Abstract
Background
Suicide is a major public health concern, and a barrier to reducing the suicide rate is the lack of objective predictors of risk. The present study considers whether quantitative sleep electroencephalography (EEG) may be a neurobiological correlate of suicidal ideation.
Methods
Participants included 84 (45 female, mean age=26.6) adults diagnosed with major depressive disorder (MDD). The item that measures thoughts of death or suicide on the Quick Inventory of Depressive Symptomatology (QIDS) was used to classify 47 participants as low suicidal ideation (24 females, mean age=26.1) and 37 as high suicidal ideation (21 females, mean age=27.3). Data were obtained from archival samples collected at the University of Michigan and University of Texas Southwestern Medical Center between 2004 and 2012. Sleep EEG was quantified using power spectral analysis, and focused on alpha, beta, and delta frequencies.
Results
Results indicated that participants with high compared to low suicidal ideation experienced 1) increased fast frequency activity, 2) decreased delta activity, and 3) increased alpha-delta sleep after adjusting for age, sex, depression, and insomnia symptoms.
Limitations
Limitations include the exclusion of imminent suicidal intent, a single suicidal ideation item, and cross-sectional archival data.
Conclusions
This is one of the first studies to provide preliminary support that electrophysiological brain activity during sleep is associated with increased suicidal ideation in MDD, and may point toward central nervous system (CNS) hyperarousal during sleep as a neurobiological correlate of suicidal ideation.
Keywords: Suicide, Depression, Hyperarousal, Polysomnography, Spectral analysis
1. Introduction
Suicide is a leading cause of death worldwide, with nearly one million incidents per year occurring at an alarming rate of one suicide every 40 s (World Health Organization, 2014). Despite being identified as a priority condition by the World Health Organization, the suicide rate has been rising. Indeed, from 2000 to 2009, the annual suicide rate increased by nearly 30% (Center for Disease Control, 2014). The evidence is clear: predictors of suicide must be identified to change the trajectory of the global suicide rate. While extant research has identified a range of predictors across various domains, the neurobiological correlates of suicide risk are not well defined. Among a number of mechanisms, sleep disturbance has emerged as an important contributor to the relationship between suicide and psychiatric disorders.
There is substantial evidence that subjective sleep disturbance is related to suicidal ideation and behavior including death from suicide (Bernert et al., 2015; Bernert and Joiner, 2007; Bernert and Nadorff, 2015; McCall et al., 2010; Perlis et al., 2015; Pigeon et al., 2012). Sleep electroencephalography (EEG) has been identified as a tool to identify potential biomarkers for disorders closely related to suicide such as major depressive disorder (Armitage et al., 2006; Benca et al., 1992; Cheng et al., 2015; Goldschmied et al., 2014; Steiger and Kimura, 2010). Further, findings from a meta-analysis indicate that sleep EEG abnormalities may also represent a transdiagnostic psychophysiological mechanism that cuts across disorders (Baglioni et al., 2016). A growing literature indicates that sleep EEG abnormalities are related to suicide. Longer sleep onset latency and alterations in REM activity have been linked previously to greater suicide risk (Agargun and Cartwright, 2003; Sabo et al., 1991; Singareddy and Balon, 2001). This research has been extended by recent studies that report less non-REM (NREM) stage 4 sleep, lower sleep efficiency, and increased awakenings among individuals with suicidal ideation (Ballard et al., 2016; Bernert et al., 2016).
The existing studies on sleep EEG abnormalities and suicide have provided critical evidence that alterations in sleep macroarchitecture, or global patterns of sleep stages, are related to suicide risk. While useful as a generalized summary of sleep, analyses of sleep macroarchitecture have been criticized for construing sleep as occurring in discrete stages (Armitage, 1995). Indeed, physiological phasic and tonic details are lost when a single stage score is assigned to several electrophysiological events that may have occurred during a single scoring period (Armitage et al., 1992). Alternatively, quantitative sleep EEG (or sleep microarchitecture) may be a more powerful method of measuring sleep as a neurobiological event, as it describes electrophysiological brain activity across different EEG frequencies, which could increase specificity in differentiating patients from healthy individuals (Armitage and Hoffmann, 2001; Augustinavicius et al., 2014; Benca et al., 1992). Measuring sleep microarchitecture could provide further evidence for a neurobiological correlate of suicidal ideation, and may help to clarify how sleep disturbance is related to suicidal ideation.
Intrusions of fast frequency EEG activity (i.e., alpha and beta activity) during sleep may be indicative of central nervous system (CNS) hyperarousal (Nofzinger et al., 2004; Perlis et al., 2001a; Riemann et al., 2010), and may be one potential mechanism related to suicidal ideation (McCall and Black, 2013). Hyperarousal has been observed in disorders associated with suicidal ideation such as MDD, insomnia, PTSD, autism spectrum disorder, and chronic pain disorders (Armitage, 1995; Cervena et al., 2014; Germain and Nielsen, 2003; Kupfer et al., 1989; Mazurek and Petroski, 2015; Merica et al., 1998; Merica and Gaillard, 1992; Moldofsky, 2001; Nofzinger, 2005a; Nofzinger et al., 2000; Perlis et al., 2001b, 1997; Riemann et al., 2010, 2001; Woodward et al., 2000). In MDD in particular, both increased whole night alpha and beta activity have been described in patients compared to healthy controls (Armitage, 1995; Armitage et al., 1992; Armitage and Hoffmann, 2001). Additionally, increased whole night power in the 10- to 28-Hz frequency range (which contains alpha and beta activity) has been observed in patients with delusional depression compared to controls (Kupfer et al., 1989). Hyperarousal, and particularly whole night beta activity, in depression appears to be linked to relative glucose metabolism in the ventromedial prefrontal cortex, which is hypothesized to interfere with brain processes related to sleep regulation (Nofzinger et al., 2000). This evidence suggests that hyperarousal is an important contributor to sleep disturbance in MDD and other disorders related to suicidal ideation. However, it remains unclear if intrusions of fast frequency EEG activity during sleep are also a neurobiological correlate of suicidal ideation.
Reduced slow frequency EEG activity (e.g., delta activity) may also reflect hyperarousal (Germain et al., 2004; Ho et al., 1996). Abnormal delta activity has consistently been observed in MDD (Armitage, 1995; Armitage et al., 2000a, 2000b; Cheng et al., 2015; Goldschmied et al., 2014; Kupfer et al., 1986; Lotrich and Germain, 2015), as well as a range of other disorders including insomnia (Buysse et al., 2008; Dijk, 2010; Merica et al., 1998), alcohol dependence (Brower et al., 2011), and schizophrenia (Hoffmann et al., 2000). Decreased delta activity may also be further compounded by the presence of fast frequency activity during periods of sleep that typically contain slow frequency delta activity. Research has begun to describe the presence of “alpha-delta” sleep in patients with MDD and chronic fatigue syndrome, which appears to be linked to physical pain symptoms and daytime impairment (Hauri and Hawkins, 1973; Jaimchariyatam et al., 2011; Manu et al., 1994). A similar phenomenon may occur in individuals with suicidal ideation whereby cortical hyperarousal combines with decreased delta activity and results in a higher ratio of alpha to delta activity over the night.
The preceding evidence suggests that hyperarousal is an important contributor to sleep disturbance in disorders closely related to suicide such as MDD or insomnia. Although there is evidence for this process in other disorders, it remains unclear whether hyperarousal during sleep may also be related to suicide. The present study aims to test whether high compared to low suicidal ideation is related to differing levels of hyperarousal during sleep among participants with MDD, above and beyond insomnia and depression symptom severity. Based on previous research in disorders related to suicide, it was hypothesized that participants with high compared to low suicidal ideation would experience (1) increased alpha and beta activity across the night, (2) decreased delta activity across the night, and (3) increased alpha-delta sleep across the night.
2. Methods
2.1. Material and methods
2.1.1. Data sourcing
Data were obtained from archival samples collected at the University of Michigan at Ann Arbor and University of Texas Southwestern Medical Center at Dallas recorded under standardized protocols examining sleep in depression between 2004 and 2012 (Armitage et al., 2000a; Cheng et al., 2015; Goldschmied et al., 2015; Liscombe et al., 2002). Archival data was used based on its value as a cost-effective method of exploring novel research questions while maximizing sample size. All participants in the original studies met criteria for MDD based upon the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) and were in a current depressive episode (First et al., 2002). All participants were free of psychiatric or sleep medications for a minimum of 2 weeks. Subjects were asked to refrain from alcohol and drug use prior to the study. Participants maintained regular sleep schedules and completed sleep diaries for a minimum of 5 days prior to overnight polysomnography (PSG). Exclusionary criteria for the original studies included psychiatric comorbidities, such as lifetime histories of substance dependence, bipolar disorder, psychosis, anorexia, and bulimia. Individuals reporting acute and imminent suicidal intent were immediately referred for clinical intervention and excluded from study participation. Individuals were also excluded for current shift-work, or sleep disorders (e.g., obstructive sleep apnea, narcolepsy, or bruxism). The research protocols described were approved by the Institutional Review Board at the respective institutions. All participants signed an informed consent document prior to undergoing study procedures.
2.1.2. Participants
The present study included 84 (45 females, mean age =26.6) adults diagnosed with major depressive disorder (MDD). Participants were included in the present sample if baseline polysomnography data and the Quick Inventory of Depressive Symptomatology (QIDS) were available. All participants had baseline PSG data and thirteen individuals excluded because of missing QIDS data.
2.1.3. Instruments
Participants were administered the 16-item self-report QIDS within two weeks of polysomnography. The QIDS assessed depressive symptoms, insomnia symptoms, and suicidal ideation (Rush et al., 2003). Item 12 (“Thoughts of Death or Suicide”) from the QIDS was used to categorize participants into suicidal ideation groups. A single suicide item from self-reported depression rating scales has been shown to be related to well-validated measures of suicidal ideation (e.g., Scale for Suicide Ideation) and number of suicide attempts (Desseilles et al., 2012). Furthermore, item 12 from the QIDS has been previously used to assess suicidal ideation (Gao et al., 2015; Huffman et al., 2016; Laje et al., 2007). The group defined as “low suicidal ideation” included participants who reported “I do not think of suicide or death” (QIDS score =0) and “I feel that life is empty or wonder if it's worth living” (QIDS score =1). The group defined as “high suicidal ideation” included participants who reported “I think of suicide or death several times a week for several minutes” (QIDS score =2) and “I think of suicide or death several times a day in some detail, or I have made specific plans for suicide or have actually tried to take my life” (QIDS score =3). This grouping method resulted in 47 participants classified as low suicidal ideation (24 females, mean age =26.1) and 37 as high suicidal ideation (21 females, mean age =27.3). Depression symptom severity was examined using a summation of the QIDS score without the sleep and suicide items (range 0–21). Insomnia symptoms were examined using a summation of the insomnia questions from the QIDS (“Falling asleep,” “Sleep during the night,” and Waking up too early;” range 0–9).
2.1.4. Polysomnography procedures
Participants spent two consecutive nights in the sleep laboratory for PSG recording. The first night served as a screening night to rule out any occult sleep disorders, and to allow habituation to a novel sleep environment. Baseline sleep parameters were collected on the second night. Participants were allowed 8 h of sleep opportunity, and sleep was scheduled based on habitual sleep (determined via self-report and 7 day sleep diary) on both nights. EEG was recorded from F3 (left frontal), F4 (right frontal), C3 (left central), C4 (right central), O1 (left occipital), and O2 (right occipital) referenced to the earlobes. The electrode montage also included left and right electro-oculogram (EOG) leads placed on both the upper and lower canthi; a bipolar, chin-cheek electromyography (EMG) lead. Leg leads, chest and abdomen respiration bands, and a nasal-oral thermistor were also included on the screening night.
EEG signals from 69 participants were recorded with Vitaport™ III digital amplifiers (described in Armitage et al., 2012), and 15 participants were recorded with a GRASS™ P511 amplifier-based paperless polygraph (described in Armitage et al., 2002). Data systems were cross-validated in three ways. First, a sine wave generator was used for signal recording on both data acquisition systems simultaneously, and then subjected to power spectral analysis to ensure that spectral profiles were identical. Second, whole night EEG data were acquired simultaneously from 10 subjects and analyzed to ensure that spectral power values fell within the 95% confidence interval of each system. EEG data were quantified at the equivalent sensitivity of 5 (50 μV, 0.5-s calibration) and a gain of 50,000. EEG filters were set at 0.3 and 30 Hz to reduce electrical noise. Sleep records were visually scored following standard criteria by research personnel trained to 90% agreement (Rechtschaffen and Kales, 1968). Sleep epochs that contained artifacts due to breathing, movement, or electrode problems were excluded from quantitative analysis. Epochs that contained power values three times above or below the interquartile range were excluded.
Power Spectral Analysis (PSA) was performed on EEG signals digitized at 256 Hz. Data were processed in 2-s epochs (512 samples every 2-s) with a Hanning window taper in the correct term. The PSA generated power (expressed as μV2) in five frequency bands: beta (16.0–32.0 Hz), sigma (12.0–15.9 Hz), alpha (8.0–11.9 Hz), theta (4.0–7.9 Hz), and delta (0.5–3.9 Hz). The PSA algorithm was based on a fast Fourier transform (Press et al., 1989). EEG frequency bands were averaged in 30 s epochs (M =884.2, SD =122.6) and averaged across recording site. Absolute and relative spectral power were calculated for each frequency band. Absolute spectral power was the value generated by the PSA algorithm. Relative spectral power was calculated as the power in each band divided by the sum of power across all bands over the entire night. Absolute power was examined in order to characterize each frequency band and relative spectral power was considered to look at the influence of individual differences in total EEG power (Krystal et al., 2002; Perlis et al., 2001b). Alpha-delta sleep was derived in a similar manner to relative spectral power by dividing power in the alpha band by power in the delta band over the entire night. This is consistent with other research that examines the ratio of alpha to delta activity (Jaimchariyatam et al., 2011; Manu et al., 1994).
2.1.5. Data analysis
Hierarchical linear models with restricted maximum likelihood estimation were used to address the aims of the study. This statistical method can appropriately account for the relationships between repeated measurements and does not have the same missing data restrictions of traditional regression analyses. The fixed part of the model included age, an indicator for sex, depression severity, insomnia severity, and an indicator variable for suicide group (low suicidal ideation as the reference). The random part of the model included crossed random effects with a random intercept for participant and sleep epoch (Baayen et al., 2008). The random intercepts were assumed to have a bivariate normal distribution with zero means and an unstructured covariance matrix. The outcome variable was sleep EEG power in the hypothesized frequencies. P-values were calculated using Satterthwaite approximation of degrees of freedom. All statistical models were tested in R using the lme4 package (Bates et al., 2015; Kuznetsova et al., 2014; R Development Core Team, 2015).
3. Results
3.1. Hyperarousal and suicidal ideation
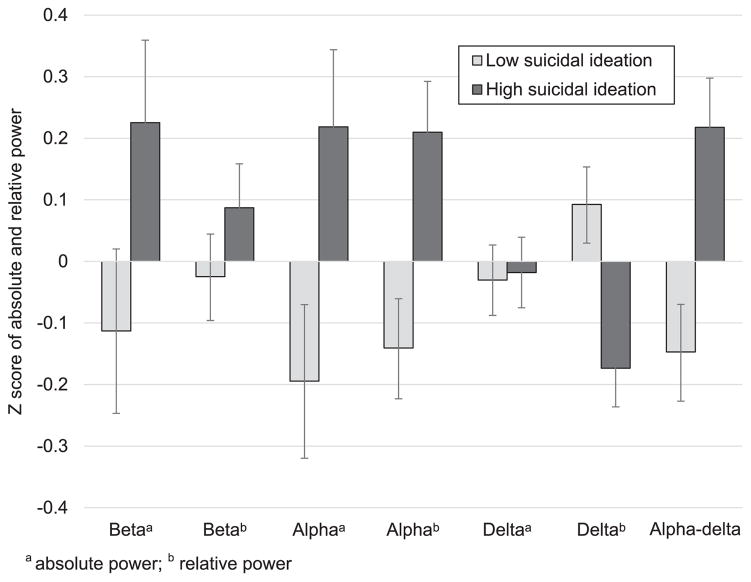
Hierarchical linear models examined the effect of suicide ideation group on absolute and relative EEG power in the alpha and beta frequency bands while also accounting for age, sex, depression symptoms, and insomnia symptoms (Table 1 and Fig. 1). Results for the alpha frequency indicated that those with high suicidal ideation experienced increased whole night absolute alpha activity compared to participants with low suicidal ideation (Table 1 and Fig. 1). Suicide group was also significantly associated with whole night relative alpha power (Table 1). Participants with high suicidal ideation experienced increased whole night relative alpha activity compared to participants with low suicidal ideation (Fig. 1). In the beta frequency, suicide group was related to absolute beta power at the trend level, and suggested that absolute beta power may be greater for participants with high compared to low suicidal ideation (Table 1 and Fig. 1). Although a marginally significant effect was observed for absolute beta power, no effect was observed for relative beta power (Table 1 and Fig. 1).
Table 1.
Means and standard deviations of sleep, clinical, and demographic variables by group.
| Low suicidal ideation
|
High Suicidal Ideation
|
|||
|---|---|---|---|---|
| (n =47) |
(n =37) |
|||
| Mean or n | SD or % | Mean or n | SD or % | |
| Age | 26.1 | 6.1 | 27.3 | 8.0 |
| Sex (n = female) | 24 | 51.1% | 21 | 56.8% |
| QIDSa | 11.2 | 3.7 | 17.4 | 4.6 |
| QIDSb | 9.8 | 3.6 | 13.7 | 4.3 |
| Insomnia symptomsc | 3.4 | 2.0 | 4.2 | 2.4 |
| Total sleep time (min) | 434.9 | 31.7 | 410.6 | 35.2 |
| Sleep latency (min) | 9.7 | 8.8 | 10.4 | 8.8 |
| Sleep efficiency (%) | 93.5% | 4.8% | 93.7% | 5.7% |
| Awake and movement (%) | 3.6% | 2.3% | 3.2% | 1.5% |
| NREM Stage 1 (%) | 4.5% | 3.4% | 3.7% | 2.8% |
| NREM Stage 2 (%) | 53.0% | 7.8% | 53.1% | 6.5% |
| NREM SWS (%) | 14.8% | 8.0% | 16.2% | 7.3% |
| REM % | 24.0% | 5.6% | 24.2% | 4.3% |
| REM Latency (min) | 87.3 | 36.0 | 74.9 | 31.0 |
QIDS: Quick Inventory of Depressive Symptomatology;
Full QIDS composite, range 0–27;
QIDS composite with sleep and suicidal items removed, range 0–21;
Insomnia symptom questions from the QIDS, range 0–9; REM: rapid eye movement sleep; NREM: non-rapid eye movement sleep; SWS: slow-wave sleep.
Fig. 1.
Predicted marginal means (standardized) of absolute and relative alpha power, absolute and relative beta power, absolute and relative delta power, and alpha-delta ratio for high low and high suicidal ideation. Marginal means were derived from hierarchical linear models a absolute power; b relative power.
3.2. Delta activity and suicidal ideation
The effect of suicide ideation group on absolute and relative delta power while also accounting for age, sex, depression symptoms, and insomnia symptoms was considered next (Table 1 and Fig. 1). No effect was observed between suicidal ideation group and whole night absolute delta power. However, results indicated that suicide group was significantly related to relative delta power (Table 2). Participants with high suicidal ideation experienced decreased whole night relative delta activity compared to participants with low suicidal ideation (Fig. 1).
Table 2.
Standardized coefficient estimates from hierarchical linear models comparing low suicidal ideation (score 0 or 1) and high suicidal ideation (score 2 or 3).
| Absolute EEG power |
||||||||
|---|---|---|---|---|---|---|---|---|
| Beta |
Alpha |
Delta |
||||||
| β | SE | β | SE | β | SE | |||
| Age | −0.109 | 0.103 | −0.045 | 0.096 | −0.001 | 0.042 | ||
| Gender | −0.018 | 0.099 | −0.036 | 0.093 | −0.040 | 0.041 | ||
| Depression severitya | 0.003 | 0.013 | 0.015 | 0.012 | −0.022*** | 0.005 | ||
| Insomnia symptomsb | −0.061 | 0.184 | −0.009 | 0.172 | 0.050 | 0.076 | ||
| High vs. low suicidal ideation | 0.338† | 0.199 | 0.413* | 0.185 | 0.012 | 0.082 | ||
| Relative EEG power | ||||||||
| Beta | Alpha | Delta | Alpha-delta | |||||
| β | SE | β | SE | β | SE | β | SE | |
| Age | −0.029 | 0.053 | −0.005 | 0.061 | 0.047 | 0.046 | −0.03 | 0.059 |
| Gender | 0.006 | 0.051 | 0.009 | 0.059 | −0.039 | 0.044 | 0.019 | 0.057 |
| Depression severitya | 0.013* | 0.007 | 0.041*** | 0.008 | −0.034*** | 0.006 | 0.042*** | 0.007 |
| Insomnia symptomsb | −0.100 | 0.095 | −0.082 | 0.111 | 0.056 | 0.083 | −0.108 | 0.107 |
| High vs. low suicidal ideation | 0.112 | 0.104 | 0.350** | 0.121 | −0.266** | 0.090 | 0.365** | 0.117 |
p < 0.05.
p < 0.01.
p < 0.001.
QIDS composite with sleep and suicidal items removed.
Composite of the QIDS insomnia items.
p < 0.10.
3.3. Alpha-delta sleep and suicide
In order to assess whether alpha-delta sleep is related to suicidal ideation, the association between suicide group and the ratio of alpha to delta power while also accounting for age, sex, depression symptoms, and insomnia symptoms was also examined (Table 1 and Fig. 1). Suicide group was significantly related to alpha-delta sleep (Table 1). Participants with high suicidal ideation experienced a higher ratio of alpha to delta sleep across the night compared to participants with low suicidal ideation (Fig. 1).
4. Discussion
The present study was an initial step toward identifying neurobiological correlates of suicidal ideation in MDD. Results from this study provide preliminary evidence that hyperarousal during sleep may be associated with higher suicidal ideation in MDD, even after adjusting for factors that may also be related to hyperarousal such as depression or insomnia symptoms. Greater alpha activity, as well as a marginally significant increase in absolute beta activity, were observed for participants with high compared to low suicidal ideation. These results are consistent with previous research in other psychiatric disorders, and may point toward CNS hyperarousal during sleep as a neurobiological correlate of suicide (Hall et al., 2000; McCall and Black, 2013; Merica et al., 1998; Perlis et al., 2001b, 1997). The potential role of hyperarousal during sleep as a contributor to suicide risk is also supported by recent work that examined sleep EEG macroarchitecture and reported more awakenings, greater NREM Stage 1 sleep, and lower sleep efficiency for individuals with suicidal ideation (Ballard et al., 2016; Bernert et al., 2016). Although there is substantial evidence demonstrating the link between waking cortical and behavioral hyperarousal and suicide (Graae et al., 1996; Iosifescu et al., 2008; Perlis et al., 2015; Steyn et al., 2013), this is one of the first studies to demonstrate that cortical hyperarousal during sleep may be associated with increased suicidal ideation in MDD. Prospective or experimental studies will be necessary to confirm that hyperarousal is related to suicidal ideation in MDD, particularly given weak evidence for increased beta activity.
These results also lend preliminary support for the hypothesis that suicide risk is associated with reduced delta activity beyond the decrease in delta activity typical of depression (Armitage, 1995; Armitage et al., 2000a, 2000b; Cheng et al., 2015; Goldschmied et al., 2014; Kupfer et al., 1986; Lotrich and Germain, 2015). Although no differences were observed for absolute whole night delta activity, relative delta activity was reduced in participants with high suicidal ideation and MDD, controlling for depression symptoms. When the influence of individual differences in EEG power are accounted for, participants with high suicidal ideation experience less delta activity across the night. In healthy sleepers, delta activity is associated with reduced activation in brain regions associated with sensory and cognitive processing such as the thalamus, anterior cingulate cortex, orbitofrontal cortex, and basal ganglia (Braun et al., 1997; Hofle et al., 1997; Nofzinger, 2005a, 2005b). Evidence from positron emission tomography studies indicates that increased activity in these regions during sleep containing delta activity is associated with depression, and may represent dual processes of sleep disturbance that includes both sleep homeostasis dysregulation and hyperarousal (Germain et al., 2004; Ho et al., 1996). Preliminary evidence for a similar process in suicidal ideation in MDD is highlighted by evidence from the present study that a higher ratio of alpha to delta sleep was observed in participants with high compared to low suicidal ideation. Although sleep homeostasis dysregulation and hyperarousal have been characterized in depression, similar mechanisms may also occur in individuals with high suicidal ideation, particularly given that the present study accounted for the influence of depression severity. Given the mixed findings in delta activity in the present study, additional research will be necessary to precisely define the relationship between sleep homeostasis dysregulation and hyperarousal in the context of suicide.
5. Limitations
Several limitations are important to consider. First, this study was limited by the use of archival data from studies that excluded individuals with imminent suicidal intent, which precludes generalizability to those at acute risk for suicide. Conducting research with individuals with a high risk of suicide requires an infrastructure of health care professionals to ensure patient safety, and should be a priority for future research. Second, the current sample was also limited to MDD without significant comorbidities such as insomnia or other forms of psychopathology that have increased risk for suicide (e.g. bipolar disorder or borderline personality disorder). Additionally this sample was limited by the low rate of participants reporting no suicidal ideation (n =13). Future studies would be enhanced by recruiting a sample of participants across a range of disorders that exhibit a broad spectrum of suicidality including the absence of suicide risk. Third, a single item was used for the assessment of suicidal ideation. While this approach has been utilized in prior studies (Desseilles et al., 2012; Gao et al., 2015; Huffman et al., 2016; Laje et al., 2007), future studies would benefit by including validated measures of suicide.
6. Conclusions
The current study provides evidence that neurophysiological hyperarousal during sleep may be associated with higher suicidal ideation in MDD. This study indicates that suicidal ideation is associated with increased fast frequency activity, decreased delta activity, and a higher ratio of alpha to delta sleep. These findings may point toward sleep as a neurobiological correlate of suicide risk such that cortical hyperarousal is associated with increased suicidal ideation. Although more efforts will be necessary to understand the clinical implications of increased high frequency activity during sleep, psychopharmacological and psychosocial interventions that reduce suicidal thoughts and behavior and target high frequency activity during sleep may be valuable research and clinical targets for understanding and preventing suicide.
Acknowledgments
The authors would like to thank the University of Michigan Department of Psychology, Department of Psychiatry, and the Depression Center for their continued support. This research was supported by the National Institute of Mental Health [grant numbers R01MH077690 (J.T.A.), R01MH061515 (R.A), and T32MH020006 (E.A.D.)]. Funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Disclosures of financial support or conflicts of interest
R.A. disclosed consulting fees from the University of Ottawa Institute of Mental Health Research from May 2013–March 2015. R.A was not a consultant when the data were collected or during the initial analysis. E.A.D., P.C., J.T.A., L.S., M.D.C., H.S.K., J.R.G., R.F.H., and P.J.D. reported no biomedical financial support or potential conflicts of interest.
Author contributions
E.A.D. completed the majority of data analyses and writing of manuscript; E.A.D. and P.C. were involved with conception and design of study; P.C. and P.D. provided data analysis support and interpretation; All authors participated in data acquisition; All authors critically revised the manuscript and approved it for publication.
References
- Agargun MY, Cartwright R. REM sleep, dream variables and suicidality in depressed patients. Psychiatry Res. 2003;119:33–39. doi: 10.1016/s0165-1781(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Armitage R. Microarchitectural findings in sleep EEG in depression: diagnostic implications. Biol Psychiatry. 1995;37:72–84. doi: 10.1016/0006-3223(94)00082-E. [DOI] [PubMed] [Google Scholar]
- Armitage R, Hoffmann RF. Sleep EEG, depression and gender. Sleep Med Rev. 2001;5:237–246. doi: 10.1053/smrv.2000.0144. [DOI] [PubMed] [Google Scholar]
- Armitage R, Roffwarg HP, Rush AJ, Calhoun JS, Purdy DG, Giles DE. Digital period analysis of sleep EEG in depression. Biol Psychiatry. 1992;31:52–68. doi: 10.1016/0006-3223(92)90006-L. [DOI] [PubMed] [Google Scholar]
- Armitage R, Hoffmann RF, Fitch T, Trivedi MH, Rush AJ. Temporal characteristics of delta activity during NREM sleep in depressed outpatients and healthy adults: group and sex effects. Sleep. 2000a;23:607–617. [PubMed] [Google Scholar]
- Armitage R, Hoffmann RF, Trivedi MH, Rush AJ. Slow-wave activity in NREM sleep: sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000b;95:201–213. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- Armitage R, Hoffmann RF, Emslie GJ, Weinberg WA, Mayes TL, Rush AJ. Sleep microarchitecture as a predictor of recurrence in children and adolescents with depression. Int J Neuropsychopharmacol. 2002;5:217–228. doi: 10.1017/S1461145702002948. [DOI] [PubMed] [Google Scholar]
- Armitage R, Hoffmann RF, Emslie G, Rintelmann J, Robert J. Sleep microarchitecture in childhood and adolescent depression: temporal coherence. Clin EEG Neurosci. 2006;37:1–9. doi: 10.1177/155005940603700103. [DOI] [PubMed] [Google Scholar]
- Armitage R, Hoffmann RF, Conroy DA, Arnedt JT, Brower KJ. Effects of a 3-hour sleep delay on sleep homeostasis in alcohol dependent adults. Sleep. 2012;35:273–278. doi: 10.5665/sleep.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinavicius JLS, Zanjani A, Zakzanis KK, Shapiro CM. Polysomnographic features of early-onset depression: a meta-analysis. J Affect Disord. 2014;158:11–18. doi: 10.1016/j.jad.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59:390–412. doi: 10.1016/j.jml.2007.12.005. [DOI] [Google Scholar]
- Baglioni C, Nanovska S, Regen W, Spiegelhalder K, Feige B, Nissen C, Reynolds CF, Riemann D. Sleep and mental disorders: a meta-analysis of polysomnographic research. Psychol Bull. 2016. [DOI] [PMC free article] [PubMed]
- Ballard ED, Vande Voort JL, Bernert RA, Luckenbaugh DA, Richards EM, Niciu MJ, Furey ML, Duncan WC, Zarate CA. Nocturnal wakefulness is associated with next-day suicidal ideation in major depressive disorder and bipolar disorder. J Clin Psychiatry. 2016;77:825–831. doi: 10.4088/JCP.15m09943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-Anal. Arch Gen Psychiatry. 1992;49:651-68-70. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Bernert RA, Joiner TE. Sleep disturbances and suicide risk: a review of the literature. Neuropsychiatr Dis Treat. 2007;3:735–743. doi: 10.2147/ndt.s1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert RA, Nadorff MR. Sleep disturbances and suicide risk. Sleep Med Clin. 2015;10:35–39. doi: 10.1016/j.jsmc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Bernert RA, Kim JS, Iwata NG, Perlis ML. Sleep disturbances as an evidence-based suicide risk factor. Curr Psychiatry Rep. 2015;17:554. doi: 10.1007/s11920-015-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert RA, Luckenbaugh DA, Duncan WC, Naomi Iwata MS, Ballard ED, Zarate CA. Sleep Architecture Parameters as a Putative Biomarker of Suicidal Ideation in Treatment-Resistant Depression. J Affect Disord. 2016. [DOI] [PMC free article] [PubMed]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Hoffmann RF, Conroy DA, Arnedt JT, Armitage R. Sleep homeostasis in alcohol-dependent, depressed and healthy control men. Eur Arch Psychiatry Clin Neurosci. 2011;261:559–566. doi: 10.1007/s00406-011-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Hall ML, Moul DE, Nofzinger EA, Begley A, Ehlers CL, Thompson W, Kupfer DJ. EEG spectral analysis in primary insomnia: nrem period effects and sex differences. Sleep. 2008;31:1673–1682. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control. Trends in Suicide Rates Among Persons Ages 10 Years and Older, by Sex, United States, 1991–2009 [WWW Document] Natl Suicide Stat a Glance. 2014. < http://www.cdc.gov/violenceprevention/suicide/statistics/trends01.html>.
- Cervena K, Espa F, Perogamvros L, Perrig S, Merica H, Ibanez V. Spectral analysis of the sleep onset period in primary insomnia. Clin Neurophysiol. 2014;125:979–987. doi: 10.1016/j.clinph.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Cheng P, Goldschmied J, Deldin P, Hoffmann R, Armitage R. The role of fast and slow EEG activity during sleep in males and females with major depressive disorder. Psychophysiology. 2015;52:1375–1381. doi: 10.1111/psyp.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desseilles M, Perroud N, Guillaume S, Jaussent I, Genty C, Malafosse A, Courtet P. Is it valid to measure suicidal ideation by depression rating scales? J Affect Disord. 2012;136:398–404. doi: 10.1016/j.jad.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Dijk DJ. Slow-wave sleep deficiency and enhancement: implications for insomnia and its management. World J Biol Psychiatry. 2010;11(Suppl 1):22. doi: 10.3109/15622971003637645. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. 2002 (SCID-I/NP) [Google Scholar]
- Gao K, Wu R, Wang Z, Ren M, Kemp DE, Chan PK, Conroy CM, Serrano MB, Ganocy SJ, Calabrese JR. Disagreement between self-reported and clinician-ascertained suicidal ideation and its correlation with depression and anxiety severity in patients with major depressive disorder or bipolar disorder. J Psychiatr Res. 2015;60:117–124. doi: 10.1016/j.jpsychires.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Germain A, Nielsen TA. Sleep pathophysiology in posttraumatic stress disorder and idiopathic nightmare sufferers. Biol Psychiatry. 2003;54:1092–1098. doi: 10.1016/S0006-3223(03)00071-4. [DOI] [PubMed] [Google Scholar]
- Germain A, Nofzinger EA, Kupfer DJ, Buysse DJ. Neurobiology of non-REM sleep in depression: further evidence for hypofrontality and thalamic dysregulation. Am J Psychiatry. 2004;161:1856–1863. doi: 10.1176/ajp.161.10.1856. [DOI] [PubMed] [Google Scholar]
- Goldschmied JR, Cheng P, Armitage R, Deldin PJ. Examining the effects of sleep delay on depressed males and females and healthy controls. J Sleep Res. 2014;23:664–672. doi: 10.1111/jsr.12174. [DOI] [PubMed] [Google Scholar]
- Goldschmied JR, Cheng P, Kim HS, Casement M, Armitage R, Deldin PJ. Slow-wave disruption enhances the accessibility of positive memory traces. Neurobiol Learn Mem. 2015;125:168–175. doi: 10.1016/j.nlm.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Graae F, Tenke C, Bruder G, Rotheram MJ, Piacentini J, Castro-Blanco D, Leite P, Towey J. Abnormality of EEG alpha asymmetry in female adolescent suicide attempters. Biol Psychiatry. 1996;40:706–713. doi: 10.1016/0006-3223(95)00493-9. [DOI] [PubMed] [Google Scholar]
- Hall M, Buysse DJ, Nowell PD, Nofzinger EA, Houck P, Reynolds CF, Kupfer DJ. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–230. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- Hauri P, Hawkins DR. Alpha-delta sleep. Electroencephalogr Clin Neurophysiol. 1973;34:233–237. doi: 10.1016/0013-4694(73)90250-2. [DOI] [PubMed] [Google Scholar]
- Ho AP, Gillin JC, Buchsbaum MS, Wu JC, Abel L, Bunney WE. Brain glucose metabolism during non-rapid eye movement sleep in major depression. A positron emission tomography study. Arch Gen Psychiatry. 1996;53:645–652. doi: 10.1001/archpsyc.1996.01830070095014. [DOI] [PubMed] [Google Scholar]
- Hoffmann RF, Hendrickse W, Rush AJ, Armitage R. Slow-wave activity during non-REM sleep in men with schizophrenia and major depressive disorders. Psychiatry Res. 2000;95:215–225. doi: 10.1016/s0165-1781(00)00181-5. [DOI] [PubMed] [Google Scholar]
- Hofle N, Paus T, Reutens D, Fiset P, Gotman J, Evans AC, Jones BE. Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. J Neurosci. 1997;17:4800–4808. doi: 10.1523/JNEUROSCI.17-12-04800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JC, Boehm JK, Beach SR, Beale EE, DuBois CM, Healy BC. Relationship of optimism and suicidal ideation in three groups of patients at varying levels of suicide risk. J Psychiatr Res. 2016;77:76–84. doi: 10.1016/j.jpsychires.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosifescu DV, Greenwald S, Devlin P, Perlis RH, Denninger JW, Alpert JE, Fava M. Pretreatment frontal EEG and changes in suicidal ideation during SSRI treatment in major depressive disorder. Acta Psychiatr Scand. 2008;117:271–276. doi: 10.1111/j.1600-0447.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- Jaimchariyatam N, Rodriguez CL, Budur K. Prevalence and correlates of alpha-delta sleep in major depressive disorders. Innov Clin Neurosci. 2011;8:35–49. [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–640. [PubMed] [Google Scholar]
- Kupfer DJ, Grochocinski VJ, McEachran AB. Relationship of awakening and delta sleep in depression. Psychiatry Res. 1986;19:297–304. doi: 10.1016/0165-1781(86)90122-8. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Reynolds CF, Ehlers CL. Comparison of EEG sleep measures among depressive subtypes and controls in older individuals. Psychiatry Res. 1989;27:13–21. doi: 10.1016/0165-1781(89)90004-8. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package) R Packag version. 2014. http://dx.doi.org/http://CRAN.R-project.org/package=lmerTest .
- Laje G, Paddock S, Manji H, Rush AJ, Wilson AF, Charney D, McMahon FJ. Genetic Markers of Suicidal Ideation Emerging During Citalopram Treatment of Major Depression. Am J Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- Liscombe MP, Hoffmann RF, Trivedi MH, Parker MK, Rush AJ, Armitage R. Quantitative EEG amplitude across REM sleep periods in depression: preliminary report. J Psychiatry Neurosci. 2002;27:40–46. [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Germain A. Decreased delta sleep ratio and elevated alpha power predict vulnerability to depression during interferon-alpha treatment. Acta Neuropsychiatr. 2015;27:14–24. doi: 10.1017/neu.2014.30. [DOI] [PubMed] [Google Scholar]
- Manu P, Lane TJ, Matthews DA, Castriotta RJ, Watson RK, Abeles M. Alpha-delta sleep in patients with a chief complaint of chronic fatigue. South Med J. 1994;87:465–470. doi: 10.1097/00007611-199404000-00008. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Petroski GF. Sleep problems in children with autism spectrum disorder: examining the contributions of sensory over-responsivity and anxiety. Sleep Med. 2015;16:270–279. doi: 10.1016/j.sleep.2014.11.006. [DOI] [PubMed] [Google Scholar]
- McCall WV, Black CG. The link between suicide and insomnia: theoretical mechanisms. Curr Psychiatry Rep. 2013;15:389. doi: 10.1007/s11920-013-0389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall WV, Blocker JN, D’Agostino R, Kimball J, Boggs N, Lasater B, Rosenquist PB. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010;11:822–827. doi: 10.1016/j.sleep.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merica H, Gaillard JM. The EEG of the sleep onset period in insomnia: a discriminant analysis. Physiol Behav. 1992;52:199–204. doi: 10.1016/0031-9384(92)90258-4. [DOI] [PubMed] [Google Scholar]
- Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–1834. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Moldofsky H. Sleep and pain. Sleep Med Rev. 2001;5:385–396. doi: 10.1053/smrv.2001.0179. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA. Neuroimaging and sleep medicine. Sleep Med Rev. 2005a;9:157–172. doi: 10.1016/j.smrv.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA. Functional neuroimaging of sleep. Semin Neurol. 2005b;25:9–18. doi: 10.1055/s-2005-867070. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Price JC, Meltzer CC, Buysse DJ, Villemagne VL, Miewald JM, Sembrat RC, Steppe DA, Kupfer DJ. Towards a neurobiology of dysfunctional arousal in depression: the relationship between beta EEG power and regional cerebral glucose metabolism during NREM sleep. Psychiatry Res. 2000;98:71–91. doi: 10.1016/s0925-4927(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–188. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001a;5:363–374. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001b;24:110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Grandner MA, Chakravorty S, Bernert RA, Brown GK, Thase ME. Suicide and sleep: is it a bad thing to be awake when reason sleeps? Sleep Med Rev. 2015;29:101–107. doi: 10.1016/j.smrv.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73:e1160–e1167. doi: 10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- Press W, Flannery B, Teukolosky S, Bettering W. Numerical Recipes in Pascal: The Art of Scientific Computing. Cambridge University Press; New York: 1989. [Google Scholar]
- R Development Core Team, R. R: A Language and Environment for Statistical Computing. R Found Stat Comput. 2015. [DOI]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. US Government Printing Office; Washington, D.C: 1968. National Institute of Health Publication No. 204. [Google Scholar]
- Riemann D, Berger M, Voderholzer U. Sleep and depression — results from psychobiological studies: an overview. Biol Psychol. 2001;57:67–103. doi: 10.1016/S0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis ML, Nissen C. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item quick Inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Sabo E, Reynolds CF, Kupfer DJ, Berman SR. Sleep, depression, and suicide. Psychiatry Res. 1991;36:265–277. doi: 10.1016/0165-1781(91)90025-K. [DOI] [PubMed] [Google Scholar]
- Singareddy RK, Balon R. Sleep and suicide in psychiatric patients. Ann Clin Psychiatry. 2001;13:93–101. doi: 10.1023/a:1016619708558. [DOI] [PubMed] [Google Scholar]
- Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. J Psychiatr Res. 2010;44:242–252. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Steyn R, Vawda N, Wyatt G, Williams J, Madu S. Posttraumatic stress disorder diagnostic criteria and suicidal ideation in a South African Police sample. Afr J Psychiatry. 2013;16:19–22. doi: 10.4314/ajpsy.v16i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward SH, Murburg MM, Bliwise DL. PTSD-related hyperarousal assessed during sleep. Physiol Behav. 2000;70:197–203. doi: 10.1016/S0031-9384(00)00271-7. [DOI] [PubMed] [Google Scholar]
- World Health Organization. A Global Imperative. Geneva: 2014. Preventing Suicide. [Google Scholar]