ABSTRACT
Heme is an essential molecule expressed in many tissues where it plays key roles as the prosthetic group of several proteins involved in vital physiological and metabolic processes such as gas and electron transport. Structurally, heme is a tetrapyrrole ring containing an atom of iron (Fe) in its center. When released into the extracellular milieu, heme exerts several deleterious effects, which make it an important player in infectious and noninfectious hemolytic diseases where large amounts of free heme are observed such as malaria, dengue fever, β-thalassemia, sickle cell disease and ischemia-reperfusion. Our recent work has uncovered an unappreciated cellular response triggered by heme or Fe, one of its degradation products, on macrophages, which is the formation of protein aggregates known as aggresome-like induced structres (ALIS). This response was shown to be fully dependent on ROS production and the activation of the transcription factor NFE2L2/NRF2. In addition, we have demonstrated that heme degradation by HMOX1/HO-1 (heme oxygenase 1) is required and that Fe is essential for the formation of ALIS, as heme analogs lacking the central atom of Fe are not able to induce these structures. ALIS formation is also observed in vivo, in a model of phenylhydrazine (PHZ)-induced hemolysis, indicating that it is an integral part of the host response to excessive free heme and that it may play a role in cellular homeostasis.
KEYWORDS: aggregation, ALIS, autophagy, ferritin, heme, iron, NRF2, oxidative stress, protein
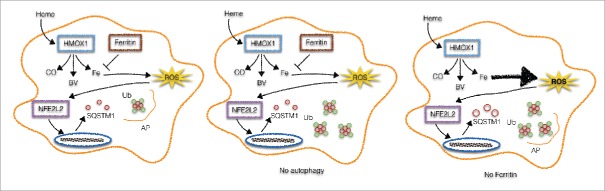
Autophagy is a crucial eukaryotic process for the maintenance of cellular homeostasis upon changes in the environment. When autophagy is activated, cytosolic components are sequestered within double-membrane vesicles, called autophagosomes, which subsequently fuse with endolysosomal vesicles leading to proteolytic degradation of a myriad of substrates such as damaged organelles and long-lived proteins. In this context, we were interest in examining the role of autophagy in the stress response induced by heme, an endogenous pro-inflammatory molecule, and how it contributed to cellular homeostasis. Interestingly, heme stimulation did not induce autophagosome formation in macrophages but, instead, perfectly round structures characterized by the presence of SQSTM1/p62 and ubiquitin were observed (Fig. 1). These structures were identified as a particular type of protein aggregate known as ALIS. Even though autophagy was not required for ALIS formation it was required for ALIS clearance.
Figure 1.
Autophagy and ferritin control ALIS formation. Upon heme stimulation, HMOX1 degrades heme into carbon oxide, biliverdin and iron (Fe). In such conditions, ferritin is upregulated and neutralizes labile Fe, restraining ROS generation and consequently SQSTM1 upregulation and ALIS formation. Autophagy (AP) is also increased to avoid the accumulation of protein aggregates (left). In autophagy- (middle) or ferritin-deficient cells (right) we observe the accumulation of ALIS or the increase in ROS that in its turn activates NFE2L2 to increase the expression levels of SQSTM1, culminating with ALIS formation.
In addition to its pro-oxidant potential, heme can also induce pro-inflammatory responses through its interaction with innate immune receptors, in particular TLR4. In our model, however, TLR4 was dispensable for heme-induced ALIS formation. We have shown that ALIS formation induced by heme is largely dependent on ROS generation by mitochondria and subsequent NFE2L2 activation. As mentioned above, SQSTM1 is a hallmark of ALIS. The Sqstm1 gene harbors an antioxidant response element binding site and, thus, is regulated by NFE2L2. Accordingly, NFE2L2-deficient macrophages display decreased levels of SQSTM1 protein and ALIS upon heme stimulation.
Because of its deleterious effects on host tissues, multiple cellular and systemic mechanisms are in place to tightly control and buffer free heme. We have shown that HMOX1, the main protein involved in heme degradation, is required for the formation of heme-induced ALIS. HMOX1-deficient macrophages display a severe impairment in the expression of SQSTM1 and ALIS formation during stimulation with heme. This suggests a role for labile Fe released during heme degradation in ALIS formation. Corroborating this hypothesis, protoporphyrin IX, a heme precursor that lacks the central Fe atom, is not able to upregulate SQSTM1 expression and ALIS formation upon macrophage stimulation. These results support the notion that Fe is the minimal molecular requirement for the formation of ALIS induced by heme. To further investigate this, macrophages were stimulated with FeSO4 (Fe2+) and FeCl3 (Fe3+), which recapitulates all the features observed in heme-induced ALIS formation, i.e., ROS production, activation of NFE2L2, increased SQSTM1 expression and ALIS formation (Fig. 1).
Upon heme degradation, labile Fe can enter Fenton reactions leading to a strong oxidant environment, which is counteracted by mechanisms induced to neutralize labile Fe. One of these mechanisms is the increased expression of FTH1 (ferritin heavy chain 1). FTH1 associates with FTL (ferritin light chain) to create a complex that is able to bind up to 4,500 atoms of Fe. We hypothesized that since FTH1 buffers intracellular labile Fe, it could also control the formation of ALIS induced by heme. Labile heme, Fe2+, Fe3+, but not protoporphyrin IX, induce a strong upregulation of ferritin protein levels. FTH1-deficient macrophages stimulated with Fe2+ present a dramatic increase in the expression of SQSTM1 accompanied by a remarkable increase in the number of ALIS. Collectively, these results indicate that ferritin limits the formation of ALIS induced by iron (Fig. 1).
Finally, to investigate whether ALIS formation induced by heme could also be observed in vivo we used a PHZ-induced hemolysis model. Our hypothesis was that the release of high amounts of free heme would induce ALIS formation in target organs. Our results show a dramatic increase in SQSTM1 expression in the kidneys, spleen and liver of PHZ-injected compared with saline-injected mice. In line with these results, the number of SQSTM1+ dots in sections from livers and spleens from PHZ-injected mice is significantly higher than that observed in saline-injected mice. These data strengthen our idea that ALIS is an important response in a context where abnormal high levels of heme are present, such as in hemolytic diseases, and likely has a role in the maintenance of homeostasis.
The formation of intracellular protein aggregates is frequently described as a deleterious event. However, the fact that a cytoprotective enzyme such as HMOX1 is essential for ALIS formation may indicate a role for ALIS as a mechanism that contributes to keep or bring cells back to homeostasis during heme stimulation. Conversely, a deleterious role of ALIS under these conditions cannot be excluded as accumulation of intracellular protein aggregates have been widely described to be the basis of several neurological diseases. Our in vivo results demonstrating the formation of ALIS during hemolysis suggest that it may play a yet to be defined role in hemolytic diseases. Further work is required to detail the role of this structure in these settings and if it can be explored as a therapeutic target.