Abstract
Infection by the rat lungworm Angiostrongylus cantonensis represents the most common cause of infectious eosinophilic meningitis in humans, causing central nervous system (CNS) angiostrongyliasis. Most of CNS angiostrongyliasis cases were described in Asia, Pacific Basin, Australia, and some limited parts of Africa and America. CNS angiostrongyliasis has been reported in the Caribbean but never in the Lesser Antilles. The primary objectives of this study were to depict the first case of CNS angiostrongyliasis in the Lesser Antilles and investigate the environmental presence of A. cantonensis in Guadeloupe, Lesser Antilles. In December 2013, a suspected case of CNS angiostrongyliasis in an 8-month-old infant in Guadeloupe was investigated by real-time polymerase chain reaction (PCR) testing on cerebral spinal fluid (CSF). The environmental investigation was performed by collecting Achatina fulica molluscs from different parts of Guadeloupe and testing the occurrence of A. cantonensis by real-time PCR. CSF from the suspected case of angiostrongyliasis was positive for A. cantonensis by real-time PCR. Among 34 collected snails for environmental investigation, 32.4% were positive for A. cantonensis. In conclusion, we report the first laboratory-confirmed case of CNS-angiostrongyliasis in the Lesser Antilles. We identified the presence and high prevalence of A. cantonensis in A. fulica in Guadeloupe. These results highlight the need to increase awareness of this disease and implement public health programs in the region to prevent human cases of angiostrongyliasis and improve management of eosinophilic meningitis patients.
Introduction
The rat lungworm Angiostrongylus cantonensis is a metastrongyloid zoonotic and parasitic nematode of rat pulmonary arteries discovered by Chen in 1935.1 The life cycle of A. cantonensis involves the rats as a definitive host (mainly Rattus norvegicus), molluscs as intermediate hosts (especially the African giant snail Achatina fulica), and crustaceans and fishes as paratenic hosts.2 Human infections are acquired by ingestion of third-stage infective larvae in raw or undercooked molluscs, paratenic hosts, and contaminated vegetables or water.3 Angiostrongylus cantonensis infection causes central nervous system (CNS) angiostrongyliasis and is the leading infectious cause of eosinophilic meningitis in humans but hematological malignancies, drugs, infectious, and inflammatory diseases can also be among prime responsible factors. Human angiostrongyliasis is commonly a self-limited meningitis syndrome, but a large spectrum of symptoms is possible.2 Clinical manifestations range from asymptomatic disease, mild headache, encephalitis, and radiculomyelitis to meningoencephalitis with permanent neurological injury or even death.2,4
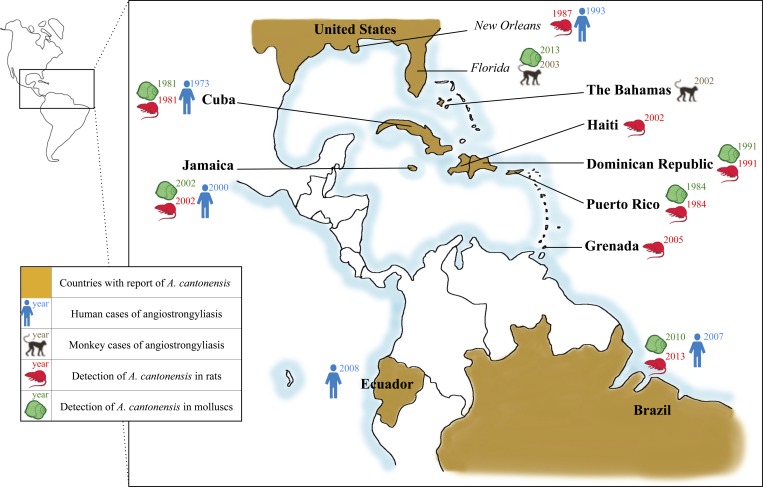
Angiostrongylus cantonensis was first detected in China,1 but its geographic distribution is gradually expanding due to rapid globalization. The spread of Angiostrongylus spp. has been driven by climate change5 as well as human activity due to transatlantic and transpacific trading. This leads to dispersion of carrier hosts along with transportation of consumable materials.6 The geographical distribution of A. cantonensis currently includes southeast Asia7; Australia8; Pacific Islands9; Indian Ocean Islands, including Madagascar, Reunion, and Mayotte10; United States of America, including Hawaii, Alabama, Louisiana, and Florida11–13; Brazil14; and Ecuador.15 In the Caribbean, the parasite has been found in wild rats in the Greater Antilles in Cuba,16 Dominican Republic,17 Haiti,18 Puerto Rico,19 Jamaica,20 and in the Lesser Antilles in Grenada only.21 Figure 1 depicts reported infections in humans and animal hosts in the Caribbean and surrounding areas to date. Several cases have been reported among humans in the Greater Antilles including Cuba,16,22 Dominican Republic,23 and Jamaica,20,24 whereas no human cases have been documented in the Lesser Antilles.
Figure 1.
Geographic distribution of angiostrongyliasis cases and presence of Angiostrongylus cantonensis in gastropods and rats in America and Antilles in the past four decades.
In December 2013, the Laboratory of Clinical Biology of Basse-Terre, Guadeloupe, was notified of a suspected case of CNS angiostrongyliasis in an 8-month-old patient who presented with eosinophilic meningitis and had never traveled outside of Guadeloupe.
Materials and Methods
Case investigation.
The infant was referred to the Pediatric Department in Basse-Terre Hospital, Guadeloupe, for clinical and biological evaluation. The mother of the infant was interviewed to characterize symptoms, date of emergence, and travel history in the weeks before symptom onset. Hygiene and food habits were also investigated to evaluate the most likely exposure.
Laboratory testing.
Blood was collected from the patient on the first day of hospitalization for hematological and serological analyses. Cerebral spinal fluid (CSF) and stool samples were collected for bacterial, viral, and parasitological analyses. Initial laboratory analyses were performed in Basse-Terre Hospital Center (CHBT) laboratory. Parasitic serologies were sent to clinical diagnostic laboratory (Cerba Healthcare, Saint-Ouen l'Aumône, France), except for schistosomiasis serology that was performed in CHBT laboratory. A CSF sample was sent to the Centers for Disease Control and Prevention (CDC, Atlanta, GA) to perform molecular detection of A. cantonensis using a species-specific internal transcribed spacer 1 (ITS1)-based TaqMan assay as described by Qvarnstrom and others.25 DNA was extracted from CSF using the DNeasy Tissue and Blood DNA Extraction Kit (Qiagen Inc., Valencia, CA) following the manufacturer's instructions for blood samples. Real-time polymerase chain reaction (PCR) was performed using 5 μL of extracted DNA sample in a 20-μL total volume containing Platinum qPCR Supermix (Invitrogen, Carlsbad, CA), 0.2 μM of each primers AcanITS1F1 (5′-TTCATGGATGGCGAACTGATAG-3′) and AcanITS1R1 (5′-GCGCCCATTGAAACATTATACTT-3′), and 0.05 μM of TaqMan probe AcanITS1P1 (5′-6-carboxyfluorescein-ATCGCATATCTACTATACGCATGTGACACCTG-BHQ-3′). The PCR was performed using standard cycling conditions for TaqMan assays. Other blood samples were taken regularly at D11, D14, and D25 after the first day of hospitalization to evaluate the normalization of eosinophils after treatment.
Environmental investigation.
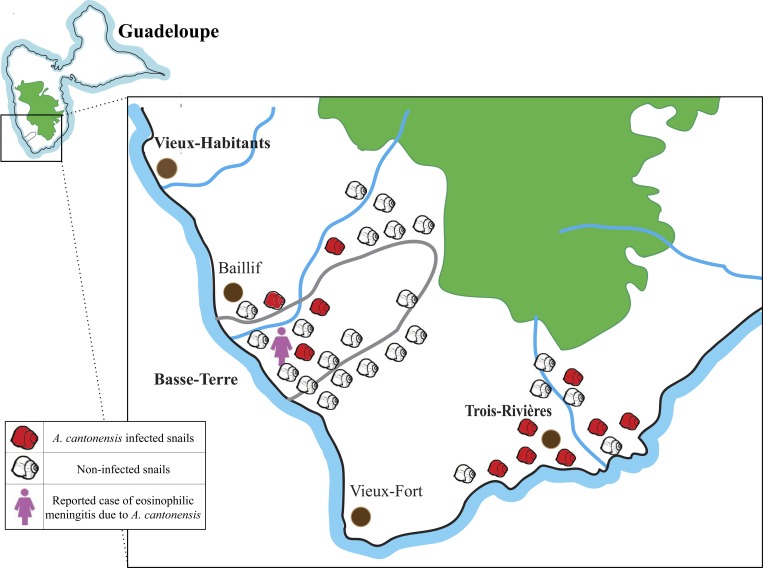
The environmental investigation was conducted for A. fulica intermediate hosts. Snails were collected from two cities of Guadeloupe , that is, Basse-Terre and Trois-Rivières, with 22 and 12 snails, respectively, in February 2014 (Figure 2 ). Some snails were collected from the garden of the patient reported in this manuscript, whereas the others were collected from the surrounding region. For identification of A. cantonensis, species-specific real-time PCR analysis was performed on snails at CDC according to the method described in Qvarnstrom and others.26 DNA was extracted from approximately 20 mg of tissue of the mantle of each snail using the DNeasy Tissue and Blood DNA Extraction Kit (Qiagen Inc.) following the manufacturer's instructions for tissue samples. PCR was performed as previously described for CSF analysis.25,26
Figure 2.
Distribution of the collected Achatina fulica in Guadeloupe for environmental investigation. The geographical map of Guadeloupe shows the distribution of collected snails for environmental investigation. In total, 34 A. fulica snails were collected from Basse-Terre (latitude: 16.01°N, longitude: 61.72°W) and Trois-Rivières (latitude: 15.97°N, longitude: 61.64°W) cities in February 2014.
Results
Case investigation.
The 8-month-old patient was born and lived in Basse-Terre City with her mother and her twin sister. The infant and her sister had no previous medical history of any severe disease. No treatment was administered to the infant at home. The patient's mother, a Haitian by origin, did not report any stay or travel outside of Guadeloupe since the patient and her twin sister were born. The family was living in a house with an adjoined garden in the center of Basse-Terre City. The patient was not provided any unusual foods and there was no exposure to pet animals. However, on further questioning, the patient's mother later revealed that the child had xposure to snails and rodents in the family garden.
The patient had a 5-day history of fluctuating fever (∼100.4°F), anorexia, vomiting, irritability, and a more recent history of diarrhea. On physical examination, the child demonstrated somnolence and slight meningismus as well as Kernig's sign. Muscular strength was normal and the eye examination revealed isochoric and photoreactive pupils.
Laboratory diagnosis.
The initial laboratory diagnosis revealed mild leukocytosis (14.1 g/L) with blood hypereosinophilia (3.6 g/L eosinophils). The initial hemogram was as follows (Sysmex® XE2100, Sysmex, Kobe, Japan): hemoglobin = 11.6 g/dL; hematocrit = 33.7%; leukocytes = 14.7 g/L: neutrophils 33.4%, eosinophils 24.8%, basophils 0.3%, lymphocytes 33.9%, monocytes 7.6%; platelets = 371 g/L. General biochemical examination including serum electrolytes, C-reactive protein (5 mg/L) and procalcitonin (0.06 ng/mL) were within reference ranges. Liver, renal, and hemostatic functions were normal as well. Initial blood culture ruled out the possibility of bacterial or mycological infections. The cerebral magnetic resonance imaging (MRI) did not show any evidence of abnormality.
Lumbar puncture demonstrated turbid CSF and intracranial hypertension. Lumbar puncture greatly relieved the child symptoms. The CSF analysis revealed 3,000 leukocytes/mm3 with 68% eosinophils, 18% neutrophils, 8% lymphocytes, 4% macrophages, and 10 red blood cells/mm3. CSF biochemical analysis (Cobas® 6000, module c501, Roche Diagnostics, Mannheim, Germany) revealed elevated protein (protein = 909 mg/L, normal range = 150–300 mg/L), low glucose (glucose = 0.91 mmol/L, normal range = 2.5–3.5 mmol/L), and normal CSF chloride (chloride = 116 mmol/L, normal range = 110–130 mmol/L). All tests for bacteria were negative, including microscopy of CSF by Gram's stain and detection of bacterial antigens by latex agglutination (Pastorex® Meningitis; Bio-Rad, Hercules, CA) for Neisseria meningitidis type A, C, Y, W135, and B; Streptococcus pneumonia; Haemophilus influenzae type b; Streptococcus B; Escherichia coli K1. Blood cultures were negative and stools collected for bacterial, viral, and parasitological examination were only found positive for rotavirus, explaining the diarrheal episode. Serologic testing for schistosomiasis (hemaglutination), toxocariasis (Novalisa® Toxocara canis IgG; Novatec, Dietzenbach, Germany), strongyloidiasis (EIA; Bordier Affinity Products, Crissier, Switzerland), and dengue (Dengue Duo®, NS1 Ag + IgG/IgM; SD BIOLINE, Standard Diagnostics, Yongin, Republic of Korea) were all negative. Angiostrongylus cantonensis-specific real-time PCR performed on CSF was positive and the patient was therefore diagnosed with CNS angiostrongyliasis.
Clinical and biological outcome.
The infant was treated with albendazole, ivermectin, and corticosteroids started on the day of hospitalization and administered for 14 days. On the second day of hospitalization, the peripheral eosinophilia reached an increased level of 4.3 g/L and subsequently normalized gradually. During hospitalization, the patient had no indication of severe complications. The patient clinically recovered and was discharged from the hospital after 11 days. Laboratory values had fully normalized on day 25 after hospitalization; leukocytes and eosinophils were 9 and 0.5 g/L, respectively.
Environmental investigation.
The diagnosis of the first confirmed case of CNS angiostrongyliasis in the Lesser Antilles prompted an environmental investigation of the presence of A. cantonensis in the local mollusc population. Out of 34 snails analyzed in Guadeloupe, 11 (32.4%) were found to be positive for presence of A. cantonensis by real-time PCR (Figure 2). The proportion of infected snails was higher in Trois-Rivières (7/12, 58.3%) than in Basse-Terre city (4/22, 18.2%) (Figure 2). The specificity of the real-time PCR was previously evaluated and reports specific detection of A. cantonensis species with no cross-reactivity with Angiostrongylus vasorum or A. costaricensis.26
Discussion
Angiostrongylus cantonensis is the most common infectious cause of eosinophilic meningitis worldwide. However, a definitive etiologic diagnosis is rarely achieved since Angiostrongylus spp. are not well known in the medical community and biological methods for detection are missing in most clinical laboratories. In majority of cases, the diagnosis is based on clinical symptoms only.4,27 Angiostrongylus cantonensis infection can be confused with other infections that cause similar symptoms. In endemic regions, individuals with clinical symptoms, including severe headache, stiff neck, nausea, vomiting, fluctuating fever, and paraesthesias should be evaluated for A. cantonensis infection. Moreover, clinical improvement postlumbar puncture is very frequently observed in eosinophilic meningitis. Parasitological and serological tests should be performed to confirm or rule out the diagnosis, however, reliable diagnostic methods are not readily available. Definitive diagnosis by the direct detection of larvae in CSF is rarely achieved due to adhesion of larvae to nerves.28 Detection of antibodies produced in response to the infection can be performed on serum or CSF using enzyme-linked immunosorbent assay or western blot techniques. The diagnostic performance of these assays varies depending on the purity of the native antigenic preparation used for detection.29 Molecular diagnosis based on PCR-detection of DNA from the parasite has recently been explored and seems promising.25 However, these methods are not yet standardized and only available in a few specialized research laboratories worldwide.
Despite the clinical presentation and improvement postlumbar puncture for the 8-month-old patient, the diagnosis was initially unclear as the Lesser Antilles have never been reported as an endemic region for this pathogen. The available literature only documents one article addressing the environmental presence of A. cantonensis in rats in Grenada Island (Lesser Antilles) in 2009.21 It may also be noted that our patient suffered from diarrhea, a symptom later explained by a rotavirus coinfection. The noninfectious etiologies of eosinophilic meningitis were unlikely including drug reaction (as the infant had not taken any other medicines before symptom onset) and hematological and inflammatory diseases (due to the young age of the patient). Although no strict treatment recommendations exist, a 14-day treatment course of dexamethasone, albendazole, and ivermectin was initiated before confirmation of A. cantonensis infection in the patient, to cover a broad range of parasitic disease etiologies. Albendazole was administered because of its ability to cross the blood–brain barrier and ivermectin was added to treat other potential causes of parasitic infections. Corticosteroid therapy was aimed at improving neurologic symptoms by limiting cranial inflammation.30,31
Cerebral MRI and hematological investigations did not yield any information about the cause of disease. Typical laboratory findings of A. cantonensis can include peripheral hypereosinophilia, increased CSF pressure, increased CSF protein, and a normal or decreased CSF glucose. CSF microscopy is usually negative for larvae, although larvae have been seen in CSF in some cases.9,25,32 This patient demonstrated these typical laboratory findings. As the child was too young to eat raw fish or molluscs, the main hypothesis was larval transmission at home either directly by licking infected molluscs or indirectly by hand transmission after contact with molluscs in the garden.
Human angiostrongyliasis is generally self-limited but pediatric cases of A. cantonensis infection seem to be more commonly associated with meningoencephalitis,4 neurologic sequelae,10,33 ophthalmic involvement,34 and death.8,33,35 It is therefore pertinent to establish an early diagnosis and promptly initiate drug therapy. Symptomatic treatment of neurologic symptoms has a major place in care for angiostrongyliasis patients, with analgesics and lumbar puncture used to decrease intracranial pressure.2 The use of antiparasitic drugs such as albendazole or mebendazole are controversial because of the possibility of exacerbating neurologic symptoms due to release of parasitic toxins.2,36 However, these drugs are widely used in some countries as they have been shown to relieve symptoms and reduce the extent of the disease in some patients.31
Our collection and identification of A. cantonensis-infected snails reflected a high prevalence of the parasite in the region. Nearly three decades back, the only reported environmental investigation in Guadeloupe was done for A. costaricensis in rats (R. rattus and in R. norvegicus), which showed a total prevalence of 7.5% in the region.37 Our environmental study was conducted directly on snails, which harbor the infective form of larvae for humans. We hereby report a high prevalence of infected snails in Trois-Rivières and Basse-Terre city, 58.3% and 18.2%, respectively, which is concerning for a region frequently visited by tourists. This is the first study that reports the occurrence of the parasite in molluscs and a probability of infection due to A. cantonensis in the Lesser Antilles. The discovery of A. cantonensis in Guadeloupe raises the possibility of its presence in islands surrounding Guadeloupe, including French West Indies (Martinique, Saint-Martin, and Saint-Barthelemy), Netherlands West Indies, UK West Indies, Dominica, Antigua, Barbuda, Barbados, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines and Trinidad, and Tobago.
The knowledge of the geographical distribution of A. cantonensis could be improved by collection of epidemiological data, including 1) surveillance of possible angiostrongyliasis and eosinophilic meningitis cases and 2) analysis of definitive and intermediate hosts to detect Angiostrongylus. This could prompt public health prevention programs and control measures to limit the risk of infection to humans. In other areas where this parasite is endemic, for example, Thailand38 and Hawaii,9 control measures have already been taken such as informing people about the risk of eating undercooked or raw molluscs. The establishment of public health awareness networks could help the medical community by providing a common protocol for the diagnosis and treatment of this disease across sentinel hospitals and related laboratories for improved diagnosis and medical care.
We hereby report the first case of CNS angiostrongyliasis in Lesser Antilles and provide evidence for the first time of the environmental presence of this parasite in Guadeloupe. This article aims to draw the attention of the medical community and health practitioners to the possible emergence of CNS angiostrongyliasis in Lesser Antilles. The presence of persistent headache, fever, and eosinophilia in travelers who return from the Lesser Antilles should alert clinicians to the possibility of eosinophilic meningitis caused by A. cantonensis.
ACKNOWLEDGMENTS
We thank the Parasitology Reference Diagnostic Laboratory, Centers for Disease Control and Prevention in Atlanta for their technical and scientific help. We also thank Carlos Graeff Teixeira and Alessandra Morassutti (Parasitology Laboratory of Biosciences University, Rio Grande do Sul, Porto Alegre, Brazil) who provided advice and support for future angiostrongyliasis immunodiagnostics in Guadeloupe. Thanks to the team of CHBT laboratory for initial diagnosis of eosinophilic meningitis. Thanks to Nicole Desbois for the helpful discussion about eosinophilic meningitis in the Lesser Antilles. Thanks to Nicolas Dard for his technical help with the snail cohort and Tahir Hussain for help with correcting the manuscript.
Footnotes
Authors' addresses: Céline Dard, Laboratoire de Biologie Médicale, Centre Hospitalier de Basse-Terre, Basse-Terre, Guadeloupe, France, and Laboratoire de Parasitologie-Mycologie, Centre Hospitalier Universitaire de Grenoble Alpes, Grenoble, France, E-mail: cdard@chu-grenoble.fr. Jean-Eudes Piloquet and Jean-Christophe Hebert, Département de Pédiatrie, Centre Hospitalier de Basse-Terre, Basse-Terre, Guadeloupe, France, E-mails: rubjep@hotmail.com and jc.hebert@ch-labasseterre.fr. Yvonne Qvarnstrom and LeAnne M. Fox, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: bvp2@cdc.gov and llf4@cdc.gov. Helmi M'kada, Didier Mattera, and Dorothée Harrois, Laboratoire de Biologie Médicale, Centre Hospitalier de Basse-Terre, Basse-Terre, Guadeloupe, France, E-mails: helmi.mkada@ch-labasseterre.fr, didier.mattera@ch-labasseterre.fr, and dorothee.harrois@ch-labasseterre.fr.
References
- 1.Chen H. A new pulmonary nematode of rats, Pulmonema cantonensis ng.nsp from Canton. Ann Parasitol. 1935;13:312–317. [Google Scholar]
- 2.Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostrongyliasis. Lancet Infect Dis. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- 3.Cowie RH. Pathways for transmission of angiostrongyliasis and the risk of disease associated with them. Hawaii J Med Public Health. 2013;72:70–74. [PMC free article] [PubMed] [Google Scholar]
- 4.Sawanyawisuth K, Chindaprasirt J, Senthong V, Limpawattana P, Auvichayapat N, Tassniyom S, Chotmongkol V, Maleewong V, Intapan PM. Clinical manifestations of eosinophilic meningitis due to infection with Angiostrongylus cantonensis in children. Korean J Parasitol. 2013;51:735–738. doi: 10.3347/kjp.2013.51.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.York EM, Butler CJ, Lord WD. Global decline in suitable habitat for Angiostrongylus (= Parastrongylus) cantonensis: the role of climate change. PLoS One. 2014;9:e103831. doi: 10.1371/journal.pone.0103831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang QP, Wu ZD, Wei J, Owen RL, Lun ZR. Human Angiostrongylus cantonensis: an update. Eur J Clin Microbiol Infect Dis. 2012;31:389–395. doi: 10.1007/s10096-011-1328-5. [DOI] [PubMed] [Google Scholar]
- 7.Punyagupta S, Juttijudata P, Bunnag T. Eosinophilic meningitis in Thailand. Clinical studies of 484 typical cases probably caused by Angiostrongylus cantonensis. Am J Trop Med Hyg. 1975;24:921–931. [PubMed] [Google Scholar]
- 8.Morton NJ, Britton P, Palasanthiran P, Bye A, Sugo E, Kesson A, Ardern-Holmes S, Snelling TL. Severe hemorrhagic meningoencephalitis due to Angiostrongylus cantonensis among young children in Sydney, Australia. Clin Infect Dis. 2013;57:1158–1161. doi: 10.1093/cid/cit444. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg NS, Park SY, Blackburn BG, Sejvar JJ, Gaynor K, Chung H, Leniek K, Herwaldt BL, Effler PV. Distribution of eosinophilic meningitis cases attributable to Angiostrongylus cantonensis, Hawaii. Emerg Infect Dis. 2007;13:1675–1680. doi: 10.3201/eid1311.070367. [DOI] [PubMed] [Google Scholar]
- 10.Graber D, Jaffar-Bandjee MC, Attali T, Poisson J, Renouil M, Alessandri JL, Combes JC. Angiostrongylus in the infant at Reunion and Mayotte. Apropos of 3 cases of eosinophilic meningitis including 1 fatal radiculomyeloencephalitis with hydrocephalus. Bull Soc Pathol Exot. 1997;90:331–332. [PubMed] [Google Scholar]
- 11.Teem JL, Qvarnstrom Y, Bishop HS, da Silva AJ, Carter J, White-McLean J. The occurrence of the rat lungworm, Angiostrongylus cantonensis, in non indigenous snails in the Gulf of Mexico region of the United States. Hawaii J Med Public Health. 2013;72:11–14. [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DY, Stewart TB, Bauer RW, Mitchell M. Parastrongylus (=Angiostrongylus) cantonensis now endemic in Louisiana wildlife. J Parasitol. 2002;88:1024–1026. doi: 10.1645/0022-3395(2002)088[1024:PACNEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Iwanowicz DD, Sanders LR, Schill WB, Xayavong MV, da Silva AJ, Qvarnstrom Y, Smith T. Spread of the Rat Lungworm (Angiostrongylus cantonensis) in Giant African Land Snails (Lissachatina fulica) in Florida, USA. J Wildl Dis. 2015;51:749–753. doi: 10.7589/2014-06-160. [DOI] [PubMed] [Google Scholar]
- 14.Morassutti AL, Thiengo SC, Fernandez M, Sawanyawisuth K, Graeff-Teixeira C. Eosinophilic meningitis caused by Angiostrongylus cantonensis: an emergent disease in Brazil. Mem Inst Oswaldo Cruz. 2014;109:399–407. doi: 10.1590/0074-0276140023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padilla-Docal B, Dorta-Contreras AJ, Moreira JM, Martini-Robles L, Muzzio-Aroca J, Alarcoìn F, Magraner-Tarrau ME, Bu-Coifiu-Fanego R. Comparison of major immunoglobulins intrathecal synthesis patterns in Ecuadorian and Cuban patients with angiostrongyliasis. Am J Trop Med Hyg. 2011;84:406–410. doi: 10.4269/ajtmh.2011.10-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguiar PH, Morera P, Pascual J. First record of Angiostrongylus cantonensis in Cuba. Am J Trop Med Hyg. 1981;30:963–965. doi: 10.4269/ajtmh.1981.30.963. [DOI] [PubMed] [Google Scholar]
- 17.Vargas M, Gomez Perez JD, Malek EA. First record of Angiostrongylus cantonensis (Chen, 1935) (Nematoda: Metastrongylidae) in the Dominican Republic. Trop Med Parasitol. 1992;43:253–255. [PubMed] [Google Scholar]
- 18.Raccurt CP, Blaise J, Durette-Desset M-C. Presence of Angiostrongylus cantonensis in Haiti [in French] Trop Med Int Health. 2003;8:423–426. doi: 10.1046/j.1365-3156.2003.01035.x. [DOI] [PubMed] [Google Scholar]
- 19.Andersen E, Gubler DJ, Sorensen K, Beddard J, Ash LR. First report of Angiostrongylus cantonensis in Puerto Rico. Am J Trop Med Hyg. 1986;35:319–322. doi: 10.4269/ajtmh.1986.35.319. [DOI] [PubMed] [Google Scholar]
- 20.Lindo JF, Waugh C, Hall J, Cunningham-Myrie C, Ashley D, Eberhard ML, Sullivan J, Bishop H, Robinson D, Holtz T, Robinson R. Enzootic Angiostrongylus cantonensis in rats and snails after an outbreak of human eosinophilic meningitis, Jamaica. Emerg Infect Dis. 2002;8:324–326. doi: 10.3201/eid0803.010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chikweto A, Bhaiyat MI, Macpherson CNL, Deallie C, Pinckney RD, Richards C, Sharma RN. Existence of Angiostrongylus cantonensis in rats (Rattus norvegicus) in Grenada, West Indies. Vet Parasitol. 2009;162:160–162. doi: 10.1016/j.vetpar.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Martiìnez-Delgado JF, Gonzaìlez-CortinÞas M, Taìpanes-Cruz TR, Ruiz-Meìndez A. Eosinophilic meningoencephalitis in Villa Clara (Cuba). A study of 17 patients [in Spanish] Rev Neurol. 2000;31:417–421. [PubMed] [Google Scholar]
- 23.Leone S, De Marco M, Ghirga P, Nicastri E, Esposito M, Narciso P. Eosinophilic meningitis in a returned traveler from Santo Domingo: case report and review. J Travel Med. 2007;14:407–410. doi: 10.1111/j.1708-8305.2007.00152.x. [DOI] [PubMed] [Google Scholar]
- 24.Slom TJ, Cortese MM, Gerber SI, Jones RC, Holtz TH, Lopez AS, Zambrano CH, Sufit RL, Sakolvaree Y, Chaicumpa W, Herwaldt BL, Johnson S. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med. 2002;346:668–675. doi: 10.1056/NEJMoa012462. [DOI] [PubMed] [Google Scholar]
- 25.Qvarnstrom Y, Xayavong M, Aramburu da Silva AC, Park SY, Whelen AC, Calimlim PS, Sciulli RH, Honda SA, Higa K, Kitsutani P, Chea N, Heng S, Johnson S. Real-time polymerase chain reaction detection of Angiostrongylus cantonensis DNA in cerebrospinal fluid from patients with eosinophilic meningitis. Am J Trop Med Hyg. 2016;94:176–181. doi: 10.4269/ajtmh.15-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qvarnstrom Y, da Silva ACA, Teem JL, Hollingsworth R, Bishop H, Graeff-Teixeira C, da Silva AJ. Improved molecular detection of Angiostrongylus cantonensis in mollusks and other environmental samples with a species-specific internal transcribed spacer 1-based TaqMan assay. Appl Environ Microbiol. 2010;76:5287–5289. doi: 10.1128/AEM.00546-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawanyawisuth K, Takahashi K, Hoshuyama T, Sawanyawisuth K, Senthong V, Limpawattana P, Intapan PM, Wilson D, Tiamkao S, Jitpimolmard S, Chotmongkol V. Clinical factors predictive of encephalitis caused by Angiostrongylus cantonensis. Am J Trop Med Hyg. 2009;81:698–701. doi: 10.4269/ajtmh.2009.09-0309. [DOI] [PubMed] [Google Scholar]
- 28.Kuberski T, Wallace GD. Clinical manifestations of eosinophilic meningitis due to Angiostrongylus cantonensis. Neurology. 1979;29:1566–1570. doi: 10.1212/wnl.29.12.1566. [DOI] [PubMed] [Google Scholar]
- 29.Nuamtanong S. The evaluation of the 29 and 31 kDa antigens in female Angiostrongylus cantonensis for serodiagnosis of human angiostrongyliasis. Southeast Asian J Trop Med Public Health. 1996;127:291–296. [PubMed] [Google Scholar]
- 30.Thanaviratananich S, Thanaviratananich S, Ngamjarus C. Corticosteroids for parasitic eosinophilic meningitis. Cochrane Database Syst Rev. 2015;2:CD009088. doi: 10.1002/14651858.CD009088.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chotmongkol V, Kittimongkolma S, Niwattayakul K, Intapan PM, Thavornpitak Y. Comparison of prednisolone plus albendazole with prednisolone alone for treatment of patients with eosinophilic meningitis. Am J Trop Med Hyg. 2009;81:443–445. [PubMed] [Google Scholar]
- 32.Chen X-G, Li H, Lun Z-R. Angiostrongyliasis, mainland China. Emerg Infect Dis. 2005;11:1645–1647. doi: 10.3201/eid1110.041338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans-Gilbert T, Lindo JF, Henry S, Brown P, Christie CDC. Severe eosinophilic meningitis owing to Angiostrongylus cantonensis in young Jamaican children: case report and literature review. Paediatr Int Child Health. 2014;34:148–152. doi: 10.1179/2046905513Y.0000000106. [DOI] [PubMed] [Google Scholar]
- 34.Malhotra S, Mehta DK, Arora R, Chauhan D, Ray S, Jain M. Ocular angiostrongyliasis in a child-first case report from India. J Trop Pediatr. 2006;52:223–225. doi: 10.1093/tropej/fmi092. [DOI] [PubMed] [Google Scholar]
- 35.Lindo JF, Escoffery CT, Reid B, Codrington G, Cunningham-Myrie C, Eberhard ML. Fatal autochthonous eosinophilic meningitis in a Jamaican child caused by Angiostrongylus cantonensis. Am J Trop Med Hyg. 2004;70:425–428. [PubMed] [Google Scholar]
- 36.Hidelaratchi MDP, Riffsy MTM, Wijesekera JC. A case of eosinophilic meningitis following monitor lizard meat consumption, exacerbated by anthelminthics. Ceylon Med J. 2005;50:84–86. doi: 10.4038/cmj.v50i2.1577. [DOI] [PubMed] [Google Scholar]
- 37.Juminer B, Roudier M, Raccurt CP, Pujol HP, Gerry F, Bonnet R. 1992. Presence of abdominal angiostrongylosis in Guadeloupe. Apropos of 2 recent cases [in French] Bull Soc Pathol Exot. 1990;85:39–43. [PubMed] [Google Scholar]
- 38.Eamsobhana P. Angiostrongyliasis in Thailand: epidemiology and laboratory investigations. Hawaii J Med Public Health. 2013;72:28–32. [PMC free article] [PubMed] [Google Scholar]