The acceptance of livers of limited quality has been the predominant way to increase the donor pool in recent years. Unfortunately, this practice increases the risk afflicted with the procedure, because these organs are more prone to preservation induced injury and ultimately allograft damage and dysfunction. Accordingly, morbidity and mortality are increased in the context of marginal liver allografts,1 and the evaluation of potential allografts for suitability for transplantation at the end of the preservation period becomes pivotal. Traditional methods include gross inspection, liver histology, and analysis of donor risk factors and fail to give precise insights to the expectable allograft damage and dysfunction.
Recently, we introduced the concept of controlled oxygenated rewarming (COR) by machine perfusion after cold storage at the end of the preservation period as a new tool for allograft reconditioning in human liver transplantation.2,3 This method uses pulsatile arterial and continuous portal perfusion of the allograft with oxygenated Custodiol-N solution (Dr. Köhler-Chemie, Bensheim, Germany). After initial hypothermic perfusion with 10°C, the temperature is gradually increased to 12°C, 16°C, and 20°C after 30, 45, and 60 minutes, respectively. In total, perfusion is carried out for 90 to 120 minutes with a maximum perfusate temperature of 20°C.
Here, we report our extended clinical experience in a larger number of donor organs (median donor risk index, 2.0 [1.6-2.5]): COR has now been applied in 15 successful human liver transplantations. Perfusion medium was collected during COR after 30, 45, 60, 90 and 120 minutes and investigated for markers of hepatocellular function (lactate clearance, pH, glucose metabolism, protein synthesis, bile production) and injury (aspartate aminotransferase [AST], alanine aminotransferase, glutamate dehydrogenase, lactate dehydrogenase). These data were correlated with the posttransplant AST peak as one of the important indicators of ischemia-reperfusion injury. AST values collected during the perfusion at 20°C after 120 minutes demonstrated an excellent linear correlation with peak AST values after transplantation (Figure 1) with an R2 of 0.9 (P < 0.001). AST values collected during the hypothermic machine perfusion period showed a less compelling correlation (R2 = 0.73). Two additional livers were perfused but discarded for the combination of donor risk factors, histology findings and macroscopic appearance. Correlation of graft determining factors (eg, macrovesicular steatosis and CIT) with AST during perfusion demonstrated only modest results, most likely due to the small patient numbers. Indeed, the discarded livers demonstrated the highest AST values during perfusion.
FIGURE 1.
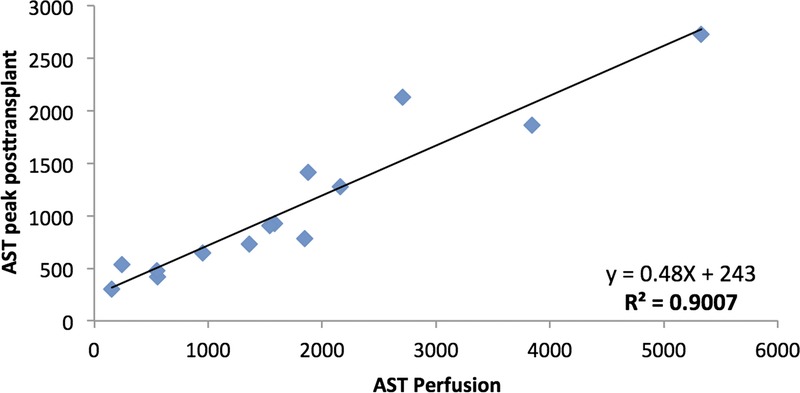
Correlation of AST values during perfusion with AST peak after transplantation (R2 = 0.9; P < 0.001).
In this context, the clinical relevance of aminotransferase peaks must be stressed: besides, being part of the recent validated definition of early allograft dysfunction,4 peak AST after liver transplantation itself correlates significantly with the outcome.5 In the presented study cohort, no patient demonstrated primary non-function and 2 patients (13.3%) presented with EAD.4
Based on clinical experience and experimental data machine perfusion by the COR protocol can be maintained for several hours. Therefore, already available point-of-care diagnostic makes the presented correlation highly attractive for clinical application as measurement of AST in the perfusion medium and according allograft assessment can be performed before any recipient procedure begins. This lifts our options of allograft assessment on a new level and potentially increases the safety of liver transplantation in the extended criteria donor setting. Furthermore, we expect to expand the donor pool as more marginal organs will be sufficiently evaluated and might be found suitable for transplantation.
One potential drawback might be that extensive injury acquired during complicated transplantation procedures in the recipient cannot be assessed by this method. However, this should affect only a minority of patients.
Footnotes
Published online 12 December 2016.
The authors declare no funding or conflicts of interest.
D.P.H. participated in research design, writing of the article, performance of research and data analysis. A.P. participated in research design, writing of the article, performance of research and data analysis. T.M. participated in research design, writing of the article, performance of research, and data analysis.
REFERENCES
- 1.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. [DOI] [PubMed] [Google Scholar]
- 2.Hoyer DP, Mathé Z, Gallinat A, et al. Controlled oxygenated rewarming of cold stored livers prior to transplantation: first clinical application of a new concept. Transplantation. 2016;100:147–152. [DOI] [PubMed] [Google Scholar]
- 3.Minor T, Efferz P, Fox M, et al. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. 2013;13:1450–1460. [DOI] [PubMed] [Google Scholar]
- 4.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943–949. [DOI] [PubMed] [Google Scholar]
- 5.Jochmans I, Monbaliu D, Pirenne J. The beginning of an end point: peak AST in liver transplantation. J Hepatol. 2014;61:1186–1187. [DOI] [PubMed] [Google Scholar]


