Abstract
The association between heavy rainfall and an increased risk of diarrhea has been well established but less is known about the effect of drought on diarrhea transmission. In 2011, the Pacific island nation of Tuvalu experienced a concurrent severe La Niña–associated drought and large diarrhea outbreak. We conducted a field investigation in Tuvalu to identify factors that contributed to epidemic transmission in the context of a drought emergency. Peak case numbers coincided with the nadir of recorded monthly rainfall, the lowest recorded since 1930. Independent factors associated with increased risk of diarrhea were households with water tank levels below 20% (odds ratio [OR] = 2.31; 95% confidence interval = 1.16–4.60) and decreased handwashing frequency (OR = 3.00 [1.48–6.08]). The resolution of the outbreak occurred after implementation of a hygiene promotion campaign, despite persistent drought and limited water access. These findings are potentially important given projections that future climate change will cause more frequent and severe droughts.
Introduction
The health impacts of drought are poorly understood despite droughts affecting up to 50 million persons annually.1 Tropical Pacific island nations are particularly susceptible to drought, in part due to the El Niño Southern Oscillation (ENSO) cycle, a natural climate event characterized by the cyclical warming (El Niño) and cooling (La Niña) of the Pacific Ocean.2 Such variability affects global climate patterns and often leads to climate emergencies such as floods, cyclones, and drought.3 Anthropogenic climate change is projected to increase the severity and volatility of ENSO events and increase the populations adversely affected by droughts.4,5
In 2011, a La Niña event triggered severe drought and state of emergency in the South Pacific island nation of Tuvalu. During the nadir of the drought in September 2011, the Pacific Syndromic Surveillance System (PSSS)6 detected a simultaneous large diarrhea outbreak.
Although previous studies have established a correlation between seasonally dry periods and an increased risk of diarrhea, only two published investigations, both from the 1970s, have explored the relationship between droughts and epidemic diarrhea transmission.7,8 The first report from Wales in 1976 describes an increase in the weekly incidence of diarrhea among schoolchildren during drought conditions in water-restricted regions compared with nonwater-restricted regions. Intermediate water restriction similarly resulted in slightly higher rates of diarrhea, although the study period lasted only 2 weeks before access to water returned to normal. In the second investigation from Port-au-Prince, Haiti, in 1977, investigators reported a nonsignificant trend toward higher diarrhea rates among families with lesser access to water during severe drought conditions. Both reports imply that drought-related water restriction was correlated with elevated rates of diarrheal disease, likely through an impact on hygiene practices.
Given that droughts are projected to become increasingly severe in many regions, and given that the role of drought on diarrheal disease is poorly described, our objectives were to 1) document the outbreaks in relation to the prevailing drought conditions, 2) present the findings of an epidemiological investigation including a case-control study on Tuvalu, and 3) explore the mechanism or mechanisms of diarrheal disease transmission in this drought context.
Materials and Methods
Study sites.
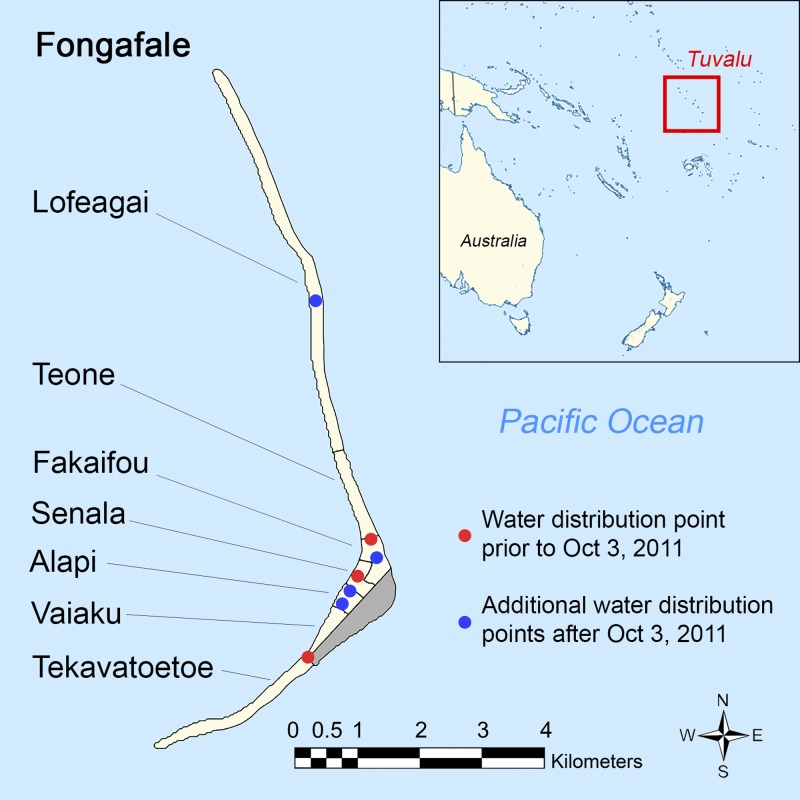
We conducted a field study in Tuvalu, a tropical island nation of 10,782 people (2012 Census) located in the South Pacific and composed of nine low-lying coral islands and atolls (Figure 1 ).9 Funafuti atoll (population 6,216), the site of the investigation, is the administrative center of Tuvalu and comprises one large island, Fongafale, with 97% of the atoll's population, and multiple small islets. Annual rainfall is approximately 3,000 mm with lower rainfall from April to October. The average temperature is 28°C with minimal seasonal variation. There are no rivers, lakes, or other natural reserves of freshwater on Funafuti. A lens of brackish water lies beneath the atoll but it is not suitable for drinking, and the population is dependent on roof-top rainwater harvesting for potable water.
Figure 1.
Map of Tuvalu, Fongafale villages, and water distribution points.
Diarrheal disease surveillance and case finding.
Cases of diarrheal disease were identified from the PSSS that since 2010 monitors four syndromes, including diarrhea, on a weekly basis from sentinel heath facilities in 23 Pacific island countries and territories including Tuvalu.6 Tuvalu started reporting through the PSSS in November 2010. This study and the PSSS define a case of diarrhea as a person reporting three or more loose stools in a 24-hour period. There is one reporting site in Tuvalu. Princess Margaret Hospital (PMH), the national reference hospital and reporting site for Tuvalu, is the only medical facility on Funafuti. In 2011, laboratory testing at PMH for the identification of diarrheal pathogens was limited to microscopy of fecal specimens for ova and parasites.
The outbreak period was defined as the weeks when the number of cases reported through the PSSS exceeded the threshold of 2 standard deviations above the historical mean before the outbreaks. To validate the PSSS case numbers and to provide a more detailed epidemiological and clinical description of the outbreak, we identified diarrhea cases from PMH outpatient (July 2011–February 2012) and inpatient (January 2010–December 2013) medical records, and extracted age, gender, clinical features, other recorded diagnoses, and outcome.
Rainfall and temperature.
We obtained rainfall data for 2000–2013 and temperature data for 2008–2013 from the Tuvalu Meteorological Service. The 2010 Tuvalu Rainwater Harvesting Survey10 provided information on the total and residential-specific water storage and the effective water harvesting capacity (EHC), the optimal volume of water that can be harvested per millimeter of rainfall. The average volume of rainwater per person per day for a given period was calculated by multiplying the cumulative rainfall during that period by the EHC, dividing by the population of Funafuti and multiplying by the number of days in the period.
Response interventions.
Information on the nature and length of humanitarian response interventions in Tuvalu was obtained from the United Nations Office for the Coordination of Humanitarian Affairs (UN OCHA)11 and by conducting semistructured interviews with organizations and agencies involved in the response including Tuvalu Government Departments (National Disaster Committee, Public Works Department, Lands Department, Media Department) and international organizations (UN OCHA, Red Cross, South Pacific Applied Geoscience Commission).
Case-control study.
During July 2014, we conducted a case-control household survey on Funafuti to assess risk factors for diarrhea during the drought. Household survey questions were related to household demographic, environmental, and hygiene variables during the drought. Cases from the outbreak period were identified from the outpatient or inpatient hospital records, counting cases only once if they were recorded in both inpatient and outpatient records, and were included for selection in the study if their current residence on Funafuti was identified. Cases were excluded if they resided in the same household as a previously selected case. We administered a standardized questionnaire to 75 case subjects, randomly selected from all patients meeting inclusion criteria using a random number generator. Since household tanks are divided into fifths by horizontal indentations, we used an ordinal scale of 20% increments to assess water tank reserves. Assessment of tank level is performed by tapping on the tanks and identifying differences in acoustics; this is a simple and common practice in Tuvalu and is particularly common during times of drought.
A sampling framework was calculated to enroll two controls per case, frequency matched by age group (< 1, 1–4, 5–14, > 14 years) in 2011. Controls were identified by screening every fifth house on Fongafale from north to south. If a control was not enrolled from a specified house after two visits (because no one was home or because a subject in the correct age group was not available or willing to participate), we selected the previous household in the sampling frame, after which we returned to the initial sampling framework. Inclusion criteria for controls included residence on Fongafale in 2011 and the presence of an adult in the household at the time of interview. Households were excluded if subjects reported that a member of the household had diarrhea during the outbreak period or if a parent or guardian was not present after two visits.
Statistical analysis.
Tuvalu census data from 2012 were used to calculate attack rates (ARs) of diarrhea.9 We evaluated the correlation between average monthly temperature and diarrhea cases by examining the value. Data for the outbreak period were analyzed in SAS for Windows version 9.3 (SAS Institute Inc., Cary, NC). We performed univariate and multivariate logistic regression to calculate crude and adjusted odds ratios for diarrhea. All survey covariates were included in the multivariate regression model. The final model was obtained using backward elimination of nonsignificant variables that decreased the model fit, evaluated by comparing akaike information criteria (AIC) scores. A difference with a P value < 0.05 was considered to be significant. Geolocalization of case and control households, based on 2011 residence, was conducted and spatiotemporal cluster analysis performed in QGIS, ArcGIS (ESRI, Redlands, CA), and SatScan (Boston, MA).
Ethical considerations.
Before enrollment, written informed consent was obtained from all study subjects or legal guardian if < 18 years. Ethical approval for this study was obtained from the Yale University Human Research Protection Program. The Tuvalu Ministry of Health (MOH) approved the study. No institutional review board exists in Tuvalu.
Results
Diarrheal disease surveillance and case description.
A total of 244 cases of diarrhea were identified in Tuvalu during the outbreak period from August 29 to October 16, 2011 (epidemiological weeks 35–41), which comprised 204 cases reported by the PSSS and 40 additional cases identified by inpatient and outpatient chart review. The mean weekly PSSS diarrhea case count between November 15, 2010 and November 15, 2015, was 6.3 cases per week, but dropped to 3.8 cases per week if we exclude the 2011 outbreak and a second nondrought-associated outbreak in 2014. To assess for potential seasonality, we evaluated the PSSS mean weekly case count during the outbreak period (epidemiological weeks 35–41) for 2011–2015—2011: 29.2 cases per week; 2012: 0.8 cases per week; 2013: 1.2 cases per week; 2014: 43.1 cases per week; and 2015: 7.6 cases per week. A diarrhea outbreak was identified during a similar period in 2014, but there is no evidence to suggest seasonality.
The outbreak peaked between September 11 and 25, 2011. The overall AR for Funafuti was 3.9%, ranging from 1.5% for > 65 years to 17.5% for 0–2 years (Table 1). The median age of cases was 6 years (range, 0–82) and 51% were male. Twenty-seven cases were hospitalized (11%) with a mean hospital stay of 2.5 days (SD ± 2.5). Children 0–2 years of age represented 32% of outpatient cases and 62% of inpatient cases. There were no deaths. Children 0–2 years of age had 12.8 (95% confidence interval [CI] = 9.3–17.7) times the risk of acquiring diarrhea and 19.9 (95% CI = 8.2–48.1) times the risk of being hospitalized for diarrhea compared with individuals age > 15 years. Other clinical features included vomiting (42/244, 17%), abdominal pain (7/244, 3%), and fever (7/244, 3%). Respiratory tract infection was reported as a codiagnosis in 8/244 (3%) of cases.
Table 1.
Age-specific attack rates and hospitalization rates during the drought-associated outbreak
| Age group (years) | Population | No. of cases (% total) | Attack rate* | No. of hospitalized cases† | Hospitalization rate* |
|---|---|---|---|---|---|
| Total | 6,216 | 244 (100) | 3.93 | 27 | 0.43 |
| 0–2 | 480 | 84 (35.29) | 17.50 | 16 | 3.33 |
| 3–5 | 459 | 33 (13.87) | 7.19 | 2 | 0.44 |
| 6–15 | 1,104 | 64 (26.89) | 5.80 | 1 | 0.09 |
| 15+ | 4,173 | 57 (23.36) | 1.37 | 7 | 0.17 |
Rates expressed per 100 people.
Mean hospital stay was 2.5 days.
Rainfall, water usage, and temperature.
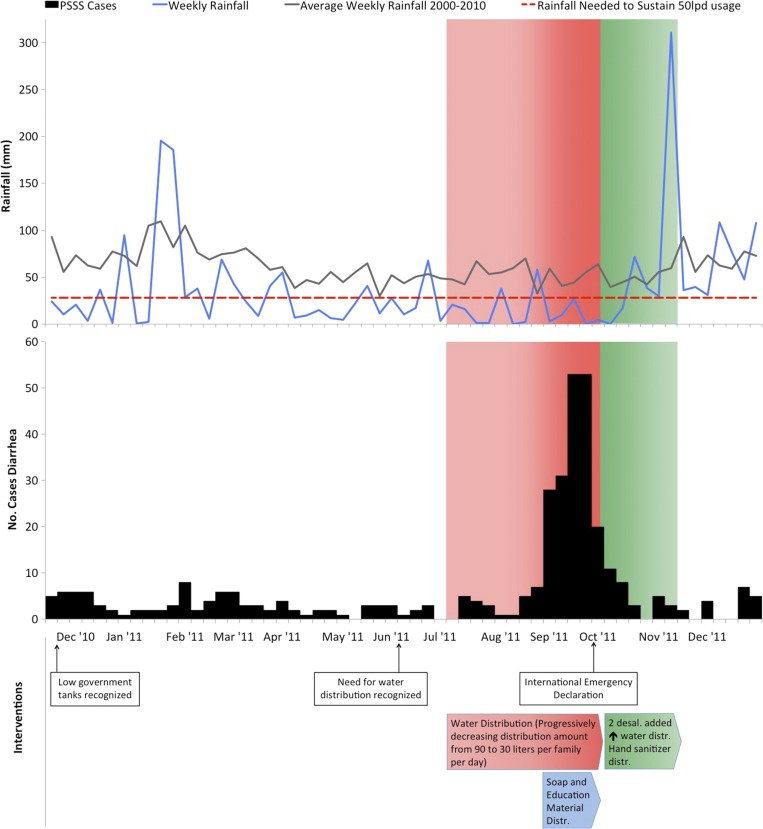
Preceding the outbreak period by 7 months, Funafuti began experiencing below-normal rainfall from early February to October 2011 (Figure 2 ). The mean weekly rainfall during this period was 22.5 mm, corresponding to 40 L per person per day (lpd). Drought conditions reached a nadir in September, coinciding with the outbreak period, when total rainfall was 38 mm (weekly mean of 9.5 mm), the lowest monthly precipitation recorded since 1930 and equating to 15.8 lpd. On October 6, government, community, and domestic reserves were estimated to be less than 850,000 L, which was 3.1% of total water storage capacity.12 Normal or above-normal rainfall returned in November. There was no correlation between average monthly temperature and increased diarrheal risk.
Figure 2.
Weekly rainfall, weekly number of cases of diarrhea from the Pacific Syndromic Surveillance System, and interventions, December 2010–December 2011 (6). Green shading designates the period of international drought relief. Distribution is abbreviated as Distr.
Response interventions.
Response interventions consisted of three phases. During the first phase, beginning in July 2011, the Tuvalu government distributed up to 90 L of water per family per day from government reserves. This decreased to 30 L per day by September because the government sought to maximize the limited water reserves in the context of worsening drought conditions and more families requiring supplemental water (Figures 1 and 2). In the second phase, beginning in early September, the MOH conducted a house-to-house and radio health promotion campaign that distributed soap and encouraged boiling all drinking water and regular handwashing with soap and water. In phase three, after a national drought emergency declaration on September 28, 2011, a range of international disaster assistance was mobilized, including bulk delivery of 450,000 L of fresh water, two additional desalinization plants, soap, hand sanitizer, and water purification tablets. By October 21, water distribution increased to 60 L per family per day and the number of water distribution points had increased from three to seven (Figure 1). The third phase continued until November 8, 2011, when sustained rainfall resumed (Figure 2).
The first indication of an outbreak occurred the week of August 29, 2011, as phase one of interventions approached its operational limit amid waning government reserves. Phase two correlated with a precipitous decrease in case rates from the peak of 53 cases on the week ending September 25, 2011, to 20 cases the week ending October 2, 2011. Arrival of international aid occurred on October 3, 2011, after case rates had dropped significantly.
Case-control study.
Of the 244 diarrhea cases, we identified a residence for 134, of which 93 were randomly selected to enroll 75 case subjects: 75/93 cases were enrolled; 17/93 had moved to another island; and 1/93 had died. No enrolled cases reported other cases of diarrhea in the household during the drought. We enrolled a single control subject from each of 141 households, from a total of 168 eligible households (83.9%) selected to identify potential control subjects. Of the remaining 27 households, 21 did not contain a household member in an eligible age category and six households did not have a parent or guardian present after two visits. Univariate analyses identified two variables significantly correlated with diarrhea: household water tank < 20% full during the drought (P < 0.01) and decreased frequency of handwashing (P < 0.01) (Table 2). Multivariate analysis identified water tank level < 20% full (odds ratio [OR] = 2.3, [95% CI = 1.2–4.6]) and decreased frequency of handwashing (OR = 3.0 [95% CI = 1.5–6.1]) as independent predictors of increased diarrhea risk (Table 3).
Table 2.
Characteristics of index case and control subjects and their households*
| Characteristics | Group | Cases† | Controls† | |
|---|---|---|---|---|
| N = 75 | N = 141 | P value‡ | ||
| Index subjects | ||||
| Mean age (years) | 15.6 ± 19.8 | 14.8 ± 18.1 | NS | |
| Age group§ | NS | |||
| 0–2 years | 27 (36.0) | 36 (25.5) | ||
| 3–5 years | 6 (8.0) | 27 (19.2) | ||
| 6–15 years | 21 (28.0) | 39 (27.7) | ||
| 16 + years | 21 (28.0) | 39 (27.7) | ||
| Male | 37 (49.3) | 65 (46.1) | NS | |
| Occupation | NS | |||
| None | 31 (41.3) | 53 (37.6) | ||
| Student | 28 (37.3) | 63 (44.7) | ||
| Government | 11 (14.7) | 10 (7.09) | ||
| Private sector | 4 (5.3) | 11 (7.8) | ||
| Clergy | 1 (1.3) | 4 (2.8) | ||
| Households | ||||
| Community | 0.033†† | |||
| Tekavatoetoe | 12 (16.0) | 11 (7.8) | ||
| Vaiaku | 12 (16.0) | 18 (12.8) | ||
| Senala | 14 (18.7) | 20 (14.2) | ||
| Alapi | 10 (13.3) | 31 (22.0) | ||
| Fakaifou | 22 (29.3) | 31 (22.0) | ||
| Teone | 1 (1.3) | 10 (7.1) | ||
| Lofeagai | 4 (5.3) | 20 (14.2) | ||
| Household members | 8.3 ± 4.2 | 7.6 ± 4.0 | NS | |
| Household income¶ | 432.2 ± 388.9 | 491.4 ± 397.5 | NS | |
| Highest education level∥ | NS | |||
| Primary | 4 (5.4) | 17 (12.1) | ||
| Secondary | 22 (29.7) | 41 (29.3) | ||
| Tertiary | 48 (64.9) | 82 (58.6) | ||
| Distance to water distribution point** | 457.62 ± 627.96 | 710.78 ± 1,036.85 | NS | |
| Tank storage capacity (L) | 29,722.7 ± 32,700.9 | 26,650.4 ± 21,765.5 | NS | |
| Tank levels less than 20% full | 40 (53.3) | 49 (34.8) | 0.008 | |
| Reported decreased frequency of handwashing | 36 (48.7) | 29 (20.6) | < 0.001 | |
| Livestock ownership | 60 (80.0) | 125 (88.7) | NS | |
Numbers may not sum to total due to missing data, and percentages may not sum to 100% due to rounding.
Data reported as no. (%) or mean ± standard deviation.
NS = nonsignificant at P > 0.05.
Controls were matched to cases by age category.
Household income in Australian Dollars per fortnight for the household in 2011.
This represents the highest level of education attained by anyone living in the household in 2011. Many individuals in Tuvalu complete certificate classes at the Tuvalu campus of the University of South Pacific and thus may have answered “Tertiary” education level mistakenly.
Mean distance in meters to a water distribution point before October 3, 2011. Case figure is based on a subset analysis of those who became ill before October 3, 2011. P value is based on a Wilcoxon rank-sum test.
Although community of residence was a significant variable in univariate analysis, we believe this statistical significance to be an artifact of our sampling method.
Table 3.
Multivariate analysis of risk factors for diarrhea during the drought
| Risk factor | OR* | 95% CI | P value† |
|---|---|---|---|
| Tank levels < 20% full | 2.31 | 1.16–4.60 | 0.018 |
| Livestock ownership | 0.43 | 0.16–1.12 | NS |
| Reported decreased frequency of handwashing | 3.00 | 1.48–6.08 | 0.002 |
CI = confidence interval; OR = odds ratio.
NS = nonsignificant at P > 0.05.
Discussion
We report an outbreak of diarrheal disease in Tuvalu in 2011 concurrent with and apparently precipitated by La Niña–associated drought conditions in the central Pacific.13 Decreased handwashing and low household water reserves during the drought were associated with an increased risk of diarrhea. Resolution of the outbreak was temporally correlated with a large health promotion campaign by the MOH that was before resolution of the drought and increased access to clean water.
From April to October 2011, rainfall was consistently below the requirement to meet the World Health Organization recommended minimum of 50 lpd (Figure 2), and in September, the total monthly rainfall was 38 mm (equating to ∼16 lpd), the lowest in Tuvalu's 81 years of climate records. During July and September, the rainfall approached the minimum rainfall needed to assure 15 lpd, the internationally recognized minimum during humanitarian emergencies as determined by the SPHERE standards14; however, given household reserves and government water distribution, average per person water availability would be higher than volumes based on rainfall only.
In addition to the drought-associated outbreak on Tuvalu, Tokelau, a territory of New Zealand located 1,000 km east of Tuvalu and comprising three low-lying atolls with a total population of 1,411 (2011 census), faced a simultaneous outbreak of diarrheal disease, also in the context of a severe drought emergency.15 Like Tuvalu, the population of Tokelau is dependent on roof-top rainwater harvesting. Tokelau reported 50 diarrhea cases through the PSSS during a 3-week outbreak period from August 29 to September 18, 2011, which represents an AR of 3.6%. The mean case count between January 9, 2011 and November 15, 2015, was 3.8 cases per week. During the outbreak period (epidemiological week 35–37) during 2011–2015, the mean PSSS case counts were 2011: 16.7 cases per week; 2012: 1.7 cases per week; 2013: 4.3 cases per week; 2014: 3.5 cases per week; and 2015: 7.0 cases per week.16 Because movement between the nations would require multiple days of travel, including a minimum of two international flights and a multiday ocean voyage, we believe that direct transmission from Tuvalu to Tokelau or vice versa was highly unlikely.
Consistent with the studies in Wales and Haiti in the 1970s, our field study in Tuvalu indicates that reduced handwashing or other hygiene practices due to limited water reserves may be a key factor driving the Tuvalu outbreak, for the following reasons. First, the risk of diarrheal disease was 2-fold higher for persons in households reporting low household water reserves during the drought. Second, low household water reserves were strongly associated with decreased handwashing (P < 0.001). Third, the risk of diarrheal disease tripled for persons reporting less frequent handwashing during the drought. Finally, the observed decrease in diarrhea disease was temporally associated with a large health and hygiene promotion campaign, suggesting improved handwashing and other hygiene practices may have been important in interrupting transmission. No other response intervention was temporally associated with outbreak resolution, including the increase in water distribution that was only implemented several weeks after outbreak resolution. Conversely, the decrease in cases may reflect a natural resolution of the outbreak unrelated to the interventions. Given that a high proportion of the children sought medical care and many more were likely infected but did not seek care due to mild or absent symptoms, it is plausible that the outbreak burned out naturally after depletion of the high risk susceptible pool.
Two other mechanisms of diarrhea due to limited rainfall or limited access to water have been previously described. First, because drought conditions limit the availability of clean water from usual and reliable sources, households may switch to untreated or less hygienic sources.1 Second, low residual volume in water storage tanks may concentrate pathogens or reduce the ability to dilute introduced pathogens, increasing the likelihood of consuming an infective dose17; some sources suggest this risk may be increased by contamination of roof-top rainfall catchment systems by bird feces and other pathogens during prolonged dry spells followed by the flushing of contaminants during heavy rains.18,19
We think it unlikely that either of the alternative pathways was the primary mechanism of the diarrheal disease outbreak on Tuvalu. First, due to the lack of fresh groundwater, the only alternative source of drinking water during the drought was government or community water tanks filled by reverse osmosis or rainwater harvesting. There was no spatiotemporal clustering of cases as would be expected if a single water source was contaminated, for example a community water storage tank or a water distribution point. The Tuvalu Public Works Department reported that water for distribution was tested for pathogens daily and none were identified. Second, although there was a small increase in rainfall in the week before the outbreak (Figure 2), potentially washing accumulated rooftop contaminants into the water tanks as discussed,20 there were multiple small increases in rainfall after prolonged dry spells in the prior and subsequent months without similar increases in diarrheal disease. In addition, ultraviolet exposure and desiccation would be expected to limit the survival of microorganisms on exposed surfaces like roof tops, particularly in settings with high ultraviolet exposure such as Tuvalu.21,22 That the majority of case (98%) and control (94%) households reported always boiling their drinking water even under drought conditions also argues against household water tank contamination.
As with many field studies attempting to retrospectively characterize and report important natural events, our community survey may be limited by recall bias. For example, none of the case households reported other household residents with diarrhea during the drought, which although possible is unlikely and may reflect the difficulty recalling a relatively common illness 3 years earlier. Despite this limitation, however, it is important to note that the key results of the case-control study were consistent with other data and information suggesting reduced hygiene practices may have been associated with an increased diarrheal risk. Recall bias, if present, may influence the case-control study findings, but not the core observation in this article that severe drought was associated with two independent, large, and simultaneous outbreaks of diarrheal disease. Limited laboratory diagnostic capacity prevented identification of a specific pathogen or pathogens, information that may have provided insight into the specific routes of transmission.
ENSO can cause severe droughts in the Western Pacific (El Niño) and Central Pacific (La Niña). During late 2015 and the first half of 2016, a strong El Niño was established in the Pacific22 and drought conditions reported from a number of countries in the Western Pacific. Given increasing evidence that climate change drives increased ENSO volatility, the current severe El Niño forewarns of future climate emergencies in the Pacific and beyond.23
Projections indicate that much of Africa and the Americans, Australia, southern Europe, the Middle East, and much of Asia and southeast Asia are at increased risk of severe drought by the middle of the 21st century.24 These projections emphasize the need to characterize and document the health impact of extreme weather events, including droughts and restricted water access, to ensure well-informed mitigation plans can be developed, particularly for countries and regions most vulnerable to climate change.5,25–27
ACKNOWLEDGMENTS
We would like to thank Kaevaa Lototele at the Tuvalu Ministry of Health and Axelle Ronsse at WHO Division of Pacific Technical Support, Fiji, for their contributions to this project and Forrest Jones for his contributions in the review of this manuscript and in friendship and support.
Footnotes
Financial support: We would like to thank the support of the National Institutes of Health (1 R01 TW009504); the Stolwijk Fellowship, Yale Global Health Initiative; and the Coca-Cola World Fund at Yale University.
Authors' addresses: Jordan P. Emont, Department of Epidemiology of Microbial Diseases, Yale University Yale School of Public Health, New Haven, CT, E-mail: jordan.emont@yale.edu. Albert I. Ko, Department of Epidemiology of Microbial Diseases, Yale University Yale School of Public Health, New Haven, CT, and Fundação Oswaldo Cruz, Centro de Pesquisas Gonçalo Moniz–FIOCRUZ, Salvador, Brazil, E-mail: albert.ko@yale.edu. Avanoa Homasi-Paelate and Nese Ituaso-Conway, Public Health, Tuvalu Ministry of Health, Funafuti, Tuvalu, E-mails: mysabs@yahoo.com and n_ituaso@yahoo.com. Eric J. Nilles, Division of Pacific Technical Support, Emerging Disease Surveillance and Response, World Health Organization Regional Office for the Western Pacific, Suva, Fiji, E-mail: ericnilles@gmail.com.
References
- 1.Stanke C, Kerac M, Prudhomme C, Medlock J, Murray V. Health effects of drought: a systematic review of the evidence. PLoS Curr. 2013 doi: 10.1371/currents.dis.7a2cee9e980f91ad7697b570bcc4b004. http://currents.plos.org/disasters/article/dis-13-0001-health-effects-of-drought-a-systematic-review-of-the-evidence/ Available at. Accessed April 4, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ropelewski CF, Halpert MS. Global and regional scale precipitation patterns associated with the El Niño/Southern Oscillation. Mon Wea Rev. 1987;115:1606–1626. [Google Scholar]
- 3.Dilley M, Heyman BN. ENSO and disaster: droughts, floods and El Niño/Southern Oscillation warm events. Disasters. 1995;19:181–193. doi: 10.1111/j.1467-7717.1995.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 4.Gergis JL, Fowler AM. A history of ENSO events since A.D. 1525: implications for future climate change. Clim Change. 2008;92:343–387. [Google Scholar]
- 5.Dai A. Drought under global warming: a review. Wiley Interdiscip Rev Clim Chang. 2011;2:45–65. [Google Scholar]
- 6.Kool JL, Paterson B, Pavlin BI, Durrheim D, Musto J, Kolbe A. Pacific-wide simplified syndromic surveillance for early warning of outbreaks. Glob Public Health. 2012;7:670–681. doi: 10.1080/17441692.2012.699536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burr ML, Davis AR, Zbijowski AG. Diarrhoea and the drought. Public Health. 1978;92:86–87. doi: 10.1016/s0033-3506(78)80034-1. [DOI] [PubMed] [Google Scholar]
- 8.Thacker SB, Music SI, Pollard RA, Berggren G, Boulos C, Nagy T, Brutus M, Pamphile M, Ferdinand RO, Joseph VR. Acute water shortage and health problems in Haiti. Lancet. 1980;1:471–473. doi: 10.1016/s0140-6736(80)91009-0. [DOI] [PubMed] [Google Scholar]
- 9.Tuvalu Statistics Population Census. 2012. http://www.spc.int/prism/tuvalu/ Available at. Accessed April 3, 2015.
- 10.Government of Tuvalu . Secretariat of the Pacific Regional Environment Programme. Integrated Waste Management, Water and Sanitation Review Action Plan; 2010. [Google Scholar]
- 11.Government of Tuvalu . Secretariat of the Pacific Community Geoscience Division. 2010. Tuvalu Rainwater Harvesting Survey. [Google Scholar]
- 12.Government of Tuvalu Tokelau Census of Population and Dwellings. 2011. http://stats.govt.nz/browse_for_stats/people_and_communities/pacific_peoples/2011-tokelau-census-landing-page.aspx Available at. Accessed October 12, 2015.
- 13.United Nations Office for the Coordination of Humanitarian Affairs Situation Update: Tuvalu Drought, 4 October 2011. 2011. http://www.pacificdisaster.net/pdnadmin/data/documents/7602.html Available at. Accessed October 14, 2015.
- 14.The SPHERE Project Water Supply Standard 1: Access and Water Quantity. 2011. http://www.spherehandbook.org/en/water-supply-standard-1-access-and-water-quantity/ Available at. Accessed October 16, 2015.
- 15.United Nations Office for the Coordination of Humanitarian Affairs Tuvalu, Tokelau, and the Cook Islands: Drought Situation Update, Friday, October 14, 2011. 2011. http://www.pacificdisaster.net/pdnadmin/data/original/OCHA_20111014_TUV_DR_situpdate.pdf Available at. Accessed October 14, 2015.
- 16.World Health Organization Pacific Public Health Surveillance Network, Pacific Syndromic Surveillance Reports. 2011. http://www.pphsn.net/Surveillance/Syndromic_reports-2011.htm Available at. Accessed October 15, 2015.
- 17.World Health Organization Guidelines for Drinking-Water Quality. 3rd edition. 2004. http://www.who.int/water_sanitation_health/dwq/fulltext.pdf Available at. Accessed Oct 14, 2015.
- 18.Tauxe RV, Holmberg SD, Dodin A, Wells JV, Blake PA. Epidemic cholera in Mali: high mortality and multiple routes of transmission in a famine area. Epidemiol Infect. 1988;100:279–289. doi: 10.1017/s0950268800067418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhavnani D, Goldstick JE, Cevallos W, Trueba G, Eisenberg JNS. Impact of rainfall on diarrheal disease risk associated with unimproved water and sanitation. Am J Trop Med Hyg. 2014;90:705–711. doi: 10.4269/ajtmh.13-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moors E, Singh T, Siderius C, Balakrishnan S, Mishra A. Climate change and waterborne diarrhoea in northern India: impacts and adaptation strategies. Sci Total Environ. 2013;468–469:S139–S151. doi: 10.1016/j.scitotenv.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed W, Hodgers L, Sidhu JPS, Toze S. Fecal indicators and zoonotic pathogens in household drinking water taps fed from rainwater tanks in southeast Queensland, Australia. Appl Environ Microbiol. 2011;78:219–226. doi: 10.1128/AEM.06554-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans CA, Coombes PJ, Dunstan RH. Wind, rain and bacteria: the effect of weather on the microbial composition of roof-harvested rainwater. Water Res. 2006;40:37–44. doi: 10.1016/j.watres.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Climate Prediction Center ENSO Diagnostic Discussion. 2015. http://www.cpc.ncep.noaa.gov/products/analysis_monitoring/enso_advisory/ensodisc.html Available at. Accessed November 5, 2015.
- 24.Barnett TP, Adam JC, Lettenmaier DP. Potential impacts of a warming climate on water availability in snow-dominated regions. Nature. 2005;438:303–309. doi: 10.1038/nature04141. [DOI] [PubMed] [Google Scholar]
- 25.Cobb KM, Westphal N, Sayani HR, Watson JT, Di Lorenzo E, Cheng H, Edwards RL, Charles CD. Highly variable El Niño-Southern Oscillation throughout the Holocene. Science. 2013;339:67–70. doi: 10.1126/science.1228246. [DOI] [PubMed] [Google Scholar]
- 26.Government of Australia Climate Variability, Extremes and Change in the Western Tropical Pacific: Pacific Climate Change Science. 2014. http://www.pacificclimatechangescience.org/publications/reports/climate-variability-.extremes-and-change-in-the-western-tropical-pacific-2014/ Available at. Accessed April 5, 2015.
- 27.Mimura N, Nurse L, McLean RF, Agard J, Brigguglio L, Lefale P, Payet R, Sem G. 16: Small Islands. Climate Change 2007: Impacts, Adaptation and Vulnerability Contribution of Working Group II in the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. 2007. pp. 687–716.http://www.ipcc.ch/pdf/assessment-report/ar4/wg2/ar4-wg2-chapter16.pdf Available at. Accessed April 5, 2015.