ABSTRACT
The microgenderome defines the interaction between microbiota, sex hormones and the immune system. Our recent research inferred support for the microgenderome by showing sex differences in microbiota-symptom associations in a clinical sample of patients with myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS). This addendum expands upon the sex-specific pattern of associations that were observed. Interpretations are hypothesized in relation to genera versus species-level analyses and D-lactate theory. Evidence of sex-differences invites future research to consider sex comparisons in microbial function even when microbial abundance is statistically similar. Pairing assessment of clinical symptoms with microbial culture, DNA sequencing and metabolomics methods will help advance our current understandings of the role of the microbiome in health and disease.
KEYWORDS: chronic fatigue syndrome, clinical symptoms, D-lactate, microbiome, myalgic encephalopathy, neurological manifestations, sex differences
Introduction
Evidence of the bidirectional role of the microbiome in human health continues to emerge. Patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) present with excessive post-exertional exhaustion and a complex array of symptoms suggestive of multi-systemic abnormalities.1 Gastrointestinal, immune and neurological symptoms in ME/CFS makes this an appropriate clinical population to examine brain-gut-microbiota interactions. The recent proposition of the ‘microgenderome’ emphasizes the potential mediating and modulatory role of sex hormones in these interactions.2 Flak et al.'s explanation and other animal studies have shown that microbiota manipulation can alter hormonal, metabolic, inflammatory and/or immune processes.3,4 Twin studies have revealed that the once similar microbial composition of opposite-sex twins becomes distinctly different after puberty when compared to same-sex twins that remain compositionally similar.5 Application of the microgenderome lens has only recently been applied to a human clinical population.6 The focus of this addendum is to provide a comprehensive summary of the original results and additional commentary on our earlier findings. We discuss further interpretations and implications related to genera compared with species-level analyses and D-lactate theory.
Our research6 indicated support for the microgenderome by showing sex-specific associations between gut microbiota and symptom presentation in ME/CFS (detailed below). Results from faecal microbial assessments and self-reported symptoms were analyzed from 274 ME/CFS patients. Sex comparisons for self-reported ME/CFS symptoms showed that females tended to report greater impairment than males. The cross-sectional design impeded clear interpretation of the reason for these observed differences. A longstanding belief is that females tend to over-report symptoms compared with under-reporting in males.7 However, accumulating evidence suggests that increased perception of symptoms in females correlates with higher circulating cytokine levels8 and more chronic health problems9 compared to males. Hence, our results may reflect gender differences in self-reporting or pathophysiological differences in ME/CFS presentation.
Culture-based methods for bacterial identification (MALDI-TOF MS) were used to measure microbial composition (see methods from original report6). The frequency and relative abundance (RA) of selected anaerobic (Bacteroides, Bifidobacterium, Clostridium, Eubacterium, and Lactobacillus) and aerobic (Escherichia, Streptococcus, and Enterococcus) genera were similar across the sexes (data available in Table S2 in the original article6). Sex-differences between self-reported symptoms in the presence of compositional similarity led to investigation of possible sex-interactions between microbiota and symptoms.
Sex differences in symptom-microbiota associations
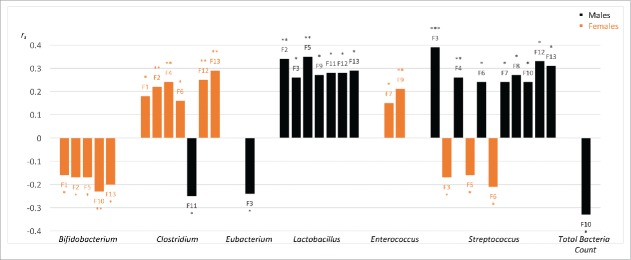
Non-parametric correlations between symptoms and the microbial abundance of specific genera showed a sex-divergent pattern of associations. Effect sizes were small to medium suggesting that microbiota-symptom interactions may reflect one piece of the complex ME/CFS puzzle. As highlighted in our original article,6 sex differences were notable for Streptococcus, Lactobacillus and Clostridium genera. Significant associations between RA of other genera and symptom factors were also shown for males and females independently. In this addendum we present all significant associations from our original analyses including genera (Enterococcus, Eubacterium) and Total Bacteria Count (defined in Fig. 1 legend) that were not previously discussed. Figure 1 clearly demonstrates a divergent pattern of associations between the sexes.
Figure 1.
Summary of significant associations between genera relative abundance (RA) and ME/CFS symptom factors (F1-F13). All significant (P ≤ 0.05) Spearman's rank order correlations (rs) are shown highlighting differences between males (black) and females (orange). RA: calculated from ratio of each genus viable count divided by t Total Bacteria Count expressed as a percentage. Total Bacteria Count: calculated from exponent value of total bacteria detectable on MALDI-TOF MS assessment. Symptom factors included: F1. Fatigue, F2. Neurocognitive, F3. Pain, F4. Sleep, F5. Neurosensory, F6. Immune, F7. Gastro-intestinal, F8. Genitourinary, F9. Sensitivities, F10. Energy Production/Transportation Impairments, F11. Mood, F12 ICC Symptom Score (sum of F1-F10), F13. Total Symptom Score (sum of F1-F11). F12 reflects diagnostic symptoms from the International Consensus Criteria (ICC) for ME/CFS. F13 also includes the mood factor (F11) as these frequently comorbid symptoms are not a diagnostic requirement. Positive correlations show that symptom factor and RA covary in the same direction i.e. either both increasing or both decreasing. An inverse monotonic association is indicated by negative correlations. Correlations can be interpreted as small (0.01), moderate (0.03) and large (0.05) effect sizes.35 *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Streptococcus and Clostridium were the only 2 genera that showed significant associations with symptom factors for both males and females. Surprisingly, an opposite direction of associations was observed. Increased streptococcal colonization was associated with more impairment in males (Fig. 1, eight significant positive correlations noted) but less impairment in females (Fig. 1, three significant negative correlations found). For females, higher levels of clostridial colonization correlated with higher symptom scores (Fig. 1, six positive significant correlations). For clostridial colonisation in males, only one significant negative correlation was identified, whereby mood symptoms and Clostridium levels were inversely related (Fig. 1). Possible reasons for these differences are merely speculative at this stage and are explored below.
For males, Eubacterium and Total Bacteria Count also showed significant negative correlations (Fig. 1). Pain symptoms (F3) correlated negatively with Eubacterium RA. Negative correlations were observed between Total Bacteria Count and all symptom factors in males. The one significant negative correlation between Total Bacteria Count and self-reported energy production/transportation impairments (F10) in males (see Fig. 1) may reflect reduced capacity for energy production with less bacterial numbers. Colonic bacterial metabolism of volatile fatty acids has been shown to account for approximately 10% of energy production in humans.10 Using metagenomics sequencing methods, decreased bacterial abundance and diversity has been shown within inflammatory bowel disease (IBD) and obesity populations.11,12 Sex differences were either not considered11 or statistically controlled12 in these studies. Inter-individual differences of bacterial abundance and diversity within healthy populations13 support the need to investigate functional difference in energy metabolism to more accurately interpret our results.
Genera analyses – A starting point
Analysis at the genus level provides a broad picture of interactions. An example of the complexity of possible interpretations is provided by examining the results for Clostridium. Clostridium RA and symptom associations highlight the potential importance of this genus in ME/CFS females. However, correlational analyses impede our ability to determine whether the increase in symptoms associated with increased Clostridium RA is causative or consequential. Further evidence of functional diversity within the Clostridium genus makes interpretation difficult and invites species-level analyses.
Varied protective roles of Clostridium species have been described within the literature. The commensal properties of the genus Clostridium have been recognized within experimental and clinical research. Atarashi and colleagues14 found that specific components of the immune system may be modulated by Clostridium, with clusters IV and XIVa promoting regulatory T cell (Treg) production and associated anti-inflammatory effects. Clostridium-abundant mice evidenced less colitis, improved bowel markers and reduced allergic response. No reference was made to the sex of the mice used in these studies, nor was reference made to the potential effect of bacterial diversity, i.e., multi-strained colonisation (46 strains of Clostridium) compared with colonisation with fewer strains (segmented filamentous bacteria, 3 strains of lactobacillus, and 16 strains of Bacteroides). Consistent with animal models, human investigations have shown lower ratios of Clostridium leptum and Clostridium coccoides in patients with IBD compared with healthy controls.15 Results to date are not causative, however, the potential beneficial role of Clostridium species in gut health warrants further investigation.
Other Clostridium species have been associated with disease. Key examples of the potentially deleterious role of Clostridium species include the well-documented neurotoxic and enterotoxic effects of many species including Clostridium botulinum and Clostridium difficile.16 Higher incidence of Clostridium species have been identified in patients with irritable bowel syndrome compared with healthy controls.17 Additionally, opportunistic species Clostridium difficile and Clostridium perfringens proliferate with increased refined sugar intake.18 Conflicting findings call for consideration of species- and host-specific effects. Genetic diversity and dietary interactions with microbiota may promote differing commensal or deleterious effects dependent on the individual.19
Results from the current sample raise more questions than answers. The associations between neurological symptoms and Clostridium in females may reflect the neurotoxic effects of specific species that may be mediated or modulated by sex hormones in a subset of ME/CFS patients. However, our interpretations are limited because hormonal and metabolic profiles were not collected for this retrospective sample and could not be correlated with bacterial composition. Species level investigations and functional microbial assessment are required to ascertain the role of Clostridium in ME/CFS presentations and why this may differ between the sexes. Increased specificity has value for all of the genera examined. Genus-level analyses provide initial insights and demand further investigation at the species-level to aid interpretation.
D-lactate theory
For males, Streptococcus was highlighted as a genus positively associated with ME/CFS symptom factors, suggesting a potential deleterious role. This result may support the application of D-lactate theory for ME/CFS. D-lactic acidosis (D-la) is a condition originally observed in ruminants.20 In humans, it is primarily reported in patients with short bowel syndrome where an increased level of D-lactate is associated with neurological symptoms reflecting encephalopathy.21 Certain species of Streptococcus, Lactobacillus, Bifidobacterium and Enterococcus produce more D-lactate (the isomer of L-lactate).22,23 Humans have the capacity to metabolise both D- and L-lactate.24,25 However, D-lactic acid can accumulate in the presence of bacterial overgrowth, triggered by carbohydrate metabolism and in individuals with impaired or reduced D-lactate metabolism.26 Increased abundance of D-lactate producing bacteria22 and symptom overlap between ME/CFS and D-la lead to the suggestion that a similar mechanism may occur for both conditions. While D-la is an acute condition, subclinical levels of D-lactate may play a role in the neurological symptoms of ME/CFS. Our team are currently investigating this possibility.
Streptococcus sanguinis has been shown to produce more D-lactic acid from glucose metabolism and is involved in maintaining a more acidic environment.22 In ruminants, greater carbohydrate intake increased Streptococcus bovis growth, reduced the pH level and encouraged the growth of Lactobacillus species.20 These mechanisms appear consistent in humans. The pH level influences bacterial composition. A more acidic environment (lower pH) encourages the growth of acid-resistant bacteria (including Lactobacilli) and increases lactic acid production.27 Several D-la case studies have shown an overgrowth of Lactobacillus species in stool samples.27-29 With no comparative control group, we could not determine whether ME/CFS patients in our sample had an overgrowth of Lactobacilli. Nonetheless, results for Lactobacillus support the application of D-lactate theory in male patients. Significant positive correlations were shown between Lactobacillus RA and neurocognitive, pain, neurosensory, gastrointestinal and mood symptoms for males (Fig. 1). These ME/CFS symptoms overlap with symptoms of D-la.30
Application of the D-lactate theory in females is less clear. No significant associations were yielded for Lactobacillus and reverse significant negative associations were found between Streptococcus and pain, neurosensory and immune symptoms (Fig. 1). While Enterococcus RA was significantly and positively correlated with gastrointestinal symptoms and sensitivities (food and chemical) in females (Fig. 1), neurological symptoms did not reach significance. These results raise the possibility of sex-differences in D-lactate metabolism. The opposing microbial-symptom associations for males and females suggests that the functional role of microbiota, and perhaps D-lactic acid, may differ between the sexes. The role of D-lactate in ME/CFS is only a theoretical proposition at this stage. Sex comparison of species-level analysis of gut microbiota, bacterial metabolites and D-lactic acid levels in ME/CFS patients will help evaluate the validity of this theory.
As discussed in our original paper,6 results for Bifidobacteria add further complexity to the argument. Sex consistency and positive microbial-symptom correlations for this genus do not support the relevance of D-lactate theory for either sex. Similar to other genera discussed, only selected species of the genus Bifidobacterium produce excess D-lactate. Investigation at the species level will clarify these unanswered questions.
Future considerations
Clinical and research settings should not underestimate the value of sex comparisons. As indicated by our results, comparison between the proportion of genera in male and female patients revealed sex-similarities. However, further analyses examining symptom-bacterial interactions suggest that merely using a surface-level comparison of bacterial composition is too simplistic. More detailed analyses of the functional differences between similar organisms are likely to provide a more comprehensive picture. Optimally, future studies will also measure sex hormone levels to advance our current understanding of the bidirectional interaction between hormones and microbial composition.
Male mice are preferentially used in animal studies.31 Historically, this has been due to the suggested variability that occurs throughout the estrous cycle.32 Recent evidence negates this proposition and encourages the inclusion of female mice in biomedical and neuroscience research.32 Our results echo the proposed policy changes by the US National Institutes of Health31 and recommend that animal research and clinical trials are designed to enable sex comparisons to accurately interpret results and establish efficacious treatments across the population.
A limitation of our results is the use of culture-based methods compared to metagenomic sequencing. Advanced sequencing technology has superior capacity to detect bacterial diversity.33 This raises the possibility that some species and genus unable to be cultured may also be relevant for ME/CFS. However, distinguishing viable genetic material can be limited using sequencing technology. Using culture methods within the context of functional and applied pathology, we have focused on a small selection of viable genera compared to the hundreds of bacterial species with unclear viability that can be revealed through sequencing methods.11 Hence our results do not exclude the relevance of other organisms not identified in this research. Nevertheless, culture methods remain valuable for gaining information about how bacteria react to other bacteria and respond to their environment.34 In fact, combining culture and sequencing methods may ensure that we continue to advance our knowledge of microbial function at the same rate as the rapidly growing identification of new bacterial species. Regardless of the selected method, examination of sex differences in bacterial function remains pertinent.
Extension of our results requires the use of metabolomics technology to accurately examine functionality of bacterial species across individuals. Concurrently with metagenomic advances, metabolomics technology allows the genetic potential of bacteria to be compared with the biological metabolites of species.34 Metabolic profiling of the gut microbiome appears to not only have localized effects. Animal studies showed that both microbiome manipulation and infection can lead to metabolic changes in multiple anatomical sites including the liver and brain.34 While the technology is still in its infancy, this information is likely to dramatically improve our understanding of mind-gut interactions and the microgenderome. The bacterial environment, related energy production and metabolism can vary according to intrinsic and extrinsic characteristics including sex, age, diet, climate, ethnicity, disease status and hormonal status. It is predicted that measurement of bacterial metabolites, including but not restricted to metabolic hormones, neurotransmitters and lactate production will advance understanding of mechanisms involved in ME/CFS. Inter-individual comparisons will enable exploration of potential sex differences and clarification of the relevance of D-lactate theory for this population.
As authors from psychology, medical and microbiology fields, we encourage inter-disciplinary collaboration and education. The brain-gut-microbial axis and our results in this ME/CFS sample suggest that some symptoms previously considered in isolation (e.g., neurological, gastrointestinal and immune symptoms) may have shared mechanisms. In conjunction with technological advances, collective insights from multiple disciplines will enhance our understanding of the complexities of the microgenderome's role in human health. If we can understand the function of the microbiota/microbiome for each individual, we can more accurately assess gut dysbiosis, metabolic abnormalities, deficiencies or accumulated toxic metabolites that may be related to disease processes. A future with more individualised assessments and targeted interventions appears within closer reach.
Abbreviations
- D-la
D-lactic acidosis
- F. prausnitzii
Faecalibacterium prausnitzii
- IBD
inflammatory bowel disease
- ME/CFS
myalgic encephalomyelitis/chronic fatigue syndrome
- RA
relative abundance
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Bioscreen (Aust.) Pty Ltd. and Victoria University provided post-graduate scholarship funding to A.W. without restriction on publication. D.B., M.B., H.B. and D.P.L. declare no competing financial interest.
Contributions
A.W. wrote the manuscript and all authors provided conceptual guidance and contributed to data interpretation, manuscript design and editing.
References
- [1].Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles ACP, Speight N, Vallings R, et al.. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med [Internet] 2011; 270:327-38; PMID:21777306; http://dx.doi.org/ 10.1111/j.1365-2796.2011.02428.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Flak MB, Neves JF, Blumberg RS. Welcome to the microgenderome. Science (80-) 2013; 339:1044-5; http://dx.doi.org/ 10.1126/science.1236226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Markle JGM, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science (80-) [Internet] 2013; 339:1084-8. Available from: http://www.sciencemag.org/content/339/6123/1084.abstract; http://dx.doi.org/ 10.1126/science.1233521 [DOI] [PubMed] [Google Scholar]
- [4].Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Article: Gender bias in autoimmunity is influenced by microbiota. Immunity [Internet] 2013; 39:400-12. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=edselp&AN=S1074761313003415&site=eds-live; PMID:23973225; http://dx.doi.org/ 10.1016/j.immuni.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yatsunenko T, Rey FE, Gordon JI, Manary MJ, Trehan I, Warner B, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, et al.. Human gut microbiome viewed across age and geography. Nature [Internet] 2012; 486:222-7. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=edselc&AN=edselc.2-52.0-84862141704&site=eds-live; PMID:22699611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wallis A, Butt H, Ball M, Lewis DP, Bruck D Support for the Microgenderome: associations in a human clinical population. Sci Rep [Internet] 2016; 6. Available from: http://www.nature.com/articles/srep19171; http://dx.doi. org/ 10.1038/srep19171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hibbard JH, Pope CR. Another look at sex differences in the use of medical care: Illness orientation and the type of morbidities for which services are used. Women Heal [Internet] 1986; 11:21-36. Available from: http://www.tandfonline.com/doi/abs/10.1300/J013v11n02_03; http://dx.doi.org/ 10.1300/J013v11n02_03 [DOI] [PubMed] [Google Scholar]
- [8].Lekander M, Elofsson S, Neve IM, Hansson LO, Unden AL. Self-rated health is related to levels of circulating cytokines [Internet]. Psychosom Med 2004; 66:559-63. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=edswsc&AN=000222858600015&site=eds-live; PMID:15272103; http://dx.doi.org/ 10.1097/01.psy.0000130491.95823.94 [DOI] [PubMed] [Google Scholar]
- [9].Malmusi D, Artazcoz L, Benach J, Borrell C. Perception or real illness? How chronic conditions contribute to gender inequalities in self-rated health. Eur J Public Health [Internet] 2012; 22:781-6 6p. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=c8h&AN=104390401&site=eds-live; PMID:22179096; http://dx.doi.org/ 10.1093/eurpub/ckr184 [DOI] [PubMed] [Google Scholar]
- [10].Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev [Internet] 1990; 70:567-90. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=2181501&site=eds-live; PMID:2181501 [DOI] [PubMed] [Google Scholar]
- [11].Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al.. A human gut microbial gene catalogue established by metagenomic sequencing. Nature [Internet] 2010; 464:59-65; PMID:20203603; http://dx.doi.org/ 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J-M, Kennedy S, et al.. Richness of human gut microbiome correlates with metabolic markers. Nature [Internet] 2013; 500:541-6; PMID:23985870; http://dx.doi.org/ 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- [13].Huttenhower C, Fah Sathirapongsasuti J, Segata N, Gevers D, Earl AM, Fitzgerald MG, Young SK, Zeng Q, Alm EJ, Alvarado L, et al.. Structure, function and diversity of the healthy human microbiome. Nature [Internet] 2012; 486:207-14. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=edselc&AN=edselc.2-52.0-84862276328&site=eds-live; PMID:22699609; http://dx.doi.org/ 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Atarashi K, Tanoue T, Taniguchi T, Honda K, Shima T, Imaoka A, Umesaki Y, Kuwahara T, Momose Y, Itoh K, et al.. Induction of colonic regulatory T cells by indigenous Clostridium species. Science (80-) [Internet] 2011; 331:337-41. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=edselc&AN=edselc.2-52.0-78650667577&site=eds-live; http://dx.doi.org/ 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier L, Beaugerie L, Cosnes J, Corthier G, Marteau P, Dore J. Low Counts of Faecalibacterium prausnitzii in Colitis Microbiota [Internet]. Inflamm Bowel Dis 2009; 15:1183-9. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=edswsc&AN=000268869000012&site=eds-live; PMID:19235886; http://dx.doi.org/ 10.1002/ibd.20903 [DOI] [PubMed] [Google Scholar]
- [16].Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev [Internet] 1990; 3:66-98. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC358141/; PMID:2404569; http://dx.doi.org/ 10.1128/CMR.3.1.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rajilić–Stojanović M, Biagi E, Heilig HGHJ, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology [Internet] 2011; 141:1792-801. Available from: http://www.gastrojournal.org/article/S0016-5085(11)01076-6/abstract; PMID:21820992; http://dx.doi.org/ 10.1053/j.gastro.2011.07.043 [DOI] [PubMed] [Google Scholar]
- [18].Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-Induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 2012; 4:1095-119; PMID:23016134; http://dx.doi.org/ 10.3390/nu4081095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fremont M, Coomans D, Massart S, De Meirleir K. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe [Internet] 2013; 22:50-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23791918; PMID:23791918; http://dx.doi.org/ 10.1016/j.anaerobe.2013.06.002 [DOI] [PubMed] [Google Scholar]
- [20].Dunlop RH, Hammond PB. D-lactic acidosis of ruminants. Ann N Y Acad Sci [Internet] 1965; 119:1109-32. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=5216443&site=ehost-live; PMID:5216443; http://dx.doi.org/ 10.1111/j.1749-6632.1965.tb47466.x [DOI] [PubMed] [Google Scholar]
- [21].Tappenden KA. Pathophysiology of short bowel syndrome: considerations of resected and residual anatomy. JPEN J Parenter Enteral Nutr [Internet] 2014; 38:14S-22S. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=24500909&site=ehost-live; PMID:24500909; http://dx.doi.org/ 10.1177/0148607113520005 [DOI] [PubMed] [Google Scholar]
- [22].Sheedy JR, Wettenhall REH, Scanlon D, Gooley PR, Lewis DP, McGregor N, Stapleton DI, Butt HL, De Meirleir KL. Increased d-lactic Acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo [Internet] 2009; 23:621-8. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=19567398&site=ehost-live; PMID:19567398 [PubMed] [Google Scholar]
- [23].Uribarri J, Oh MS, Carroll HJ. D-lactic acidosis. A review of clinical presentation, biochemical features, and pathophysiologic mechanisms. Medicine (Baltimore) [Internet] 1998; 77:73-82. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=9556700&site=eds-live; PMID:9556700; http://dx.doi.org/ 10.1097/00005792-199803000-00001 [DOI] [PubMed] [Google Scholar]
- [24].de Vrese M, Koppenhoefer B, Barth CA. D-lactic acid metabolism after an oral load of DL-lactate. Clin Nutr [Internet] 1990; 9:23-8. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=16837316&site=ehost-live; PMID:16837316 [DOI] [PubMed] [Google Scholar]
- [25].Hove H, Mortensen PB. Colonic lactate metabolism and D-lactic acidosis. Dig Dis Sci [Internet] 1995; 40:320-30. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=7851197&site=ehost-live; PMID:7851197; http://dx.doi.org/ 10.1007/BF02065417 [DOI] [PubMed] [Google Scholar]
- [26].Petersen C. D-lactic acidosis. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr [Internet] 2005; 20:634-45. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=16306301&site=ehost-live; http://dx.doi.org/ 10.1177/0115426505020006634 [DOI] [PubMed] [Google Scholar]
- [27].Caldarini MI, Pons S, D'Agostino D, DePaula JA, Greco G, Negri G, Ascione A, Bustos D. Abnormal fecal flora in a patient with short bowel syndrome. An in vitro study on effect of pH on D-lactic acid production. Dig Dis Sci [Internet] 1996; 41:1649-52. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=8769294&site=ehost-live; PMID:8769294; http://dx.doi.org/ 10.1007/BF02087915 [DOI] [PubMed] [Google Scholar]
- [28].Day AS, Abbott GD. D-lactic acidosis in short bowel syndrome. N Z Med J [Internet] 1999; 112:277-8. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=10472893&site=ehost-live; PMID:10472893 [PubMed] [Google Scholar]
- [29].Kaneko T, Bando Y, Kurihara H, Satomi K, Nonoyama K, Matsuura N. Fecal microflora in a patient with short-bowel syndrome and identification of dominant lactobacilli. J Clin Microbiol [Internet] 1997; 35:3181-5. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=9399516&site=ehost-live; PMID:9399516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kowlgi NG, Chhabra L. D-lactic acidosis: an underrecognized complication of short bowel syndrome. Gastroenterol Res Pract [Internet] 2015; 2015:476215. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=25977687&site=ehost-live; PMID:25977687; http://dx.doi.org/ 10.1155/2015/476215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature [Internet] 2014; 509:282-3. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=mnh&AN=248345&site=eds-live [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research [Internet]. Neurosci Biobehav Rev 2014; 40:1-5. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=edswsc&AN=000333786900001&site=eds-live; PMID:24456941; http://dx.doi.org/ 10.1016/j.neubiorev.2014.01.001 [DOI] [PubMed] [Google Scholar]
- [33].Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010; 90:859-904; PMID:20664075; http://dx.doi.org/ 10.1152/physrev.00045.2009 [DOI] [PubMed] [Google Scholar]
- [34].Preidis GA, Hotez PJ. doi: 10.1371/journal.pntd.0003382. (2015) The Newest “Omics”—Metagenomics and Metabolomics—Enter the Battle against the Neglected Tropical Diseases. PLoS Negl Trop Dis 9(2): e0003382. doi:10.1371/ journal.pntd.0003382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fritz CO, Morris PE, Richler JJ. Effect size estimates: Current use, calculations, and interpretation. J Exp Psychol Gen [Internet] 2012; 141:2-18. Available from: http://0-search.ebscohost.com.library.vu.edu.au/login.aspx?direct=true&db=psyh&AN=2011-16756-001&site=eds-live; PMID:21823805; http://dx.doi.org/ 10.1037/a0024338 [DOI] [PubMed] [Google Scholar]