Abstract
Leishmania (Viannia) braziliensis is the main causative species of tegumentary leishmaniasis in Brazil. In this study, we evaluated the susceptibility of 16 clinical isolates of L. (V.) braziliensis from different regions of Brazil to miltefosine in vitro. Half-maximal inhibitory concentrations of miltefosine varied from 22.9 to 144.2 μM against promastigotes and from 0.3 to 4.2 μM against intracellular amastigotes. No significant differences were found between isolates of different geographical origins. A clear correlation between the EC50 against promastigotes and amastigotes within each isolate was found. These findings contribute to the evaluation of miltefosine's potential and limitations for the treatment of tegumentary leishmaniasis in Brazil.
Tegumentary leishmaniasis is a disease of importance in Brazil, where it is mainly caused by Leishmania (Viannia) braziliensis. The efficacy of the first-line drug, meglumine antimoniate, for the treatment of cutaneous leishmaniasis in areas of L. (V.) braziliensis predominance in Brazil can be as low as 53%.1 New therapeutic alternatives are highly desirable.
Miltefosine (MF) (hexadecylphosphatidylcholine) was approved for the treatment of visceral leishmaniasis in India in 2002, where pentavalent antimony was already considered as ineffective due to widespread parasite resistance.2 This oral drug has also been approved for the treatment of tegumentary leishmaniasis in Colombia, after the demonstration of equivalent efficacy to antimony, and in other countries in South America.3 However, the response is heterogeneous in areas of high prevalence of L. (V.) braziliensis: for example, a clinical trial showed 83% efficacy for MF in cutaneous leishmaniasis in Bolivia, whereas a 53% cure rate was observed in Guatemala.3,4 In Brazil, 70% success rates were observed in two MF clinical trials of cutaneous leishmaniasis due to L. (V.) braziliensis and Leishmania (V.) guyanensis.1,5
The aim of this work was to characterize the MF susceptibility of L. (V.) braziliensis clinical isolates from Brazilian patients with tegumentary leishmaniasis from two geographically distinct regions.
Eight clinical isolates were obtained from lesion biopsies of patients with tegumentary leishmaniasis attending the Anuar Auad Tropical Diseases Hospital, Goiânia, Goiás, Brazil (Leishbank),6 and eight isolates were obtained through needle aspiration of skin lesions from patients attending the health post of Corte de Pedra, Bahia, Brazil. After the isolation, cultures were frozen and recovered to perform this study. This study was approved by the Ethical Committee of the Hospital das Clínicas of the Goiás Federal University and by the Ethical Committee for Human Research of the Bahia Federal University (CEP/MCO/UFBA-Par/Res 034/2007). Consent was obtained from all the subjects enrolled in the study.
The clinical isolates and two L. (V.) braziliensis reference strains (MHOM/BR/75/M2903 and MHOM/BR/94/H3227) were grown in 199 medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% heat inactivated fetal calf serum, 2% sterile male human urine, and 0.005% hemin.
The isolates were typed by polymerase chain reaction of internal transcribed spacer of ribosomal DNA and hsp70 gene followed by restriction analysis using Hae III, as described.7,8 The M2903 reference strain and 16 clinical isolates produced the expected profile for L. (V.) braziliensis (data not shown).
The activity of MF against promastigotes was evaluated by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) assay as previously described.9 Approximately 2 × 106 log-phase parasites were incubated in the presence of 25–200 μM MF diluted from a 10 mM stock solution in water. After 24 hours, cell viability was determined by incubation with MTT. Results were expressed as the mean percentage reduction of parasite numbers compared with untreated control wells. Half-maximal and 90% effective concentrations (EC50 and EC90) were determined by sigmoidal regression curves using Graph Pad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA) and the activity index was obtained by the ratio between the clinical isolate's EC50 and the reference strain M2903.
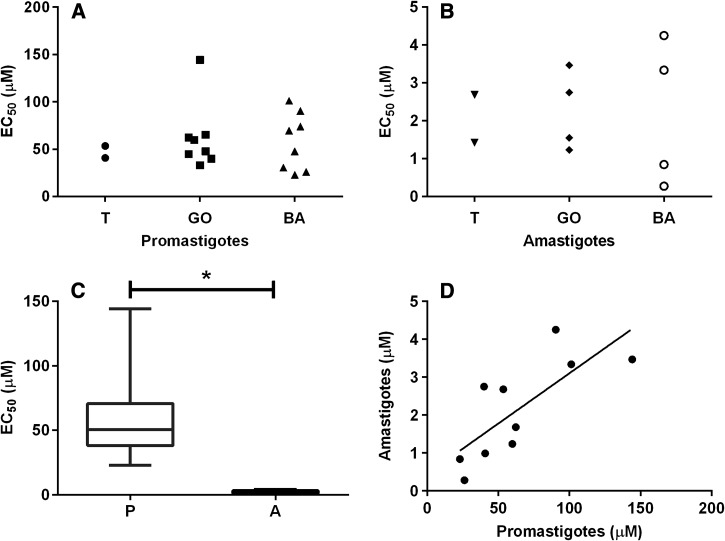
The EC50 of MF against promastigotes of 16 isolates and two reference strains ranged from 22.9 ± 3.7 to 144.2 ± 16.1 μM (Figure 1 and Table 1) with a median of 47.8 μM. The EC50 for the reference strains M2903 and H3227 were 53.5 ± 6.6 and 40.7 ± 8.5 μM, respectively. The EC50 for the most and the least susceptible isolates (henceforth called polar isolates) varied 6.3-fold. The differences between the EC50 for polar isolates and the M2903 reference strain were statistically significant (P < 0.0001 analysis of variance (ANOVA) and Tukey's multiple comparison test). On the other hand, no significant differences were detected between isolates of different geographic origins (Figure 1A).
Figure 1.
Susceptibility of promastigotes and intracellular amastigotes of Leishmania (V.) braziliensis isolates to miltefosine. (A) EC50 determined against promastigotes. (B) EC50 determined against intracellular amastigotes. “T” indicates the type strains M2903 and H3227, “GO” isolates from Goiás, and “BA” isolates from Bahia. (C) Comparison between the EC50 against promastigotes and amastigotes. The box indicates the 25th–75th percentiles. The line in the middle of the box indicates the median; * P < 0.0001 (Mann–Whitney test). (D) Correlation between the EC50 determined against promastigotes and amastigotes for each clinical isolate. Spearman coefficient r = 0.793; P = 0.008.
Table 1.
Susceptibility of Leishmanis (Viannia) braziliensis clinical isolates to MF
| Isolate | Promastigotes* | Infection rate (%)** | Amastigotes† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Origin‡ | Clinical form | Age | Treatment§ | Clinical cure¶ | EC50 (μm) | EC90 (μm) | AI∥ | EC50 (μm) | EC90 (μm) | AI∥ | ||
| MHOM/BR/1975/2903 | Ceará | – | – | – | – | 53.5 ± 6.6 | 70.21 ± 7.7 | – | 77 ± 7 | 2.7 ± 0.2 | 5.1 ± 1.1 | – |
| MHOM/BR/1994/H3227 | Pará | – | – | – | – | 40.7 ± 8.5 | 69.5 ± 5.5 | 0.76 | 50 ± 6 | 1.0 ± 0.0†† | 8.7 ± 1.9 | 0.37 |
| MHOM/BR/2006/GDL | Goiás | CL | 60 | Gluc | Yes | 45.0 ± 7.8 | 57.1 ± 5.1 | 0.84 | ||||
| MHOM/BR/2006/BES | Goiás | CL | 41 | Gluc | No | 62.2 ± 13.5 | 92.9 ± 13.3 | 1.16 | 40 ± 6 | 1.7 ± 0.5 | 4.8 ± 1.3 | 0.62 |
| MHOM/BR/2006/EFSF | Goiás | CL | 44 | Gluc | Yes | 144.2 ± 16.1 | 277.0 ± 16.3 | 2.70 | 54 ± 8 | 3.5 ± 0.6 | 11.9 ± 1.1 | 1.29 |
| MHOM/BR/2006/UAF | Goiás | CL | 29 | Gluc | Yes | 47.8 ± 6.6 | 58.3 ± 6.9 | 0.89 | ||||
| MHOM/BR/2005/WSS | Goiás | CL | 22 | Gluc | NR | 39.9 ± 4.2 | 75.9 ± 3.0 | 0.75 | 71 ± 6 | 2.7 ± 0.4 | 6.9 ± 0.3 | 1.02 |
| MHOM/BR/2006/HPV | Goiás | CL | 46 | Gluc | Yes | 65.2 ± 3.7 | 78.4 ± 2.1 | 1.22 | ||||
| MHOM/BR/2006/PPS | Goiás | MCL | 69 | NR | NR | 59.8 ± 4.7 | 73.6 ± 4.8 | 1.12 | 58 ± 6 | 1.2 ± 0.4 | 8.2 ± 1.6 | 0.46 |
| MHOM/BR/2006/TBM | Goiás | CL | 15 | Gluc | Yes | 33.1 ± 4.7 | 56.9 ± 13.2 | 0.62 | ||||
| MHOM/BR/2006/LTCP 16907 | Bahia | CL | 27 | Gluc | Yes | 101.2 ± 6.0 | 133.6 ± 12.0 | 1.89 | 33 ± 1 | 3.3 ± 0.4 | 11.5 ± 0. 4 | 1.24 |
| MHOM/BR/2010/LTCP 20221 | Bahia | CL | 2 | Gluc | No | 69.8 ± 9.0 | 102.9 ± 14.6 | 1.30 | ||||
| MHOM/BR/2009/LTCP 19512 | Bahia | CL | 58 | Gluc | No | 26.1 ± 1.6 | 41.5 ± 3.0 | 0.49 | 86 ± 8 | 0.3 ± 0.1 | 1.8 ± 0.6 | 0.10 |
| MHOM/BR/2003/LTCP 15344 | Bahia | MCL | 23 | Gluc | No | 47.8 ± 7.9 | 67.4 ± 3.9 | 0.89 | ||||
| MHOM/BR/2009/LTCP 19446 | Bahia | CL | 28 | Gluc | Yes | 90.4 ± 5.2 | 140.3 ± 16.4 | 1.69 | 70 ± 3 | 4.2 ± 0.2 | 8.4 ± 2.1 | 1.58 |
| MHOM/BR/2006/LTCP 16596 | Bahia | CL | 16 | Gluc | No | 74.1 ± 4.0 | 106.4 ± 16.9 | 1.39 | ||||
| MHOM/BR/2005/LTCP 16012 | Bahia | CL | 21 | Gluc | Yes | 22.9 ± 3.7 | 30.9 ± 6.4 | 0.42 | 45 ± 5 | 0.8 ± 0.1 | 2.0 ± 0.2 | 0.31 |
| MHOM/BR/2010/LTCP 20190 | Bahia | CL | 30 | Gluc | Yes | 30.5 ± 2.3 | 61.8 ± 6.3 | 0.57 | ||||
CL = cutaneous leishmaniasis; MCL = mucocutaneous leishmaniasis; MF = miltefosine; SD = standard deviation.
Inhibitory concentrations against promastigotes, EC50 ± SD and EC90 ± SD. Experiments were performed three times in triplicate.
Inhibitory concentrations against intracellular amastigotes, EC50 ± SD and EC90 ± SD. Experiments were performed three times in duplicate.
State in Brazil where the infection was most likely acquired.
NR = did not return for treatment; Gluc = Glucantime 20 mg/kg for 20 days.
Clinical cure at 180 days posttreatment; NR = patient did not return for follow up.
AI = activity index (ratio between the isolate's EC50 and M2903 reference strain's EC50).
Percentage of infected macrophages after 72h ± SD (%) (N = 6).
EC50 values for intracellular amastigotes were reported previously.18
Based on the EC50 values against promastigotes, we selected the three most susceptible isolates, the two least susceptible and three isolates with intermediate EC50 to evaluate the in vitro susceptibility of intracellular amastigotes (Table 1).
The sensitivity of intracellular amastigotes to MF was evaluated as reported previously.9 Bone marrow derived macrophages (BMDM) were plated on round glass coverslips in 24-well plates (3 × 105 cells per well). Infections were performed with L. (V.) braziliensis stationary-phase promastigotes (30 parasites per macrophage) for 3 hours at 33°C. Noninternalized parasites were removed by washing with warmed phosphate-buffered saline (PBS), followed by the addition of medium containing increasing MF concentrations, varying from 0.25 to a maximum of 40 μM, since 50% cytotoxicity determined against BMDM was calculated as 46.5 ± 3.9 μM. After 72 hours, the cells were washed with PBS, fixed in methanol, and stained with the panoptical Instant Prov kit (Newprov, Pinhais, Paraná, Brazil). The EC50 was determined based on the infection index (number of infected macrophages multiplied by the number of amastigotes per infected macrophage) in sigmoidal regression curves as described earlier.
The infection rates varied between 33% and 86% (Table 1). Compared with promastigotes, intracellular amastigotes were more susceptible to MF (P < 0.0001 ANOVA) (Figure 1B and C). The EC50 against intracellular amastigotes was also heterogeneous between the isolates varying between 0.3 ± 0.1 and 4.2 ± 0.2 μM (Table 1). The EC50 for the reference strain M2903 was 2.7 ± 0.2 μM and the median of EC50 values was 2.1 μM for these isolates. The EC50 for the less susceptible isolate was approximately 15-fold greater than the EC50 against amastigotes of the more susceptible isolate.
The data available for each isolate as well as EC50 and EC90 values determined for promastigotes and intracellular amastigotes are summarized in Table 1. Fourteen patients presented localized cutaneous lesions, one had simultaneous cutaneous and mucosal lesions and one was a mucosal leishmaniasis patient. Fifteen patients were treated with meglumine antimoniate. Of these, five failed to cure after the first course of treatment and one did not return for follow up. One patient was lost to follow up before treatment. The therapeutic failure after antimonial did not correlate with susceptibility to MF.
A clear correlation between the susceptibility of promastigotes and intracellular amastigotes was observed (r = 0.793; P = 0.008 Spearman's correlation test) (Figure 1D). Similar findings were also observed in Leishmania donovani isolates,10 and indicate that in vitro susceptibility of promastigotes may be considered a surrogate of susceptibility of intracellular amastigotes. Therefore, in vitro assays using promastigotes are useful to evaluate the susceptibility of clinical isolates to MF.
The characterization of MF susceptibility of eight Peruvian L. braziliensis isolates found EC50 for intracellular amastigotes in the range of 52 to greater than 73 μm,11 therefore markedly higher than EC50 values determined in this work for Brazilian isolates, emphasizing the existence of considerable diversity in susceptibility to MF within this species.
Since the isolation of parasites used in this study occurred before treatment, there was no previous exposition to MF or any other antileishmanial drug, indicating that this differential susceptibility is an intrinsic characteristic of these isolates.
The correlation between MF treatment failure or success and in vitro susceptibility to the drug is still unresolved, with some evidence pointing to selection of less tolerant parasites with drug exposure but without the accompanying relationship to cure rates.12–16 One of the limitations of the present study was to lack the evaluation of a relationship between in vitro susceptibility with in vivo response to MF. However, these isolates were not from patients who used MF.
Considering that MF's efficacy against cutaneous leishmaniasis due to Leishmania (Viannia) species varies from 53% to 91%,3,4,17,18 and in Brazil, MF was effective against L. (V.) braziliensis in 75% of patients,1 it would be interesting to investigate whether clinical isolates from unresponsive patients presented low susceptibility to MF in vitro.
MF is not yet approved for the treatment of tegumentary leishmaniasis in Brazil and this study may contribute to the evaluation of MF's treatment potential in the country.
ACKNOWLEDGMENTS
We thank Maria Jania Teixeira from Universidade Federal do Ceará for providing the MHOM/BR/1994/H3227 strain.
Footnotes
Financial support: This work was supported by grants 2011/20484-7 and 2015/09080-2, São Paulo Research Foundation (FAPESP), grant 473343/2012-6, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). CRE (2015/05130-5) and ACC (2012/14629-5) were supported by FAPESP fellowships. SRBU and EMC receive senior researcher scholarships from CNPq. FR-D is a research fellow of CNPq.
Authors' addresses: Caroline R. Espada, Jenicer K. U. Yokoyama-Yasunaka, and Silvia R. B. Uliana, Laboratório de Leishmanioses, Departamento de Parasitologia, Instituto de Ciências Biomédicas, Universidade de São Paulo, São Paulo, Brazil, E-mails: caroline.respada@gmail.com, jenicerk@usp.br, and srbulian@icb.usp.br. Fátima Ribeiro-Dias, Miriam L. Dorta, and Ledice Inácia de Araújo Pereira, Laboratório de Imunobiologia das Leishmanioses, Instituto de Patologia Tropical e Saúde Pública, Universidade Federal de Goiás, Goiânia, Brazil, E-mails: fatimardias@gmail.com, mledorta@gmail.com, and ledicepereira@gmail.com. Edgar M. de Carvalho, Paulo R. Machado, and Albert Schriefer, Serviço de Imunologia, Faculdade de Medicina, Universidade Federal da Bahia, Salvador, Brazil, E-mails: edgar@ufba.br, prlmachado@hotmail.com, and nab.schriefer@gmail.com. Adriano C. Coelho, Departamento de Biologia Animal, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, Brazil, E-mail: accoelho@unicamp.br.
References
- 1.Machado PR, Ampuero J, Guimaraes LH, Villasboas L, Rocha AT, Schriefer A, Sousa RS, Talhari A, Penna G, Carvalho EM. Miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis in Brazil: a randomized and controlled trial. PLoS Negl Trop Dis. 2010;4:e912. doi: 10.1371/journal.pntd.0000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med. 2002;347:1739–1746. doi: 10.1056/NEJMoa021556. [DOI] [PubMed] [Google Scholar]
- 3.Soto J, Toledo J, Valda L, Balderrama M, Rea I, Parra R, Ardiles J, Soto P, Gomez A, Molleda F, Fuentelsaz C, Anders G, Sindermann H, Engel J, Berman J. Treatment of Bolivian mucosal leishmaniasis with miltefosine. Clin Infect Dis. 2007;44:350–356. doi: 10.1086/510588. [DOI] [PubMed] [Google Scholar]
- 4.Soto J, Arana BA, Toledo J, Rizzo N, Vega JC, Diaz A, Luz M, Gutierrez P, Arboleda M, Berman JD, Junge K, Engel J, Sindermann H. Miltefosine for new world cutaneous leishmaniasis. Clin Infect Dis. 2004;38:1266–1272. doi: 10.1086/383321. [DOI] [PubMed] [Google Scholar]
- 5.Chrusciak-Talhari A, Dietze R, Chrusciak Talhari C, da Silva RM, Gadelha Yamashita EP, de Oliveira Penna G, Lima Machado PR, Talhari S. Randomized controlled clinical trial to access efficacy and safety of miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania (Viannia) guyanensis in Manaus, Brazil. Am J Trop Med Hyg. 2011;84:255–260. doi: 10.4269/ajtmh.2011.10-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira MA, Pires Ada S, de Bastos RP, Lima GM, Pinto SA, Pereira LI, Pereira AJ, Abrahamsohn Ide A, Dorta ML, Ribeiro-Dias F. Leishmania spp. parasite isolation through inoculation of patient biopsy macerates in interferon gamma knockout mice. Rev Inst Med Trop Sao Paulo. 2010;52:83–88. doi: 10.1590/s0036-46652010000200004. [DOI] [PubMed] [Google Scholar]
- 7.Cupolillo E, Grimaldi G, Jr, Momen H, Beverley SM. Intergenic region typing (IRT): a rapid molecular approach to the characterization and evolution of Leishmania. Mol Biochem Parasitol. 1995;73:145–155. doi: 10.1016/0166-6851(95)00108-d. [DOI] [PubMed] [Google Scholar]
- 8.Montalvo AM, Fraga J, Maes I, Dujardin JC, Van der Auwera G. Three new sensitive and specific heat-shock protein 70 PCRs for global Leishmania species identification. Eur J Clin Microbiol Infect Dis. 2012;31:1453–1461. doi: 10.1007/s10096-011-1463-z. [DOI] [PubMed] [Google Scholar]
- 9.Zauli-Nascimento RC, Miguel DC, Yokoyama-Yasunaka JK, Pereira LI, Pelli de Oliveira MA, Ribeiro-Dias F, Dorta ML, Uliana SR. In vitro sensitivity of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis Brazilian isolates to meglumine antimoniate and amphotericin B. Trop Med Int Health. 2010;15:68–76. doi: 10.1111/j.1365-3156.2009.02414.x. [DOI] [PubMed] [Google Scholar]
- 10.Kumar D, Kulshrestha A, Singh R, Salotra P. In vitro susceptibility of field isolates of Leishmania donovani to miltefosine and amphotericin B: correlation with sodium antimony gluconate susceptibility and implications for treatment in areas of endemicity. Antimicrob Agents Chemother. 2009;53:835–838. doi: 10.1128/AAC.01233-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yardley V, Croft SL, De Doncker S, Dujardin JC, Koirala S, Rijal S, Miranda C, Llanos-Cuentas A, Chappuis F. The sensitivity of clinical isolates of Leishmania from Peru and Nepal to miltefosine. Am J Trop Med Hyg. 2005;73:272–275. [PubMed] [Google Scholar]
- 12.Bhandari V, Kulshrestha A, Deep DK, Stark O, Prajapati VK, Ramesh V, Sundar S, Schonian G, Dujardin JC, Salotra P. Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS Negl Trop Dis. 2012;6:e1657. doi: 10.1371/journal.pntd.0001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prajapati VK, Mehrotra S, Gautam S, Rai M, Sundar S. Am J Trop Med Hyg. 2012;87:655–657. doi: 10.4269/ajtmh.2012.12-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prajapati VK, Sharma S, Rai M, Ostyn B, Salotra P, Vanaerschot M, Dujardin JC, Sundar S. In vitro susceptibility of Leishmania donovani to miltefosine in Indian visceral leishmaniasis. Am J Trop Med Hyg. 2013;89:750–754. doi: 10.4269/ajtmh.13-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obonaga R, Fernandez OL, Valderrama L, Rubiano LC, Castro Mdel M, Barrera MC, Gomez MA, Gore Saravia N. Treatment failure and miltefosine susceptibility in dermal leishmaniasis caused by Leishmania subgenus Viannia species. Antimicrob Agents Chemother. 2014;58:144–152. doi: 10.1128/AAC.01023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrickx S, Eberhardt E, Mondelaers A, Rijal S, Bhattarai NR, Dujardin JC, Delputte P, Cos P, Maes L. Lack of correlation between the promastigote back-transformation assay and miltefosine treatment outcome. J Antimicrob Chemother. 2015;70:3023–3026. doi: 10.1093/jac/dkv237. [DOI] [PubMed] [Google Scholar]
- 17.Soto J, Berman J. Treatment of New World cutaneous leishmaniasis with miltefosine. Trans R Soc Trop Med Hyg. 2006;100((Suppl 1)):S34–S40. doi: 10.1016/j.trstmh.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Rubiano LC, Miranda MC, Muvdi Arenas S, Montero LM, Rodriguez-Barraquer I, Garcerant D, Prager M, Osorio L, Rojas MX, Perez M, Nicholls RS, Gore Saravia N. Noninferiority of miltefosine versus meglumine antimoniate for cutaneous leishmaniasis in children. J Infect Dis. 2012;205:684–692. doi: 10.1093/infdis/jir816. [DOI] [PMC free article] [PubMed] [Google Scholar]