person with VL caused by L. siamensis.
Sandfly Distribution and Potential Vector
A few surveys of sandfly species were conducted in different parts of Thailand.37–47 A recent review of sandfly distribution in Thailand indicated that at least 27 species of the four genera, Sergentomyia, Phlebotomus, Idiophlebotomus, and Chinius were identified.42,43 Certain species such as Sergentomyia gemmea are the most predominant species in the north33 as well as in the south.24,42,44 The prevalence of S. gemmea was as high as 85–95% in the northern and southern areas. However, almost 50% of limestone cave-dwelling sandfly species in a hilly area in the north was Nemopalpus vietnamensis, of which Sergentomyia species was found less than 1%.41
The studies of potential vectors were conducted around the affected areas in southern provinces.24,42 Using the PCR-ITS1 and PCR-hsp70, DNA of Leishmania was detected in Sergentomyia (Neophlebotomus) gemmea and Sergentomyia (Parrotomyia) barraudi. As shown in Table 2, analysis of nucleotide sequence compared with the reference GenBank database of ITS1 (JQ866907) and hsp-70 (JX852708) revealed that S. gemmea and S. barraudi could serve as potential vectors of L. martiniquensis in the south.24,25 Studies of the natural sandfly vector are very important for control disease transmission; thus, large-scale surveys of sandfly species in affected areas are needed. Due to limited knowledge on vector competency of Sergentomyia, more studies are required on their host-preference behavior and blood meal feeding including their breeding habitat.
Animal Reservoirs
Transmission of leishmaniasis in Thailand is most likely to be a zoonotic cycle, in which animal reservoirs play an important role for disease transmission in nature. Recently, DNA of L. martiniquensis, previously identified as L. siamensis lineage PG25 was detected in the blood, liver, and spleen of black rats (Rattus rattus) captured around the patients' house, which could serve as a natural reservoir host. Moreover, evidence of zoonotic transmission has been demonstrated when CL caused by L. martiniquensis was reported in farm animals, that is, horses in Germany,16 bovines in Switzerland,17 and one horse in Florida,21 of which sequence analysis of the ITS1 of the SSU-rRNA gene of Leishmania DNA was similar to the species of Leishmania reported in Thailand.15 Thus, zoonotic transmission of leishmaniasis in Thailand could be similar to that of L. infantum in Europe and the Mediterranean Basin.
Conclusion
The recent reported leishmaniasis cases raise awareness among clinicians and public health personnel, as well as the need for public alertness concerning this emerging disease in Thailand. The current evidence has confirmed two species of causative agents, that is, L. martiniquensis and L. siamensis, which are closely related to Leishmania enrietti complex. L. martiniquensis infection in humans and animal populations has been reported from different geographical areas, that is, the Caribbean Island, Central Europe, Florida, Thailand, and Myanmar, whereas L. siamensis infection was only reported in one Thai patient with AIDS.
The information on biology and epidemiology regarding these two species is still lacking. True prevalence, incidence, and risk factors of the disease especially in a high-risk, HIV/AIDS population need to be identified. In addition, studies of natural vectors and reservoir hosts are very important to prevent and control disease transmission.
ACKNOWLEDGMENTS
We would like to thank Mohamed Kasbari and Francine Pratlong from the French Agency for Health and Safety and the French Reference Centre on Leishmaniasis, respectively, for the preliminary results of isoenzyme analysis.
Footnotes
Authors' addresses: Saovanee Leelayoova, Phunlerd Piyaraj, and Mathirut Mungthin, Department of Parasitology, Phramongkutklao College of Medicine, Bangkok, Thailand, E-mails: sleelayoova@gmail.com, p_phunlerd@yahoo.com, and mathirut@hotmail.com. Suradej Siripattanapipong, Jipada Manomat, and Peerapan Tan-ariya, Department of Microbiology, Faculty of Science, Mahidol University, Bangkok, Thailand, E-mails: suradejs@rocketmail.com, manomat.j@gmail.com, and peerapan.tan@mahidol.ac.th. Lertwut Bualert, Department of Medicine, Trang Hospital, Trang Province, Thailand, E-mail: dr_lertwut_clinic@yahoo.com.
References
- 1.Lainson R, Ward RD, Shaw JJ. Leishmania in phlebotomid sandflies: VI. Importance of hindgut development in distinguishing between parasites of the Leishmania mexicana and L. braziliensis complexes. Proc R Soc Lond B Biol Sci. 1977;199:309–320. doi: 10.1098/rspb.1977.0141. [DOI] [PubMed] [Google Scholar]
- 2.Belo VS, Struchiner CJ, Barbosa DS, Nascimento BW, Horta MA, da Silva ES, Werneck GL. Risk factors for adverse prognosis and death in American visceral leishmaniasis: a meta-analysis. PLoS Negl Trop Dis. 2014;8:e2982. doi: 10.1371/journal.pntd.0002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bañuls AL, Bastien P, Pomares C, Arevalo J, Fisa R, Hide M. Clinical pleiomorphism in human leishmaniases, with special mention of asymptomatic infection. Clin Microbiol Infect. 2011;17:1451–1461. doi: 10.1111/j.1469-0691.2011.03640.x. [DOI] [PubMed] [Google Scholar]
- 4.Cota GF, de Sousa MR, Rabello A. Predictors of visceral leishmaniasis relapse in HIV-infected patients: a systematic review. PLoS Negl Trop Dis. 2011;5:e1153. doi: 10.1371/journal.pntd.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alemayehu M, Wubshet M, Mesfin N. Magnitude of visceral leishmaniasis and poor treatment outcome among HIV patients: meta-analysis and systematic review. HIV AIDS (Auckl) 2016;8:75–81. doi: 10.2147/HIV.S96883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Leishmaniasis. 2016. http://www.who.int/mediacentre/factsheets/fs375/en/ Available at. Accessed August 2016.
- 8.Grimaldi G, Jr, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am J Trop Med Hyg. 1989;41:687–725. doi: 10.4269/ajtmh.1989.41.687. [DOI] [PubMed] [Google Scholar]
- 9.Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, Sereno D. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis. 2016;10:e0004349. doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leelayoova S, Siripattanapipong S, Hitakarun A, Kato H, Tan-ariya P, Siriyasatien P, Osatakul S, Mungthin M. Multilocus characterization and phylogenetic analysis of Leishmania siamensis isolated from autochthonous visceral leishmaniasis cases, southern Thailand. BMC Microbiol. 2013;18:13–60. doi: 10.1186/1471-2180-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desbois N, Pratlong F, Quist D, Dedet JP. Leishmania (Leishmania) martiniquensis n. sp. (Kinetoplastida: Trypanosomatidae), description of the parasite responsible for cutaneous leishmaniasis in Martinique Island (French West Indies) Parasite. 2014;21:12. doi: 10.1051/parasite/2014011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dedet JP, Roche B, Pratlong F, Cales-Quist D, Jouannelle J, Benichou JC, Huerre M. Diffuse cutaneous infection caused by a presumed monoxenous trypanosomatid in a patient infected with HIV. Trans R Soc Trop Med Hyg. 1995;89:644–646. doi: 10.1016/0035-9203(95)90427-1. [DOI] [PubMed] [Google Scholar]
- 13.Noyes H, Pratlong F, Chance M, Ellis J, Lanotte G, Dedet JP. A previously unclassified trypanosomatid responsible for human cutaneous lesions in Martinique (French West Indies) is the most divergent member of the genus Leishmania sp. Parasitology. 2002;124:17–24. doi: 10.1017/s0031182001008927. [DOI] [PubMed] [Google Scholar]
- 14.Bualert L, Charungkiattikul W, Thongsuksai P, Mungthin M, Siripattanapipong S, Khositnithikul R, Naaglor T, Ravel C, El Baidouri F, Leelayoova S. Autochthonous disseminated dermal and visceral leishmaniasis in an AIDS patient, southern Thailand, caused by Leishmania siamensis. Am J Trop Med Hyg. 2012;86:821–824. doi: 10.4269/ajtmh.2012.11-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sukmee T, Siripattanapipong S, Mungthin M, Worapong J, Rangsin R, Samung Y, Kongkaew W, Bumrungsana K, Chanachai K, Apiwathanasorn C, Rujirojindakul P, Wattanasri S, Ungchusak K, Leelayoova S. A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int J Parasitol. 2008;38:617–622. doi: 10.1016/j.ijpara.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Müller N, Welle M, Lobsiger L, Stoffel MH, Boghenbor KK, Hilbe M, Gottstein B, Frey CF, Geyer C, von Bomhard W. Occurrence of Leishmania sp. in cutaneous lesions of horses in Central Europe. Vet Parasitol. 2009;166:346–351. doi: 10.1016/j.vetpar.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Lobsiger L, Müller N, Schweizer T, Frey CF, Wiederkehr D, Zumkehr B, Gottstein B. An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet Parasitol. 2010;169:408–414. doi: 10.1016/j.vetpar.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Suankratay C, Suwanpimolkul G, Wilde H, Siriyasatien P. Autochthonous visceral leishmaniasis in a human immunodeficiency virus (HIV)-infected patient: the first in Thailand and review of the literature. Am J Trop Med Hyg. 2010;82:4–8. doi: 10.4269/ajtmh.2010.09-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chusri S, Hortiwakul T, Silpapojakul K, Siriyasatien P. Consecutive cutaneous and visceral leishmaniasis manifestations involving a novel Leishmania species in two HIV patients in Thailand. Am J Trop Med Hyg. 2012;87:76–80. doi: 10.4269/ajtmh.2012.11-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phumee A, Kraivichian K, Chusri S, Noppakun N, Vibhagool A, Sanprasert V, Tampanya V, Wilde H, Siriyasatien P. Detection of Leishmania siamensis DNA in saliva by polymerase chain reaction. Am J Trop Med Hyg. 2013;89:899–905. doi: 10.4269/ajtmh.12-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuss SM, Dunbar MD, Calderwood Mays MB, Owen JL, Mallicote MF, Archer LL, Wellehan JF., Jr Autochthonous Leishmania siamensis in horse, Florida, USA. Emerg Infect Dis. 2012;18:1545–1547. doi: 10.3201/eid1809.120184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osatakul S, Mungthin M, Siripattanapipong S, Hitakarun A, Kositnitikul R, Naaglor T, Leelayoova S. Recurrences of visceral leishmaniasis caused by Leishmania siamensis after treatment with amphotericin B in a seronegative child. Am J Trop Med Hyg. 2014;90:40–42. doi: 10.4269/ajtmh.13-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noppakun N, Kraivichian K, Siriyasatien P. Disseminated dermal leishmaniasis caused by Leishmania siamensis in a systemic steroid therapy patient. Am J Trop Med Hyg. 2014;91:869–870. doi: 10.4269/ajtmh.13-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanjanopas K, Siripattanapipong S, Ninsaeng U, Hitakarun A, Jitkaew S, Kaewtaphaya P, Tan-ariya P, Mungthin M, Charoenwong C, Leelayoova S. Sergentomyia (Neophlebotomus) gemmea, a potential vector of Leishmania siamensis in southern Thailand. BMC Infect Dis. 2013;13:333. doi: 10.1186/1471-2334-13-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chusri S, Thammapalo S, Chusri S, Thammapalo S, Silpapojakul K, Siriyasatien P. Animal reservoirs and potential vectors of Leishmania siamensis in southern Thailand. Southeast Asian J Trop Med Public Health. 2014;45:13–19. [PubMed] [Google Scholar]
- 26.Phumee A, Chusri S, Kraivichian K, Wititsuwannakul J, Hortiwakul T, Thavara U, Silpapojakul K, Siriyasatien P. Multiple cutaneous nodules in an HIV-infected patient. PLoS Negl Trop Dis. 2014;8:e3291. doi: 10.1371/journal.pntd.0003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pothirat T, Tantiworawit A, Chaiwarith R, Jariyapan N, Wannasan A, Siriyasatien P, Supparatpinyo K, Bates MD, Kwakye-Nuako G, Bates PA. First isolation of Leishmania from northern Thailand: case report, identification as Leishmania martiniquensis and phylogenetic position within the Leishmania enriettii complex. PLoS Negl Trop Dis. 2014;8:e3339. doi: 10.1371/journal.pntd.0003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiewchanvit S, Tovanabutra N, Jariyapan N, Bates MD, Mahanupab P, Chuamanochan M, Tantiworawit A, Bates PA. Chronic generalized fibrotic skin lesions from disseminated leishmaniasis caused by Leishmania martiniquensis in two patients from northern Thailand infected with HIV. Br J Dermatol. 2015;173:663–670. doi: 10.1111/bjd.13812. [DOI] [PubMed] [Google Scholar]
- 29.Siriyasatien P, Chusri S, Kraivichian K, Jariyapan N, Hortiwakul T, Silpapojakul K, Pym AM, Phumee A. Early detection of novel Leishmania species DNA in the saliva of two HIV-infected patients. BMC Infect Dis. 2016;16:89. doi: 10.1186/s12879-016-1433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liautaud B, Vignier N, Miossec C, Plumelle Y, Kone M, Delta D, Ravel C, Cabié A, Desbois N. First case of visceral leishmaniasis caused by Leishmania martiniquensis. Am J Trop Med Hyg. 2015;92:317–319. doi: 10.4269/ajtmh.14-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garin YJ, Sulahian A, Méneceur P, Pratlong F, Prina E, Gangneux J, Dedet JP, Derouin F. Experimental pathogenicity of a presumed monoxenous trypanosomatid isolated from humans in a murine model. J Eukaryot Microbiol. 2001;48:170–176. doi: 10.1111/j.1550-7408.2001.tb00299.x. [DOI] [PubMed] [Google Scholar]
- 32.Thisyakorn U, Jongwutiwes S, Vanichsetakul P, Lertsapcharoen P. Visceral leishmaniasis: the first indigenous case report in Thailand. Trans R Soc Trop Med Hyg. 1999;93:23–24. doi: 10.1016/s0035-9203(99)90166-9. [DOI] [PubMed] [Google Scholar]
- 33.Kongkaew W, Siriarayaporn P, Leelayoova S, Supparatpinyo K, Areechokchai D, Duang-ngern P, Chanachai K, Sukmee T, Samung Y, Sridurongkathum P. Autochthonous visceral leishmaniasis: a report of a second case in Thailand. Southeast Asian J Trop Med Public Health. 2007;38:8–12. [PubMed] [Google Scholar]
- 34.Kattipathanapong P, Akaraphanth R, Krudsood S, Riganti M, Viriyavejakul P. The first reported case of autochthonous cutaneous leishmaniasis in Thailand. Southeast Asian J Trop Med Public Health. 2012;43:17–20. [PubMed] [Google Scholar]
- 35.Viriyavejakul P, Viravan C, Riganti M, Punpoowong B. Imported cutaneous leishmaniasis in Thailand. Southeast Asian J Trop Med Public Health. 1997;28:558–562. [PubMed] [Google Scholar]
- 36.Maharom P, Siripattanapipong S, Mungthin M, Naaglor T, Sukkawee R, Pudkorn R, Wattana W, Wanachiwanawin D, Areechokchai D, Leelayoova S. Visceral leishmaniasis caused by Leishmania infantum in Thailand. Southeast Asian J Trop Med Public Health. 2008;39:988–990. [PubMed] [Google Scholar]
- 37.Apiwathnasorn C, Sucharit S, Rongsriyam Y, Leemingsawat S, Kerdpibule V, Deesin T, Surathin K, Vutikes S, Punavuthi N. A brief survey of phlebotomine sandflies in Thailand. Southeast Asian J Trop Med Public Health. 1989;20:429–432. [PubMed] [Google Scholar]
- 38.Apiwathnasorn C, Sucharit S, Surathin K, Deesin T. Anthropophilic and zoophilic phlebotomine sand flies (Diptera, Psychodidae) from Thailand. J Am Mosq Control Assoc. 1993;9:135–137. [PubMed] [Google Scholar]
- 39.Polseela R, Apiwathnasorn C, Samung Y. Seasonal variation of cave-dwelling phlebotomine sandflies (Diptera: Psychodidae) in Phra Phothisat Cave, Saraburi Province, Thailand. Southeast Asian J Trop Med Public Health. 2007;38:1011–1015. [PubMed] [Google Scholar]
- 40.Polseela R, Apiwathnasorn C, Samung Y. Seasonal distribution of phlebotomine sand flies (Diptera: Psychodidae) in Tham Phra Phothisat temple, Saraburi province, Thailand. Trop Biomed. 2011;28:366–375. [PubMed] [Google Scholar]
- 41.Polseela R, Vitta A, Nateeworanart S, Apiwathnasorn C. Distribution of cave-dwelling phlebotomine sand flies and their nocturnal and diurnal activity in Phitsanulok Province, Thailand. Southeast Asian J Trop Med Public Health. 2011;42:1395–1404. [PubMed] [Google Scholar]
- 42.Apiwathnasorn C, Samung Y, Prummongkol S, Phayakaphon A, Panasopolkul C. Cavernicolous species of phlebotomine sand flies from Kanchanaburi Province, with an updated species list for Thailand. Southeast Asian J Trop Med Public Health. 2011;42:1405–1409. [PubMed] [Google Scholar]
- 43.Polseela R, Depaquit J, Apiwathnasorn C. Description of Sergentomyia phadangensis n. sp. (Diptera, Psychodidae) of Thailand. Parasit Vectors. 2016;15:9–21. doi: 10.1186/s13071-016-1300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sukra K, Kanjanopas K, Amsakul S, Rittaton V, Mungthin M, Leelayoova S. A survey of sandflies in the affected areas of leishmaniasis, southern Thailand. Parasitol Res. 2013;112:297–302. doi: 10.1007/s00436-012-3137-x. [DOI] [PubMed] [Google Scholar]
- 45.Polseela R, Vitta A, Apiwathnasorn C. Distribution of phlebotomine sand flies (Diptera: Psychodidae) in limestone caves, Khao Pathawi, Uthai Thani province, Thailand. Southeast Asian J Trop Med Public Health. 2015;46:425–433. [PubMed] [Google Scholar]
- 46.Chittsamart B, Samruayphol S, Sungvorayothin S, Pothiwat R, Samung Y, Apiwathnasorn C. Phlebotomine sand flies of edible-nest swiftlet cave of Lang Ga Jiew Island, Chumphon province, Thailand. Trop Biomed. 2015;32:402–406. [PubMed] [Google Scholar]
- 47.Panthawong A, Chareonviriyaphap T, Phasuk J. Species diversity and seasonality of phlebotomine sand flies (Diptera: Psychodidae) in Satun province, Thailand. Southeast Asian J Trop Med Public Health. 2015;46:857–865. [PubMed] [Google Scholar]