Abstract
The aim of the present study was to assess the frequency of intestinal parasitic infection among patients with gastrointestinal tract disorders from the Greater Cairo region, Egypt. In addition, a comparison was made of the accuracy of direct thin and thick smear, formol-ether sedimentation (FEC), centrifugal flotation (CF), and mini-FLOTAC techniques in the diagnosis of infection. Out of 100 patients, the overall prevalence of parasitic infection was 51%. Only 6% had dual infection. Giardia lamblia was the most common parasite (26%), followed by Hymenolepis nana (20%), Entamoeba coli (8%), and Enterobius vermicularis (3%). Except the statistically significant association between E. vermicularis infection and perianal itching and insomnia (P < 0.001), age, gender, and complaints of the examined individuals had no association with prevalence of parasitic infection. Both FEC and CF were equally the most accurate techniques (accuracy = 98.2%, confidence interval [CI] = 0.95–1.0, and κ index = 0.962), whereas the Kato-Katz method was the least accurate (accuracy = 67.5%, CI = 0.57–0.78, and κ index = 0.333). However, mini-FLOTAC-ZnSO4 was the most accurate for diagnosis of helminthic infection, and FEC was more accurate for diagnosis of protozoal infection (accuracy = 100%, CI = 1.0–1.0, and κ index = 1).
Introduction
Intestinal parasitic infection represents a large and serious medical and public health problem in developing countries. The World Health Organization (WHO) has estimated that intestinal parasites infect more than 3.5 billion individuals, and that 450 million develop illness because of infection.1 In Egypt, intestinal parasitic infection represents a public-health problem. The rate of infection in certain areas may reach up to more than 50% of the asymptomatic population.2
A trustworthy evaluation of the pervasiveness of parasitic diseases relies upon using suitable and accurate methods of diagnosis. A wide variety of laboratory methods, including parasitological, molecular, serologic, and culture approaches, have been developed for the diagnosis of intestinal parasite infection. The choice of a particular technique is influenced by its affordability, sensitivity, simplicity, cost, and the level of technical skills involved.3 Novel advanced methods like DNA probes, polymerase chain reaction, and direct fluorescent antibody methods, which are highly sensitive, are too costly to be used routinely in developing countries, where the health centers have minimal laboratory equipment. Direct wet mount preparations, the Kato-Katz technique, the formol-ether sedimentation (FEC) technique, and flotation techniques, in the form of stool microscopy, offer many advantages over other diagnostic methods for detecting intestinal parasites. If performed correctly, these approaches are sensitive, simple, and economical.4 However, the presence of polyparasitism, particularly in developing countries,5,6 and the paucity of diagnostic tools that are able to detect multiple-species parasitic infection with a high level of accuracy, make it necessary to use more than one method to detect different parasitic forms, especially under conditions of low parasite burden.7 Therefore, there is a need for sensitive diagnostic tools that are inexpensive, simple to apply, and concurrently detect different intestinal parasite species in the same stool sample.
The present study aimed to evaluate the prevalence rate of intestinal parasitic infection among patients with gastrointestinal tract (GIT) disorders from the Greater Cairo region, Egypt. In addition, a comparison was made of the accuracy of direct thin and thick smear, FEC, centrifugal flotation (CF), and mini-FLOTAC techniques.
Materials and Methods
Sample collection.
This comparative cross-sectional study was carried out in the period from September 2014 to April 2015. All the practical work was done in the Department of Parasitology, Faculty of Medicine, Benha University, Egypt.
One hundred fresh stool samples were collected from patients of different ages, and of both sexes, attending the outpatient clinics of Benha Educational Hospital and Abu El-Resh Hospital, Greater Cairo region, Egypt. The patients were complaining of various GIT manifestations. Stool samples were collected according to WHO8 guidelines in clean, labeled, wide-mouth plastic containers with tight-fitting lids. The samples were protected from contamination with water, soil, and urine. As soon as the sample received, qualitative macroscopic examination was done regarding consistency, color, and presence of mucous and/or blood. Stool samples were homogenized by mixing after delivery to the laboratory. The specimens were neither incubated nor frozen and processed within 1 hour after delivery. Each container was labeled with the patient's name, the date of specimen collection, and a serial number.
Parasitological methods.
All specimens were processed by five techniques. 1) Direct thin smear examination done by preparing two wet mounts before processing the specimen further. One wet mount was prepared with physiologic saline and the other was prepared with Lugol's iodine solution.8 2) Kato-Katz technique (direct thick smear) using a template delivering about 41.7 mg of feces.9 Slides were examined within 1 hour of preparation to avoid over clarification of some helminth eggs. The number of eggs per gram of feces was calculated by multiplying the number of observed eggs by 24. 3) FEC technique using 1 g of stool suspended in 10% formalin and centrifuged at 3,000 rpm.10 4) Modified CF accomplished according to the method of Traunt and others11 using zinc sulfate solution of specific gravity 1.35. 5) Mini-FLOTAC technique using two different flotation solutions, zinc sulfate heptahydrate with specific gravity 1.35 (mini-FLOTAC-ZnSO4), and saturated sodium chloride with specific gravity 1.18 (mini-FLOTAC-NaCl).12
Statistical analysis.
The collected data were tabulated and analyzed using SPSS version 16 software (SPSS Inc., Chicago, IL). Categorical data are presented as number and percentage, whereas quantitative data are expressed as median and quartiles. Fisher's exact test with the Monte Carlo method for tables larger than 2 × 2 was used to analyze the differences between categorical variables. It is used when sample size is small. The z test was used to compare proportion between two groups of qualitative data. The Friedman test followed by post hoc multiple comparisons using Bonferroni adjusted Wicoxon test was used to analyze the difference between more than two medians of matched groups. The kappa test of significance (κ) was used to test the degree of agreement between the results of the studied techniques and the gold standard (the combined results of the techniques were taken as the gold standard6). κ measures were interpreted as follows: < 0, poor agreement; 0–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.0, almost perfect agreement.13 The accepted level of significance in this work was stated as 0.05 (P < 0.05 was considered significant).
Ethical considerations.
The study was approved by Parasitology Department Research Committee and the Ethical Committee at the Faculty of Medicine, Benha University. Oral consents were taken from the participants or their parents (in the case of children) at the hospitals.
Results
The current study showed that about half of the study group suffered from parasitic infection (P > 0.05). Most of the infected population had a single parasitic infection, whereas only 6% had a multiple parasitic infection (P < 0.001). Giardia lamblia was the most common parasitic infection, followed by Hymenolepis nana. Other parasitic infections found were significantly less common (P < 0.001) (Table 1). In addition, our results showed that the age, gender, and complaint of the examined individuals had no statistically significant effect on the overall prevalence of parasitic infection, although most of the infected persons (70%) were in the school-age group and 82.4% of complaints were of abdominal pain. Age and gender did not have a statistically significant association with the type of the infecting parasite. However, some complaints had a high frequency with intestinal parasitic infection: 100% of patients with Enterobius vermicularis infection had perianal itching and insomnia, and 100%, 88.5%, and 85% of patients with Entamoeba coli, G. lamblia, and H. nana infection, respectively, had abdominal pain. However, statistical analysis showed that except for E. vermicularis, there is no association between the patient's complain and the prevalence of H. nana, G. lamblia, and E. coli. Moreover, the current study showed that there was no significant association between the macroscopic criteria of the stool sample and parasitic infection.
Table 1.
Intestinal parasitic infections according to the study population characteristics
| Population characteristics | Total examined no. = 100 | Hymenolepis nana | Enterobius vermicularis | Giardia lamblia | Entamoeba coli | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | P value | No. (%) | P value | No. (%) | P value | No. (%) | P value | No. (%) | P value | ||
| Age (years) | 0.114 | 0.450 | 0.143 | 0.161 | 0.66 | ||||||
| 2–5 | 27 | 8 (29.6) | 2 (7.4) | 3 (11.1) | 1 (3.7) | 12 (44.4)* | |||||
| 6–15 | 62 | 12 (19.4) | 1 (1.6) | 20 (32.3) | 5 (8.1) | 34 (54.8)† | |||||
| > 15 | 11 | 0 (0) | 0 (0) | 3 (27.3) | 2 (18.2) | 5 (45.5) | |||||
| Gender | 0.424 | 1.0 | 0.111 | 0.697 | 0.697 | ||||||
| Male | 53 | 9 (17) | 2 (3.8) | 17 (32.1) | 3 (5.7) | 28 (52.8)‡ | |||||
| Female | 47 | 11 (23.4) | 1 (2.1) | 9 (19.1) | 5 (10.6) | 23 (48.9)‡ | |||||
| Symptoms | 0.861 | < 0.001 | 0.364 | 0.253 | 0.253 | ||||||
| Diarrhea | 12 | 3 (25) | 0 (0) | 3 (25) | 0 (0) | 6 (50) | |||||
| Abdominal pain | 81 | 17 (21) | 0 (0) | 23 (28.4) | 8 (9.9) | 42 (51.9)§ | |||||
| Perianal itching + insomnia | 3 | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 3 (100) | |||||
| Macroscopic visualization of stool | 0.308 | 1.0 | 0.469 | 0.114 | 0.289 | ||||||
| Color | |||||||||||
| Brown | 82 | 19 (23.2) | 3 (3.7) | 23 (28) | 6 (7.3) | 45 (54.9)§ | |||||
| Green | 15 | 1 (6.7) | 0 (0) | 3 (20) | 2 (13.3) | 6 (40) | |||||
| Yellow | 3 | 0 (0.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Consistency | 0.375 | 0.716 | 0.861 | 0.523 | 0.523 | ||||||
| Formed | 22 | 2 (9.1) | 0 (0) | 7 (31.8) | 2 (9.1) | 9 (40)* | |||||
| Soft | 63 | 14 (22.2) | 3 (4.8) | 16 (25.4) | 6 (9.5) | 35 (55.6)† | |||||
| Loose | 6 | 1 (16.7) | 0 (0) | 1 (16.7) | 0 (0) | 2 (33.3) | |||||
| Watery | 9 | 3 (33.3) | 0 (0) | 2 (22.2) | 0 (0) | 5 (55.6) | |||||
| Stool with mucous | 6 | 0 (0) | 0 (0) | 2 (33.3) | 1 (16.7) | 3 (50) | 1.0 | ||||
Two, three, four, sex cases have mixed infection, respectively.
Given that the sum of positive results from the combination of all the techniques was used as a gold standard in this study, its sensitivity and specificity were 100%. Our results showed that, for the diagnosis of parasitic infection using a single technique, both FEC and CF were equally the most accurate techniques (accuracy = 98.2% and κ index = 0.962), whereas the Kato-Katz method was the least accurate technique (accuracy = 67.5% and κ index = 0.333). However, mini-FLOTAC-ZnSO4 was the most accurate for the diagnosis of helminthic infection, and FEC was more accurate in the diagnosis of protozoal infection (accuracy = 100% and perfect agreement with the gold standard [κ index = 1]; Table 2).
Table 2.
Performance of direct stool diagnostic techniques in the detection of intestinal parasites
| Techniques | Positive cases | Sensitivity | NPV | Accuracy (%) | 95% CI | κ index* |
|---|---|---|---|---|---|---|
| Thin smear | ||||||
| Helminths | 17 | 73.9 | 90.7 | 87 | 0.76–0.98 | 0.814 |
| Protozoa | 32 | 94.1 | 96.1 | 97.1 | 0.92–1.0 | 0.955 |
| Total | 49 | 86.0 | 86 | 93 | 0.88–0.98 | 0.850 |
| FEC | ||||||
| Helminths | 21 | 91.3 | 96.1 | 95.7 | 0.89–1.0 | 0.942 |
| Protozoa | 34 | 100 | 100 | 100 | 1.0–1.0 | 1.0 |
| Total | 55 | 96.5 | 96.1 | 98.2 | 0.95–1.0 | 0.962 |
| CF | ||||||
| Helminths | 22 | 95.7 | 98 | 97.8 | 0.93–1.0 | 0.971 |
| Protozoa | 33 | 97.1 | 98 | 98.5 | 0.95–1.0 | 0.978 |
| Total | 55 | 96.5 | 96.1 | 98.2 | 0.95–1.0 | 0.962 |
| Kato-Katz | ||||||
| Helminths | 20 | 87.0 | 94.2 | 93.5 | 0.93–1.0 | 0.911 |
| Protozoa | NA | NA | NA | NA | NA | NA |
| Total | 20 | 35.1 | 43 | 67.5 | 0.57–0.78 | 0.333 |
| Mini-FLOTAC-ZnSO4 | ||||||
| Helminths | 23 | 100 | 100 | 100 | 1.0–1.0 | 1.0 |
| Protozoa | 28 | 82.4 | 89.1 | 91.2 | 0.83–0.99 | 0.860 |
| Total | 51 | 89.5 | 89.1 | 94.7 | 0.90–0.99 | 0.887 |
| Mini-FLOTAC-NaCl | ||||||
| Helminths | 22 | 95.7 | 98 | 97.8 | 0.93–1.0 | 0.971 |
| Protozoa | 19 | 55.9 | 76.6 | 77.9 | 0.67–0.89 | 0.626 |
| Total | 41 | 72.0 | 75.4 | 86 | 0.79–0.93 | 0.703 |
CF = centrifugal flotation; CI = confidence interval; FEC = formol-ether sedimentation; NA = not applicable; NPV = negative predictive value. Combined results from all diagnostic techniques were considered as “gold standard.”
κ measures were interpreted as follows: < 0, poor agreement; 0–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.0, almost perfect agreement.
Regarding the efficiency of the techniques used according to genus of the parasite, Table 3 shows that mini-FLOTAC-ZnSO4 and FEC were the most efficient for the diagnosis H. nana and E. coli, respectively (100% sensitivity, 100% efficiency, and κ index = 1), whereas the three flotation techniques used are the most efficient for the diagnosis of E. vermicularis, and FEC and CF methods are equally the most efficient for G. lamblia (100% sensitivity, 100% efficiency, and κ index = 1).
Table 3.
Species-specific sensitivity and efficiency of direct techniques in detection of intestinal parasites
| Techniques | Hymenolepis nana | Enterobius vermicularis | Giardia lamblia | Entamoeba coli | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Efficiency | κ index | Sensitivity | Efficiency | κ index | Sensitivity | Efficiency | κ index | Sensitivity | Efficiency | κ index | |
| Direct smear | 85% | 97% | 0.901 | 0.0% | 0.0% | 0.0 | 96.2% | 99% | 0.974 | 87.5% | 99% | 0.928 |
| Formol-ether sedimentation | 95 | 99 | 0.968 | 66.6 | 99 | 0.795 | 100 | 100 | 1.0 | 100 | 100 | 1.0 |
| Centrifugal flotation | 95 | 99 | 0.968 | 100 | 100 | 1.0 | 100 | 100 | 1.0 | 87.5 | 99 | 0.928 |
| Kato-Katz | 90 | 98 | 0.935 | 66.6 | 99 | 0.795 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mini-FLOTAC-ZnSO4 | 100 | 100 | 1.0 | 100 | 100 | 1.0 | 84.6 | 96 | 0.891 | 75.0 | 98 | 0.847 |
| Mini-FLOTAC-NaCl | 95 | 99 | 0.968 | 100 | 100 | 1.0 | 57.7 | 89 | 0.669 | 50.0 | 96 | 0.648 |
Combined results from all diagnostic techniques were considered as “gold standard.” κ index was interpreted as follows: < 0, poor agreement; 0–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.0, almost perfect agreement.
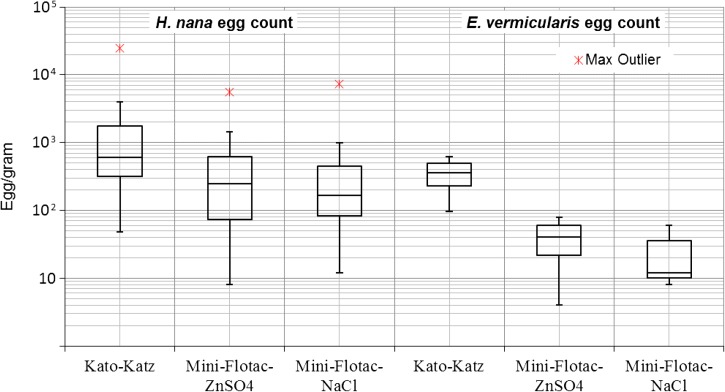
The box plot of egg count/gram for H. nana and E. vermicularis (Figure 1 ) revealed that Kato-Katz technique was statistically significantly (P < 0.001) able to detect more H. nana eggs (median = 612, interquartile range [IQR] = 1,458) than either mini-FLOTAC-ZnSO4 technique (median = 248, IQR = 554) or mini-FLOTAC-NaCl (median = 168, IQR = 360). However, no significant difference was found in comparison of the two flotation solutions used in performing the mini-FLOTAC technique. Quantitative diagnosis of E. vermicularis showed no significant difference (P > 0.05) between Kato-Katz technique (median = 360, IQR = 264) and either mini-FLOTAC-ZnSO4 technique (median = 40, IQR = 38) or mini-FLOTAC-NaCl (median = 12, IQR = 26).
Figure 1.
Box plot of Hymenolepis nana and Enterobius vermicularis egg counts of the same stool samples (log scale) using Kato-Katz, mini-FLOTAC-ZnSO4, and mini-FLOTAC-NaCl techniques.
Discussion
Intestinal parasitic infection is a major health problem in many developing countries where parasites are endemic. The present study shows that, in the Greater Cairo region, about half of the patients (51%) complaining of GIT symptoms had parasitic infection. A more or less similar prevalence (48.8%) was reported in the Canal region, east of Egypt,14 whereas a higher rate of infection (60.9–67.1%) was reported in the North Nile Delta.15,16 A lower rate of infection (25%) was recorded among diarrheic children in Benha City (Greater Cairo region) by Abed and others.17 This discrepancy among different studies could be ascribed to many factors, including dissimilarity in the selection of the enrolled study population, the sensitivity of the diagnostic technique used or the proficiency of the investigator. Our data showed that 6% of the examined individuals had a dual parasitic infection. Polyparasitism is not an uncommon finding,2,16 and interaction between parasites may result in more nutritional and pathological consequences than single infection.5 The current study showed that G. lamblia was the most predominant parasitic infection. This corroborates the findings of other studies, which emphasizes that giardiasis is one of the most common parasitic infections among Egyptian patients complaining of GIT disorders.18,19 The significantly low percentage of E. vermicularis infection (3%), in comparison with other Egyptian studies that used perianal swab in diagnosis,20,21 emphasizes that stool examination is not sensitive enough to be used in diagnosis of entrobiasis.22
The relationship between the prevalence of parasitic infection and either age or gender of the population is controversial. Although our present study and those of Gelaw and others23 and Abate and others24 deny any statistically significant effect on the overall prevalence of parasitic infection, other studies found a significant relation of age and gender with the prevalence of intestinal parasitic infection.25–27
Patients with parasitic infection may be either asymptomatic or present to physicians with a variety of clinical manifestations that may mimic nonparasitic diseases. Assessment of the contribution of parasitic infection and sequentially developing guidelines for the management of such a clinical situation may be useful. This syndromic approach is believed to be useful in communities with limited resources, where access to adequate diagnostic tools is not always available. The current study did not show an association between the presence of GIT disturbances and prevalence of parasitic infection among patient enrolled in the study. However, when analyzing this association for each parasite separately, a different pattern of relation appeared. Our results showed a highly significant association between E. vermicularis infection and perianal itching and insomnia, a result that corroborates that of Pezzani and others28 and partially with that of Acosta and others29 who emphasized this relation for perianal itching but not for insomnia. However, although the high frequency of abdominal pain among patients infected with G. lamblia, H. nana, and E. coli infection (88.5%, 85%, and 100% of positive patients, respectively), there was no statistically significant association between the prevalence of infection and the patient's complain (P > 0.05). Previous studies conducted in Pakistan, Kashmir, and Mexico reported abdominal pain as a very common complain among G. lamblia30,31 and H. nana32 infected patients. Entamoeba coli is a nonpathogenic intestinal protozoa and the biased misleading presumed association with abdominal pain reported in our study may be due to absence of a control normal group to compare with. Neither diarrhea nor the macroscopic criteria of the stool samples was found to have significant association with any of the parasites detected. Diarrhea was suggested as a potential risk factor that should draw attention to the presence of G. lamblia and H. nana infection.33–35
For many years, microscopy has been the main tool for the detection of intestinal parasitic infection. This is predominantly true in countries with limited economic resources, including Egypt. Moreover, in these countries, examination of one stool sample using single methods is the traditional trend due to its affordability, simplicity, and low cost. This trend is justified by the results of previous studies that assumed that single stool sample examination could be enough to diagnose up to 93% of positive cases.36,37 In the current work, given that the sum of positive results from the combination of all the techniques was used as a gold standard, data analysis showed that both FEC and CF were equally the most accurate techniques for diagnosis of parasitic infection (accuracy = 98.2%, confidence interval [CI] = 0.95–1.0, and κ index = 0.962), whereas the Kato-Katz method is the least accurate technique (accuracy = 67.5%, CI = 0.57–0.78, and κ index = 0.333). These results partially agree with previous studies that claimed a greater accuracy of FEC in the diagnosis of overall parasitic infection when compared with direct wet mount,38 flotation,39 and Kato-Katz40 methods. However, mini-FLOTAC-ZnSO4 was the most accurate for diagnosis of helminthic infection, and FEC was more accurate in the diagnosis of protozoal infection (accuracy = 100%, CI = 1.0–1.0, and perfect agreement with the gold standard, κ index = 1), a result that corresponds to that of Barda and others.41
Quantitative diagnosis of H. nana infections showed that the Kato-Katz technique significantly detected more eggs than mini-FLOTAC techniques (P < 0.001). However, no significant difference was found in quantitative diagnosis of E. vermicularis egg count (P = 4). No significant difference was found in comparison between the two flotation solutions used in performing the mini-FLOTAC technique.
The results reported in our study are limited by the small number of enrolled cases, the absence of a healthy asymptomatic control group, and the absence of a true gold standard to compare with.
In conclusion, intestinal parasites are common among patients complaining of GIT disorders in the Greater Cairo region. No single test is regarded the gold standard for diagnosing parasitic infection. However, FEC or CF is recommended to be used alternatively in the routine diagnosis of parasitic infection in Egypt, and mini-FLOTAC-ZnSO4 may be a substitute when infection with only helminths is suspected.
Footnotes
Authors' addresses: Atef H. Hussein, Samia M. Rashed, Ibrahim A. El-Hayawan, Nagwa S. M. Aly, Eman A. Abou Ouf, and Amira T. Ali, Department of Parasitology, Faculty of Medicine, Benha University, Benha, Egypt, E-mails: atef.abdelhamid@fmed.bu.edu.eg, samia.rashed@fmed.bu.edu.eg, ibrahim.alhywan@fmed.bu.edu.eg, nagwa.ali@fmed.bu.edu.eg, emanabououf@fmed.bu.edu.eg, and amera.ali@fmed.bu.edu.eg.
References
- 1.WHO . Division of Control of Tropical Diseases: Progress Report 1997. Geneva, Switzerland: WHO; 1998. [Google Scholar]
- 2.Hegazy AM, Younis NT, Aminou HA, Badr AM. Prevalence of intestinal parasites and its impact on nutritional status among preschool children living in Damanhur City, El-Behera Governorate, Egypt. J Egypt Soc Parasitol. 2014;44:517–524. doi: 10.12816/0006490. [DOI] [PubMed] [Google Scholar]
- 3.Garcia LS. Diagnostic Medical Parasitology. 4th edition. Washington, DC: ASM Press; 2001. p. 1069. [Google Scholar]
- 4.Parija SC, Srinivasa H. Viewpoint: the neglect of stool microscopy for intestinal parasites and possible solutions. Trop Med Int Health. 1999;4:522–524. doi: 10.1046/j.1365-3156.1999.00434.x. [DOI] [PubMed] [Google Scholar]
- 5.Pullan R, Brooker S. The health impact of polyparasitism in humans: are we under-estimating the burden of parasitic diseases? Parasitology. 2008;135:783–794. doi: 10.1017/S0031182008000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinmann P, Du ZW, Wang LB, Wang XZ, Jiang JY, Li LH, Marti H, Zhou XN, Utzinger J. Extensive multiparasitism in a village of Yunnan province, People's Republic of China, revealed by a suite of diagnostic methods. Am J Trop Med Hyg. 2008;78:760–769. [PubMed] [Google Scholar]
- 7.Sudré AP, Macedo HW, Peralta RHS, Peralta JM. Diagnóstico da estrongiloidíase humana: importância e técnicas. Rev Patol Trop. 2006;35:173–184. [Google Scholar]
- 8.WHO . Basic Laboratory Methods in Medical Parasitology. Geneva, Switzerland: WHO; 1991. [Google Scholar]
- 9.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 10.Allen AVH, Ridley DS. Further observations on the formol-ether concentration technique for faecal parasites. J Clin Pathol. 1970;23:545–546. doi: 10.1136/jcp.23.6.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truant AL, Elliott SH, Kelly MT, Smith JH. Comparison of formalin-ethyl ether sedimentation, formalin-ethyl acetate sedimentation, and zinc sulfate floatation techniques for detection of intestinal parasites. J Clin Microbiol. 1981;13:882. doi: 10.1128/jcm.13.5.882-884.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cringoli G. Copromicroscopic diagnosis of dicrocoeliosis: what's new? Mappe Parasitologiche. 2012;18:41. [Google Scholar]
- 13.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 14.Rayan HZ, Ismail OA, El Gayar EK. Prevalence and clinical features of Dientamoeba fragilis infections in patients suspected to have intestinal parasitic infection. J Egypt Soc Parasitol. 2007;37:599–608. [PubMed] [Google Scholar]
- 15.Abdel-Hafeez EH, Belal US, Abdellatif MZ, Naoi K, Norose K. Breast-feeding protects infantile diarrhea caused by intestinal protozoan infections. KJP. 2013;51:519–524. doi: 10.3347/kjp.2013.51.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayoumy AM, Mohammed KA, Shahat SA, Ghannam MM, Gazy-Mel S. Role of parasites among chronic diarrheic patients. J Egypt Soc Parasitol. 2010;40:679–698. [PubMed] [Google Scholar]
- 17.Abed NT, Mohamed NS, Abdel-Gawad ER, Ibrahim SG. Vitamin D status in children with recurrent acute diarrhea. Int J Curr Microbiol App Sci. 2014;3:858–868. [Google Scholar]
- 18.Banisch DM, El-Badry A, Klinnert JV, Ignatius R, El-Dib N. Simultaneous detection of Entamoeba histolytica/dispar, Giardia duodenalis and cryptosporidia by imunochromatographic assay in stool samples from patients living in the Greater Cairo Region, Egypt. World J Microbiol Biotechnol. 2015;31:1251–1258. doi: 10.1007/s11274-015-1875-5. [DOI] [PubMed] [Google Scholar]
- 19.Eldash HH, Bekhit OE, Algameel AA. Impact of Helicobacter pylori-giardiasis coinfection on children with recurrent abdominal pain. J Egypt Soc Parasitol. 2013;43:509–516. doi: 10.12816/0006407. [DOI] [PubMed] [Google Scholar]
- 20.Hussien SM, Taha MA, Omran EKh. Relationship between Enterobius vermicularis infection and pelvic inflammatory diseases in children at Sohag Governorate, Egypt. J Egypt Soc Parasitol. 2015;45:633–638. doi: 10.12816/0017931. [DOI] [PubMed] [Google Scholar]
- 21.El Sahn AA, Hassan MH, Ftohy EM, Abou-El Ela NE, Eassa SM. Parasitic infections and maternal awareness of preschool children in Karmouz district, Alexandria. J Egypt Public Health Assoc. 2000;75:1–29. [PubMed] [Google Scholar]
- 22.WHO . Quality Assurance in Bacteriology and Immunology. Geneva, Switzerland: World Health Organization, Regional Office for South-East Asia; 2012. SEARO Regional Publication No. 47. [Google Scholar]
- 23.Gelaw A, Anagaw B, Nigussie B, Silesh B, Yirga A, Alem M, Endris M, Gelaw B. Prevalence of intestinal parasitic infections and risk factors among school-children at the University of Gondar Community School, Northwest Ethiopia: a cross-sectional study. BMC Public Health. 2013;13:304. doi: 10.1186/1471-2458-13-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abate A, Kibret B, Bekalu E, Abera S, Teklu T, Yalew A, Endris M, Worku L, Tekeste A. Cross-sectional study on the prevalence of intestinal parasites and associated risk factors in Teda Health Centre, northwest Ethiopia. ISRN Parasitology. 2013;2013:1–5. doi: 10.5402/2013/757451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira D, Ferreira FS, Atouguia J, Fortes F, Guerra A, Centeno-Lima S. Infection by intestinal parasites, stunting and anemia in school aged children from southern Angola. PLoS One. 2015;10:e0137327. doi: 10.1371/journal.pone.0137327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chikukwa S, Lukas T. Prevalence and aetiological agents of parasitic infestation among population in northern Namibia. Int Sci Technol J Namibia. 2015;5:97–103. [Google Scholar]
- 27.El-Sherbini GT, Abosdera MM. Risk factors associated with intestinal parasitic infections among children. J Egypt Soc Parasitol. 2013;43:287–294. doi: 10.12816/0006385. [DOI] [PubMed] [Google Scholar]
- 28.Pezzani BC, Minvielle MC, de Luca MM, Córdoba MA, Apezteguía MC, Basualdo JA. Enterobius vermicularis infection among population of General Mansilla, Argentina. World J Gastroenterol. 2004;10:2535–2539. doi: 10.3748/wjg.v10.i17.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acosta M, Cazorla D, Garvett M. Enterobiasis among school children in a rural population from Estado Falcón, Venezuela, and its relation with socioeconomic level. Invest Clin. 2002;43:173–181. [PubMed] [Google Scholar]
- 30.Buch NA, Ahmad SM, Ahmed SZ, Ali SW, Charoo BA, Hassan MU. Recurrent abdominal pain in children. Indian Pediatr. 2002;39:830–834. [PubMed] [Google Scholar]
- 31.Younas M, Shah S, Talaat A. Frequency of Giardia lamblia infection in children with recurrent abdominal pain. J Pak Med Assoc. 2008;58:171–174. [PubMed] [Google Scholar]
- 32.Romero-Cabello R, Godínez-Hana L, Gutiérrez-Quiroz M. Clinical aspects of hymenolepiasis in pediatrics. Bol Med Hosp Infant Mex. 1991;48:101–105. [PubMed] [Google Scholar]
- 33.Muhsen K, Levine MM. A systematic review and meta-analysis of the association between Giardia lamblia and endemic pediatric diarrhea in developing countries. Nephrol Dial Transplant. 2012;55((Suppl 4)):S271–S293. doi: 10.1093/cid/cis762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdel Hamid MM, Eljack IA, Osman MK, Elaagip AH, Muneer MS. The prevalence of Hymenolepis nana among preschool children of displacement communities in Khartoum state, Sudan: a cross-sectional study. Travel Med Infect Dis. 2015;13:172–177. doi: 10.1016/j.tmaid.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Abrar Ul Haq K, Gul NA, Hammad HM, Bibi Y, Bibi A, Mohsan J. Prevalence of Giardia intestinalis and Hymenolepis nana in Afghan refugee population of Mianwali district, Pakistan. Afr Health Sci. 2015;15:394–400. doi: 10.4314/ahs.v15i2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senay H, MacPherson D. Parasitology: diagnostic yield of stool examination. CMAJ: Canadian Medical Association Journal. 1989;140:1329–1331. [PMC free article] [PubMed] [Google Scholar]
- 37.Morris AJ, Wilson ML, Reller LB. Application of rejection criteria for stool ovum and parasite examinations. J Clin Microbiol. 1992;30:3213–3216. doi: 10.1128/jcm.30.12.3213-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oguoma VM, Ekwunife CA. The need for a better method: comparison of direct smear and formol-ether concentration techniques in diagnosing intestinal parasites. Int J Trop Med. 2006;3:1–6. [Google Scholar]
- 39.Assefa LM, Crellen T, Kepha S, Kihara JH, Njenga SM, Pullan RL, Brooker SJ. Diagnostic accuracy and cost-effectiveness of alternative methods for detection of soil-transmitted helminths in a post-treatment setting in western Kenya. PLoS Negl Trop Dis. 2014;8:2843. doi: 10.1371/journal.pntd.0002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mergani MH, Mohammed MA, Khan N, Bano M, Khan A. Detection of intestinal protozoa by using different methods. Dent Med Res. 2014;2:28–32. [Google Scholar]
- 41.Barda BD, Rinaldi L, Ianniello D, Zepherine H, Salvo F, Sadutshang T, Cringoli G, Clementi M, Albonico M. Mini-FLOTAC, an innovative direct diagnostic technique for intestinal parasitic infections: experience from the field. PLoS Negl Trop Dis. 2013;7:e2344. doi: 10.1371/journal.pntd.0002344. [DOI] [PMC free article] [PubMed] [Google Scholar]