Abstract
Background
NUT midline carcinoma (NMC) is a rare and aggressive genetically characterized subtype of squamous cell carcinoma frequently arising from the head and neck (HN). HNNMC characteristics and optimal management are unclear.
Methods
We performed a retrospective review of all known cases of HNNMC in the International NMC Registry, data as of December 31, 2014. Of 48 consecutive patients treated from 1993–2014, clinicopathologic variables and outcomes from 40 patients were available for analyses, the largest cohort of HN NMC studied to date. Overall survival (OS) and progression-free survival (PFS) according to patient characteristics and treatment were analyzed.
Results
We identified a five-fold increase in diagnosis of HNNMC from 2011 to 2014. Median age was 21.9 years (range 0.1–81.7), male:female was 40%:60%, and 86% had BRD4-NUT fusion. Initial treatment was initial surgery (S) +/− adjuvant chemoradiation (CRT) or adjuvant radiation (RT) (56%), initial RT +/− chemotherapy (C) (15%), or initial C +/− S or RT (28%). Median PFS was 6.6 months (range 4.7–8.4). Median OS was 9.7 months (range 6.6–15.6). Two-year PFS was 26% (95% CI, 13%–40%). Two-year OS was 30% (95% CI, 16%–46%). Initial S +/− post-operative CRT or RT (p=0.04), and complete resection with negative margins (p=0.01) were significant predictors of improved OS even after adjustment for age, tumor size and neck lymphadenopathy. Initial RT or C, and NUT translocation type were not associated with outcome.
Conclusions
HNNMC portends a poor prognosis. Aggressive initial surgical resection +/− post-operative CRT or RT was associated with significantly enhanced survival. C or RT alone is often inadequate.
Keywords: NUT protein, BRD4, BRD3, NUT midline carcinoma, head and neck cancer
Introduction
NUT midline carcinoma (NMC) is a rare and aggressive genetically defined subtype of squamous cell carcinoma characterized by chromosomal rearrangement of the NUT gene (a.k.a. NUTM1, Chr15orf55).1 The characteristic somatic t(15:19) translocation that positions NUT in-frame with BRD4, a ubiquitously expressed transcriptional coactivator, was first identified in 2003.2 About 30% of NMCs lack BRD4 rearrangement and are termed NUT variants, whereby NUT is often fused to BRD3,3 or NUT is fused to a non-BRD gene such as NSD3.4 NMCs are poorly differentiated tumors that display variable degrees of squamous differentiation. Diagnosis is made by demonstration of NUT rearrangement by molecular analysis, including reverse transcriptase-PCR (RT-PCR), fluorescence in situ hybridization (FISH), or cytogenetic analysis.1 Alternatively, an immunohistochemical (IHC) stain with monoclonal antibody to NUT (C52, Cell Signaling) has been shown to be 100% specific and 87% sensitive5 for the diagnosis of NMC. Thus, a positive NUT IHC stain by itself is diagnostic of NMC.6
NMC is considered the most clinically aggressive type of squamous carcinoma and the majority of patients will succumb to rapid disease progression with early metastases to locoregional and distant sites. Over 80 % of patients will die within one year of diagnosis despite intensive treatment, underscoring the need for effective treatment of this disease.7 NMC typically arises from the midline structures of the thorax, or from the head and neck (HN). NMC was initially described in children and adolescents, however in recent years there appears to be an increasing diagnosis in adults.7 The actual NMC incidence is unclear, and is almost certainly under diagnosed. Up to 18% of undifferentiated carcinomas of the head and neck are in fact NMC.8, 9
In vitro studies of NMC have demonstrated that NUT fusion proteins drive tumor growth and blockade of differentiation through aberrant histone acetylation in a manner dependent on the targeting of MYC and TP63 genes by BRD bromodomains.3, 10–12 In a unique mechanism, the acetyl-histone binding bromodomains of BRD4 tether NUT to chromatin, driving acetylation of massive regions of chromatin through recruitment of p300, a histone acetyl-transferase, by NUT. These so-called ‘megadomains’ trigger transcription of underlying genes both directly and through activation of their entire regulatory domains, including enhancers, enforcing the expression of key oncoproteins such as MYC, p63, and MED24.10 Small molecule BET bromodomain inhibitors targeting BRD4-NUT are in development and are enrolling NMC patients in clinical trials. While an initial report indicates that at least one of these these drugs, OTX015 (OncoEthix/Merck), appears to have some efficacy as single agent in human NMC, it is unclear at this stage, due to limited patient numbers and lack of clinical trial data, whether this drug class alone is more effective than other conventional strategies.13
For over a decade, we have served as the primary diagnostic center for NMC, and in 2010 we established an international NMC Registry to analyze clinical and pathologic data in aggregate to inform natural history, therapeutic interventions and outcomes. We previously reported on 63 cases of NMC in which the overall median survival was 6.7 months.7 Slightly better outcomes were observed for patients with HNNMC (n=19) compared to thoracic NMC in that cohort.7 Since 2012 the registry has accrued more NMC cases, particularly arising from the head and neck (n=29). To define preferred management strategies, we sought to determine the clinical presentation, treatment and outcome of 48 patients with HNNMC from the Registry. The clinico-pathologic features, treatment regimens and outcomes of 40 evaluable patients with HNNMC are reported herein.
Materials and Methods
NUT midline carcinoma registry
The International NMC Registry was created in 2010, and NMC patients in this study who were enrolled were identified by referral as part of consultation for clinical care either for diagnosis or treatment. Patients were not identified by literature searches or added from published cases. NMC patients prior to 2010 were identified similarly through referral, or through screening of archival pathology specimens at one of our institutions,9, 14 and retrospectively enrolled into the NMC Registry. The number of patients retrospectively enrolled before 2010 is 19, and patients prospectively enrolled 2010 or later is 29. The Registry is international; enrolled patients have come from North America, South America, Europe, Asia, and Australia. There are no geographic restrictions to enrollment in the Registry.
Patients
From January 1993 to December 2014, we identified 48 patients with HNNMC amongst a total of 107 patients (45%) in the International NMC Registry. HNNMC patients were defined as those with tumors originating in the head and neck, exhibiting aberrant NUT expression demonstrated by IHC, NUT rearrangement shown by fluorescent in situ hybridization (FISH), BRD4-NUT fusion by reverse transcriptase (RT)-PCR, or cytogenetic t(15;19) in the setting of carcinoma.1 Histology and immunohistochemistry was reviewed for all cases by Dr. French. Of these 48 patients, 19 were reported in our previous study7 and 29 were not previously reported.
A registry questionnaire was sent to treating physicians inquiring about demographic, clinical, treatment, and outcome variables. Outcome data were provided for 40 patients. Approval for the International NMC Registry (www.nmcregistry.org), including the retrospective and prospective analysis of NMC patient data, was obtained from the Institutional Review Board of the Dana-Farber Cancer Institute (Boston, MA). Written informed consent was obtained from all study participants.
Patient data including demographics, clinical staging data (site of primary tumor, lymph node involvement, and location of metastasis), therapeutic interventions, and response to treatment were abstracted from questionnaires completed by the treating physician and analyzed in aggregate. Initial therapy was defined as treatment administered from initial diagnosis until first relapse or progression. Surgical extent was classified as complete resection with negative margins (R0 resection), gross total resection (resection of all gross visible disease however microscopic residual disease present), debulking (gross residual disease present). Chemotherapy was categorized into regimens containing either platinum, or regimens containing anthracyclines and nonplatinum alkylating agents. Progression-free survival (PFS) was measured as the time from initial diagnosis of NMC until the time of first disease relapse, progression or death, or until last contact if none of these events occurred. Overall survival (OS) was defined as the time from diagnosis until the time of death or until last contact. Clinical responses to initial therapies were classified as complete or partial responses, stable disease, or progressive disease according to the clinical judgment of the treating physician. Confirmation of diagnosis was obtained by pathology reports and actual histology was reviewed when available. Cases were classified into 3 histopathologic categories: carcinoma with squamous differentiation, carcinoma without squamous differentiation, and other histology.
Statistical analysis
Analyses were conducted to investigate factors potentially associated with PFS or OS, using Kaplan Meier plots and log-rank tests. Two-year PFS and OS point estimates were reported with 95% confidence intervals (CI). Cox proportional hazards regression modeling to predict PFS and OS was conducted to generate unadjusted and adjusted results where individual predictors of PFS and OS were analyzed with and without simultaneously including age and tumor size in the model. Hazard ratios and 95% CI’s were reported. SAS version 9.4 was used.
Results
Demographic and Tumor Characteristics
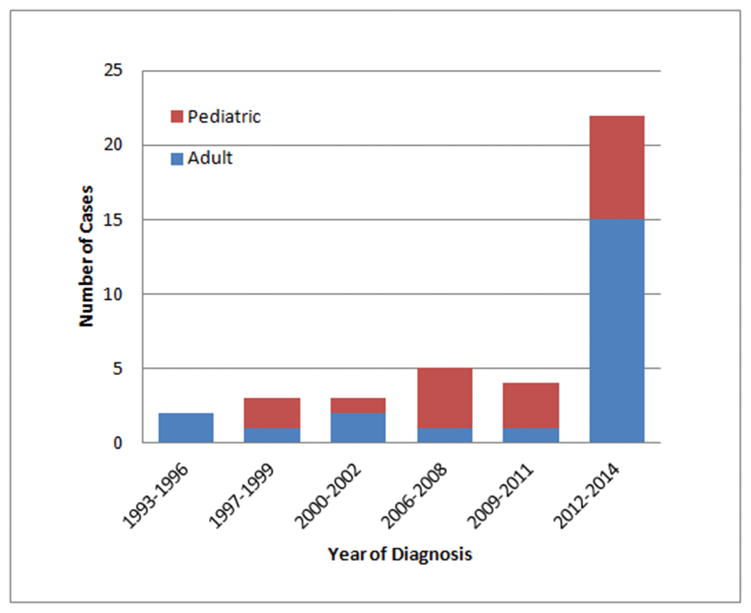
Demographic and tumor characteristics were available for all 48 patients. The diagnosis of HNNMC has increased dramatically since 2012 with an increasing proportion of adult cases (diagnosed at 18 years or older) (Figure 1). Median age was 21.9 years (range 0.1–81.7), and there was a female predominance (1.5:1). Tumor site was sinonasal origin in 57% and other sites included nasopharynx (n=3), oropharynx (n=1), hypopharynx (n=1), larynx (n=1), salivary gland (n=2) and unknown primary (n=5). The BRD4-NUT fusion was found in 86%. Histology was classified in 49% of patients as carcinoma with squamous differentiation, whereas 43% had carcinoma without squamous differentiation and 8% had other histology. Regarding NMC diagnosis, we found that 16 of 46 (35%) patients for whom the initial and final diagnoses are known, were in fact initially diagnosed with NMC. The original diagnosis in the remaining 30 (65%) patients are listed in Supplemental Table 1 and included most commonly ‘poorly differentiated carcinoma’ (n=8), ‘poorly differentiated squamous carcinoma’ (n=6) and sinonasal undifferentiated carcinoma (n=5). At diagnosis, 26% had regional nodal metastases, 6% had distant metastases and 12% had both. The average primary tumor size was 5.2cm at diagnosis.
Figure 1.
Diagnosis of HNNMC in adult and pediatric patients per year.
Treatment
Details of treatment were available for 39 of 48 patients. All patients received surgery, radiation or chemotherapy either as single agents, or in combination as part of their initial management. Because no established treatment regimen exists for NMC, treatment was selected based on physician discretion and individual factors. Of the 24 patients who underwent surgery, five patients had complete tumor resection with negative margins, nine had gross total resection, and 10 underwent debulking (subtotal resection). Twenty-nine patients received radiotherapy. Thirty-three patients received chemotherapy, and of these, 27 received a platinum agent.
The majority of patients received intensive initial multimodality therapy (n=28, 72%) consisting of various combinations of surgery, chemotherapy and radiotherapy. For the purposes of this study, treatment was classified into three main categories according to the initial sequencing strategy of therapeutic modalities: 1) Initial surgery (S) with or without adjuvant chemoradiation (CRT) or adjuvant radiation (RT) (n=22, 56%), 2) initial RT with or without concurrent chemotherapy (CRT) (n=6, 15%), or 3) initial chemotherapy alone or followed by S, RT or CRT (n=11, 28%). Of the 22 patients who underwent initial surgery, one patient had surgery alone without adjuvant therapy, 19 received post-operative adjuvant chemoradiation with concurrent chemotherapy utilizing agents such as cisplatin, and two patients received post-operative adjuvant radiation alone. Six patients underwent initial definitive radiation based therapy: three of these patients received radiation alone, and three patients received radiation concurrent with chemotherapy. Eleven patients received initial chemotherapy: seven received chemotherapy alone, two received subsequent surgery, one received subsequent radiation alone, and one went on to receive chemoradiation.
Outcomes
Outcome data were available for 40 of 48 cases (Table 1). Median progression-free survival (PFS) was 6.6 months (range 4.7–8.4). Median overall survival (OS) was 9.7 months (range 6.6–15.6). The 2-year PFS was 26% (95% CI, 13–40). The 2-year OS was 30% (95% CI, 16–46). Median follow-up was 8.3 months (range 2.1–30.0) for the entire cohort, and 19.2 months (range 2.0–79.0) for living patients. There was no statistically significant difference in PFS or OS by age, gender, tumor location, size, histology, presence of neck lymph node involvement, or BRD4-NUT translocation (Table 1).
Table 1.
Estimated two-year PFS and OS by patient characteristics and treatment
| N | 2y PFS % (95% CI) | P* | 2y OS % (95% CI) | P* | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | 40 | 26 (13, 40) | 30 (16, 46) | |||
|
| ||||||
| Age | 17 years or younger | 16 | 31 (11, 54) | 0.35 | 41 (17, 65) | 0.11 |
| 18 years or older | 22 | 19 (6, 38) | 21 (7, 41) | |||
|
| ||||||
| Sex | Female | 24 | 31 (14, 50) | 0.41 | 38 (18, 57) | 0.42 |
| Male | 16 | 19 (5, 40) | 23 (7, 46) | |||
|
| ||||||
| Site | Nasal cavity & Paranasal sinus | 17 | 26 (8, 48) | 0.24 | 23 (7, 46) | 0.08 |
| Other | 12 | 42 (15, 67) | 49 (15, 77) | |||
|
| ||||||
| Size | Less than 6cm | 18 | 33 (14, 55) | 0.17 | 42 (20, 64) | 0.24 |
| 6cm or greater | 10 | 12 (1, 40) | 22 (3, 51) | |||
|
| ||||||
| Histology | Carcinoma with squamous diff | 14 | 36 (13, 59) | 0.61 | 36 (13, 59) | 0.84 |
| Carcinoma without squamous diff or other histology | 14 | 8 (1, 29) | 8 (0.5, 29) | |||
|
| ||||||
| NUT translocation | BRD4-NUT | 29 | 18 (7, 34) | 0.25 | 20 (7, 37) | 0.28 |
| BRD3-NUT | 2 | 100 (100, 100) | 100 (100, 100) | |||
| NSD3-NUT | 2 | 0 | 50 (1, 91) | |||
| NUT-variant | 1 | 0 | 0 | |||
|
| ||||||
| Distant metastases at diagnosis | Absent | 30 | 31 (16, 48) | 0.02 | 36 (18, 54) | 0.06 |
| Present | 5 | 0 | 0 | |||
|
| ||||||
| Neck lymph nodes at diagnosis | Absent | 24 | 35 (17, 54) | 0.26 | 39 (20 58) | 0.54 |
| Present | 13 | 15 (3, 39) | 23 (4, 51) | |||
|
| ||||||
| Initial Treatment strategy | Surgery +/− subsequent chemoradiation or radiation | 22 | 43 (22, 63) | 0.07 | 50 (27, 79) | 0.04 |
| Chemotherapy +/− subsequent surgery or radiation | 11 | 9 (1, 33) | 18 (3, 44) | |||
| Radiation +/− concurrent chemotherapy | 6 | 0 | 0 | |||
|
| ||||||
| Surgery | Yes | 24 | 44 (23, 63) | 0.005 | 50 (28, 69) | 0.003 |
|
|
|
|||||
| No | 15 | 0 | 7 (0, 26) | |||
|
| ||||||
| Extent of surgical resection | None | 15 | 0 | 0.01 | 7 (0, 26) | 0.01 |
|
|
|
|||||
| Debulking (subtotal resection) | 10 | 23 (4, 52) | 37 (7, 69) | |||
|
|
|
|||||
| Gross total | 9 | 44 (14, 72) | 44 (14, 72) | |||
|
|
|
|||||
| Complete with negative margins (R0) | 5 | 80 (20, 97) | 80 (20, 97) | |||
|
| ||||||
| Extent of margins | No negative margins | 18 | 24 (8, 46) | 0.03 | 30 (9, 55) | 0.06 |
|
|
|
|||||
| Negative margins | 5 | 80 (20, 97) | 80 (20, 97) | |||
|
| ||||||
| Radiation | Yes | 29 | 29 (14, 46) | 0.37 | 34 (16, 53) | 0.12 |
|
|
|
|||||
| No | 10 | 20 (3, 48) | 20 (3, 48) | |||
|
| ||||||
| Chemotherapy | Yes | 33 | 25 (12, 41) | 0.79 | 30 (14, 48) | 0.70 |
|
|
|
|||||
| No | 6 | 33 (5, 68) | 33 (5, 68) | |||
|
| ||||||
| Chemotherapy type | Anthracycline | 6 | 33 (5, 68) | 0.76 | 67 (20, 90) | 0.56 |
|
|
|
|||||
| No Anthracycline | 25 | 25 (10, 43) | 23 (7, 43) | |||
|
| ||||||
| Platinum | 27 | 23 (10, 41) | 0.59 | 25 (10, 45) | 0.49 | |
|
|
|
|||||
| No Platinum | 6 | 33 (4, 67) | 50 (11, 80) | |||
|
| ||||||
| Best response to initial treatment strategy | Complete Response | 10 | 80 (41, 95) | <0.0001 | 80 (41, 95) | <0.0001 |
|
|
|
|||||
| Partial Response | 9 | 11 (1, 39) | 35 (6, 67) | |||
|
|
|
|||||
| Stable Disease | 0 | 0 | 0 | |||
|
|
|
|||||
| Progression | 19 | 5 (0, 21) | 5 (0, 21) | |||
by log-rank test
The presence of distant metastases was associated with a 2-year PFS and 2-year OS of 0%. The pattern of treatment failure at first relapse or progression was evaluable for 21 cases; three patients (14%) had isolated locoregional disease, seven (33%) had isolated distant disease, and 11 (52%) developed both locoregional and distant disease.
Clinical response to initial therapy was reported in 38 patients. Best response to initial therapy was complete response in 10 patients, partial response in nine patients, and progressive disease in 19 patients. Of the 10 patients with complete response, eight patients were alive at last follow up with no evidence of disease (NED): one patient had surgery alone (NED at 23 months), one patient had surgery then adjuvant radiation (NED at 72 months), and six patients had surgery followed by post-operative adjuvant chemoradiation (NED at 14, 15, 17, 18, 35, 78 months). The remaining two patients with complete response to initial therapy had progression at 6 months after diagnosis and died from disease (OS 8 and 9 months) and both patients had completed treatment with surgery followed by post-operative adjuvant chemoradiation. By comparison, of the nine patients with partial response, only three were alive at last follow-up, and of the 19 patients with progressive disease, none were alive at last follow-up. Best response to initial therapy was associated with a statistically significantly higher PFS (p<0.0001) and OS (p<0.0001) (Table 1). Response was not significantly associated with age, gender, tumor histology, NUT translocation subtype, tumor location or neck lymph node involvement.
Impact of Therapy
Surgical resection, the extent of surgical resection, negative margins, and best response to initial treatment strategy were significantly associated with improved PFS and OS (Table 1) in our retrospective analysis of this small patient cohort. Patients who underwent surgery had a 2-year OS of 50%, whereas those who did not had a 2-year OS of 7%, p=0.003. Notably, the extent of surgical resection was significantly associated with PFS and OS (Table 1) in a graded fashion; the 2-year OS for patients who achieved negative margins was 80%, gross total resection with positive margins was 44%, debulking was 37% and no surgery was 7% (Table 1). Radiotherapy and chemotherapy, including the type of chemotherapy (anthracycline or cisplatin), was not associated with differences in PFS or OS.
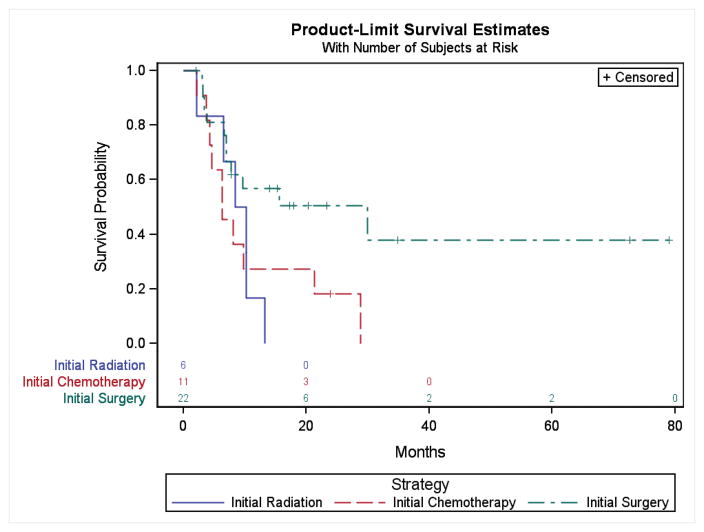
The sequencing of the initial treatment strategy was also statistically significantly associated with survival. Patients who underwent initial surgery with or without subsequent radiation based therapy had a 2-year OS of 50% (11/22) (95% CI 27–79%), whereas patients who had initial chemotherapy followed by subsequent surgery or radiation had a 2-year OS of 18% (2/11) (95% CI 3–44%), and patients who underwent initial radiation with or without chemotherapy had a 2-year OS of 0% (0/6) (p=0.04) (Figure 2).
Figure 2.
Overall survival according to initial treatment strategy. The probability of OS is presented for patients with HNNMC according to the initial treatment strategy consisting of either initial radiation, initial chemotherapy or initial surgery.
To identify factors independently prognostic of PFS and OS, a multivariate analysis of selected predictors of PFS and OS was performed with and without adjustment for age and tumor size (Table 2). The initial treatment strategy incorporating initial surgery remained predictive of OS even after adjustment for age and tumor size (HR=0.35 (95% CI 0.13–0.90) p=0.03) (Table 2). Initial surgery was also independently prognostic for OS (HR=0.36 (95% CI 0.15–0.83) p=0.01) and PFS (HR=0.24 (95% CI 0.09–0.68) (p=0.007) after adjustment for neck lymph node involvement. Complete resection with negative margins was also independently predictive of PFS and OS after adjustment for age and tumor size (Table 2).
Table 2.
Hazard Ratios for selected predictors for PFS and OS, unadjusted and adjusted for age and tumor size
| PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR* (95% CI) | P value | Adjusted HR** (95% CI) | P value | Unadjusted HR* (95% CI) | P value | Adjusted HR** (95% CI) | P value | |
| Age 17 years or younger | 0.70 (0.33, 1.50) | 0.36 | 0.51 (0.22, 1.17) | 0.11 | ||||
| Tumor size 6cm or greater | 1.87 (0.76, 4.59) | 0.17 | 1.75 (0.68, 4.49) | 0.24 | ||||
| Neck lymph nodes | 1.54 (0.72, 3.32) | 0.27 | 2.09 (0.83, 5.30) | 0.12 | 1.28 (0.58, 2.82) | 0.54 | 1.85 (0.68, 5.05) | 0.23 |
| Male | 1.35 (0.66, 2.78) | 0.41 | 1.6 (0.52, 4.91) | 0.41 | 1.36 (0.65, 2.86) | 0.42 | 1.32 (0.41, 4.32) | 0.64 |
| Nasal cavity & Paranasal sinus location | 1.74 (0.68, 4.48) | 0.25 | 2.25 (0.54, 9.28) | 0.26 | 2.48 (0.86, 7.12) | 0.09 | 8.90 (1.27, 62.50) | 0.03 |
| Carcinoma with squamous differentiation histology | 0.80 (0.34, 1.88) | 0.61 | 1.06 (0.32, 3.57) | 0.92 | 0.92 (0.39, 2.14) | 0.84 | 1.14 (0.33, 3.91) | 0.83 |
| Distant Metastases | 3.16 (1.14, 8.77) | 0.03 | 2.82 (0.89, 8.92) | 0.08 | 2.74 (0.90, 8.30) | 0.08 | 2.31 (0.62, 8.59) | 0.21 |
| Initial surgery +/− subsequent chemoradiation or radiation | 0.42 (0.20, 0.90) | 0.03 | 0.44 (0.18, 1.09) | 0.07 | 0.38 (0.17, 0.84) | 0.02 | 0.35 (0.13, 0.90) | 0.03 |
| Complete resection with negative margins (R0) | 0.27 (0.11, 0.68) | 0.005 | 0.33 (0.12, 0.89) | 0.03 | 0.29 (0.12, 0.74) | 0.01 | 0.32 (0.12, 0.88) | 0.03 |
| Partial resection | 1.74 (0.65, 4.67) | 0.27 | 2.43 (0.64, 9.19) | 0.19 | 1.59 (0.55, 4.59) | 0.39 | 2.34 (0.46, 11.95) | 0.31 |
| Anthracycline chemotherapy | 0.77 (0.29, 2.05) | 0.6 | 1.05 (0.24, 4.58) | 0.95 | 0.66 (0.22, 1.97) | 0.45 | 0.87 (0.16, 4.61) | 0.88 |
| Platinum chemotherapy | 0.79 (0.33, 1.88) | 0.6 | 0.79 (0.19, 3.34) | 0.74 | 1.01 (0.41, 2.53) | 0.98 | 1.13 (0.21, 5.94) | 0.89 |
Single predictor in the model
Single predictor plus age and tumor size in the model.
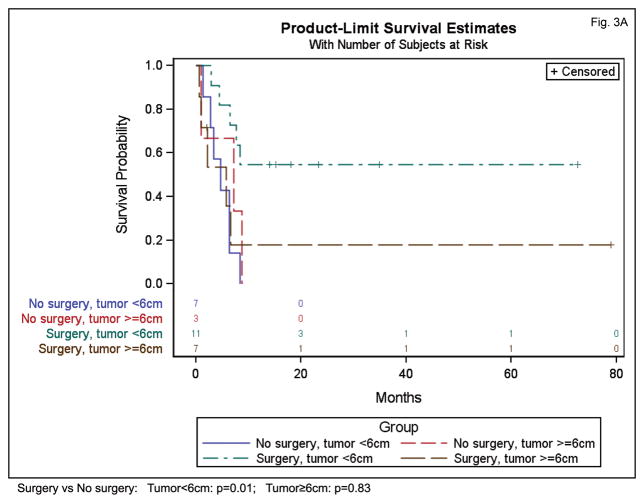
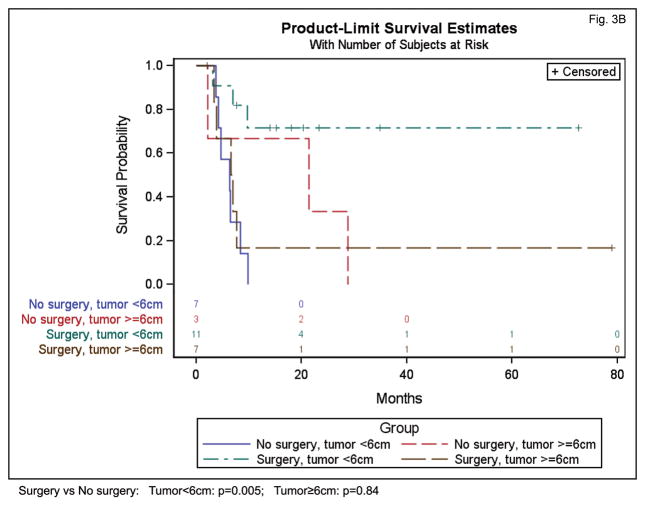
In an exploratory analysis, we examined the interaction between initial surgery and tumor size to determine if the effect of surgery may depend on the size of the tumor. An interaction between initial surgery and tumor size was present for PFS (p=0.06) and OS (p=0.02), indicating that the impact of initial surgery depended on tumor size. In our patient cohort, initial surgery appeared beneficial for smaller tumors but not for larger tumors. For example, for tumors under 6 cm in size, initial surgery was associated with significantly higher PFS (HR=0.21 (95% CI 0.06–0.69) p=0.01) and OS (HR=0.13 (95% CI 0.03–0.5) p=0.005) (Figure 3). For tumors 6 cm and larger, initial surgery was not associated with a significant protective effect on PFS (HR=1.17 (95% CI 0.27–5.16) p=0.83) or OS (HR=1.2 (95% CI 0.26–5.13), p=0.84) (Figure 3). While intriguing, we recognize that strong conclusions cannot be drawn due to the small sample size.
Figure 3.
Influence of surgical resection on PFS (A) and OS (B) for patients with HNNMC according to primary tumor size (smaller than 6cm, or greater than or equal to 6cm).
Discussion
NMC is an extremely aggressive and rare genetically defined subtype of squamous carcinoma arising from the head and neck in approximately 45% of cases. To define clinical presentation and optimal treatment approaches, we performed a retrospective analysis of all HNNMC cases in the International NMC Registry. Our study represents the largest cohort of HNNMC reported to date, as the existing literature regarding HNNMC has been primarily restricted to isolated case reports with limited treatment or follow up data.15–21 The frequency of diagnosis of HNNMC appears to be increasing, particularly since 2012, and the proportion of adults with this diagnosis is also rising. This may be an effect of reporting bias since the recent description of NMC in the head and neck8, 9, 22 and improved diagnostic availability of a simple highly sensitive and specific, immunohistochemical stain for the NUT gene product using a commercially available clinical antibody.5 The increasing diagnosis, coupled with the fact that nearly two-thirds of HNNMC cases in this cohort were initially misdiagnosed, suggests that NMC remains under recognized. We found that the most common incorrect initial histologic diagnoses preceding the subsequent diagnosis of HNNMC were poorly differentiated carcinoma (n=8) and poorly differentiated squamous carcinoma (n=6). This suggests that clinicians should consider NMC in any HN carcinoma with a poorly differentiated component, or with clinically aggressive behavior. Most HNNMC patients present with locally advanced disease and the average tumor size is over 5cm at the time of diagnosis. Unlike most HN cancers, HNNMC appears to affect women more than men for unclear reasons. Survival outcomes are poor with a median survival of 9.7 months, although this appears to be better than thoracic NMC by historical comparison.7
Survival appears to be impacted by treatment selection and initial therapeutic sequencing strategy. Initial surgical resection, and the extent of surgical resection (negative margins), was significantly associated with progression free and overall survival. In our cohort, surgical resection appeared to be beneficial for survival for most patients, particularly those with tumors less than 6 cm in size, and this association was independent of patient age and neck lymph node involvement. This data should be interpreted with caution given the limited size of this cohort, and lack of prospective comparison. It is possible that, because enrollment in the International NMC Registry is voluntary and does not involve systematic testing of all HN squamous cell carcinomas for NUT rearrangement, patients in this registry cohort may not fully represent the behavior of all HNNMCs.
Initial radiation or chemotherapy were not associated with significantly improved survival outcomes, however either may be important as adjuvant therapy. The incremental benefit and role of post-operative adjuvant therapy is unclear as all but one patient in our cohort who underwent surgical resection received post-operative adjuvant radiation or chemoradiation. Our findings are in keeping with accepted treatment paradigms used for other aggressive sinonasal tumors, such as sinonasal undifferentiated carcinomas (SNUC), in which better survival outcomes and local control rates are achieved when complete surgical resection is incorporated into multimodality treatment.23–25 HNNMC appears to be more aggressive (2-year OS rate 30% in our series) compared to SNUC (2-year OS rate of ~47%24, 5-year OS rate of 45%26), highlighting the need for prompt multidisciplinary evaluation in specialized centers (HN surgical, radiation and medical oncology) for all patients diagnosed with HNNMC.
Our findings reveal that initial treatment selection and sequencing in HNNMC is critical based on the observation that response to initial therapy was associated with improved survival. In our cohort, only 10 patients achieved complete response to initial therapy, all of whom underwent initial surgery. The only long term survivors in our series (survival of 35, 72, 78 months) were those who received initial surgery. Again, despite these compelling findings, interpretation should be made with caution given the small size and retrospective nature of the study.
No other clinical or pathologic features including NUT translocation type, or treatment approach, including type of chemotherapy regimen, were associated with survival outcomes. The lack of association of outcome with translocation type may be due to lack of sufficient statistical power, because the majority of patients in our series had a BRD4-NUT translocation (86%), leaving a minority with BRD3-NUT (n=2), NSD3-NUT (n=2) and NUT-variant (n=2) fusions. Notably two of the three long-term survivors had BRD3-NUT tumors, and a borderline statistically significant difference in PFS and OS was observed when comparing patients with BRD3-NUT to all other translocation types (p=0.05). In our series, many systemic therapy regimens including platinums, anthracyclines, alkylating agents in various combinations were used, however no agent was associated with improved outcomes.
The overall unsatisfactory treatment outcomes in this genomically driven tumor highlights the rationale for molecularly targeted therapies as a promising therapeutic strategy. Acetyl-histone mimetic drugs, termed BET or bromodomain inhibitors, act by competitively inhibiting binding of BRD-NUT to chromatin, preventing its ability to activate these oncogenic target genes.10, 27 First-in-class, direct-acting BET inhibitors are active in NMC xenograft models and humans.13, 27 At least three phase I clinical trials in the U.S. (NCT01587703, NCT01987362, NCT02431260) and Europe (NCT02259114, NCT01587703) are presently evaluating bromodomain inhibitors in patients with NMC. In addition, histone deacetylase inhibitors have exhibited pre-clinical and clinical activity in NMC and there is a clinical trial in the U.S. enrolling NMC patients who fail bromodomains inhibitors (NCT02307240).12, 28 If deemed effective, it will be critical to incorporate these novel agents into HNNMC treatment paradigms.
Supplementary Material
Acknowledgments
Funding sources: NIH grant (2R01CA124633) (C.A.F.)
We thank the patients and families for their enrollment into the Registry, and contribution of data and material without which this work would not be possible. We thank the treating oncologists and surgeons for agreeing to contribute to the Registry and completing the Registry questionnaires.
This work was supported by a grant from the NIH: 2R01CA124633 (Supplement to C.A.F.), which provided partial salary support for the clinical research coordinator (P.H.) and biostatistics (S.H.); as well as the NUT Midline Carcinoma Registry (www.NMCRegistry.org) in which all patient data was collected and stored (to N.G.C., D.E.B., C.S.L., C.R.G., S.E.S., J.E.B., and C.A.F.). The NIH did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The members of the NUT Midline Carcinoma Registry were involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Conflicts of interest: none
Author Contributions: Nicole G. Chau**: Conceptualization, methodology, software, validation, investigation, resources, data curation, writing–original draft, writing–review and editing, visualization, supervision, and project administration. Shelley Hurwitz: Methodology, software, validation, formal analysis, data curation, writing–review and editing, visualization and project administration. Chelsey M. Mitchell: Conceptualization, methodology, investigation, data curation, and writing–review and editing. Alexandra Aserlind: Methodology, investigation and data curation, and writing–review and editing. Noam Grunfeld: Methodology, investigation, data curation, and writing–review and editing. Leah Kaplan: Investigation, and writing–review and editing. Peter Hsi: Investigation, and writing–review and editing. Daniel E. Bauer: Conceptualization, methodology, writing–review and editing, and visualization. Christopher S. Lathan: Conceptualization, methodology, writing–review and editing, and visualization. Carlos Rodriguez-Galindo: Conceptualization, methodology, writing–review and editing, and visualization. Roy B. Tishler: Conceptualization, methodology, writing–review and editing, and visualization. Robert I. Haddad, MD: Conceptualization, methodology, writing–review and editing, and visualization. Stephen E. Sallan: Conceptualization, methodology, writing–review and editing, visualization, and supervision. James E. Bradner: Conceptualization, methodology, writing–review and editing, and visualization. Christopher A. French**: Conceptualization, methodology, validation, investigation, resources, data curation, writing–original draft, writing–review and editing, visualization, supervision, project administration and funding acquisition. (**N.G.C. and C.A.F. are responsible for the overall content)
References
- 1.French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol. 2012;7:247–265. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 2.French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–307. [PubMed] [Google Scholar]
- 3.French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 4.French CA, Rahman S, Walsh EM, et al. NSD3-NUT Fusion Oncoprotein in NUT Midline Carcinoma: Implications for a Novel Oncogenic Mechanism. Cancer Discov. 2014;4:928–941. doi: 10.1158/2159-8290.CD-14-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT Midline Carcinoma Using a NUT-specific Monoclonal Antibody. Am J Surg Pathol. 2009;33:984–991. doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chirieac LR, French CA, Sholl L, Yatabi Y WHO. Classification of Tumours of Lung, Pleura, Thymus and Heart. Lung: Other and unclassified carcinomas. (4th) 2015:97–98. [Google Scholar]
- 7.Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18:5773–5779. doi: 10.1158/1078-0432.CCR-12-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36:1216–1221. doi: 10.1097/PAS.0b013e318254ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 10.Alekseyenko AA, Walsh EM, Wang X, et al. The oncogenic BRD4-NUT chromatin regulator drives aberrant transcription within large topological domains. Genes Dev. 2015;29:1507–1523. doi: 10.1101/gad.267583.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayson AR, Walsh EM, Cameron MJ, et al. MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene. 2014;33:1736–1742. doi: 10.1038/onc.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz BE, Hofer MD, Lemieux ME, et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res. 2011;71:2686–2696. doi: 10.1158/0008-5472.CAN-10-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stathis AZE, Bekradda M, Gomez-Roca C, Delord JP, de La Motte Rouge T, Uro-Coste E, de Braud F, Pelosi G, French CA. Clinical Response of Carcinomas Harboring the BRD4-NUT Oncoprotein to the Targeted Bromodomain Inhibitor OTX015/MK-8628. Cancer Discov. 2016 doi: 10.1158/2159-8290.CD-15-1335. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22:4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 15.den Bakker MA, Beverloo BH, van den Heuvel-Eibrink MM, et al. NUT Midline Carcinoma of the Parotid Gland With Mesenchymal Differentiation. Am J Surg Pathol. 2009;33:1253–1258. doi: 10.1097/PAS.0b013e3181abe120. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh MS, French CA, Liang CW, Hsiao CH. NUT midline carcinoma: case report and review of the literature. Int J Surg Pathol. 2011;19:808–812. doi: 10.1177/1066896909353600. [DOI] [PubMed] [Google Scholar]
- 17.Rutt AL, Poulik J, Siddiqui AH, et al. NUT midline carcinoma mimicking tonsillitis in an eight-year-old girl. Ann Otol Rhinol Laryngol. 2011;120:546–549. doi: 10.1177/000348941112000810. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S, Kurabe N, Minato H, et al. A rare Japanese case with a NUT midline carcinoma in the nasal cavity: a case report with immunohistochemical and genetic analyses. Pathol Res Pract. 2014;210:383–388. doi: 10.1016/j.prp.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Vargas SO, French CA, Faul PN, et al. Upper respiratory tract carcinoma with chromosomal translocation 15;19: evidence for a distinct disease entity of young patients with a rapidly fatal course. Cancer. 2001;92:1195–1203. doi: 10.1002/1097-0142(20010901)92:5<1195::aid-cncr1438>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Vulsteke C, Lurquin E, Debiec-Rychter M, et al. First evidence of treatment efficacy in metastatic carcinoma of the parotid gland with BRD4/NUT translocation. J Chemother. 2015 doi: 10.1179/1973947815Y.0000000046. 1973947815Y0000000046. [DOI] [PubMed] [Google Scholar]
- 21.Ziai J, French CA, Zambrano E. NUT gene rearrangement in a poorly-differentiated carcinoma of the submandibular gland. Head Neck Pathol. 2010;4:163–168. doi: 10.1007/s12105-010-0174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stelow EB, French CA. Carcinomas of the upper aerodigestive tract with rearrangement of the nuclear protein of the testis (NUT) gene (NUT midline carcinomas) Adv Anat Pathol. 2009;16:92–96. doi: 10.1097/PAP.0b013e31819923e4. [DOI] [PubMed] [Google Scholar]
- 23.Chen AM, Daly ME, El-Sayed I, et al. Patterns of failure after combined-modality approaches incorporating radiotherapy for sinonasal undifferentiated carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2008;70:338–343. doi: 10.1016/j.ijrobp.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 24.Musy PY, Reibel JF, Levine PA. Sinonasal undifferentiated carcinoma: the search for a better outcome. Laryngoscope. 2002;112:1450–1455. doi: 10.1097/00005537-200208000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Reiersen DA, Pahilan ME, Devaiah AK. Meta-analysis of treatment outcomes for sinonasal undifferentiated carcinoma. Otolaryngol Head Neck Surg. 2012;147:7–14. doi: 10.1177/0194599812440932. [DOI] [PubMed] [Google Scholar]
- 26.Gray ST, Herr MW, Sethi RK, et al. Treatment outcomes and prognostic factors, including human papillomavirus, for sinonasal undifferentiated carcinoma: a retrospective review. Head Neck. 2015;37:366–374. doi: 10.1002/hed.23606. [DOI] [PubMed] [Google Scholar]
- 27.Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maher OM, Christensen AM, Yedururi S, Bell D, Tarek N. Histone deacetylase inhibitor for NUT midline carcinoma. Pediatr Blood Cancer. 2015;62:715–717. doi: 10.1002/pbc.25350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.