Abstract
Background
Identifying highly sensitive and reliable neurological exam components are crucial in recognizing clinical deficiencies. This study aimed to investigate finger tapping performance differences between patients with CNS demyelinating lesions and healthy control subjects.
Methods
Twenty-three patients with multiple sclerosis or clinically isolated syndrome with infratentorial and/or cervical cord lesions on MRI, and 12 healthy controls were videotaped while tapping the tip of the index finger against the tip and distal crease of the thumb using both the dominant and non-dominant hand. Videos were assessed independently by 10 evaluators (three MS neurologists, four neurology residents, three advanced practice providers). Sensitivity and inter-evaluator reliability of finger tapping interpretations were calculated.
Results
A total of 1400 evaluations (four videos per each of the 35 subjects evaluated by 10 independent providers) were obtained. Impairments in finger tapping against the distal thumb crease of the non-dominant hand, identified by neurologists, had the greatest sensitivity (84%, p < 0.001) for detecting impairment. Finger tapping against the thumb crease was more sensitive than the thumb tip across all categories of providers. The best inter-evaluator reliability was associated with neurologists’ evaluations for the thumb crease of the non-dominant hand (kappa = 0.83, p < 0.001).
Conclusions
Impaired finger tapping against the distal thumb crease of the non-dominant hand was a more sensitive technique for detecting impairments related to CNS demyelinating lesions. Our findings highlight the importance of precise examinations of the non-dominant side where impaired fine motor control secondary to an upper motor injury might be detectable earlier than the dominant side.
Keywords: Finger tapping, Neurological examination, Upper motor neuron, Multiple sclerosis, Demyelinating, Magnetic resonance imaging
Background
The neurological examination remains the cornerstone of neuroanatomic localization and diagnosis of nervous system disorders, despite breakthrough advances in neuroimaging [1]. However, an unmet need exists for high quality research on neurological examination techniques aimed at identifying the most pragmatic, time-effective, and reliable components with a high degree of sensitivity for appreciating central nervous system (CNS) abnormalities that may be utilized by a wide variety of healthcare providers [2].
Repetitive rapid finger tapping is a common test of fine motor control of the upper extremities. Normal finger tapping requires the functional integrity of the corticospinal tract, cerebellar motor circuitry, and proprioceptive pathways [3]. Tasks involving the tapping of fingers, with varying techniques, have been widely studied in various domains such as neuropsychiatry and behavioral neurology (as a predictor of IQ and reaction time) [4, 5], traumatic brain injury and stroke (as an indicator of motor recovery) [6, 7], and perhaps most commonly in Parkinsonism (as an index of bradykinesia and hypokinesia) [8]. Upper extremity dysfunction has been reported in up to 80% of patients with multiple sclerosis (MS), the most common demyelinating disease of the central nervous system (CNS) [9]. Impairments of manual fine motor skills can significantly impact the quality of life of patients with MS. A number of studies have specifically examined upper extremity-related fine motor control tasks in patients with MS [10–12]. Evaluating upper extremity dysfunction is more difficult compared to the lower limbs given the higher complexity and multidimensionality of manual tasks [10]. The complex neural substrate for motor control in demyelinating diseases remains to be fully understood. Recent neuroimaging evidence from patients with long-standing MS suggests that the mean upper cervical cord area, and the presence of cervical and infratentorial lesions, appear to be predictors of motor dysfunction, having the potential to be easily used in the real-world clinic practice as predictive metrics for physical function [13].
Here, we studied the validity of the repetitive finger tapping test (in both the dominant and non-dominant hand) in subjects with and without inflammatory demyelinating lesions within the infratentorial region and cervical spinal cord. Three categories of providers (i.e. MS neurologists, neurology residents, and advanced practice providers (APPs)) with different levels of training and skills were included to account for inter-evaluator variability with respect to the interpretation of the finger tapping tests. We also assessed for performance impairments in tapping of the index finger against the thumb tip in comparison to the distal interphalangeal joint of the thumb (distal thumb crease), two different techniques commonly used within the clinical setting. We hypothesized that finger tapping is a sensitive test to identify CNS demyelinating lesions and its sensitivity could be affected by the technique used as well as the provider performing the test.
Methods
Design and study population
This study included 23 adult patients (age > 18 years) with MS or clinically isolated syndrome (CIS) [14], and 12 healthy adult control subjects. Patients were consecutively recruited from the UT Southwestern Clinical Center for Multiple Sclerosis between June 2014 and October 2014. Selection criteria for patients required the presence of ≥ 1 infratentorial (brainstem, cerebellar peduncle, or cerebellar lesions) or cervical spinal cord lesions on MRI based on the formal interpretation of brain and cervical spinal cord MRI by a neuroradiologist with subsequent verification by an MS neurologist (see Figure 1). Healthy controls had no known history of neurological disorders, and were recruited from clinic staff or family members and friends of patients enrolled over the same time period.
Fig. 1.
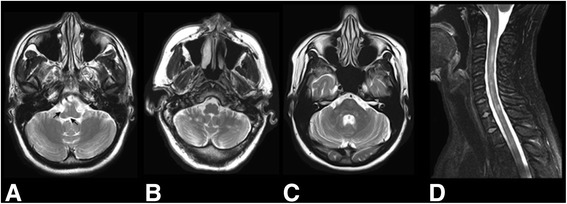
Axial T2-weighted MRI images of the infratentorial region from three unique patients demonstrating foci of T2-hyperintensity at the brainstem (right pontomedullary sulcus and medial vestibular nucleus (arrows)) (a), bilateral cerebellar lobes (b), and brainstem and bilateral cerebellar peduncle regions (c). Sagittal T2-weighted MRI image of the cervical spinal cord revealing multi-focal regions of T2-hyperintensity within the parenchyma (d)
Data collection
Each of the 35 study subjects were separately videotaped and asked to perform the finger tapping test by tapping the i) index finger on the thumb tip using the dominant hand, ii) index finger on the thumb tip using the non-dominant hand, iii) index finger on the distal thumb crease using the dominant hand, and iv) index finger on the distal thumb crease using the non-dominant hand. Participants were provided with instructions to repetitively tap the index finger on the thumb for at least 20 s, an adopted and modified technique from the Unified Parkinson’s Disease Rating Scale (UPDRS) instructions which require tapping as quickly and as big as possible [8]. Each hand was tested separately. A total of 140 videos (four videos per each of the 35 subjects) of finger tapping performance were subsequently collected. All videos were edited to capture only the first 10 s of finger tapping performance in order to better simulate how finger tapping is evaluated in the real-world clinical practice. The sequence of videos was randomized and a standardized test module was created for evaluators.
The videos were assessed independently by 10 evaluators of different levels of experience, and skills in neurology (three MS neurologist, four neurology residents, and three APPs) who were all blinded to the study subjects. The evaluators were first trained on how to score finger tapping using the UPDRS [8], the most commonly used rating scale for finger tapping performance, by viewing sample videos. Since younger age, male sex, and the dominant hand have been suggested by some studies to be associated with higher tapping rates [15], information on patient’s sex, age and hand dominance were provided to the evaluators before assessing each video. Repeated viewing of the videos was allowed if the evaluator was not able to rate the finger tapping on the first attempt.
Outcomes, and comparisons
The main outcome included the identification of impairments in finger tapping performance related to the presence of inflammatory demyelinating infratentorial and cervical spinal cord lesions. Separate comparisons with sensitivity determinations were made when finger tapping was performed against the tip in comparison to the distal crease of the thumb, with the dominant and non-dominant hand, and also across three categories of providers (MS neurologists, neurology residents, and APPs). Other measures of validity including specificity and accuracy were also analyzed. Results were also compared when findings for the dominant and non-dominant hands were combined (i.e. when finger tapping was considered abnormal if either or both of the dominant and non-dominant hands were evaluated as abnormal). In addition, we assessed the inter-evaluator reliability of finger tapping test within each category of the providers. We also looked into the sensitivity of other methods of assessing motor function including manual muscle testing (graded 0 to 5) performed by an MS specialist [3].
Statistics
Baseline characteristics of patients and healthy control subjects were compared using Fisher’s exact test for categorical variables and t-test for quantitative variables. For the purpose of calculating measures of validity, all finger tapping interpretations were classified in a binary manner as normal (score 0 on UPDRS) vs. abnormal (score ≥1). Chi-square or Fisher’s exact tests (where appropriate), and the associated probability values, were used to calculate sensitivities and specificities. Inter-evaluator reliability of finger tapping interpretation among the providers within the same category was calculated by determining the level of agreement between interpretations using Cohen’s kappa coefficient. Kappa statistics were graded as follows based on Fleiss’s scale [16]: values <0.40 poor agreement; 0.40-0.75 fair to good agreement; ≥0.75 excellent agreement.
All statistical tests were two-sided, and p < 0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS Inc. Chicago, Illinois, version 16.0).
Results
A total of 35 subjects (23 patients with MS or CIS, and 12 healthy control subjects) were included. Table 1 shows the baseline characteristics of the patients and healthy controls. Of the 23 patients, 19 had MS, and four had CIS at the time of the study. The majority of the patients (65%) had both infratentorial and cervical spinal cord lesions. There was no significant difference in handedness between the patients and controls.
Table 1.
Baseline characteristics of patients with infratentorial or cervical spinal cord demyelinating lesions in comparison to healthy control subjects
| Baseline characteristics | Patients (N = 23) |
Healthy controls (N = 12) |
P value |
|---|---|---|---|
| Sex, N(%) | |||
| Male | 6 (26%) | 8 (67%) | 0.31a |
| Female | 17 (74%) | 4 (33%) | |
| Age (years) | |||
| Mean (SD) | 44.8 (14.6) | 37.4 (14.5) | 0.87b |
| Range | 22 – 74 | 22 – 62 | |
| Ethnicity, N(%) | |||
| White | 22 (96%) | 11 (92%) | 0.57a |
| African American | 1 (4%) | 0 (0%) | |
| Middle Eastern | 0 (0%) | 1 (8%) | |
| Handedness, N(%) | |||
| Right | 21 (91%) | 11 (92%) | 0.73a |
| Left | 2 (9%) | 1 (8%) | |
| Disease subtype, N(%) | |||
| CIS | 4 | ||
| RRMS | 17 | ||
| SPMS | 1 | ||
| PPMS | 1 | - | - |
| Age at onset of disease symptoms (years) | |||
| Mean (SD) | 36.5 (14.7) | - | - |
| Range | 16 – 68 | ||
| Disease duration from onset of symptoms (years) | |||
| Mean (SD) | 8.7 (9.1) | - | - |
| Range | 0 – 39 | ||
| Lesion location on MRI, Nc | |||
| Infra-tentorial | 20 | - | - |
| Brainstem | 15 | ||
| Cerebellum | 18 | ||
| Cervical spinal cord | 18 | ||
aTwo-sided Fisher’s exact test
bTwo sided t-test
cData reflect the presence of multi-focal lesions within the CNS
A total of 1400 evaluations (4 videos corresponding to four settings of the test per each of the 35 subjects viewed by 10 evaluators) were analyzed including 420 evaluations by neurologists, 560 evaluations by neurology residents, and 420 evaluations by APPs. Table 2 shows the sensitivity, specificity, and accuracy of the finger tapping test in identifying infratentorial and cervical cord demyelinating lesions in four different settings of finger tapping depending on whether the test was performed using the dominant vs. non-dominant hand, and against thumb tip versus the distal thumb crease across different categories of providers. Finger tapping against the thumb tip using the dominant hand was found to have an overall sensitivity of 69.6% (p = 0.003), 45.7% (p = 0.859), and 42% (p = 0.004) among neurologist, neurology residents, and APPs, respectively. When tapping was performed over the distal thumb crease of the dominant hand, the corresponding sensitivity values increased to 78.3% (p < 0.001), 71.7% (p = 0.026), and 62.3% (p < 0.001) for neurologists, neurology residents, and APPs, respectively. When results for non-dominant hands were analyzed, finger tapping using the thumb tip of the non-dominant hand was found to have a sensitivity of 78.3% (p < 0.001), 56.5% (p = 0.021), and 43.5% (p < 0.001) among neurologists, neurology residents, and APPs. When tapping was performed over the distal thumb crease of the non-dominant hand, the corresponding sensitivity values increased to 84.1% (p < 0.001), 70.7% (p = 0.006), and 72.5% (p < 0.001) for neurologist, neurology residents, and APPs. Overall when evaluations by all categories of providers were combined, finger tapping using the distal thumb crease of the non-dominant hand was still found to have the highest sensitivity value compared to other settings (Table 2). In addition, interpretations of finger tapping test made by neurologists were found to be associated with higher sensitivities compared to neurology residents and APPs (Table 2).
Table 2.
Validity of the finger tapping test for identifying infratentorial and cervical cord demyelinating lesions in four different settings based on handedness, and location of tap (i.e. thumb tip versus distal thumb crease) across different categories of providers (neurologists, neurology residents, and advanced practice providers)
| Dominant hand, Thumb tip |
Dominant hand, Distal thumb crease |
Non-dominant hand, Thumb tip |
Non-dominant hand, Distal thumb crease |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | P valuea | Sensitivity | Specificity | Accuracy | P valuea | Sensitivity | Specificity | Accuracy | P valuea | Sensitivity | Specificity | Accuracy | P valuea | |
| Neurologist 1 | 69.6% | 66.7% | 68.6% | 0.040b | 87.0% | 58.3% | 77.1% | 0.015 | 78.3% | 75.0% | 77.1% | 0.004 | 82.6% | 75.0% | 80.0% | 0.002 |
| Neurologist 2 | 60.9% | 83.3% | 68.6% | 0.013b | 82.6% | 83.3% | 82.9% | <0.001 | 78.3% | 100.0% | 85.7% | <0.001b | 82.6% | 66.7% | 77.1% | 0.007 |
| Neurologist 3 | 52.2% | 91.7% | 65.7% | 0.013 | 87.0% | 58.3% | 77.1% | 0.015 | 73.9% | 100.0% | 82.9% | <0.001b | 91.3% | 75.0% | 85.7% | <0.001 |
| All neurologists | 69.6% | 61.1% | 66.7% | 0.003b | 78.3% | 66.7% | 74.3% | <0.001b | 78.3% | 86.1% | 81.0% | <0.001b | 84.1% | 83.3% | 83.8% | <0.001b |
| Neurology resident 1 | 43.5% | 75.0% | 54.3% | 0.463 | 73.9% | 83.3% | 77.1% | 0.001b | 56.5% | 75.0% | 62.9% | 0.076b | 82.6% | 66.7% | 77.1% | 0.007 |
| Neurology resident 2 | 47.8% | 41.7% | 45.7% | 0.555b | 69.6% | 41.7% | 60.0% | 0.709 | 47.8% | 58.3% | 51.4% | 0.728b | 65.2% | 50.0% | 60.0% | 0.477 |
| Neurology resident 3 | 43.5% | 33.3% | 40.0% | 0.193b | 73.9% | 41.7% | 62.9% | 0.451 | 56.5% | 58.3% | 57.1% | 0.404b | 65.2% | 33.3% | 54.3% | 1.000 |
| Neurology resident 4 | 47.8% | 58.3% | 51.4% | 0.728b | 69.6% | 25.0% | 54.3% | 1.000 | 65.2% | 66.7% | 65.7% | 0.072b | 69.6% | 66.7% | 68.6% | 0.040b |
| All neurology residents | 45.7% | 52.1% | 47.9% | 0.859b | 71.7% | 47.9% | 63.6% | 0.026b | 56.5% | 64.6% | 59.3% | 0.021b | 70.7% | 54.2% | 65.0% | 0.006b |
| APP 1 | 52.2% | 91.7% | 65.7% | 0.013 | 56.5% | 100% | 71.4% | 0.001 | 39.1% | 100% | 60.0% | 0.015 | 73.9% | 91.7% | 80.0% | <0.001b |
| APP 2 | 34.8% | 91.7% | 54.3% | 0.121 | 69.6% | 100% | 80.0% | <0.001b | 47.8% | 91.7% | 62.9% | 0.027 | 82.6% | 66.7% | 77.1% | 0.007 |
| APP 3 | 39.1% | 75.0% | 51.4% | 0.476 | 60.9% | 91.7% | 71.4% | 0.003b | 43.5% | 83.3% | 57.1% | 0.149 | 60.9% | 100.0% | 74.3% | 0.001 |
| All APPs | 42.0% | 86.1% | 57.1% | 0.004b | 62.3% | 97.2% | 74.3% | <0.001b | 43.5% | 91.7% | 60.0% | <0.001b | 72.5% | 86.1% | 77.1% | <0.001b |
| Overall | 51.7% | 65.0% | 56.3% | 0.003b | 70.9% | 68.3% | 70.0% | <0.001b | 59.1% | 79.2% | 66.0% | <0.001b | 75.2% | 72.5% | 74.3% | <0.001b |
APP Advanced practice provider
aTwo-sided Fisher’s exact test unless indicated otherwise
bTwo-sided chi-square test
Table 3 displays the inter-evaluator reliability of the finger tapping test within each of the three categories of providers across different techniques. Overall, the highest kappa values were associated with evaluations by neurologists (0.83 with p < 0.001 for the distal thumb crease of the non-dominant hand, and 0.82 with p < 0.001 for the distal thumb crease of the dominant hand), indicating an excellent inter-evaluator agreement. Among neurologists and APPs, the kappa values associated with the distal thumb crease were consistently higher than the ones associated with the thumb tip for both the dominant and non-dominant hands. However, among neurology residents, this effect was only found for the non-dominant hand.
Table 3.
Inter-evaluator reliability of the finger tapping test in four different settings based on handedness and location of tap (thumb tip versus distal thumb crease) within different categories of providers (neurologists, neurology residents, and advanced practice providers)
| Dominant hand, Thumb tip |
Dominant hand, Distal thumb crease |
Non-dominant hand, Thumb tip |
Non-dominant hand, Distal thumb crease |
|||||
|---|---|---|---|---|---|---|---|---|
| Kappa | P value | Kappa | P value | Kappa | P value | Kappa | P value | |
| Neurologists (N = 3) | 0.73 | <0.001 | 0.82 | <0.001 | 0.69 | <0.001 | 0.83 | <0.001 |
| Neurology residents (N = 4) | 0.53 | <0.001 | 0.38 | <0.001 | 0.48 | <0.001 | 0.64 | <0.001 |
| APPs (N = 3) | 0.56 | <0.001 | 0.72 | <0.001 | 0.38 | 0.002 | 0.54 | <0.001 |
| Overall (N = 10) | 0.47 | <0.001 | 0.43 | <0.001 | 0.38 | <0.001 | 0.56 | <0.001 |
APP advanced practice provider
The specificity of finger tapping using the thumb tip of the dominant hand increased from 61.1% (p = 0.003) to 66.7% (p < 0.001) for the distal thumb crease of the dominant hand among neurologists, and from 86.1% (p = 0.004) to 97.2% (p <0.001) among APPs; however, this effect was not consistently seen in other settings of the tests (Table 2). With respect to other methods of assessing motor function, impaired manual muscle testing (graded 0 to 5) was found to have a sensitivity of 45.5% (p = 0.053) for identifying upper motor neuron lesions.
Discussion
Our findings suggest that the specific technique of finger tapping against the distal thumb crease was more sensitive for identifying upper motor neuron injury due to demyelinating lesions when compared to tapping against the thumb tip. We also identified that finger tapping using the non-dominant hand was associated with greater sensitivities amongst all healthcare providers in identifying a CNS demyelinating focus as compared to when the technique was executed with the dominant hand. Finger tapping against the distal thumb crease of the non-dominant hand, when evaluated by neurologists, was associated with the highest sensitivity (84%, p < 0.001), and inter-evaluator reliability (kappa = 0.83, p < 0.001), when compared to all the other techniques of performing the test, and other categories of providers (neurology residents and APPs).
Repetitive rapid finger tapping is viewed classically as an assessment of fine motor skills of the upper extremities that requires coordinated alternating activity of distal flexor and extensor muscles. Fine independent finger movements are crucial for many motor skills of everyday life. Quantitative assessment of a repetitive finger to-thumb tip-opposition task was recently found to be highly valuable in discriminating MS patients with very low Expanded Disability Status Scale (EDSS) from healthy controls [12]. While finger tapping is traditionally examined in clinical practice by repetitive tapping of the index finger against the thumb tip, our study represents a first effort to look into an infrequently utilized variation involving tapping against the distal thumb crease. The observation of a higher sensitivity of finger tapping against the distal thumb crease compared to the thumb tip for detection of CNS demyelinating lesions could possibly suggest greater recruitment of finger and wrist extensor muscles when tapping is performed against the distal thumb crease. Kinematics and muscle activation pattern of finger tapping have been the subject of several previous studies [17, 18]. However, these prior studies simplified the task by tapping with the index finger on a computer keyswitch [17], making the extrapolation of findings to our study challenging. Our findings do provide evidence to suggest the need for refinement of existing traditional assessments as well as the clinical training delivered to healthcare providers.
The finding that tapping with the non-dominant hand was associated with an overall higher sensitivity for identifying CNS demyelinating lesions compared to the dominant hand (regardless of whether tapping was performed against the thumb tip or the distal thumb crease), may suggest a lower “reserve capacity” in the motor networks responsible for controlling the non-dominant side. Historically, within the cognitive sciences, particularly Alzheimer’s disease research, the general concept of “reserve capacity” has been proposed based on the observations of a discordance between the severity of brain pathology and the cognitive manifestations [19–22]. Yet, this concept may be relevant to any situation when the nervous system sustains injury [20]. The neural mechanisms of reserve capacity are not fully understood. However, differences in the resilience of pre-existing networks may play a role such that an individual with more efficient or higher capacity networks could have a greater capacity in tolerating neuropathologic processes [23]. In the context of handedness, one can postulate that the motor networks responsible for controlling the more dexterous dominant hand are perhaps more efficient with a greater functional capacity being present as a result of use-dependent plasticity compared to the networks associated with controlling the non-dominant hand. Interestingly, it has been recently shown that patients with dominant-side onset Parkinson’s disease had fewer motor deficits compared to those with non-dominant-side onset disease despite similar dopamine reductions on dopamine transporter (DAT) imaging [24]. Likewise, patients with affected dominant hands by cerebrovascular accidents were found to have less motor impairment post-stroke compared to those with the non-dominant hand affected [25]. Our results therefore appear to be consistent with prior observations from neurological disorders. Our findings also have important implications for clinical practice, highlighting the importance of closer examination of the less dexterous non-dominant hand where impairments of fine motor control may be more discernable at an earlier stage of the disease process.
Our findings of greater inter-evaluator reliabilities amongst neurologists compared to neurology residents and APPs is not unexpected, given a greater level of experience in the field and familiarity with the assessment. This emphasizes the importance of enhancing training in neurological exam skills both for neurology residents, as emerging future neurologists, and for the growing number of APPs being integrated within neurological practices to improve the identification of clinical abnormalities [26, 27].
Motor dysfunction in MS, while having a complex substrate which cannot be attributed to a single neuroimaging correlate, has been suggested to be the consequence of infratentorial and spinal cord lesions, as well as damage to the corticospinal tract [13]. In particular, the mean upper cervical cord area, and the presence of cervical and infratentorial lesions can be used relatively easily in the real-world practice setting to predict motor dysfunction [13]. We therefore focused on infratentorial and cervical spinal cord demyelinating lesions in this study.
Our study represents a rigorous attempt to systematically evaluate the value of a classical neurological examination test for detecting infratentorial or cervical spinal cord demyelinating lesions. The other strengths include blinding of evaluators to the study subjects and their clinical and radiological findings, the participation of three categories of healthcare providers in neurology with different levels of training, and experience and a consistent test platform used to acquire our data. Limitations to this effort include a modest number of study subjects that was dictated principally by the anticipated duration of testing (average time for the assessment of 140 videos by each evaluator was 1.5 h), the lack of a formalized rating scale describing finger tapping impairments in MS, utilization of the UPDRS (designed for measuring motor impairments in Parkinson’s disease, not impairments related to CNS demyelinating lesions), and evaluator bias as all healthcare providers were selected from a tertiary academic medical center. One may argue the contribution of lesion laterality in justifying our findings. While we agree that it would have been informative to look into the impact of lesion laterality on finger tapping impairments if we had a larger sample size, we do not believe that lesion laterality can explain our findings given the majority of our patients had multifocal lesions, and given the complexity of the motor networks involved when performing fine motor tasks. It would have also been nice to have an additional control group including MS patients without infratentorial or cervical spinal cord lesions, given the non-specific nature of finger tapping test with respect to the precise lesion location.
Attempts to improve upon classic neurological examination techniques have been previously described. A previous study which questioned the reliability and accuracy of the sign of Babinski was performed, suggesting that the speed of foot tapping may serve as a better predictor of upper motor neuron weakness compared to the plantar reflex [28]. Although this finding provoked criticism [29], it encouraged others to further re-evaluate the reliability of the Babinski sign [30], and some other elements of the neurological exam [31]. Our study was an attempt to address the need for scientific evaluation of neurological exam components by focusing on an important test of fine motor control in upper extremities, and its relationship to neuroimaging findings.
Conclusions
We found that finger tapping against the distal thumb crease of the non-dominant hand, when evaluated by neurologists, was associated with the highest sensitivity for identifying infratentorial and cervical cord demyelinating lesions. Our findings highlight the importance of precise examinations of the non-dominant side where impaired fine motor control secondary to an upper motor injury might be detectable earlier than the dominant side. These data also encourage several lines of future research. Despite being an easy to perform test in clinical practice, repetitive finger tapping task has a complex nature not only from a neural aspect [32, 33] but also from modeling perspective [34]. Characterization of finger tapping by tapping rate, amplitude or rhythm may not reveal all the information that this valuable test can provide [34, 35]. Future research is therefore warranted to improve upon the characterization of tapping features in relation to neurological diseases.
Acknowledgements
We would like to thank all study subjects and providers who dedicated their valuable time to participate in this study. We also thank Dr. Pravin Khemani for his valuable comments.
Funding
None
Availability of data and materials
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
DO conceived the original idea for this study. AS, BN, and DO contributed to the design of the study. BN and DO collected the study data. AS carried out the statistical analyses, and drafted the original manuscript. AS, BN, and DO contributed to interpretations of findings. BN and DO provided revisions to the manuscript. DO supervised the study and verified the statistical analyses. All the authors read, commented, and approved the final version of the manuscript.
Competing interests
A.S. and B.N. report no disclosures. D.O. received lecture fees from Acorda Therapeutics, Genzyme, and TEVA Neuroscience, consulting and advisory board fees from Genentech, Genzyme, Novartis, and TEVA Neuroscience, and research support from Biogen.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the UT Southwestern Medical Center Institutional Review Board. Written informed consent was obtained from all study subjects. Patient privacy and data confidentiality were ensured.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- APP
Advanced practice provider
- CNS
Central nervous system
- DAT
Dopamine transporter
- EDSS
Expanded disability status scale
- MS
Multiple sclerosis
- UPDRS
Unified Parkinson’s disease rating scale
Contributor Information
Afsaneh Shirani, Email: Afsaneh.Shirani@phhs.org.
Braeden D. Newton, Email: Braeden.Newton@UTSouthwestern.edu
Darin T. Okuda, Phone: (214) 645-0558, Email: Darin.Okuda@UTSouthwestern.edu
References
- 1.Nicholl DJ, Appleton JP. Clinical neurology: why this still matters in the 21st century. J Neurol Neurosurg Psychiatry. 2015;86:229–33. doi: 10.1136/jnnp-2013-306881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston SC, Hauser SL. The beautiful and ethereal neurological exam: an appeal for research. Ann Neurol. 2011;70:A9–A10. doi: 10.1002/ana.22542. [DOI] [PubMed] [Google Scholar]
- 3.Campbell W. DeJong’s the neurologic examination. 7. Philadelphia: Lippincott Williams & Wilkins; 2013. Motor strength and power. [Google Scholar]
- 4.Sundqvist M, Johnels JA, Lindh J, Laakso K, Hartelius L. Syllable repetition vs. finger tapping: aspects of motor timing in 100 healthy adults. Mot Control. 2016;20:233–54. doi: 10.1123/mc.2014-0068. [DOI] [PubMed] [Google Scholar]
- 5.Dean AC, Victor TL, Boone KB, Arnold G. The relationship of IQ to effort test performance. Clin Neuropsychol. 2008;22:705–22. doi: 10.1080/13854040701440493. [DOI] [PubMed] [Google Scholar]
- 6.Haaland KY, Temkin N, Randahl G, Dikmen S. Recovery of simple motor skills after head injury. J Clin Exp Neuropsychol. 1994;16:448–56. doi: 10.1080/01688639408402655. [DOI] [PubMed] [Google Scholar]
- 7.de Groot-Driessen D, van de Sande P, van Heugten C. Speed of finger tapping as a predictor of functional outcome after unilateral stroke. Arch Phys Med Rehabil. 2006;87:40–4. doi: 10.1016/j.apmr.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society - unified Parkinson’s disease rating scale (MDS-UPDRS) Mov Disord. 2008;23:2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 9.Kraft GH, Amtmann D, Bennett SE, et al. Assessment of upper extremity function in multiple sclerosis: review and opinion. Postgrad Med. 2014;126:102–8. doi: 10.3810/pgm.2014.09.2803. [DOI] [PubMed] [Google Scholar]
- 10.Lamers I, Kelchtermans S, Baert I, et al. Upper limb assessment in multiple sclerosis: a systematic review of outcome measures and their psychometric properties. Arch Phys Med Rehabil. 2014;95:1184–200. doi: 10.1016/j.apmr.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Schenk T, Walther EU, Mai N. Closed- and open-loop handwriting performance in patients with multiple sclerosis. Eur J Neurol. 2000;7:269–79. doi: 10.1046/j.1468-1331.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 12.Bonzano L, Sormani MP, Tacchino A, et al. Quantitative assessment of finger motor impairment in multiple sclerosis. PLoS One. 2013;8:e65225. doi: 10.1371/journal.pone.0065225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daams M, Steenwijk MD, Wattjes MP, et al. Unraveling the neuroimaging predictors for motor dysfunction in long-standing multiple sclerosis. Neurology. 2015;85:248–55. doi: 10.1212/WNL.0000000000001756. [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubel KA, Reed B, Yund EW, Herron TJ, Woods DL. Computerized measures of finger tapping: effects of hand dominance, age, and sex. Percept Mot Skills. 2013;116:929–52. doi: 10.2466/25.29.PMS.116.3.929-952. [DOI] [PubMed] [Google Scholar]
- 16.Gwet KL. Handbook on interrator reliability: The definitive guide to measuring the extent of agreement among multiple raters. 3rd ed. Gaithersburg: Advanced Analytics; 2012.
- 17.Kuo PL, Lee DL, Jindrich DL, Dennerlein JT. Finger joint coordination during tapping. J Biomech. 2006;39:2934–2942. doi: 10.1016/j.jbiomech.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Arunachalam R, Weerasinghe VS, Mills KR. Motor control of rapid sequential finger tapping in humans. J Neurophysiol. 2005;94:2162–70. doi: 10.1152/jn.01173.2004. [DOI] [PubMed] [Google Scholar]
- 19.Stern Y. The concept of cognitive reserve: a catalyst for research. J Clin Exp Neuropsychol. 2003;25:589–93. doi: 10.1076/jcen.25.5.589.14571. [DOI] [PubMed] [Google Scholar]
- 20.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–60. doi: 10.1017/S1355617702813248. [DOI] [PubMed] [Google Scholar]
- 21.Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology. 1993;43:13–20. doi: 10.1212/WNL.43.1_Part_1.13. [DOI] [PubMed] [Google Scholar]
- 22.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–10. doi: 10.1001/jama.1994.03510370056032. [DOI] [PubMed] [Google Scholar]
- 23.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–12. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ham JH, Lee JJ, Kim JS, Lee PH, Sohn YH. Is dominant-side onset associated with a better motor compensation in Parkinson’s disease? Mov Disord. 2015;30:1921–5. doi: 10.1002/mds.26418. [DOI] [PubMed] [Google Scholar]
- 25.Harris JE, Eng JJ. Individuals with the dominant hand affected following stroke demonstrate less impairment than those with the nondominant hand affected. Neurorehabil Neural Repair. 2006;20:380–9. doi: 10.1177/1545968305284528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwarz HB, Fritz JV, Govindarajan R, et al. Neurology advanced practice providers: A position paper of the Ameircan Academy of Neurology. Neurol Clin Pract. 2015;5:333–7. [DOI] [PMC free article] [PubMed]
- 27.Ringel SP, Vickrey BG, Keran CM, Bieber J, Bradley WG. Training the future neurology workforce. Neurology. 2000;54:480–4. doi: 10.1212/WNL.54.2.480. [DOI] [PubMed] [Google Scholar]
- 28.Miller TM, Johnston SC. Should the Babinski sign be part of the routine neurologic examination? Neurology. 2005;65:1165–8. doi: 10.1212/01.wnl.0000180608.76190.10. [DOI] [PubMed] [Google Scholar]
- 29.Landau WM. Plantar reflex amusement: misuse, ruse, disuse, and abuse. Neurology. 2005;65:1150–1. doi: 10.1212/01.wnl.0000184609.15196.4f. [DOI] [PubMed] [Google Scholar]
- 30.Araujo R, Firmino-Machado J, Correia P, et al. The plantar reflex. A study of observer agreement, sensitivity, and observer bias. Neurol Clin Pract. 2015;5:309–16. [DOI] [PMC free article] [PubMed]
- 31.Sullivan SJ, Hammond-Tooke GD, Schneiders AG, Gray AR, McCrory P. The diagnostic accuracy of selected neurological tests. J Clin Neurosci. 2012;19:423–7. doi: 10.1016/j.jocn.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. NeuroImage. 2008;42:343–56. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhamala M, Pagnoni G, Wiesenfeld K, Zink CF, Martin M, Berns GS. Neural correlates of the complexity of rhythmic finger tapping. NeuroImage. 2003;20:918–26. doi: 10.1016/S1053-8119(03)00304-5. [DOI] [PubMed] [Google Scholar]
- 34.Austin D, McNames J, Klein K, Jimison H, Pavel M. A statistical characterization of the finger tapping test: modeling, estimation, and applications. IEEE J Biomed Health Inform. 2015;19:501–7. doi: 10.1109/JBHI.2014.2384911. [DOI] [PubMed] [Google Scholar]
- 35.Barut C, Kiziltan E, Gelir E, Kokturk F. Advanced analysis of finger-tapping performance: a preliminary study. Balkan Med J. 2013;30:167–71. doi: 10.5152/balkanmedj.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.


