Abstract
Background: Pregnancy is a risk factor for sleep disordered breathing, including obstructive sleep apnea (OSA). Progesterone, one of the key hormones in pregnancy, a known respiratory drive stimulant, increases ventilation and may protect against OSA. We aimed to examine the relationship between circulating progesterone and OSA, after accounting for body weight and gestational age.
Methods: A case control study was conducted of pregnant women with OSA and those at low risk for the disorder. Cases were identified by ICD-9 code and review of medical record. Controls were identified if they scored zero (never) for snoring, apnea, and gasping on the multivariable apnea prediction index questionnaire immediately following delivery. Subjects with available stored first and/or second trimester residual serum samples were then included in this study and serum analyzed for progesterone. Raw progesterone levels were adjusted for the effects of gestational age and maternal weight.
Results: Twenty-seven cases and 64 controls with available serum were identified. Women with OSA had greater maternal weight and higher rates of related comorbidities, compared to controls. Progesterone levels correlated positively with gestational age and negatively with greater weight. Progesterone levels, adjusted for gestational age and maternal weight and expressed as multiples of median (MoM), were significantly lower in OSA cases compared to controls in both the first trimester (MoM = 0.71, confidence interval [95% CI] 0.60–0.83) relative to the MoM in controls of 1.00. In the second trimester levels were also lower in OSA cases (MoM = 0.84, 95% CI 0.73–0.96) compared to the MoM of 1.00 in controls.
Conclusions: Progesterone levels, after accounting for weight and gestational age, were lower in women with OSA than controls. Progesterone may play a protective role against OSA.
Keywords: : obesity, obstructive sleep apnea, pregnancy, progesterone
Introduction
Obstructive sleep apnea (OSA) is a sleep related breathing disorder in which the upper airway is repeatedly obstructed during sleep. OSA is characterized by various degrees of airflow limitation, intermittent hypoxemia, and repeated arousals. Sleep disordered breathing, a spectrum of disorders that encompasses snoring, OSA, and other disorders, is more prevalent in pregnant than in nonpregnant women (35% vs. 9%).1,2 Several factors associated with physiologic changes of pregnancy may contribute to this higher prevalence. Nasal congestion,3 reduction in upper airway size,4 more advanced Mallampati scores,5 and decreased functional residual capacity6 are all factors that may contribute to the de novo development or worsening of preexisting sleep disordered breathing. Sleep disordered breathing, including OSA, is associated with an elevated risk of adverse pregnancy outcomes such as gestational hypertensive disorders and gestational diabetes, possibly leading to adverse neonatal outcomes such as intrauterine growth restriction and premature delivery.7–10
Progesterone is a crucial reproductive hormone in pregnancy with levels reaching 6- to 8-fold preconception levels due to placental contribution in its secretion.11 It is also a key factor in stimulating ventilation at the level of the central chemoreceptors on the ventrolateral surface of the medulla,12 leading to increased minute ventilation, decreased end-tidal carbon dioxide, and lowered upper airway resistance.13,14 As such, it is possible that progesterone may play a protective role against sleep disordered breathing.1 There are multiple proposed mechanisms by which increased progesterone levels may inhibit the development of sleep disordered breathing. Ventilatory drive stimulation and increased minute ventilation may be one of the protective mechanisms.15 Furthermore, progesterone enhances the responsiveness of the upper airway dilator muscles to chemical stimuli during sleep, reducing upper airway resistance.16,17 Though increased progesterone levels create a state of respiratory alkalosis that may theoretically favor central apneas, we recently demonstrated that pregnant women were unlikely to be diagnosed with central sleep apnea compared to matched nonpregnant controls.18
Studies involving exogenous administration of progesterone directly suggest a protective role of progesterone on breathing control and the prevalence of obstructive events. In postmenopausal women with chronic hypercapnic respiratory and partial airway obstruction during sleep, administration of medroxyprogesterone has been associated with improved carbon dioxide levels.19,20
Given this potential protective role, we hypothesized that OSA status would impact levels of progesterone in pregnancy. The main aims of this study were to examine the relationship between progesterone levels and body mass index (BMI) and assess the association between OSA status and progesterone levels.
Materials and Methods
This is a case control study aimed at evaluating progesterone levels in women with OSA and low risk controls that were enrolled at two large teaching hospitals. The study was approved by the Institutional Review Boards for Human Studies at Women and Infants Hospital of Rhode Island and Rhode Island Hospital in Providence, RI, as a collaborative project, IRB No. 254193, approved on August 11, 2011.
Identification of pregnant women with OSA
Women with OSA (cases) were identified by a review of ICD-9 codes for OSA and delivery between 2003 and 2013. Individual medical records were reviewed to confirm a diagnosis of OSA (i.e., positive polysomnography report or documentation of an outpatient continuous positive airways pressure [CPAP] prescription for sleep apnea). Given the limited documentation regarding details of OSA diagnosis and treatment in the available obstetric medical records on retrospective review, an accurate timing of OSA diagnosis in relation to timing of pregnancy (diagnosis preceding or during pregnancy) could not be determined. Apnea hypopnea index (AHI) is defined as the number of apneas and hypopneas per hour of sleep. AHI ≥5 events per hour was considered abnormal. Disease severity was defined as mild (AHI 5–15), moderate (15–30), and severe (>30). Participants with multiple gestation pregnancies were excluded from this study. Of the women meeting these criteria, 27 (48%) also had sufficient first and/or second trimester residual serum samples for progesterone testing.
Identification of pregnant controls
At delivery, a convenience sample of English-speaking women was screened for sleep disordered breathing by trained study personnel. Survey was conducted between 2007 and 2009. Informed consent was obtained from all participants and detailed methods of this survey-based screening test have been published.7,21 Briefly, women were asked to rate the frequency of three symptoms (snoring, gasping, and witnessed apneas) over their last 3 months of pregnancy in the following categories: never (scored 0), rarely (1), sometimes (2), often (3), and always (4).22 Of the 321 patients who scored 0 for all three symptoms, 64 (20%) had available first and/or second trimester residual serum and were considered controls. Given that sleep disordered breathing symptoms worsen rather than improve during the course of pregnancy23 women who denied symptoms in late pregnancy per questionnaire were also unlikely to have had symptoms earlier in pregnancy—at the time of sampling for progesterone—and thus were considered to be adequate controls. Similarly to cases, participants with multiple gestation pregnancies were excluded from this study. The same source population was used for controls as for cases, and controls were proportionally sourced from the same two large teaching hospitals as cases.
Laboratory testing
Serum samples were originally drawn for Down syndrome screening in the first and/or second trimester of pregnancy. First trimester stored serum samples were collected between days 71 and 98 of gestation (10–13 completed weeks), while second trimester samples were collected between days 105 and 159 (15–22 completed weeks).
Residual first and second trimester serum samples from case and control subjects were retrieved from −20°C freezer storage, thawed, and tested. Progesterone levels were determined using an automated immunoassay (Immulite 2000 Siemens, Los Angeles CA). Progesterone coefficient of variance was 5% at 19.7 ng/mL. Maternal weight for both cases and controls was provided by one-time provider report at the initial time of sample collection. Height was obtained from the medical record.
Statistical analysis
Descriptive statistical analysis, including mean, median, standard deviation, and range, was performed using SAS version 9.2 (SAS Institute, Cary, NC). Categorical variables were compared between cases and controls using two-tailed Fisher's exact test and reported as count and percent. Continuous variables were compared between cases and controls using the Student's t-test and reported as mean and standard deviation. Progesterone measurements were reported as multiples of the median (MoM) to account for gestational age and maternal weight. The use of MoM (multiple of the median) was first described in 1977 by Wald et al.24 and is currently the standard for reporting prenatal screening tests. MoM is a measure of how far an individual value deviates from the median value obtained in a control, reference population. Given the dynamic nature of screening markers results in pregnancy, variation in analytical performance of immunoassays used for testing, and specific population characteristics, an individual's test result may vary widely; hence MoM can normalize an individual test by comparing it to a representative population's median value. Therefore, a patient's MoM would then be reported as patient result/population median. A single set of log-linear medians was fitted to the combined first and second trimester data for controls. The progesterone values were converted to MoM levels by dividing by the expected median value for a given gestational age. These MoM levels were then regressed against maternal weight and a reciprocal model fitted.25 All progesterone values, including those from cases, were then converted to gestational age and maternal weight adjusted MoM levels. The Wilcoxon rank sum test was used to report significant differences between levels of hormones between OSA cases and controls. BMI values were divided in categories according to data from the National Health and Nutrition Examination Survey to identify women, but they were analyzed as a continuous variable. Correlation of progesterone and BMI was examined using the Pearson correlation test. Data were not adjusted for the effect of CPAP therapy as records from the obstetric hospital were inconsistent in the documentation of CPAP prescription along with duration or adequacy of therapy.
Results
Patient characteristics
A total of 27 pregnant women with confirmed OSA and 64 pregnant controls were identified with available residual serum. Table 1 lists selected maternal and pregnancy outcome characteristics of women included in the study. Women with OSA had significantly higher BMI (p < 0.001), and significantly higher rates of gestational diabetes (p < 0.001), preeclampsia (p = 0.0025), pregestational type 2 diabetes (p = 0.0016), and chronic hypertension (p < 0.001), as compared to controls. Gestational age at delivery was significantly lower in OSA cases as compared to controls (p = 0.012).
Table 1.
Characteristics of Enrolled Women and Their Pregnancy, Stratified by Presence (Cases) or Absence (Controls) of Obstructive Sleep Apnea
| Characteristic | Cases | Controls | p |
|---|---|---|---|
| Number of women | 27 | 64 | |
| Mean age in years (SD) | 29.7 (6.2) | 27.6 (6.4) | 0.15 |
| Parity | |||
| Primiparous, n (%) | 11 (41) | 24 (38) | 0.82 |
| Multiparous, n (%) | 16 (59) | 40 (63) | |
| Mean BMI (kg/m2) at sampling (SD) | 41.4 (8.9) | 25.9 (5.6) | <0.001 |
| Missing data for 2 control subjects, N = 62 | |||
| Mean weight in Kg at sampling (SD) | 113 (28) | 69.1 (14) | <0.001 |
| Mean gestational age at sampling (SD) | |||
| First trimester, n, mean (SD) | 20, 12.0 (1.0) | 44, 11.9 (1.0) | 0.92 |
| Second trimester, n, mean (SD) | 26, 17.5 (1.9) | 59, 17.3 (1.7) | 0.62 |
| Gestational diabetes, n (%) | 10 (37) | 3 (5) | <0.001 |
| Pregestational diabetes type II, n (%) | 5 (19) | 0 (0) | 0.0016 |
| Chronic hypertension, n (%) | 13 (48) | 0 (0) | <0.001 |
| Preeclampsia, n (%) | 6 (22) | 1 (1.6) | 0.0025 |
| Maternal race | |||
| Black, n (%) | 2 (7) | 10 (15) | 0.50 |
| Non-Black, n (%) | 25 (93) | 54 (84) | |
| Mean gestational age at delivery (SD) | 36.8 (3.6) | 39.1 (1.1) | 0.012 |
| Mean birthweight in grams (SD) | 3644 (887) | 3379 (458) | 0.33 |
| Missing data for 4 case subjects, N = 23 | |||
| Birthweight <2500 g, n (%) | 4 (17) | 1 (1.6) | 0.0162 |
| Birthweight >4500 g, n (%) | 2 (8) | 1 (1.6) | 0.17 |
BMI, body mass index; SD, standard deviation.
Women with no available serum sample were compared to women with available serum samples who were included in the study for demographics and comorbidities and pregnancy outcomes. There were no significant differences observed in age, weight, and BMI at sampling, history of pregestational diabetes and chronic hypertension, development of gestational diabetes or hypertension during the index pregnancy, and gestational age at delivery and mean birthweight. There were no significant differences in any of these variables between the two groups suggesting minimal selection bias (Data not shown.)
Raw progesterone levels
Raw progesterone values documented in this study fell within the expected population progesterone norms. In the first trimester, the average raw progesterone value in our study across all subjects was 21.6 ng/mL, with a reference range of 9–33 ng/mL. In the second trimester, the average raw progesterone value across all subjects was 33 ng/mLwith a reference range of 29–50 ng/mL.
Conversion of progesterone levels to weight adjusted multiples of the median
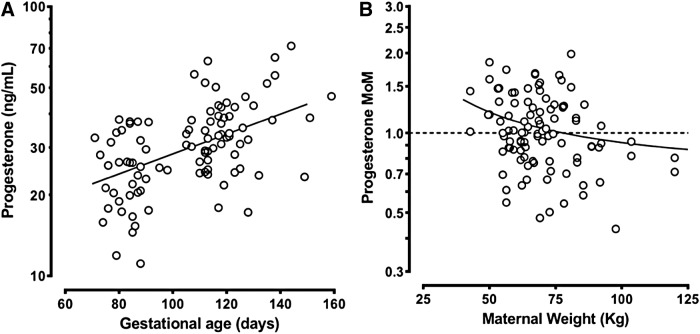
To examine the relationship of progesterone levels and gestational age and maternal weight, progesterone levels from control pregnancies were plotted. Progesterone levels in the 64 control pregnancies showed the expected positive association with gestational age (Fig. 1A). Linear regression was used to estimate the median levels, after a logarithm transformation of progesterone results. The resulting day-specific regression equation was then used to transform the results in mass units to MoM. These progesterone MoM levels were then regressed against maternal weight (Fig. 1B). Progesterone MoM adjusted for gestational age was negatively associated with maternal weight, with heavier women having lower gestational age-adjusted progesterone. In two women, the maternal weight was missing and no weight adjustment was possible. Using the expected gestational age and maternal weight-specific medians computed in controls, progesterone results in cases were also converted to weight-adjusted MoM levels. The resulting MoM levels in all study subjects were then divided by 0.93 (the observed weight adjusted MoM in all control results), so that the overall median MoM in control pregnancies was exactly 1.00.
FIG. 1.
Association of progesterone level with gestational age and maternal weight in 64 control women. (A) Raw progesterone levels in mass units in control women are shown on a logarithmic vertical axis with gestational age shown on the horizontal axis. (B) Progesterone MoM (adjusted for gestational age) in control women are shown on a logarithmic vertical axis with maternal weight shown on the horizontal axis. MoM, multiples of the median.
Progesterone levels and OSA status
Progesterone levels were significantly lower in OSA cases than in controls across both trimesters. In the first trimester, the median progesterone MoM level in cases was 0.71 MoM (confidence interval [95% CI] 0.60–0.83, p < 0.001), relative to the MoM in controls of 1.00. In the second trimester, the median progesterone MoM level in cases was 0.84 MoM (95% CI 0.73–0.96, p = 0.001). Figure 2 shows the gestational age and maternal weight adjusted progesterone results expressed in MoM in cases and controls in both the first and second trimesters of pregnancy.
FIG. 2.
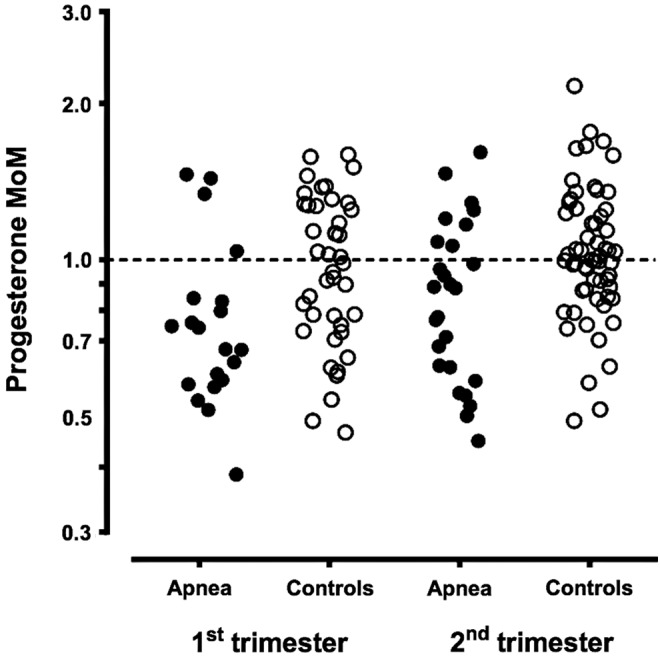
Adjusted progesterone levels in women with and without obstructive sleep apnea, stratified by trimester of pregnancy. The gestational age and weight-corrected progesterone levels, expressed as MoM, are shown on the vertical axis. Samples associated with women diagnosed with sleep apnea are shown as filled circles while levels in control women are shown as open circles. The left side shows levels in the first trimester, while the right side shows levels in the second trimester.
Discussion
To our knowledge, this is the first study to evaluate the relationship between progesterone and OSA status during pregnancy, with consideration of gestational age at sample collection and maternal weight. We found a positive correlation of progesterone with gestational age, as expected, and a negative correlation with maternal weight. Convention in prenatal screening is to express maternal serum markers as MoM to account for gestational age and weight effects, as for example, when discussing alpha-fetoprotein, human chorionic gonadotropin, and unconjugated estriol26; however, this has not been the case for earlier studies of progesterone and sleep disordered breathing research. Expressing progesterone as MoM in accordance with convention for other maternal serum markers, as performed in this study, should be strongly considered for future clinical use and analysis, since interpretation of progesterone as raw, unadjusted values may not provide an accurate assessment of adequate progesterone secretion.
Progesterone levels remain a valuable way to assess pregnancy health, as deficiency may play a role in specific pathologies such as recurrent pregnancy loss.27 In current clinical practice, progesterone levels are interpreted as raw values, and patient characteristics such as gestational age and maternal weight are not factored into the interpretation. In this study, we have confirmed the expected increase in progesterone levels with increasing gestational age. We have also demonstrated that higher weight in pregnant women is, in general, associated with lower progesterone levels and vice versa. This negative correlation may be due to a simple dilution from the larger blood volume of heavier women, as is routinely observed for Down syndrome screening markers. In prenatal screening for Down syndrome and open neural tube defects, adjusting levels of maternal serum markers by covariates such as maternal weight has been shown to improve screening performance.28 We have controlled for weight in this study by using linear regression.
As hypothesized, our study has shown significantly lower progesterone levels in women with OSA compared to controls. Our progesterone MoM of 0.71 for cases in the first trimester indicates that progesterone levels in women with OSA are 29% lower than matched controls during this period. Similarly, a progesterone MoM of 0.84 in the second trimester indicates that levels in women with OSA are 16% lower than matched controls during this time. These findings suggest that women with higher levels of progesterone may be protected against OSA. As lower progesterone levels are associated with heavier weight, it is possible that lower progesterone levels may in part mediate the association between obesity and OSA in pregnancy. However, progesterone levels remained lower in women with OSA even after correction for maternal weight.
We cannot discount the possibility that OSA may have impacted placental secretion of progesterone. This putative effect may occur via oxidative stress, sympathetic stimulation, or inflammation. Our previous work showed that women with OSA had alterations in placental secretory function evidenced by altered pregnancy associated plasma protein A and estriol levels as compared to low risk controls.29,30 We have also demonstrated evidence of fetal normoblastemia—a marker of chronic hypoxia on placental histopathology—and cellular hypoxia evidenced by altered carbonic anhydrase IX in placentas of women with OSA.31 It is therefore possible that reduced progesterone occurs in response to placental damage from OSA, rather than, or in addition to having a causative effect.
As it was difficult to ascertain the timing of OSA development in relation to pregnancy in our cohort, our data cannot determine a temporal relationship between OSA and progesterone secretion to help address the question of causality. The association of OSA and progesterone levels may also differ depending on the timing of OSA development and diagnosis in relation to pregnancy. We did, however, find a somewhat larger effect in the first compared to the second trimester. Additional studies are needed to confirm this finding, especially since only a single sample was evaluated in each trimester. Other possible confounding variables of progesterone secretion, such as circadian rhythm, pulsatile secretion, and metabolic clearance could be addressed with multiple samples from each subject within a trimester. While progesterone levels have not appeared to follow consistent circadian patterns, levels may fluctuate with pulsatile secretion and decrease after meals.32–34 Lastly, our data are complicated by the fact that meaningful data on continuous airway pressure therapy use, duration, or compliance could not be reliably ascertained by a review of the obstetrical medical record among our subjects due to poor documentation, prohibiting any useful analysis of the effect of therapy on the described association. However, in this study, CPAP prescription among OSA cases may have perhaps led to a reduction in effect size and ultimately an underestimation of study results.
This study was limited by the use of stored convenience samples. Hence, only a limited subset of subjects had residual serum samples available for progesterone testing and inclusion in this study (48% of subjects with confirmed OSA diagnosis and 20% of controls had residual samples for testing). Given the large proportion of controls excluded from this study, selection bias in determining the study control group in particular is possible. However, comparison of included controls with available residual serum and excluded controls without available residual serum shows overall similar maternal demographics, comorbidities, and similar maternal and neonatal outcomes. Additionally, our control subjects at low risk for OSA originated from a convenience sample screened by questionnaires rather than polysomnography, due to lack of funding to perform polysomnography on asymptomatic women. Therefore, it is possible that a small number of control subjects could have had undiagnosed OSA not picked up by the multivariable apnea prediction index survey. If present, this would lead to a reduced estimate of the effect size.
Conclusions
Our study demonstrated a positive association of progesterone levels with gestational age through the first and second trimesters and a negative correlation between progesterone levels and maternal weight. In addition, lower progesterone levels in women with OSA, after accounting for gestational age and maternal weight, may suggest a protective role of this hormone against OSA.
Ultimately, the relationship between low progesterone levels and OSA is important to confirm, as progesterone may serve as an inexpensive method to identify pregnant women at higher risk of OSA and may be used in conjunction with other clinical predictors to triage women for diagnostic testing. In addition, as exogenous progesterone supplementation has been shown to improve upper airway obstruction during sleep and lower carbon dioxide levels by improving ventilation in the nonpregnant population, this therapy could also be evaluated in pregnancy. The use of such therapy would be simplified by the already approved use of progesterone in women with a history of preterm labor. Other future research may expand the focus to other hormones with a potential to impact respiratory control and upper airway obstruction, such as leptin.35,36
Acknowledgments
J.L.: Grant funding from the NIH NHLBI (T35HL094308). G.B. was funded by NICHD R01HD078515-01A1 at the time of this study. Data from this study have been presented at the European Respiratory Society Meeting in Barcelona (2013) and at the Congress for Women's Health in Washington DC (2015). The authors would like to acknowledge Beth Hott for her help with article preparation and all the patients who have consented to be part of the study. Dr. G.B. received funding from the Perkins Charitable Foundation. The Perkins Foundation had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bourjeily G, Ankner G, Mohsenin V. Sleep-disordered breathing in pregnancy. Clin Chest Med 2011;32:175–189 [DOI] [PubMed] [Google Scholar]
- 2.O'Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: Prospective cohort study. Am J Obstet Gynecol 2012;207:487.e1–e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camann WR, Ostheimer GW. Physiological adaptations during pregnancy. Int Anesthesiol Clin 1990;28:2–10 [DOI] [PubMed] [Google Scholar]
- 4.Izci B, Vennelle M, Liston WA, Dundas KC, Calder AA, Douglas NJ. Sleep-disordered breathing and upper airway size in pregnancy and post-partum. Eur Respir J 2006;27:321–327 [DOI] [PubMed] [Google Scholar]
- 5.Pilkington S, Carli F, Dakin MJ, et al. Increase in Mallampati score during pregnancy. Br J Anaesth 1995;74:638–642 [DOI] [PubMed] [Google Scholar]
- 6.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005;172:1363–1370 [DOI] [PubMed] [Google Scholar]
- 7.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J 2010;36:849–855 [DOI] [PubMed] [Google Scholar]
- 8.Micheli K, Komninos I, Bagkeris E, et al. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology 2011;22:738–744 [DOI] [PubMed] [Google Scholar]
- 9.Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep 2014;37:843–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol 2012;206:136.e1–e5 [DOI] [PubMed] [Google Scholar]
- 11.Halasz M, Szekeres-Bartho J. The role of progesterone in implantation and trophoblast invasion. J Reprod Immunol 2013;97:43–50 [DOI] [PubMed] [Google Scholar]
- 12.Edwards N, Middleton PG, Blyton DM, Sullivan CE. Sleep disordered breathing and pregnancy. Thorax 2002;57:555–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol 2008;164:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol 2010;202:261.e1–e5 [DOI] [PubMed] [Google Scholar]
- 15.Cugell DW, Frank NR, Gaensler EA, Badger TL. Pulmonary function in pregnancy. I. Serial observations in normal women. Am Rev Tuberc 1953;67:568–597 [DOI] [PubMed] [Google Scholar]
- 16.Parisi RA, Santiago TV, Edelman NH. Genioglossal and diaphragmatic EMG responses to hypoxia during sleep. Am Rev Respir Dis 1988;138:610–616 [DOI] [PubMed] [Google Scholar]
- 17.Wheatley JR, White DP. The influence of sleep on pharyngeal reflexes. Sleep 1993;16:S87–S89 [DOI] [PubMed] [Google Scholar]
- 18.Bourjeily G, Sharkey KM, Mazer J, Moore R, Martin S, Millman R. Central sleep apnea in pregnant women with sleep disordered breathing. Sleep Breath 2015;19:835–840 [DOI] [PubMed] [Google Scholar]
- 19.Saaresranta T, Polo-Kantola P, Irjala K, Helenius H, Polo O. Respiratory insufficiency in postmenopausal women: Sustained improvement of gas exchange with short-term medroxyprogesterone acetate. Chest 1999;115:1581–1587 [DOI] [PubMed] [Google Scholar]
- 20.Saaresranta T, Polo-Kantola P, Rauhala E, Polo O. Medroxyprogesterone in postmenopausal females with partial upper airway obstruction during sleep. Eur Respir J 2001;18:989–995 [DOI] [PubMed] [Google Scholar]
- 21.Bourjeily G, Raker C, Chalhoub M, Miller M. Excessive daytime sleepiness in late pregnancy may not always be normal: Results from a cross-sectional study. Sleep Breath 2013;17:735–740 [DOI] [PubMed] [Google Scholar]
- 22.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep 1995;18:158–166 [DOI] [PubMed] [Google Scholar]
- 23.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep 2005;28:1299–1305 [DOI] [PubMed] [Google Scholar]
- 24.Wald NJ, Cuckle H, Brock JH, Peto R, Polani PE, Woodford FP. Maternal serum-alpha-fetoprotein measurement in antenatal screening for anencephaly and spina bifida in early pregnancy. Report of U.K. collaborative study on alpha-fetoprotein in relation to neural-tube defects. Lancet 1977;1:1323–1332 [PubMed] [Google Scholar]
- 25.Neveux LM, Palomaki GE, Larrivee DA, Knight GJ, Haddow JE. Refinements in managing maternal weight adjustment for interpreting prenatal screening results. Prenat Diagn 1996;16:1115–1119 [DOI] [PubMed] [Google Scholar]
- 26.Reynolds TM, Vranken G, Van Nueten J. Weight correction of MoM values: Which method? J Clin Pathol 2006;59:753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ku CW, Allen JC, Jr., Malhotra R, et al. How can we better predict the risk of spontaneous miscarriage among women experiencing threatened miscarriage? Gynecol Endocrinol 2015;31:647–651 [DOI] [PubMed] [Google Scholar]
- 28.Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down's syndrome. Health Technol Assess 1998;2:i–iv:1–112. [PubMed] [Google Scholar]
- 29.Bourjeily G, Curran P, Butterfield K, Maredia H, Carpenter M, Lambert-Messerlian G. Placenta-secreted circulating markers in pregnant women with obstructive sleep apnea. J Perinat Med 2015;43:81–87 [DOI] [PubMed] [Google Scholar]
- 30.Bourjeily G, Butterfield K, Curran P, Lambert-Messerlian G. Obstructive sleep apnea is associated with alterations in markers of fetoplacental wellbeing. J Matern Fetal Neonatal Med 2015;28:262–266 [DOI] [PubMed] [Google Scholar]
- 31.Ravishankar S, Bourjeily G, Lambert-Messerlian G, He M, De Paepe ME, Gundogan F. Evidence of placental hypoxia in maternal sleep disordered breathing. Pediatr Dev Pathol 2015;18:380–386 [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto VY, Clifton DK, Cohen NL, Soules MR. Variability of serum prolactin and progesterone levels in normal women: The relevance of single hormone measurements in the clinical setting. Obstet Gynecol 1990;76:71–78 [PubMed] [Google Scholar]
- 33.van Kerkhof LW, Van Dycke KC, Jansen EH, et al. Diurnal variation of hormonal and lipid biomarkers in a molecular epidemiology-like setting. PLoS One 2015;10:e0135652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima ST, McAuliffe T, Gibson M. The 24-hour pattern of the levels of serum progesterone and immunoreactive human chorionic gonadotropin in normal early pregnancy. J Clin Endocrinol Metab 1990;71:345–353 [DOI] [PubMed] [Google Scholar]
- 35.Pho H, Hernandez AB, Arias RS, et al. The effect of leptin replacement on sleep-disordered breathing in the leptin-deficient ob/ob mouse. J Appl Physiol (1985) 2016;120:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Q, Pho H, Kirkness J, et al. Localizing effects of leptin on upper airway and respiratory control during sleep. Sleep 2016;39:1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]