Abstract
Purpose
To test the effects of rearing light intensity on retinal function and morphology in the retinoschisis knockout (Rs1-KO) mouse model of X-linked retinoschisis, and whether it affects functional outcome of RS1 gene replacement.
Methods
Seventy-six Rs1-KO mice were reared in either cyclic low light (LL, 20 lux) or moderate light (ML, 300 lux) and analyzed at 1 and 4 months. Retinal function was assessed by electroretinogram and cavity size by optical coherence tomography. Expression of inward-rectifier K+ channel (Kir4.1), water channel aquaporin-4 (AQP4), and glial fibrillary acidic protein (GFAP) were analyzed by Western blotting. In a separate study, Rs1-KO mice reared in LL (n = 29) or ML (n = 27) received a unilateral intravitreal injection of scAAV8-hRs-IRBP at 21 days, and functional outcome was evaluated at 4 months by electroretinogram.
Results
At 1 month, no functional or structural differences were found between LL- or ML-reared Rs1-KO mice. At 4 months, ML-reared Rs1-KO mice showed significant reduction of b-wave amplitude and b-/a-wave ratio with no changes in a-wave, and a significant increase in cavity size, compared to LL-reared animals. Moderate light rearing increased Kir4.1 expression in Rs1-KO mice by 4 months, but not AQP4 and GFAP levels. Administration of scAAV8-hRS1-IRBP to Rs1-KO mice showed similar improvement of inner retinal ERG function independent of LL or ML rearing.
Conclusions
Rearing light conditions affect the development of retinal cavities and post-photoreceptor function in Rs1-KO mice. However, the effect of rearing light intensity does not interact with the efficacy of RS1 gene replacement in Rs1-KO mice.
Keywords: Rs1-KO mouse, rearing light, electroretinogram, OCT, gene therapy
Light exposure conditions are well known to modify the natural history of inherited and acquired retinal degenerations.1–5
This is not surprising, as photoreceptors are the main photosensitive cells in the retina by virtue of their high content of visual pigment, which makes them susceptible to light-induced damage and apoptotic death (See Ref. 6 for review). Defects in photoreceptor-specific genes further increase the sensitivity to light damage, and light restriction is protective in many animal models of retinal degenerations caused by mutations in photoreceptor-specific genes.2,5 It is not clear, however, whether light also alters the time course of retinal diseases affecting the inner retina.
X-linked retinoschisis (XLRS) is a degenerative retinal disease caused by genetic defects in retinoschisin, an extracellular protein that participates in cell adhesion.7–10 Retinoschisin is also critical for integrity of the synapse between photoreceptors and bipolar cells.11–13 Early pathology in XLRS patients affects predominantly the inner retinal layers, which become structurally disorganized by the formation of cavities, whereas photoreceptors degenerate quite slowly.14–16
The hallmark disease features of human XLRS are recapitulated in the Rs1-KO mouse.17,18 As in XLRS patients, Rs1-KO mice show early formation of cavities in the inner retinal layers and have a characteristic “electronegative” electroretinogram (ERG) in which the b-wave, from bipolar cell activity, is greatly reduced in amplitude with respect to the a-wave, which arises from photoreceptors, reflecting dysfunction of the proximal retina.17
During our studies on the Rs1-KO mouse, we suspected that lighting conditions in the vivarium were affecting the retinal phenotype and we began a systematic study of the effects of light rearing (20 or 300 lux) on the XLRS mouse retinal function and morphology. We also evaluated whether lighting conditions influenced the efficacy of RS1 gene replacement19 and found that the effects of light rearing and gene therapy were additive.
Materials and Methods
Animal Handling and Light Conditions
Animal procedures were conducted in accord with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were reviewed and approved by the National Eye Institute Animal Care and Use Committee. The retinoschisin knockout (Rs1-KO) mouse model was generated as described previously.20 These mice have been backcrossed for at least 20 generations onto the C57BL/6J line (Jackson Laboratory, Bar Harbor, ME, USA), and the phenotype and natural history of this animal are well described.11,17,18 Approximately 200 male Rs1-KO mice were used in this study. Four independent groups of mice were reared in low light (LL) or moderate light (ML) and analyzed by ERG and optical coherence tomography (OCT) at either 1 or 4 months. In a second experiment, two groups of Rs1-KO mice were reared in LL or ML and treated at 21 days with an intravitreal injection of scAAV8-hRs-IRBP vector. An electroretinogram was recorded in both groups at 4 months of age. Light conditions were controlled by rearing mice from birth in two different rooms at either 20 lux (LL) or 300 lux (ML) (measured outside and in front of the cage) from an overhead fluorescent light, on either a 12/12 (LL) or 14/10 (ML) hour light/dark cycle. Food and water were available ad libitum. Animals were weaned at 3 weeks of age, and then housed 5 or fewer per cage. Rs1-KO mice were genotyped from tail DNA to verify that they carried the Rs1-KO construct.
Electroretinogram
The ERG procedures were described previously.17 Briefly, mice were dark-adapted overnight and all subsequent procedures were performed in dim red light or darkness. Animals were anesthetized by intraperitoneal ketamine (100 mg/kg) and xylazine (10 mg/kg), pupils were dilated with a mixture of 0.5% tropicamide and 0.5% phenylephrine, and 0.5% tetracaine topical anesthetic drops were applied before placing the recording electrodes. After anesthesia and pupil dilation, mice were placed on a heating pad at 37°C, and gold wire electrodes were placed on the center of the cornea with a drop of methylcellulose for corneal hydration. Electroretinogram recordings were averaged (1–20 responses) to 10-ms full-field flashes (Grass Photic Stimulator PS33; Astro-Med, Inc., West Warwick, RI, USA) over a range of intensities eliciting threshold through maximum isolated rod responses and mixed rod–cone responses. The ERG signals were amplified 5000 times and bandpass filtered (0.1–1 kHz, 3 db/decade) with a 60-Hz line filter. Oscillatory potentials were not removed before measurement. Electroretinogram amplitudes in response to the maximum stimulus intensity of 0.6 log cd·s/m2 were used to compare mice reared in ML and LL. The a-wave was measured from the 50-ms prestimulus baseline to the maximum value of the negative trough preceding the b-wave. The b-wave amplitude was measured from the a-wave trough to the maximum peak immediately following the a-wave. The a-wave reflects the activation phase of the rod and cone photoresponse; the b-wave results from the response of bipolar cells that are transynaptically activated by photoreceptors. The b-wave amplitude was divided by the a-wave (b-/a-wave ratio) to normalize for the activation of bipolar cells by photoreceptors and better estimate the gain in the bipolar cell response.
Optical Coherence Tomography
Optical coherence tomography images were obtained as described previously,18 with the Envisu R2200 SD-OCT ophthalmic imaging system (Bioptigen, Durham, NC, USA). Animals were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in a custom holder. The pupils were dilated with a mixture of 0.5% tropicamide and 0.5% phenylephrine. Artificial tears were used throughout the procedure to maintain corneal hydration and clarity. Radial volume scans consisting of 10 B-scans (1000 A-scans per B-scan) were collected at 18° angular intervals and were each an average of five frames. The cavity size was measured from bitmap images created by averaging 10 frames of linear B-scans from the same horizontal plane of the optic nerve (ImageJ, http://imagej.nih.gov/ij; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA). In each image, the width of the inner nuclear layer plus the outer plexiform layer was measured perpendicularly to the retinal surface at the level of largest cavity on both sides of the optic nerve. This was done in four radial scans through the optic nerve head: nasal to temporal, inferior to superior, nasal inferior quadrant to temporal superior quadrant, and temporal inferior quadrant to nasal superior quadrant. The average of these eight measurements was used as the cavity size for that retina. This method was found to produce results comparable to area measurements of cavities isolated by using intensity threshold segmentation in ImageJ.18
Western Blotting
Retinal cell lysates were prepared in RIPA buffer, pH 7.4 (50 mM Tris-HCl, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, and protease inhibitor cocktail), and their protein concentrations were determined by bicinchoninic acid (BCA) reagent kit (Thermo Scientific, Rockford, IL, USA). For each light condition and time point, three independent samples were prepared, each being a pool of three retinas from three different mice. Cell lysates corresponding to 10 μg total protein were loaded on a 10% SDS-PAGE gel and transferred to polyvinylidene difluoride membrane (Bio-Rad Hercules, CA, USA) for subsequent immunoblotting. The membranes were incubated in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE, USA) for 1 hour and later incubated overnight with one of the indicated primary antibodies diluted in PBS containing 0.05% Tween 20 (PBST), pH 7.5, at 4°C: anti–inward rectifier K+ ion channel 4.1 (Kir4.1, 1:400; Alomone Labs, Jerusalem, Israel), anti–water channel aquaporin-4 (AQP4, 1:1000; Alomone Labs), anti–glial fibrillary acidic protein (GFAP, 1:5000; Sigma-Aldrich Corp., St. Louis, MO, USA), and anti–β-actin (mouse monoclonal, 1:6000; Sigma-Aldrich). After overnight incubation, membranes were rinsed three times in PBST and incubated with one of the corresponding infrared (IR) dye-conjugated secondary antibodies: IR Dye 800CW–conjugated goat (polyclonal) anti-rabbit IgG (from LI-COR Biosciences); or Alexa Fluor 680–conjugated goat anti-mouse IgG (Thermo Scientific). Blots were scanned on LI-COR Odyssey Infrared Imaging System (model 9120, LI-COR Biosciences), and intensity of bands corresponding to AQP4 (28 kDa), Kir4.1 (30 kDa), and GFAP (50 kDa) were analyzed and normalized against an internal control protein β-actin (43 kDa), using Odyssey software. The final values represent the average of three independent samples each being a pool of three retinas from three different Rs1-KO mice. The wild type (WT) group contained two independent samples each with a pool of two retinas.
Intravitreal Injection of scAAV8-hRS1-IRBP
Recombinant AAV-RS1 gene therapy vector was administered into one eye of 55 Rs1-KO mice on postnatal day 21 to 26 by intravitreal injection at 2.5 × 109 vector genomes per eye, as described previously21; the contralateral eye remained untouched. The viral vector was injected into the vitreous body through the sclera on the nasal side of the eye approximately 1 mm posteriorly to the limbus with a 10-μL Nanofil syringe and a removable 35-gauge needle (World Precision Instruments, Inc., Sarasota, FL, USA). After injection, triple antibiotic ophthalmic ointment (neomycin, polymyxin B, and bacitracin) was applied to the eye.
Statistical Analysis
Electroretinogram parameters (a-wave and b-wave amplitude and b-/a-wave ratio) and cavity size measured from OCT images were compared by 1-way ANOVA between groups of 1- and 4-month-old Rs1-KO mice reared in either 20 or 300 lux. A post hoc Tukey test was used for multiple between-group comparisons. One eye from each mouse was randomly selected to be included in the analysis.
Two-way ANOVA was used to assess effects of light rearing on the ERG parameters of Rs1-KO mice after uniocular gene therapy. Both treated and untreated eyes were included in the analysis and compared to each other. The ERG parameters were the dependent variables. Light exposure and treatment were independent variables, set as between-subjects and within-subjects variables, respectively. We considered P < 0.05 as threshold significance.
Results
Low-Light Rearing Preserves Inner Retinal Structure and Function in Rs1-KO Mice
Rs1-KO mice reared in different light intensities for 4 months showed substantial differences in retinal structure and function (Fig. 1). On OCT analysis, Rs1-KO mice reared in 300 lux had increased retinal thickness, compared to Rs1-KO mice reared at 20 lux, owing to extensive schisis cavities, which spanned the outer plexiform and inner nuclear layers. Rs1-KO mice reared in 20 lux had fewer cavities and the inner retina appeared structurally more organized. Rs1-KO mice reared in 300 lux had a smaller ERG b-wave and b/a ratio indicating greater impairment of inner retinal function. The a-wave was similar for the two lighting conditions.
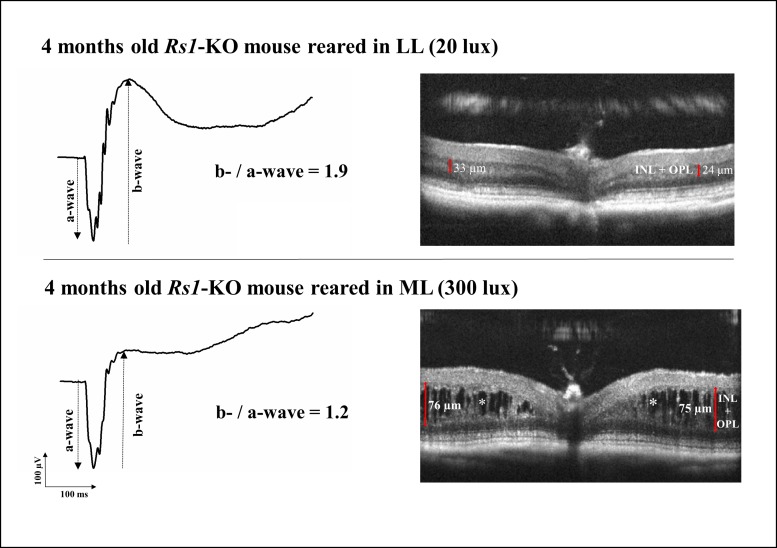
Figure 1.
Retinal function and morphology in two representative 4-month-old Rs1-KO mice reared in low light (LL) and moderate light (ML) conditions. At 4 months, Rs1-KO mice reared in ML showed substantial differences in both retinal morphology and function, compared to Rs1-KO mice reared in LL. ML-reared Rs1-Ko mice showed large schisis cavities in the outer plexiform and inner nuclear layers, which were less numerous and smaller in LL-reared Rs1-KO mice (asterisks). Consistent with a severe structural impairment of the inner retina, the ERG b-wave amplitude in the ML Rs1-KO mouse was considerably reduced, resulting in a typical electronegative ERG. Compared with the ML-reared Rs1-KO mouse, LL-reared Rs1-KO mouse had a much larger b-/a-wave ratio (1.9 vs. 1.2), indicating a better post-photoreceptor function. Calipers indicate the thickness of the inner nuclear layer plus the outer plexiform layer at the level of the largest cavity on each side of the retinal section. INL, inner nuclear layer; OPL, outer plexiform layer.
The changes in retinal function and structure over time were systematically explored by comparing the ERG and the cavity size in Rs1-KO mice reared in 20 and 300 lux at 1 and 4 months of age (Fig. 2).
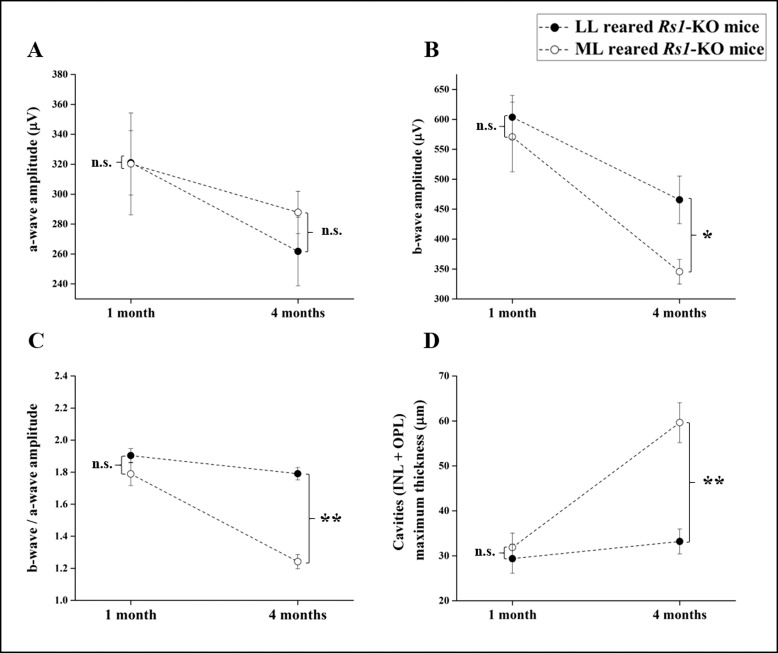
Figure 2.
Electroretinogram findings and cavity size in 1- and 4-month-old Rs1-KO mice reared at either 20 lux (LL) or 300 lux (ML) light intensity. (A) A-wave amplitude did not change significantly between 1 and 4 months for both light intensities and no significant difference was found between LL and ML mice at any time point. B-wave amplitude (B) declined significantly in ML and LL Rs1-KO mice by 4 months and at 4 months was significantly smaller in ML condition than LL. When we normalized the b-wave by the a-wave, b-/a-wave amplitude ratio (C) decreased significantly only in the ML group and was unchanged in LL. By 4 months of age, cavity size (D) increased significantly in the ML-reared Rs1-KO mice, whereas it remained unchanged respect to 1 month in LL-reared animals. In each graph, dot is the mean value and error bars indicate standard errors. *P < 0.05, **P < 0.001. n.s., not significant.
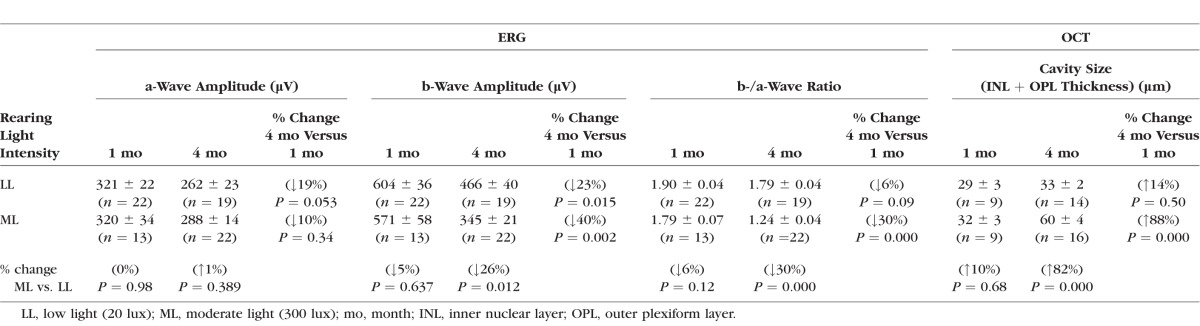
At 1 month of age, no effect of rearing light intensity was identified on either retinal function or structure. The ERG a-wave, b-wave, and b/a ratio of LL- and ML-reared mice were not significantly different (P = 0.98, P = 0.637, and P = 0.12, respectively). The OCT showed mild schisis cavities in the inner retina of both LL- and ML-reared mice but did not show any difference in size (P = 0.68).
At 4 months of age, no significant change in a-wave amplitude was observed for either LL- or ML-reared mice with respect to 1 month (Table 1), suggesting no effect of aging and light exposure on photoreceptor function. As expected by the natural progressive decline of post-photoreceptor function in Rs1-KO mice,17 the b-wave declined significantly from 1 to 4 months in both LL and ML groups (by 23%, P = 0.015 and by 40%, P = 0.002, respectively). When the two light conditions were compared, however, ML-reared mice had significantly smaller b-waves than Rs1-KO mice reared in LL (P = 0.012), indicating a faster decline in post-photoreceptor function in mice reared in 300 lux. The effect of a brighter light on inner retinal function was even more evident in the b-/a-wave ratio, which estimates the gain in signaling between photoreceptors and bipolar cell.19 Between 1 and 4 months, the b-/a-wave ratio remained unchanged in the LL-reared Rs1-KO mice (P = 0.09), whereas it was reduced by 30% in the ML-reared animals (P = 0.000).
Table 1.
ERG and OCT Parameters in Rs1-KO Mice Reared in Either LL or ML
Consistent with these functional findings, OCT analysis (Fig. 2; Table 1) showed that the cavity size was essentially unaltered between 1 and 4 months in LL-reared mice (P = 0.50), whereas cavities increased in size by 88% in mice reared in ML (P = 0.000).
Kir4.1 Channels but Not Aquaporin-4 Channels Are Upregulated in Rs1-KO Mice Reared in ML
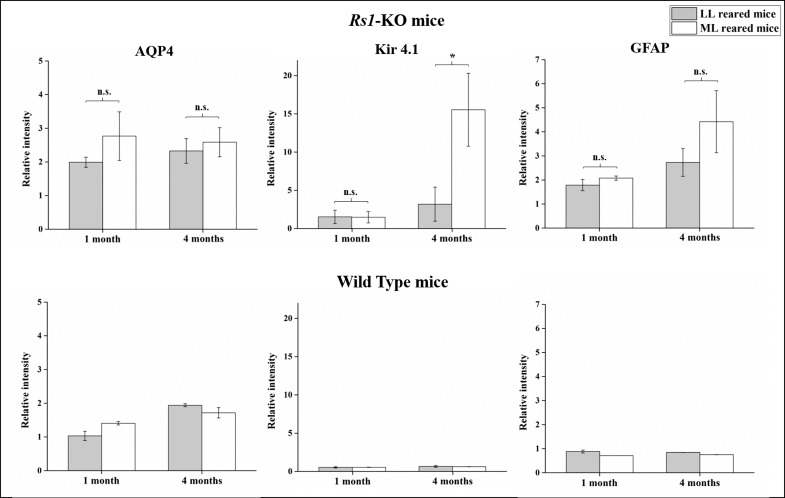
Fluid homeostasis in the inner retina and outer plexiform layer is regulated by Müller glial cells.22 We investigated whether effects of light rearing on cavity formation might be mediated by changes in expression of ion and water channels such as Kir4.1 and AQP4 on Müller cells. AQP4 channel expression remained unchanged between 1 and 4 months in both LL and ML Rs1-KO mice, and no difference was identified between the two light intensities. Kir4.1 expression, however, was affected significantly by exposure to a brighter light. Compared to LL-reared Rs1-KO mice in which Kir4.1 expression remained unchanged between 1 and 4 months, ML rearing caused Kir4.1 levels to increase 7-fold by 4 months (P < 0.05) (Fig. 3). Glial fibrillary acidic protein levels, an indicator of Müller cell activation,23 were not significantly different between LL- and ML-reared Rs1-KO mice at either 1 or 4 months, though there was a trend toward an increase of GFAP between 1 and 4 months in both groups, and especially for ML-reared mice. AQP4, Kir4.1, and GFAP levels were unchanged in WT mice irrespective of light conditions and age, but their levels were overall higher in Rs1-KO mice than in WT animals, likely as a result of Müller cell response to the presence of intraretinal cavities.
Figure 3.
Effect of light rearing on AQP-4, Kir4.1, and GFAP levels in 1- and 4-month-old Rs1-KO and WT mice. Expression levels of AQP4, Kir4.1, and GFAP normalized to internal control protein actin are presented as mean ± standard error for 1- and 4-month-old Rs1-KO and WT mice reared in either LL or ML. Rs1-KO mice showed no significant difference in AQP4 and GFAP levels between LL and ML conditions at 1 and 4 months (A, C). Kir4.1 levels were unchanged between LL and ML Rs1-KO mice at 1 month, but at 4 months they were significantly increased by 7-fold in ML-reared Rs1-KO mice compared to those reared in LL (B). AQP4, Kir4.1, and GFAP levels were unchanged in WT mice irrespective of light conditions and age (D–F) but their levels were overall higher in Rs1-KO mice than in WT animals, likely as a result of Müller cell response to the presence of intraretinal cavities. *P < 0.05. n.s., not significant.
Rearing Light Intensity Does Not Interact With RS1 Gene Replacement Efficacy in Rs1-KO Mice
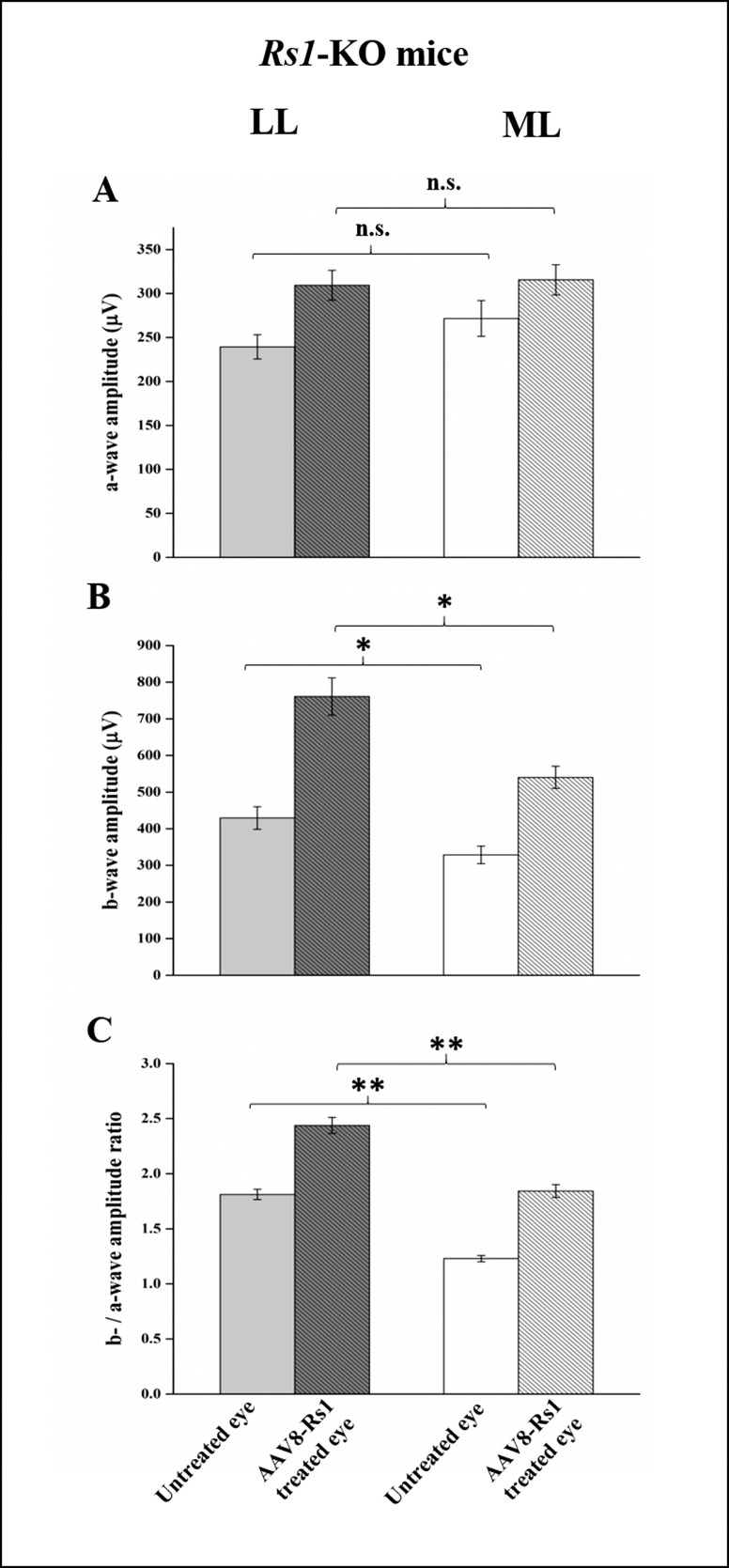
We also tested whether rearing light conditions affected the efficacy of RS1 gene replacement. Two groups of Rs1-KO mice were reared at 20 (n = 26) or 300 lux (n = 29) and were treated at 21 days with an intravitreal injection of scAAV8-hRs-IRBP vector as we have done previously.19 As we found in the previous set of Rs1-KO mice, functional ERG recordings at 4 months showed that untreated eyes of Rs1-KO mice reared in LL had significantly larger b-wave amplitudes and b-/a-wave ratios than untreated eyes of mice reared in ML (P = 0.012 and P = 0.000, respectively). No significant difference in a-wave amplitude was observed between the two groups (Fig. 4).
Figure 4.
Effect of light rearing on the functional outcome after AAV8-mediated RS1 gene replacement in Rs1-KO mice. Average ERG a-wave (A), b-wave (B), and b-/a-wave ratio (C) in two groups of 4-month-old Rs1-KO mice reared in low light (LL) (n = 26) and moderate light (ML) (n = 29) and treated with AAV8-RS1 at 21 days. Although LL mice showed significantly larger b-wave amplitude and b-/a-wave ratio than ML mice in the AAV8-Rs1–treated eye, 2-way ANOVA analysis did not show significant interaction between treatment and rearing light exposure, indicating that AAV8-mediated RS1 expression improved inner retinal function in LL and ML reared mice by the same extent. Untreated eyes in LL-reared Rs1-KO mice had significantly larger b-wave and b-/a-wave than ML mice. This indicates that gene therapy and dark rearing have an additive beneficial effect. In each graph, error bars indicate standard errors. *P < 0.05, **P < 0.001. n.s., not significant.
Gene replacement significantly increased the b-wave amplitude and the b-/a-wave ratio in the Rs1-KO–treated eyes for mice reared in ML (by 65%, P < 0.001 and 50%, P < 0.001, respectively) and LL (by 77%, P < 0.001 and 35%, P < 0.001, respectively) (Table 2). Low light–reared mice had significantly larger b-wave amplitudes and b-/a-wave ratios than ML-reared mice, though there was no significant interaction between light rearing and treatment on the ERG parameters (2-way ANOVA, P > 0.05 for both). This indicates that replacing RS1 protein improved inner retinal function in both LL- and ML-reared mice independently of effects from the rearing light intensity.
Table 2.
ERG Parameters in LL- or ML-Reared Rs1-KO Mice After Treatment With AAV8-RS1

Discussion
This study demonstrated that the light intensity in which Rs1-KO mice are reared exerts a significant effect on the inner retinal phenotype of these animals. Rearing them in LL retards the progression of the XLRS disease as judged by cavity size and post-photoreceptor ERG function.
Our previous natural history studies in Rs1-KO mice have shown schisis cavities as early as 3 weeks and then a significant increase in their size between 1 and 4 months.17,18 Cavity formation in the inner retina is accompanied by considerable impairment in post-photoreceptor ERG b-wave function with little effect on the a-wave, causing the prototypical “electronegative ERG.”17,20 We observed similar changes in ML-reared animals in the present study. However, in LL-reared mice, both cavity size and inner retinal function, (i.e., b-/a-wave ratio) remained unchanged between 1 and 4 months, indicating that light restriction was beneficial for evolution of inner retinal pathology in Rs1-KO mice.
In other animal models of retinal degeneration, exposure to bright light can promote photoreceptor degeneration.6 However, the light intensities have been usually much higher (e.g., 5000 lux for 8 hours with dilated pupils6) than those used in this study in which the ML condition was standard colony room lighting. So, it is not surprising that ML rearing did not increase the rate of photoreceptor loss in Rs1-KO mice. However, ML rearing exacerbated the inner retinal phenotype of cavity formation and decreased ERG responses. This effect of relatively modest lighting on post-photoreceptor structure and function is surprising, since visible light is absorbed by photoreceptors and retinal pigmented epithelium, and no photosensitive elements are known to be present at the outer plexiform layer where schisis cavities specifically develop.17,18
There is some evidence that light environment during rearing can influence the inner retinal structure and/or function, in some cases without detectable effects on the outer retina.24–28 For example, Vistamehr and Tian27 have shown that dark rearing suppresses ERG oscillatory potentials, which represent the activity of rod bipolar, amacrine, and retinal ganglion cells,29,30 in mature animals, without suppressing the ERG a- or b-waves. We do not have a clear picture of how light influences inner retinal pathology in Rs1-KO mice, but one hypothesis is that a brighter light environment may alter inner retinal metabolism, ion gradients, and extracellular fluid movement, exerting more stress on retinal structure than in an LL environment. In this context the extracellular adhesion properties of retinoschisin may play a role in maintaining structural integrity under these conditions and its lack may result in cavity formation.
Müller cells contribute to retinal fluid homeostasis.31 Photoreceptors and RPE primarily control outer retinal homeostasis, while Müller cells regulate ion and water homeostasis of the inner retina through specific membrane channels.22 Specifically, AQP4 channels control water flux across the Müller cell membrane, and Kir4.1 buffers K+ ions released into the extracellular space by synaptic activity during retinal illumination.31,32 These two channels colocalize on the Müller cell membrane and are thought to couple water transport with potassium currents.22 We found that by 4 months, Kir4.1 was strongly upregulated in Rs1-Ko mice reared in ML, indicating dysregulation of K+ balance in the inner retina. This upregulation may also contribute to cavity formation in Rs1-KO mice by accumulation of ions and fluid in the extracellular space. However, as Kir4.1 channels mainly mediate the K+ efflux from Müller cells into the vitreous, blood vessels, and subretinal space, increased Kir4.1 levels may represent compensatory activity of Müller cells to augment K+ clearance from the intraretinal extracellular space in conjunction with removal of fluid from the schisis cavities.
Despite AQP4 and Kir4.1 channel activity being coupled to extracellular fluid reabsorption, we did not observe an increase of AQP4 expression along with Kir4.1 in ML 4-month-old Rs1-Ko mice. In fact, AQP4 levels were not affected by either age or light condition, though Rs1-KO mice had higher levels of AQP4 expression than WT mice in both LL and ML conditions at 1 month. At this age, Rs1-KO mice already show retinal cavities, and it may be that the increase of AQP4 channels on Müller cells as a compensatory mechanism for fluid accumulation is already saturated in 1-month-old mice. Consequently, further increase in intraretinal fluid in ML rearing cannot be compensated for, causing cavities to increase. A recent study in AQP4-null mice has shown that cellular K+ reuptake from the extracellular space is impaired when AQP4 channels are not expressed.33 Likewise, saturation of AQP4 expression in Rs1-KO mice may increase the extracellular K+ and result in the compensatory increase in Kir4.1 channel expression in 4-month-old Rs1-KO mice.
Photoreceptor Maturation
Effects of ML exposure on inner retinal pathology were not evident in 1-month-old Rs1-KO mice, as both LL- and ML-reared animals showed comparable cavity size and similar degree of inner retinal dysfunction. Differences became apparent only in 4-month-old animals. It could be that, as the ML intensity differed by approximately only 1 log from the LL intensity, the light-induced changes were not immediate but took longer to develop.
We have previously observed that photoreceptors in Rs1-KO mouse are not fully matured by 1 month of age, and their outer segments require additional weeks to reach normal length. There is also a delay in development of some elements of the transduction cascade.17,34 Photoreceptors were more mature by 4 months, and outer segments reached normal length, which would enhance the synaptic signaling to the post-photoreceptor pathway. We postulate that this may exacerbate the effects of a brighter light environment on the inner retina, leading to cavity formation.
Gene Therapy Versus Light Rearing Effect
We have shown that delivering a normal RS1 gene by using an adeno-associated viral vector significantly improves retinal structure and function in Rs1-KO mice with reduction of the size and number of cavities and an increase in the b-wave, and b-/a-wave ratio.18,19 In the present study, we found that the rearing light intensity does not interfere with the functional improvement of RS1 gene therapy. Both LL- and ML-reared Rs1-KO mice showed comparable improvements in b-wave amplitude and b-/a-wave ratio at 4 months of age relative to fellow untreated eyes. Gene therapy and LL rearing effects were independent and had additive beneficial effect on inner retinal function of Rs1-KO mice. Thus, after gene therapy both b-wave amplitude and b-/a-wave ratio were larger in LL- than ML-reared animals.
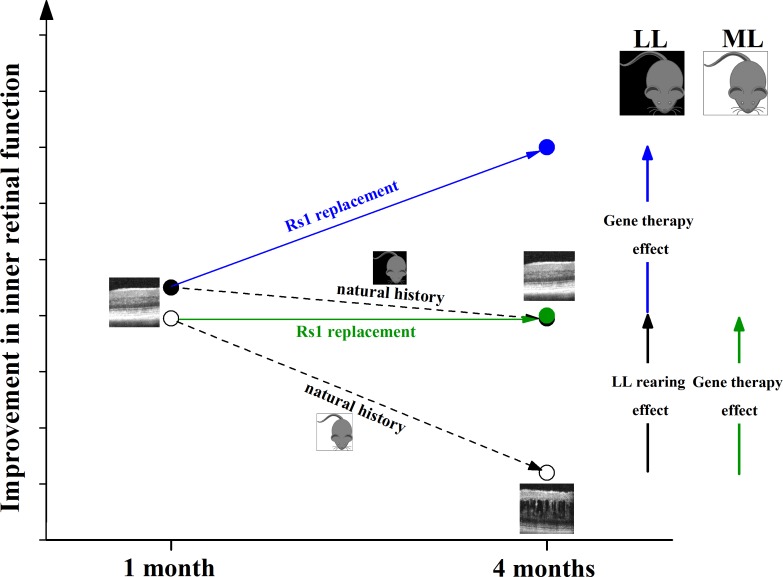
Figure 5 compares the natural history of LL and ML Rs1-KO mice with respect to those which were treated with RS1 gene therapy. Although after treatment the inner retinal function increased by the same amount in LL- and ML-reared Rs1-KO mice, replacing RS1 further improved the inner retinal function in LL-reared mice, whereas it preserved function in ML-reared mice with respect to levels at 1 month of age. At 4 months, LL-reared animals showed an increase in b-/a-wave ratio after treatment, whereas the latter was unchanged in Rs1-KO mice reared in ML.
Figure 5.
Effects of RS1 gene therapy on low light (LL)- and moderate light (ML)-reared Rs1-KO mice natural history. RS1 gene replacement increased the inner retinal function (b-/a-wave ratio) by the same extent in 4-month-old LL- and ML-reared Rs1-KO mice. When compared to 1-month-old Rs1-KO mice, gene therapy improved inner retinal function in 4-month-old LL-reared animals but only preserved inner retinal function in ML-reared animals. Since LL-reared Rs1-KO mice do not show any change in cavity size between 1 and 4 months, RS1 gene transfer improves inner retinal function independently from an effect on retinal cavities, most likely by restoring synapse integrity between photoreceptors and bipolar cells.
We have previously shown that replacing RS1 improves retinal function and reduces cavity size.19 The fact that treating LL-reared Rs1-KO mice produces a functional improvement, although no change in cavity size occurs naturally at this light intensity between 1 and 4 months, indicates that RS1 gene replacement can improve the inner retinal function independently from its beneficial effect on cavities. This is consistent with our previous finding that retinoschisin acts as a synaptic stabilizer element.11,13 When Rs1-KO mice are reared in ML, despite RS1 gene transfer producing the same degree of functional improvement, light exposure itself or the development of cavities may irreversibly impact the inner retina and prevent them from reaching the same level of retinal function as in LL conditions.
In conclusion, this study showed that rearing light intensities contribute to the severity of the Rs1-KO phenotype and that even a moderate light intensity significantly impacts the pathology of the inner retina. We do not know whether the ambient illumination has a similar effect on human XLRS. If it does, environmental exposure to different light conditions may contribute to heterogeneity of the human XLRS phenotype, and light restriction could be considered as a potential prophylactic intervention.
Acknowledgments
The authors thank Zhijian Wu, PhD, and Suja Hiriyanna, MS, MPhil (National Eye Institute, Ocular Gene Therapy Core, National Institutes of Health, Bethesda, MD, USA) for providing the viral vector used in the gene therapy studies.
Supported by the Intramural Research Program of the National Institute on Deafness and Other Communication Disorders and National Institutes of Health (Bethesda, MD, USA).
Disclosure: D. Marangoni, None; Z. Yong, None; S. Kjellström, None; C. Vijayasarathy, None; P.A. Sieving, None; R.A. Bush, None
References
- 1. Penn JS,, Williams TP. Photostasis: regulation of daily photon-catch by rat retinas in response to various cyclic illuminances. Exp Eye Res. 1986; 43: 915–928. [DOI] [PubMed] [Google Scholar]
- 2. Paskowitz DM,, LaVail MM,, Duncan JL. Light and inherited retinal degeneration. Br J Ophthalmol. 2006; 90: 1060–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valter K,, Kirk DK,, Stone J. Optimising the structure and function of the adult P23H-3 retina by light management in the juvenile and adult. Exp Eye Res. 2009; 89: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 4. Khan JC,, Shahid H,, Thurlby DA,, et al. Age related macular degeneration and sun exposure, iris colour, and skin sensitivity to sunlight. Br J Ophthalmol. 2006; 90: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cronin T,, Lyubarsky A,, Bennett J. Dark-rearing the rd10 mouse: implications for therapy. Adv Exp Med Biol. 2012; 723: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wenzel A,, Grimm C,, Samardzija M,, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005; 24: 275–306. [DOI] [PubMed] [Google Scholar]
- 7. Grayson C,, Reid SN,, Ellis JA,, et al. Retinoschisin, the X-linked retinoschisis protein, is a secreted photoreceptor protein, and is expressed and released by Weri-Rb1 cells. Hum Mol Genet. 2000; 9: 1873–1879. [DOI] [PubMed] [Google Scholar]
- 8. Sauer CG,, Gehrig A,, Warneke-Wittstock R,, et al. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet. 1997; 17: 164–170. [DOI] [PubMed] [Google Scholar]
- 9. Wu WW,, Wong JP,, Kast J,, Molday RS. RS1, a discoidin domain-containing retinal cell adhesion protein associated with X-linked retinoschisis, exists as a novel disulfide-linked octamer. J Biol Chem. 2005; 280: 10721–10730. [DOI] [PubMed] [Google Scholar]
- 10. Tolun G,, Vijayasarathy C,, Huang R,, et al. Paired octamer rings of retinoschisin suggest a junctional model for cell-cell adhesion in the retina. Proc Natl Acad Sci U S A. 2016; 113: 5287–5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takada Y,, Vijayasarathy C,, Zeng Y,, Kjellstrom S,, Bush RA,, Sieving PA. Synaptic pathology in retinoschisis knockout (Rs1-/y) mouse retina and modification by rAAV-Rs1 gene delivery. Invest Ophthalmol Vis Sci. 2008; 49: 3677–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vijayasarathy C,, Takada Y,, Zeng Y,, Bush RA,, Sieving PA. Retinoschisin is a peripheral membrane protein with affinity for anionic phospholipids and affected by divalent cations. Invest Ophthalmol Vis Sci. 2007; 48: 991–1000. [DOI] [PubMed] [Google Scholar]
- 13. Ou J,, Vijayasarathy C,, Ziccardi L,, et al. Synaptic pathology and therapeutic repair in adult retinoschisis mouse by AAV-RS1 transfer. J Clin Invest. 2015; 125: 2891–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sikkink SK,, Biswas S,, Parry NR,, Stanga PE,, Trump D. X-linked retinoschisis: an update. J Med Genet. 2007; 44: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sieving PA,, MacDonald IM,, Meltzer M,, Smaoui N. X-linked juvenile retinoschisis. GeneReviews®. Seattle, WA: University of Washington; 1993–2017. Available at: https://www.ncbi.nlm.nih.gov/books/NBK1222/. [Google Scholar]
- 16. Vincent A,, Robson AG,, Neveu MM,, et al. A phenotype-genotype correlation study of X-linked retinoschisis. Ophthalmology. 2013; 120: 1454–1464. [DOI] [PubMed] [Google Scholar]
- 17. Kjellstrom S,, Bush RA,, Zeng Y,, Takada Y,, Sieving PA. Retinoschisin gene therapy and natural history in the Rs1h-KO mouse: long-term rescue from retinal degeneration. Invest Ophthalmol Vis Sci. 2007; 48: 3837–3845. [DOI] [PubMed] [Google Scholar]
- 18. Zeng Y,, Petralia RS,, Vijayasarathy C,, et al. Retinal structure and gene therapy outcome in retinoschisin-deficient mice assessed by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2016; 57: OCT277–OCT287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bush RA,, Zeng Y,, Colosi P,, et al. Preclinical dose-escalation study of intravitreal AAV-RS1 gene therapy in a mouse model of X-linked retinoschisis: dose-dependent expression and improved retinal structure and function. Hum Gene Ther. 2016; 27: 376–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeng Y,, Takada Y,, Kjellstrom S,, et al. RS-1 gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-linked retinoschisis. Invest Ophthalmol Vis Sci. 2004; 45: 3279–3285. [DOI] [PubMed] [Google Scholar]
- 21. Marangoni D,, Wu Z,, Wiley HE,, et al. Preclinical safety evaluation of a recombinant AAV8 vector for X-linked retinoschisis after intravitreal administration in rabbits. Hum Gene Ther Clin Dev. 2014; 25: 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bringmann A,, Pannicke T,, Grosche J,, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006; 25: 397–424. [DOI] [PubMed] [Google Scholar]
- 23. Dyer MA,, Cepko CL. Control of Müller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000; 3: 873–880. [DOI] [PubMed] [Google Scholar]
- 24. Birch DG,, Jacobs GH. The effects of prolonged dark exposure on visual thresholds in young and adult rats. Invest Ophthalmol Vis Sci. 1979; 18: 752–756. [PubMed] [Google Scholar]
- 25. Tian N,, Copenhagen DR. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron. 2001; 32: 439–449. [DOI] [PubMed] [Google Scholar]
- 26. Tian N,, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003; 39: 85–96. [DOI] [PubMed] [Google Scholar]
- 27. Vistamehr S,, Tian N. Light deprivation suppresses the light response of inner retina in both young and adult mouse. Vis Neurosci. 2004; 21: 23–37. [DOI] [PubMed] [Google Scholar]
- 28. Giovannelli A,, Di Marco S,, Maccarone R,, Bisti S. Long-term dark rearing induces permanent reorganization in retinal circuitry. Biochem Biophys Res Commun. 2008; 365: 349–354. [DOI] [PubMed] [Google Scholar]
- 29. Kolb H,, Nelson R. Amacrine cells of the cat retina. Vision Res. 1981; 21: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 30. Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998; 17: 485–521. [DOI] [PubMed] [Google Scholar]
- 31. Kofuji P,, Biedermann B,, Siddharthan V,, et al. Kir potassium channel subunit expression in retinal glial cells: implications for spatial potassium buffering. Glia. 2002; 39: 292–303. [DOI] [PubMed] [Google Scholar]
- 32. Newman EA. Regional specialization of retinal glial cell membrane. Nature. 1984; 309: 155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Padmawar P,, Yao X,, Bloch O,, Manley GT,, Verkman AS. K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator. Nat Methods. 2005; 2: 825–827. [DOI] [PubMed] [Google Scholar]
- 34. Ziccardi L,, Vijayasarathy C,, Bush RA,, Sieving PA. Loss of retinoschisin (RS1) cell surface protein in maturing mouse rod photoreceptors elevates the luminance threshold for light-driven translocation of transducin but not arrestin. J Neurosci. 2012; 32: 13010–13021. [DOI] [PMC free article] [PubMed] [Google Scholar]