Abstract
Objective
The objective of this study was to assess whether there are racial and ethnic disparities in the time to development of a pressure ulcer and number of pressure ulcer treatments in individuals aged 65 and older after nursing home admission.
Method
Multi-level predictors of time to a pressure ulcer from three national surveys were analyzed using Cox proportional hazards regression for White Non-Hispanic residents. Using the Peters–Belson method to assess for disparities, estimates from the regression models were applied to American Indians/Alaskan Natives, Asians/ Pacific Islanders, Blacks, and Hispanics separately resulting in estimates of expected outcomes as if they were White Non-Hispanic, and were then compared with their observed outcomes.
Results
More Blacks developed pressure ulcers sooner than expected. No disparities in time to a pressure ulcer disadvantaging other racial/ethnic groups were found. There were no disparities in pressure ulcer treatment for any group.
Discussion
Reducing disparities in pressure ulcer development offers a strategy to improve the quality of nursing home care.
Keywords: pressure ulcer, health disparity, nursing home
Pressure ulcers remain a foremost concern in nursing homes (NHs). Pressure ulcers and their treatment are costly in terms of physical and emotional suffering for the patients as well as in actual treatment dollars and the intensity of staff oversight required for nursing care. A few studies have observed disparities in pressure ulcer prevalence and incidence among Black NH residents compared with White Non-Hispanics (Baumgarten et al., 2004; Howard & Taylor, 2009). One study of NH residents in the southeast United States (Alabama, Florida, Georgia, Kentucky, Minnesota, North Carolina, South Carolina, Tennessee) found a 90-day incidence of 4.7% for Blacks and 3.4% for White Non-Hispanics (Howard & Taylor, 2009). Adjusting for the same follow-up time, a study of NHs in Maryland estimated a higher incident rate of 13.8% for Blacks versus 8.6% for White Non-Hispanics (Baumgarten et al., 2004; Howard & Taylor, 2009).
Studies of pressure ulcer prevalence suggest that factors beyond individual resident characteristics are associated with disparities. For example, higher percentages of Hispanic (Gerardo, Teno, & Mor, 2009) or Black (Cai, Mukamel, & Temkin-Greener, 2010; Li, Yin, Cai, Temkin-Greener, & Mukamel, 2011) residents in NHs were associated with higher prevalence of pressure ulcers in the corresponding minority group. A concentration of minorities in a NH has been associated with a poorer community surrounding the NH, fewer resources, and lower quality of care by the NHs (Grabowski, 2004; Mor, Zinn, Angelelli, Teno, & Miller, 2004). In one study reporting pressure ulcer incidence by gender and race in a set of southeastern U.S. NHs, higher patient to staff ratios, more health deficiencies, and location in a non-rural area were significant risk factors for pressure ulcer development in Black females (Howard & Taylor, 2009). There are limited data on pressure ulcer epidemiology after NH admission for racial and ethnic groups of residents other than Blacks, and most studies do not consider NH/community-level factors. Because racial-ethnic disparities in some treatments for NH residents have been reported (Allsworth, Toppa, Palin, & Lapane, 2005; Harada, Chun, Chiu, & Pakalniskis, 2000; Hudson, Cody, Armitage, Curtis, & Sullivan, 2005), we also investigated whether there were disparities in treatment of incident pressure ulcers, as no studies to our knowledge have done so.
The purpose of this study was to assess racial and ethnic disparities in the time to development of a pressure ulcer and in the number of pressure ulcer treatments in a cohort of older individuals after their admission to NHs considering potential risk factors at the individual, NH, and community levels.
Method
Design and Data Files
The study had a prospective cohort design. The following data files were linked and analyzed: (a) the Minimum Data Set (MDS) Version 2.0 containing demographic and comprehensive health assessment data of individual residents of a national for-profit chain of NHs; (b) the Online Survey, Certification, and Reporting (OSCAR) containing measures of NH staffing, quality of care, and the care environment from years 2000 to 2002; and (c) the 2000 U.S. Census containing and socioeconomic (SES) and sociodemographic (SDS) measures of the Census tract of the community surrounding the NHs. The Minnesota Population Center at the University of Minnesota in Minneapolis, MN, identified the census tracts of the NHs. The Institutional Review Board of the University of Minnesota determined the study was exempt from review because data were de-identified.
Cohorts, Outcomes, and Predictors
The cohort for analyzing disparities in time to developing a pressure ulcer included older adults (aged 65 years or more) who were admitted to one of the NHs without a Stage 2, 3, or 4 pressure ulcer per their first full/admission MDS record. The cohort for modeling disparities in the number of treatments of a pressure ulcer included those admissions that subsequently developed a pressure ulcer. Race and ethnicity groups were formed according to MDS definitions: American Indian, Asian, Black non-Hispanic (referred to as Black), Hispanic, and White non-Hispanic. An incident pressure ulcer was defined from the first report of a Stage 2, 3, or 4 pressure ulcer on MDS records after the admission MDS (MDS Item M2, highest stage of a pressure ulcer in the last 7 days). Stage 1 pressure ulcers were not included because of the known difficulty in their identification and classification by nursing staff (Bethell, 2003; Dealey & Lindholm, 2006). Each resident’s MDS records were followed until the date of the MDS with the incident pressure ulcer or their last observed MDS record, whichever came first. Pressure ulcer treatments were taken from the same MDS as the incident pressure ulcer, and included the total number of the following found in MDS Section M5: pressure ulcer relieving device(s) for chair or bed, turning/repositioning program, nutrition or hydration intervention to manage skin problems, ulcer care, and application of ointments/medications (other than to feet). Relevant predictors of each outcome were identified using the literature and expertise of the investigators and clinical consultants. Potential predictors (see Tables 1 and 2) were defined using individual items of the data records and established scales with good psychometric properties as multiple items on a record are often related to the same concept. Where no scale existed and a single item was insufficient, composite measures were developed. Construction of composite measures followed previously established statistical procedures (Savik, Fan, Bliss, & Harms, 2005) and consultation from clinical and research experts. Variables were screened for inclusion in a model using bivariate associations with an outcome, and those with an association at p< .05 were considered model candidates. Bivariate associations between variables were also performed to avoid collinearity. If an individual-level variable and NH/ community-level variable were highly correlated, the individual-level variable was included in the model due to its greater specificity.
Table 1.
Characteristics by Race and Ethnicity of Older Nursing Home Admissions Free of a Pressure Ulcer at Admission Followed for Developing One.
| Variable/scale
|
MDS item (range of score) | American Indian/Alaskan Native
|
Asian/Pacific Islander
|
Black Non-Hispanic
|
Hispanic
|
White Non-Hispanic
|
|---|---|---|---|---|---|---|
| No. of admissions | n = 440 | n = 1,490 | n = 7,113 | n = 1,511 | n = 80,021 | |
| Demographics | ||||||
| Age at admissiona | AA3, AB1 | 78.8 (8.3) | 82.6 (7.2) | 80.13 (8.25) | 79.9 (8.2) | 81.9 (7.5) |
| Female gender | AA2 | 247 (56.1) | 904 (60.7) | 4,396 (61.8) | 852 (56.4) | 53,475 (66.8) |
| ≥High school education | AB7 | 156 (35.5) | 780 (52.3) | 2,437 (34.3) | 483 (32.0) | 49,704 (62.1) |
| Function/mobility | ||||||
| Activities of daily living deficit score (J. N. Morris, Fries, & Morris, 1999)a | G1aA, G1bA, G1eA, G1gA, G1hA, G1iA, G1jA (Range 0–28) | 13.0 (8.3) | 17.8 (6.4) | 16.0 (8.3) | 15.3 (7.9) | 14 (7.6) |
| Bedfast/transfer dependency | G6a, G6d | 68 (15.5) | 152 (10.2) | 1,254 (17.7) | 229 (15.2) | 7,855 (9.8) |
| Plegia or pareses—any | I1v, I1x, I1z | 27 (6.1) | 292 (19.6) | 770 (10.8) | 170 (11.3) | 4,551 (5.7) |
| Restraint use—any | P4c-e | 11 (2.5) | 113 (7.6) | 334 (4.7) | 75 (5.0) | 2,536 (3.2) |
| Side rail use—any | P4a-b | 203 (46.1) | 1,208 (81.1) | 4,973 (69.9) | 1,108 (73.4) | 49,449 (61.8) |
| Vision impairment | D1, D2a, D2b | |||||
| Number of indicators | ||||||
| 1 | 99 (22.5) | 362 (24.3) | 1,905 (26.8) | 337 (22.3) | 17,314 (21.6) | |
| 2 | 40 (9.1) | 162 (10.9) | 761 (10.7) | 140 (9.3) | 6,242 (7.8) | |
| ≥3 | 46 (10.5) | 110 (7.4) | 736 (10.3) | 117 (7.7) | 4,654 (5.8) | |
| Cognition/emotion | ||||||
| Cognitive deficits score MDS–Cognition Scale (Hartmaier, Sloane, Guess, & Koch, 1994)a | B2a, B2b, B3b, B3d, B3e, B4, C4, G1gA (Range 0–10) | 3.0 (2.9) | 3.6 (2.8) | 3.9 (3.0) | 3.3 (3.1) | 2.9 (2.9) |
| Communication difficulties score adapted from Hopper, Bayles, Harris, & Holland (2001)a | C1, C5, C6, C3b-f, (Range 0–9) | 1.4 (1.7) | 2.1 (1.9) | 1.4 (1.7) | 1.5 (1.7) | 1.2 (1.5) |
| Delirium | B5a-f, B6, E5 | |||||
| Minimum Data Set-Confusion Assessment Method (MDS-CAM) (Dosa, Intrator, McNicoll, Cang, & Teno, 2007) | ||||||
| Subsyndromal delirium level 1 | 82 (18.6) | 273 (18.3) | 983 (13.8) | 209 (13.8) | 11,521 (14.4) | |
| Subsyndromal delirium level 2 | 38 (8.6) | 230 (15.4) | 622 (8.7) | 97 (6.4) | 8,095 (10.1) | |
| Full delirium | 3 (0.7) | 14 (0.9) | 48 (0.7) | 5 (0.3) | 764 (1.0) | |
| Depression—any (Burrows, Morris, Simon, Hirdes, & Phillips, 2000) | E1a, E1d, E1f, E1h, E1i, E1l, E1m | 120 (27.3) | 212 (14.2) | 1,470 (20.7) | 300 (19.9) | 22,144 (27.7) |
| Discomfort behavior score (Stevenson, Brown, Dahl, Ward, & Brown, 2006)a | E1c, E1k, E1l-p, E4a-eA, E4a-eB, (Range 0–102) | 5.3 (9.6) | 3.4 (7.6) | 4.1 (8.8) | 3.8 (8.2) | 4.3 (8.8) |
| Physical | ||||||
| Acute condition—any | J5b | 92 (20.9) | 466 (31.3) | 1,366 (19.2) | 277 (18.3) | 22,287 (27.9) |
| Body mass indexa | K2a-b | 25.5 (5.1) | 22.9 (3.7) | 25.5 (5.4) | 25.8 (5.0) | 25.4 (5.1) |
| Bowel problems | I2b, H2b-d | 65 (14.8) | 500 (33.6) | 850 (11.9) | 238 (15.8) | 15,698 (19.6) |
| Catheter—indwelling | H3d | 53 (12.0) | 210 (14.1) | 910 (12.8) | 236 (15.6) | 11,490 (14.4) |
| Comorbidity index Charlson Index (Charlson, Pompei, Ales, & MacKenzie, 1987)a | I3a-e, and/or I1, (Range 0–30) | 2.1 (1.6) | 2.2 (1.8) | 2.3 (1.7) | 2.3 (1.8) | 1.8 (1.6) |
| Fever in last 7 days | J1h | 13 (3.0) | 86 (5.8) | 209 (2.9) | 33 (2.2) | 3,062 (3.8) |
| Incontinence—any (Bliss et al., 2013) | H1a-H1b | 182 (41.4) | 921 (61.8) | 4,233 (59.5) | 763 (50.5) | 33,942 (42.4) |
| Incontinence—dual | H1a and H1b | 106 (24.1) | 636 (42.7) | 3,004 (42.2) | 496 (32.8) | 19,442 (24.3) |
| Incontinence—only fecal | H1a | 32 (7.3) | 95 (6.4) | 588 (8.3) | 127 (8.4) | 4,884 (6.1) |
| Incontinence—only urinary | H1b | 44 (10.0) | 190 (12.8) | 641 (9.0) | 140 (9.3) | 9,616 (12.0) |
| Mortality risk | J1c, J1g, J1l, J1o, K3a, K4c, | 1.4 (1.19) | 2.1 (1.0) | 1.5 (1.1) | 1.5 (1.1) | 1.8 (1.1) |
| Changes in Health, End-stage disease and Symptoms and Signs (CHESS) (Hirdes, Frijters, & Teare, 2003)a | J5c, B6, G9 (Range 0–5) | |||||
| Medications, Number per weeka | O4a-e (Range 0–35) | 4.9 (5.4) | 2.9 (4.3) | 5.2 (5.6) | 4.9 (5.6) | 6.4 (6.0) |
| Oxygenation problems, No. of indicators | J1b, J1k-l, P1ag P1ai-j, P1al, P1bdA | |||||
| 1 | 39 (8.9) | 152 (10.2) | 678 (9.5) | 165 (10.9) | 9,961 (12.4) | |
| 2 | 38 (8.6) | 60 (4.0) | 400 (5.6) | 109 (7.2) | 6,382 (8.0) | |
| ≥3 | 21 (4.8) | 67 (4.5) | 266 (3.7) | 61 (4.0) | 4,994 (6.2) | |
| Pads—any use | H3g | 202 (45.9) | 92 (62.2) | 4,255 (59.8) | 746 (49.4) | 35,301 (44.1) |
| Perfusion problems | J1a, J1c-d, J1g | |||||
| No. of indicators | ||||||
| 1 | 94 (21.4) | 435 (29.2) | 1,703 (23.9) | 310 (20.5) | 22,130 (27.7) | |
| ≥2 | 3 (0.7) | 59 (4.0) | 121 (1.7) | 32 (2.1) | 2,376 (3.0) | |
| Poor nutrition | K3a, K4c | |||||
| No. of indicators | ||||||
| 1 | 141 (32.0) | 677 (45.4) | 2,586 (36.4) | 527 (34.9) | 33,402 (41.7) | |
| 2 | 33 (7.5) | 152 (10.2) | 367 (5.2) | 98 (6.5) | 6,040 (7.5) | |
| Tube feeding | K5b | 17 (3.9) | 180 (12.1) | 676 (9.5) | 139 (9.2) | 3,152 (3.9) |
Note. Values are presented as n (%) unless noted otherwise. MDS = Minimum Data Set.
Means (SDs).
Table 2.
Characteristics of Nursing Homes and Their Surrounding Communities.
| Nursing home staffing and care quality
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M (SD) | Licensed nurses (FTE/resident)
|
Licensed nurses (hr/resident/day)
|
CNA (FTE/resident)
|
CNA (hr/resident/day)
|
No. of deficienciesa
|
Quality of care indexb
|
% Residents on Medicaid
|
|||
| 0.22 (0.10) | 1.10 (0.48) | 0.44 (0.43) | 2.20 (2.13) | 3.83 (2.32) | 7.70 (6.29) | 73.86 (15.89) | ||||
|
| ||||||||||
| Census tract community characteristic | American Indians/ Asians/Pacific lslandersc | Black non- Hispanics | Hispanics | White non- Hispanics | Below poverty | Working class | Residing inside an urban area | Residing inside a rural area | Median household income | |
|
| ||||||||||
| Level (%) of community characteristic | Nursing homes, n (%) | Level of community characteristic | ||||||||
| <25 | 434 (97.0)d | 388 (87.0) | 406 (91.0) | 16 (4.0) | 406 (9 1.0) | 2 (0.5) | 210 (47.0) | 342 (77.0) | <$25,000 | 41 (9.0) |
| 25 to <50 | 10 (2.5) | 34 (8.0) | 28 (6.0) | 41 (9.0) | 38 (8.5) | 46 (10.0) | 1 (0.2) | 36 (8.0) | $25,000 to <$50,000 | 327 (73.0) |
| 50 to <75 | 0 (0.0) | 17 (4.0) | 9 (2.0) | 74 (17.0) | 2 (0.5) | 332 (74.5) | 13 (2.8) | 9 (2.0) | $50,000 to <$75,000 | 63 (14.0) |
| >75 | 2 (0.5) | 7 (1.0) | 3 (1.0) | 314 (70.0) | 0 (0.0) | 66 (15.0) | 222 (50.0) | 59 (13.0) | >$75,000 | 15 (4.0) |
Note. FTE = full-time equivalent; CNA = certified nursing assistant.
Selected deficiencies.
Scope/severity of selected quality of care deficiencies relevant to outcome.
Racial/ethnic categories are according to U.S. Census.
For example, 97% of NHs (n = 446) in our sample were located in Census tracts with <25% American Indian/Asian/Pacific Islander population.
Potential NH- and community-level predictors were developed and screened for inclusion in the models. NH-level predictors included proportions of NH residents with various health conditions or receiving Medicaid, NH quality deficiencies, staffing, and percentages of admissions with characteristics of interest, such as gender, race, and incontinence. Proportions of NH residents with various health conditions were calculated in seven areas: limitations in activities of daily living (ADLs), bowel/bladder incontinence, mobility/falls, mental status deficits, skin integrity problems, psychiatric medications, and weight loss. Composite predictor variables for NH quality deficiencies were constructed in four areas (resident behavior-facility, practices-dignity, quality of care, and resident assessment-nursing services) by summarizing the scope and severity levels of the respective deficiencies in a NH. In addition, the total number of deficiencies of interest by NH comprised a fifth composite. Nine staffing composite predictor variables were developed and screened for inclusion in the models: activity staff, certified nursing assistants/medication aides (CNAs), dietitians, dentists, licensed nurse (LN; including registered nurses [RNs] and licensed practical nurses [LPNs]), nursing administrators/directors of nursing, pharmacists, physicians/physician extenders, and therapists (physical, occupational, etc.). Staffing full-time equivalents (FTEs) per resident were calculated by dividing the total of the type of staff reported for a 2-week period (including full-time, part-time, and contract positions) by the total number of residents in a NH.
The SES and SDS of the tract surrounding each NH were described using seven Census-level variables in their original form and 16 variables that were converted into fractions of the Census tract population. Table 2 describes key predictors at the NH and community levels. The following additional community SES/SDS variables not listed in Table 2 were assessed for inclusion in the models: proportion of males or females aged <65 years and 65 and older in the tract, proportion of tract population in an urban cluster, proportion of tract population with 1 to 8 years or 9 to 16 years of education, proportion of tract population at <50%, 50% to 99%, or ≥100% of poverty, median home value, and poverty rank of the tract population.
The model for time to a pressure ulcer in White Non-Hispanic admissions, from which disparities for the minority groups were estimated, included the following potential predictors: score of deficits in ADLs, comorbidity index, cognitive deficit score, having fecal incontinence only, having dual incontinence, being bedfast/transfer dependent, number of indicators of poor nutrition, proportion of NH admissions that were White Non-Hispanic, the percentage of residents who were on Medicaid, licensed nurse staffing, CNA staffing, proportions of the community around a NH that had 9 to 16 years of education, was male, was greater than 65 years of age (representing the percentage of the community older aged), was below poverty level, or was in an urban area, and the Census divisions in which NHs were located. The model for the number of treatments for a pressure ulcer in White Non-Hispanic admissions, from which disparities for the minority groups were estimated, included the predictors of score of deficits in ADLs, comorbidity index, number of indicators of poor nutrition, having fecal incontinence only, NH quality of care, proportion of NH admissions that were White Non-Hispanic, proportion of the NH community in an urban area or below poverty level, and Census division of each NH.
Statistical Analysis
Data were summarized using descriptive statistics appropriate to their level, but differences were not formally tested for significance as the very large sample size renders even the smallest differences as statistically significant; hence no p values were generated.
Health disparities have typically been analyzed using traditional regression methods where pressure ulcers would be regressed against the race and ethnicity of NH admissions and other relevant characteristics. In these models, disparities are quantified by the predicted racial/ethnic group differences (given the observed characteristics) overall or within levels of those characteristics if interactions were included. The Peters–Belson method used in this study is a two-staged approach in which outcomes for disadvantaged groups (presumed to be racial-ethnic minority NH admissions in this study) are first predicted based on the regression model for an advantaged group (presumed to be White Non-Hispanic NH admissions in this study) and then compared with their own observed outcomes. In other words, the Peters–Belson method tests whether observed outcomes of a minority group differ from their predicted outcomes based on a regression model of outcomes for White Non-Hispanics.
In implementing the Peters–Belson method, each of the racial/ethnic groups was analyzed separately. Because the cohort is clustered within NHs, we controlled for unmeasured NH effects by insuring that residents of each racial/ethnic minority of interest were in the same NHs as White Non-Hispanics whose modeling coefficients were applied to their group; these are hence referred to as mixed race NHs. For time to a pressure ulcer, data for each group of White Non-Hispanic residents were analyzed using proportional hazards regression including individual- and NH/community-level factors. The estimated regression coefficients (beta weights) of the White Non-Hispanic model were applied to each minority group to calculate predicted time without a pressure ulcer. For visual comparison, Kaplan–Meier curves for each minority group’s predicted and observed time without a pressure ulcer were plotted together. Using a one-sample two-sided log-rank test, these two survival curves were compared (Eberly et al., 2013). When the log-rank statistic using resident and NH/community predictors was significant, this was defined as a significant disparity based on race or ethnicity. There is no racial-ethnic disparity when the difference between the observed and expected outcome is not significant or when the minority group has a better than expected outcome.
For ease of explaining the findings, Figure 1 shows observed and predicted proportion of admissions that developed a pressure ulcer over time. For each minority group, example proportions of residents who were observed to and expected to develop a pressure ulcer and the proportion of observed pressure ulcers in White Non-Hispanics were calculated at selected time points over the follow-up time. Overall disparity is the difference in the observed proportion between minority versus White Non-Hispanic admissions who developed an outcome. The percent of disparity explained by predictors in the model is calculated as ([Expected proportion of minority group − Observed proportion of White Non-Hispanics] / [Observed proportion of minority group − Observed proportion of White Non-Hispanics])*100.
Figure 1.
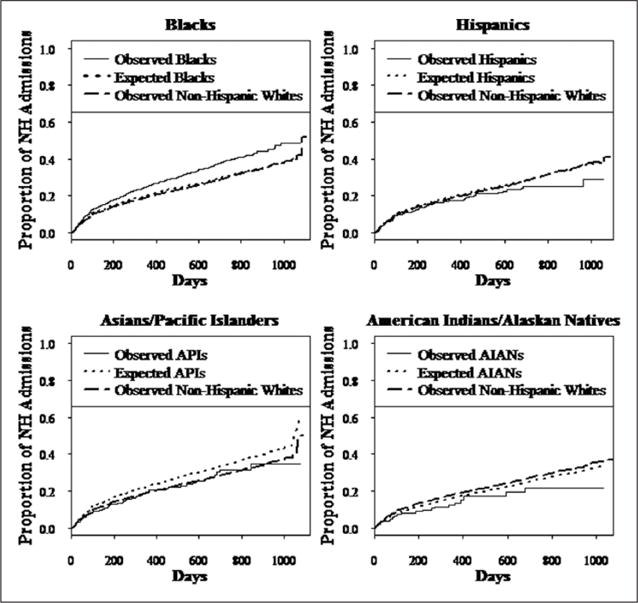
Survival curves of time to a pressure ulcer after nursing home admission.
Note. Compared with the proportion of each race/ethnic group that is expected to develop a pressure ulcer (dotted line), the observed proportion of Black admissions (solid line) was significantly more likely to develop a pressure ulcer and develop one sooner (p< .001). Hispanic (p = .04) and Asian/Pacific Islander (Asian; p = .01) admissions were less likely to develop a pressure ulcer and developed one later than expected. There was no significant difference in the observed versus expected proportion of American Indians/Alaskan Native (American Indian; p = .18) who developed a pressure ulcer over time. The proportion of White Non-Hispanic residents in the mixed race nursing homes observed to develop a pressure ulcer is also shown (dashed-dotted line). Analyses are adjusted for individual and nursing home/community predictors.
Among those with incident pressure ulcer, a similar method was used for the number of treatments for pressure ulcers. Clinical predictors were modeled in multiple regressions among White Non-Hispanics in the mixed race NHs, one model per minority racial/ethnic group. From these models, the expected number of treatments for each member of each disadvantaged group was calculated separately. These expected counts were then compared with the observed counts; significance was determined using a one-sample two-sided z test for each disadvantaged group. All steps of the Peters-Belson (PB) analysis were performed using R 2.14. Data management and descriptive statistics of the racial/ethnic groups’ characteristics and the NHs and their census tracts were conducted using SAS 9.2 (SAS Institute Inc., Cary, North Carolina, USA) and SPSS Version 18 (SPSS, Chicago, Illinois, USA). Final results were considered significant at the p< .05 level.
Results
Characteristics of the Cohort
The characteristics at NH admission of each racial/ethnic group that was followed for development of a pressure ulcer are presented in Table 1. In a previous study, we showed that this admission cohort is highly comparable with admitted older residents in all Medicare-/Medicaid-certified NHs in the United States during the same time period (Bliss et al., 2013). Greater percentages of White Non-Hispanic and Asian admissions were female, had a high school education, and were among the oldest age on average compared with the other admission groups. Regarding function/mobility, White Non-Hispanic and American Indian admissions had fewer deficits in ADLs, and fewer were restrained compared with the other admission groups. Fewer White Non-Hispanics had vision impairments. More Asian admissions suffered from some form of plegia or paresis while a higher percentage of Blacks were bedfast/dependent in transferring. In terms of cognitive and emotional status, cognitive deficits seemed relatively similar among all groups while delirium was present in a higher percentage of Asian admissions. White Non-Hispanics had the least communication difficulty. More White Non-Hispanics and American Indians were depressed. Asians showed the least discomfort behavior.
Regarding physical status, fewer Hispanics had an acute condition or a fever. Asians had the lowest body mass index on average and poorer nutrition, and more were tube fed. A lower percentage of White Non-Hispanic and American Indian admissions had incontinence of any type and fewer used pads. Fecal incontinence and dual incontinence were most common among Black admissions. Having only urinary incontinence was more common among Asians and White Non-Hispanics. An indwelling catheter was most common among Hispanics. Asians and White Non-Hispanics had more perfusion problems. White Non-Hispanics had more indicators of oxygen problems and received more medications.
Characteristics of the NHs and Surrounding Communities
Staffing of the NHs by licensed nurses had a mean of 1.09 (SD = 0.48) hr/ resident/day. Staffing by CNAs was approximately twice that of licensed nurses. The average number of deficiencies per NH was fairly low. Seventy-four percent of residents in the NHs were on Medicaid. The majority of NHs (70%) was located in a community whose population was mostly White Non-Hispanic, but 6% to 9% of the NHs were in communities whose populations were 25% to 50% Black or Hispanic (see Table 2). A high percentage of NHs (91%) was in a community that had <25% of its population below poverty level, and about half of the NHs (47%) was in a community with a high (50%–75%) percentage of working class. Half of the NHs were in a community that was mostly urban.
Overall Incidence of Pressure Ulcers and Treatment
Overall, a total of 7.7% of older people (6,989/90,580) developed at least one pressure ulcer after NH admission during the 3-year period of follow-up. Of those, 99% (6,917/6,989) received treatment for their pressure ulcer.
Disparities in Time to Developing a Pressure Ulcer
Black admissions
There was a significant disparity in time to a pressure ulcer among older Black individuals admitted to a NH, that is, more Blacks were observed to develop a pressure ulcer and sooner than expected had they been White Non-Hispanic. In Figure 1, this disparity is shown by a substantially lower dotted line representing the proportion of Blacks expected to develop a pressure ulcer had they been White Non-Hispanic compared with the solid line representing the observed proportion of Blacks who did develop a pressure ulcer. The total disparity, which is the difference between pressure ulcers observed in Blacks and those observed in White Non-Hispanics, is illustrated in Figure 1 by the gap between the solid line and the dashed-dotted line. The percent of the total disparity unexplained by the available predictors is shown by the space between the dotted line, which represents the proportion of Blacks expected to develop a pressure ulcer, and the solid line representing the pressure ulcers actually observed in Blacks. Figure 1 also shows that the proportion of Blacks expected to develop a pressure ulcer (dotted line) was slightly higher than the proportion of White Non-Hispanics observed to develop one (dashed-dotted line).
Table 3 shows examples of percentages of minority groups that were expected and observed to develop a pressure ulcer and the percentage of White Non-Hispanics observed to develop a pressure ulcer at selected time points of follow-up. The overall disparity in time to a pressure ulcer between Black and White Non-Hispanic admissions was 3% at 3 months and increased to 5.8% at 6 months and 7.7% at 18 months.
Table 3.
Observed Percentage of White Non-Hispanic Older Adults and Observed and Expected Percentages of Minority Adults Who Developed a Pressure Ulcer at Selected Time Points After Nursing Home Admission Adjusting for Individual- and Nursing Home/Community-Level Predictors.
| Racial/ethnic group | Months after admission | Percentage of White Non-Hispanics observed to develop a pressure ulcera | Percentage of racial/ethnic group expected to develop a pressure ulcer | Percentage of racial/ ethnic group observed to develop a pressure ulcer | Percentage overall disparityb | Percentage explained disparityc |
|---|---|---|---|---|---|---|
| Black | 3 | 8.75 | 9.45 | 11.69 | 2.94 | 23.81 |
| 6 | 13.19 | 14.11 | 16.93 | 3.74 | 24.60 | |
| 12 | 19.26 | 20.34 | 28.66 | 5.79 | 18.65 | |
| Hispanic | 3 | 8.45 | 8.91 | 7.50 | ||
| 6 | 13.01 | 13.59 | 11.44 | |||
| 12 | 18.73 | 19.38 | 17.19 | |||
| A/PI | 3 | 8.62 | 10.17 | 7.63 | ||
| 6 | 13.04 | 15.32 | 12.04 | |||
| 12 | 19.06 | 22.56 | 19.52 | |||
| AI/AN | 3 | 8.41 | 7.39 | 6.35 | ||
| 6 | 12.38 | 10.81 | 8.16 | |||
| 12 | 18.06 | 16.15 | 12.51 |
Note. A/PI = Asian/Pacific Islander; AI/AN = American Indian/Alaskan Native.
Pressure ulcer (Stages 2–4).
(Observed proportion of minority group − Observed proportion of White Non-Hispanics)*100.
([Expected proportion of minority group − Observed proportion of White Non-Hispanics]/[Observed proportion of minority group − Observed proportion of White Non-Hispanics])*100.
Predictors for time to a pressure ulcer that were significant in White Non-Hispanics and used in determining the disparity for Blacks were deficits in ADLs, being bedfast or dependent in transfer, having fewer cognitive deficits, greater comorbidities, fecal incontinence only, or poor nutrition, number of NH quality care deficiencies, and being in Census Division 3 (states of Indiana, Illinois, Michigan, Ohio, Wisconsin; see Table 4). Having fecal incontinence only was associated with the greatest hazard for developing a pressure ulcer followed by having poor nutrition or being bedfast/transfer dependent. Predictors in the model only explained a moderate percentage (~20%–25%) of the disparity in time to developing a pressure ulcer seen for Blacks (see Table 3). The explanatory ability of the predictors, which were measured at NH admission, declined over time.
Table 4.
Significant Predictors of Time to a Pressure Ulcer for White Non-Hispanic Admissions to Mixed Race Nursing Homes Used to Model Disparity in Blacks.
| Predictors | Individual level
|
Nursing home
|
Community
|
|||||
|---|---|---|---|---|---|---|---|---|
| Activities of daily living deficits | Bedfast/transfer dependency | Comorbidity index | Cognitive deficit score | Incontinence fecal only | Poor nutrition | Quality of care deficiencies | Census Division 3 | |
| HR [95% CI] | 1.06* [1.05, 1.06] | 1.13** [1.03, 1.23] | 1.08* [1.06, 1.10] | 0.95* [0.93, 0.97] | 1.55* [1.39, 1.73] | 1.16* [1.11, 1.22] | 1.01*** [1.002, 1.01] | 1.26** [1.08, 1.47] |
Note. CI = confidence interval. HR = hazard ratio.
p< .001.
p< .01.
p< .05.
Other minority admissions
There was no significant disparity in time to develop a pressure ulcer disadvantaging Asian, Hispanic, and American Indian admissions. In Figure 1, the curves that represent the proportion of admissions of each minority group who are expected to develop a pressure ulcer (dotted line) are above (Hispanics and Asians) or similar (American Indian) to the curve representing the proportion who did develop one. More Hispanics and American Indians (higher solid lines) were observed to develop a pressure ulcer compared with White Non-Hispanics (dashed-dotted lines; see Figure 1), but after controlling for risk factors for a pressure ulcer, no disparity based on race or ethnicity was found. The absence of disparity is shown by the following example: At 12 months after admission, 20.4% of Hispanics were expected to develop a pressure ulcer, but only 17.2% did.
Disparities in Treatment of Pressure Ulcers
There were no disparities in treatment of a pressure ulcer for any of the minority groups. Table 5 shows the average number of treatments for White Non-Hispanics and each minority group, which are similar. American Indians received slightly fewer treatments on average than all other groups.
Table 5.
Observed and Expected Mean Number of Treatments for Pressure Ulcers for White Non-Hispanic Admissions in Mixed Race Nursing Homes Used to Model Disparity due to Race or Ethnicity.
| Racial/Ethnic Group | Observed number of pressure ulcer treatments of minority group | Expected number of pressure ulcer treatments of minority group | Observed number of pressure ulcer treatments of White Non-Hispanics in respective mixed race nursing home |
|---|---|---|---|
| Black | 4.6 | 4.7 | 4.6 |
| Hispanic | 4.6 | 4.8 | 4.5 |
| A/PI | 5.1 | 4.9 | 4.7 |
| American Indian | 3.5 | 4.0 | 4.6 |
Note. A/PI = Asian/Pacific Islander; AI/AN = American Indian/Alaskan Native.
Discussion
The overall incidence of pressure ulcers, approximately 8%, is consistent with previously reported rates (Baumgarten et al., 2004; Howard & Taylor, 2009). The results of this study show the importance of analyzing pressure ulcer incidence by race and ethnicity as there were significant disparities for older Black individuals after they were admitted to a NH, supporting the findings of others (Baumgarten et al., 2004; Howard & Taylor, 2009). Our results add new information that Blacks developed a pressure ulcer sooner than expected, as a time to event model was used. Our study is among the first to provide information about time to incident pressure ulcers in minority groups other than Blacks. No disparity was found in the time to a pressure ulcer for the other racial/ethnic groups investigated. Other positive findings were that 99% of incident pressure ulcers were treated among all racial/ethnic groups, and no disparities were found in the number of treatments for pressure ulcers. NH residents with a pressure ulcer received an estimated three to five treatments on average.
As shown in the description of the health status of the racial/ethnic groups at NH admission, all significant predictors for time to a pressure ulcer at the individual level for White Non-Hispanics in mixed race NHs, except for having fewer indicators of poor nutrition, indicated poorer health in Blacks than White Non-Hispanics. Black admissions had greater comorbidities and worse functional or cognitive status than White Non-Hispanics, and a greater percentage of Blacks had fecal incontinence. These findings are consistent with other reports that Black admissions have greater care needs when they enter NHs due to poorer health (Buchanan, Rosenthal, Graber, Wang, & Kim, 2008; Davis & Lapane, 2004; Weintraub et al., 2000). A significant predictor at the NH level of time to a pressure ulcer was a higher number of quality care deficiencies of the NH. There is increasing evidence that Blacks are admitted to NHs that have lower quality of care (Grabowski, 2004; Mor et al., 2004), which our results appear to support. Being admitted to a NH in Census Division 3 (including states of Indiana, Illinois, Michigan, Ohio, Wisconsin) versus Census Division 1 (including Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont), the reference group, was a significant community-level predictor, suggesting that there is regional variation in nursing care practices that influence the development of pressure ulcers. Results suggest that even after controlling for these differences, Blacks develop a pressure ulcer sooner.
An advantage of the Peters–Belson method used in this study is that, unlike traditional regression methods, it does not assume that the estimated disparity should be the same for all members of the disadvantaged/minority group (Rao, Graubard, Breen, & Gastwirth, 2004). The application of the regression coefficients from the White Non-Hispanics’ model is made to the values of the predictors for each individual member of the minority group being modeled, enabling an estimate of outcomes for each minority member “as if they were White Non-Hispanic.” The Peters–Belson method also provides a quantification of the simple and intuitive ideas of “How much of the observed group disparity is explained and unexplained by the available characteristics?” The explained disparity quantifies how close outcomes of a minority group are to White Non-Hispanic outcomes if the minority group had the same predictor– outcome associations as White Non-Hispanics. One of the unique contributions of this study is the revelation that predictors typically used for assessing racial-ethnic disparity in pressure ulcers explain only about a quarter of the disparity. The unexplained disparity estimated by the Peters–Belson method represents the race-/ethnicity-based disparity between the White Non-Hispanic and minority group, that is, how far are the minority group’s observed outcomes from what we would predict based on the model for the White Non-Hispanics. The unexplained disparity in time to a pressure ulcer in Blacks suggests that discriminatory practices are a possibility (Graubard, Sowmya Rao, & Gastwirth, 2005; Rao et al., 2004). The unexplained disparity also represents factors that may be missing from the model or have a differential effect for the minority group (Eberly et al., 2013). In assessing disparity, our analysis screened a comprehensive set of potential predictors at the individual, NH, and community levels. Nonetheless, due to the high collinearity among variables that measure various aspects of health, there are limitations in the number of variables that can be included in a statistical model and in procedures for selecting them that could have resulted in some missing variables that could have improved the percentage of explained disparity.
Interventions aimed at changing known modifiable predictors identified by the Peters–Belson method can be expected to reduce the disparity in time to a pressure ulcer for Blacks. These include improving residents’ functional/ADL abilities, maintaining adequate nutritional status, reducing fecal incontinence, timely or careful assistance in transferring bedfast or dependent residents, and/or use of pressure-relieving devices in these residents. Another area for improvement is to reduce care deficiencies and thus raise the quality of care provided by NHs. The development of management and leadership skills of directors of nursing (Siegel, Mueller, Anderson, & Dellefield, 2010) and improving how nursing care is organized (Arling, Kane, Mueller, Bershadsky, & Degenholtz, 2007) are strategies recommended to improve quality of NH care in general.
The moderate percentage of disparity explained by the model suggests a need for additional interventions to reduce pressure ulcer incidences in Blacks. Possible factors that could contribute to the development of pressure ulcers that were not part of the model are inadequate risk screening for residents at risk of pressure ulcers and early identification of pressure-related damage on darker toned skin. Both interventions could have triggered prevention measures. The Braden Scale, which identifies residents at risk of pressure ulcers, has been shown to have good predictive validity for older Black persons in a hospital setting (Lyder et al., 1999) and offers potential benefit for preventing pressure ulcer onsets for NH residents. However, Wipke-Tevis et al. (2004) reported that pressure ulcer risk assessment tools, including the Braden Scale, are underutilized by NHs. There is no instrument with good psychometric qualities to guide nursing staff in identifying pressure-related damage in darker skinned individuals. Results suggest a need for efforts to make pressure ulcer risk assessment more universal and routine, to increase the expertise of nursing staff to assess darker toned skin for damage, and to develop appropriate tools to use for this assessment. Pressure ulcer prevention is another factor that could not be measured; however, an evaluation of the use and effectiveness of prevention measures seems warranted.
Chin et al. (2012) developed a set of general recommendations for reducing racial-ethnic disparities in health care for health care organizations. The main steps indicated a comprehensive approach including the recognition of the racial-ethnic disparities and an organizational commitment to reducing them, the implementation of a quality improvement process, and the design, implementation, evaluation, and sustainment of interventions that address the disparities. Some common interventions relevant to NHs include staff education and training in disparity reduction, using reminders and feedback to promote adherence to best practices and clinical guidelines, incentivizing/ rewarding positive behaviors with money or subsidized goods or services, restructuring care teams/care delivery models to achieve equitable care delivery, using technology to enhance quality improvement, and improving communication among staff.
Recent trends show that the diversity in NHs is rising with substantial increases in Black, Hispanic, and Asian residents due to a combination of social, demographic, and economic factors (Feng, Fennell, Tyler, Clark, & Mor, 2011) Forecasts predict greater disabilities among NH admissions as well as greater minority admissions (J. L. Angel, Angel, Aranda, & Miles, 2004; Lakdawalla et al., 2003). Identifying disparities in NH outcomes, as these findings do, highlights the importance of policies and efforts to prevent increases in disparities as the diversity of NHs rises and to eliminate disparities altogether. Eradicating racial and ethnic disparities in health and health care is a priority objective of several national agencies (Centers for Medicare and Medicaid Services, 2013; U.S. Department of Health and Human Services, 2011).
Although this study focused on racial and ethnic disparity in pressure ulcer incidences and ways of reducing the gap between minority and White Non-Hispanic admissions, the findings also highlight the need to lower the incidence of pressure ulcers in White Non-Hispanics. Since the initial model of risk factors for time to a pressure ulcer in the Peters–Belson method is done for White Non-Hispanics in mixed race NHs, suggested interventions might benefit White Non-Hispanic residents as well.
There are limitations in our study. Our data are from for-profit NHs and may not be representative of all NHs. The characteristics of our admission cohort, however, are comparable with those of all U.S. NHs (Bliss et al., 2013) of which 69% are for-profit (Centers for Medicare & Medicaid Services, 2012). There is limited generalizability of results to NHs whose residents are only of one race or to Stage 1 pressure ulcers. Although there were no disparities in pressure ulcer treatment, we are unable to know whether specific treatments were equally appropriate for each racial/ethnic group. The percentage of disparity explained by the predictors in the model may have been influenced in part by the necessity of including pressure ulcers of various stages and body locations in one outcome due to the limited specificity of the MDS items on pressure ulcers. It may be possible that the influence of risk factors might differ by pressure ulcer stage and location. As for all observational studies, not all relevant predictors of the outcome may be known or possible to include in our models.
In summary, there were disparities in the time to a pressure ulcer among older Black NH residents. Predictors that explained part of the disparity suggest factors to target to reduce the disparity. Combined with guidelines that promote equitable care within NH organizations, recommendations from this study offer potential to reduce disparities in pressure ulcer development and improve care of all NH residents. A positive outcome was that no disparities in treatment of a pressure were found.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by National Institute of Nursing Research, NIH, 1R01NR010731-01A2 and support was received from the Minnesota Supercomputing Institute at the University of Minnesota, Minneapolis, MN.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Allsworth JE, Toppa R, Palin NC, Lapane KL. Racial and ethnic disparities in the pharmacologic management of diabetes mellitus among long-term care facility residents. Ethnicity & Disease. 2005;15:205–212. [PubMed] [Google Scholar]
- Angel JL, Angel RJ, Aranda MP, Miles TP. Can the family still cope? Social support and health as determinants of nursing home use in the old Mexican-Origin population. Journal of Aging Health. 2004;16:338–354. doi: 10.1177/0898264304264203. [DOI] [PubMed] [Google Scholar]
- Arling G, Kane RL, Mueller C, Bershadsky J, Degenholtz HB. Nursing effort and quality of care for nursing home residents. Gerontologist. 2007;47:672–682. doi: 10.1093/geront/47.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten M, Margolis D, Van Doorn C, Gruber-Baldini AL, Hebel JR, Zimmerman S, Magaziner J. Black/White differences in pressure ulcer incidence in nursing home residents. Journal of the American Geriatrics Society. 2004;52:1293–1298. doi: 10.1111/j.1532-5415.2004.52358.x. [DOI] [PubMed] [Google Scholar]
- Bethell E. Controversies in classifying and assessing grade I pressure ulcers. Journal of Wound Care. 2003;12:33–36. doi: 10.12968/jowc.2003.12.1.26453. [DOI] [PubMed] [Google Scholar]
- Bliss DZ, Harms S, Garrard JM, Savik K, Gurvich O, Wyman JF, Cunanan K. Prevalence of incontinence by race and ethnicity of older people admitted to nursing homes. Journal of the American Medical Directors Association. 2013;14:451.e1–451.e7. doi: 10.1016/j.jamda.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RJ, Rosenthal M, Graber DR, Wang S, Kim MS. Racial and ethnic comparisons of nursing home residents at admission. Journal of the American Medical Directors Association. 2008;9:568–579. doi: 10.1016/j.jamda.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Burrows AB, Morris JN, Simon SE, Hirdes JP, Phillips C. Development of a minimum data set-based depression rating scale for use in nursing homes. Age and Ageing. 2000;29:165–172. doi: 10.1093/ageing/29.2.165. [DOI] [PubMed] [Google Scholar]
- Cai S, Mukamel DB, Temkin-Greener H. Pressure ulcer prevalence among Black and White nursing home residents in New York state: Evidence of racial disparity? Medical Care. 2010;48:233–239. doi: 10.1097/MLR.0b013e3181ca2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services. Nursing home data compendium 2012. 2012 Retrieved from http://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/downloads/nursinghomedatacompendium_508.pdf.
- Centers for Medicare and Medicaid Services. CMS Quality Strategy 2013–Beyond. 2013 Retrieved from http://www.ahrq.gov/workingforquality/agency-plans/cms-quality-strategy.pdf.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chin MH, Clarke AR, Nocon RS, Casey AA, Goddu AP, Keesecker NM, Cook SC. A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. Journal of General Internal Medicine. 2012;27:992–1000. doi: 10.1007/s11606-012-2082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Lapane KL. Do characteristics associated with nursing home residents vary by race/ethnicity? Journal of Health Care for the Poor and Underserved. 2004;15:251–266. doi: 10.1353/hpu.2004.0022. [DOI] [PubMed] [Google Scholar]
- Dealey C, Lindholm C. Pressure ulcer classification. In: Romanelli M, editor. Science and practice of pressure ulcer management. London, England: Springer; 2006. pp. 37–41. [Google Scholar]
- Dosa D, Intrator O, McNicoll L, Cang Y, Teno J. Preliminary derivation of a nursing home confusion assessment method based on data from the minimum data set. Journal of the American Geriatrics Society. 2007;55:1099–1105. doi: 10.1111/j.1532-5415.2007.01239.x. [DOI] [PubMed] [Google Scholar]
- Eberly LE, Hodges JS, Savik K, Gurvich O, Bliss DZ, Mueller C. Extending the Peters-Belson approach for assessing disparities to right censored time-to-event outcomes. Statistics in Medicine. 2013;32:4006–4020. doi: 10.1002/sim.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Fennell ML, Tyler DA, Clark M, Mor V. The care span: Growth of racial and ethnic minorities in US nursing homes driven by demographics and possible disparities in options. Health Affairs (Project Hope) 2011;30(7):1358–1365. doi: 10.1377/hlthaff.2011.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardo MP, Teno JM, Mor V. Not so black and white: Nursing home concentration of Hispanics associated with prevalence of pressure ulcers. Journal of the American Medical Directors Association. 2009;10:127–132. doi: 10.1016/j.jamda.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski D. The admission of Blacks to high-deficiency nursing homes. Medical Care. 2004;42:456–464. doi: 10.1097/01.mlr.0000124307.17380.df. [DOI] [PubMed] [Google Scholar]
- Graubard BI, Sowmya Rao R, Gastwirth JL. Using the Peters–Belson method to measure health care disparities from complex survey data. Statistics in Medicine. 2005;24:2659–2668. doi: 10.1002/sim.2135. [DOI] [PubMed] [Google Scholar]
- Harada ND, Chun A, Chiu V, Pakalniskis A. Patterns of rehabilitation utilization after hip fracture in acute hospitals and skilled nursing facilities. Medical Care. 2000;38:1119–1130. doi: 10.1097/00005650-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Hartmaier SL, Sloane PD, Guess HA, Koch GG. The MDS Cognition Scale: A valid instrument for identifying and staging nursing home residents with dementia using the minimum data set. Journal of the American Geriatrics Society. 1994;42:1173–1179. doi: 10.1111/j.1532-5415.1994.tb06984.x. [DOI] [PubMed] [Google Scholar]
- Hirdes JP, Frijters DH, Teare GF. The MDS-CHESS scale: A new measure to predict mortality in institutionalized older people. Journal of the American Geriatrics Society. 2003;51:96–100. doi: 10.1034/j.1601-5215.2002.51017.x. [DOI] [PubMed] [Google Scholar]
- Hopper T, Bayles KA, Harris FP, Holland A. The relationship between minimum data set ratings and scores on measures of communication and hearing among nursing home residents with dementia. American Journal of Speech-Language Pathology. 2001;10:370–381. [Google Scholar]
- Howard DL, Taylor YJ. Racial and gender differences in pressure ulcer development among nursing home residents in the southeastern United States. Journal of Women & Aging. 2009;21:266–278. doi: 10.1080/08952840903284594. [DOI] [PubMed] [Google Scholar]
- Hudson TJ, Cody M, Armitage TL, Curtis MA, Sullivan G. Disparities in use of antipsychotic medications among nursing home residents in Arkansas. Psychiatric Services. 2005;56:749–751. doi: 10.1176/appi.ps.56.6.749. [DOI] [PubMed] [Google Scholar]
- Lakdawalla D, Goldman DP, Bhattacharya J, Hurd MD, Joyce GF, Panis WA. Forecasting the nursing home population. Medical Care. 2003;41:8–20. doi: 10.1097/00005650-200301000-00003. [DOI] [PubMed] [Google Scholar]
- Li Y, Yin J, Cai X, Temkin-Greener J, Mukamel DB. Association of race and sites of care with pressure ulcers in high-risk nursing home residents. Journal of the American Medical Association. 2011;306:179–186. doi: 10.1001/jama.2011.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyder CH, Yu C, Emerling J, Mangat R, Stevenson D, Empleo-Frazier O, McKay J. The Braden Scale for pressure ulcer risk: Evaluating the predictive validity in Black and Latino/Hispanic elders. Applied Nursing Research. 1999;12:60–68. doi: 10.1016/s0897-1897(99)80332-2. [DOI] [PubMed] [Google Scholar]
- Mor V, Zinn J, Angelelli J, Teno JM, Miller SC. Driven to tiers: Socioeconomic and racial disparities in the quality of nursing home care. Milbank Quarterly. 2004;82:227–256. doi: 10.1111/j.0887-378X.2004.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. Journals of Gerontology, Series A: Biological Sciences & Medical Sciences. 1999;54:M546–M553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- Rao RS, Graubard BI, Breen N, Gastwirth JL. Understanding the factors underlying disparities in cancer screening rates using the Peters-Belson approach: Results from the 1998 National Health Interview Survey. Medical Care. 2004;42:789–800. doi: 10.1097/01.mlr.0000132838.29236.7e. [DOI] [PubMed] [Google Scholar]
- Savik K, Fan Q, Bliss D, Harms S. Preparing a large data set for analysis: Using the minimum data set to study perineal dermatitis. Journal of Advanced Nursing. 2005;52:399–409. doi: 10.1111/j.1365-2648.2005.03604.x. [DOI] [PubMed] [Google Scholar]
- Siegel EO, Mueller C, Anderson KL, Dellefield ME. The pivotal role of the director of nursing in nursing homes. Nursing Administration Quarterly. 2010;34:110–121. doi: 10.1097/NAQ.0b013e3181d91813. [DOI] [PubMed] [Google Scholar]
- Stevenson KM, Brown RL, Dahl JL, Ward SE, Brown MS. The Discomfort Behavior Scale: A measure of discomfort in the cognitively impaired based on the Minimum Data Set 2.0. Research in Nursing & Health. 2006;29:576–587. doi: 10.1002/nur.20168. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. HHS action plan to reduce racial and ethnic health disparities. A nation free of disparities in health and health care. 2011 Retrieved from http://minorityhealth.hhs.gov/npa/files/Plans/HHS/HHS_Plan_complete.pdf.
- Weintraub D, Raskin A, Ruskin PE, Gruber-Baldini AL, Zimmerman SI, Hebel JR, Magaziner J. Racial differences in the prevalence of dementia among patients admitted to nursing homes. Psychiatric Services. 2000;51:1259–1264. doi: 10.1176/appi.ps.51.10.1259. [DOI] [PubMed] [Google Scholar]
- Wipke-Tevis DD, Williams DA, Rantz MJ, Popejoy LL, Madsen RW, Petroski GF, Vogelsmeier AA. Nursing home quality and pressure ulcer prevention and management practices. Journal of the American Geriatrics Society. 2004;52:583–588. doi: 10.1111/j.1532-5415.2004.52166.x. [DOI] [PubMed] [Google Scholar]


