Abstract
Obesity is rampant across the spectrum of age, gender, and race in the Unites States. Paralleling this epidemic, kidney stone prevalence is also rising, affecting nearly 1 in 11 individuals. Bariatric surgical procedures, such as Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG), are the most effective weight loss options for morbidly obese or severely obese individuals with comorbidities. A number of studies have linked kidney stone development to bariatric surgical history, particularly RYGB, which portends up to a threefold increase in calcium oxalate stone risk compared with age-matched, obese controls. Stone development after malabsorptive (RYGB) and restrictive (SG) bariatric procedures are driven primarily by alterations in 24-h urine profiles, such as increased urinary oxalate, decreased urine volume, and reduced urinary citrate levels—all of which have been linked to increased kidney stone risk. What clinical recommendations, if any, can be given to reduce kidney stone risk in bariatric kidney stone patients? This review provides not only updated stone incidence and 24-h urine data in this population, but also reassurance—the metabolic alterations that result from bariatric surgery can be successfully mitigated by increased provider awareness, patient education, and a combination of dietary and pharmacological adjustments.
Keywords: : kidney stones, calcium oxalate, Roux-en-Y gastric bypass, hyperoxaluria, hypocitraturia, 24-h urine
Introduction
Obesity is a worldwide public health concern. In the United States, one out of three adults is obese, accounting for 16.5% ($168 billion) of all U.S. health expenses annually.1–3 In morbidly obese (body mass index [BMI] >40 kg/m2) or severely obese individuals (BMI >35 kg/m2) with complications, bariatric surgery continues to be the preferred intervention to attain successful long-term weight reduction and effectively lower obesity-associated mortality and comorbidities, such as diabetes, cardiovascular disease, and hypertension.4–6 Despite its potential complications, bariatric surgery is extremely successful in weight and morbidity reduction, leading to a steady number of procedures performed annually in the United States (54.2/100,000 adults, ∼200,000/year).7,8
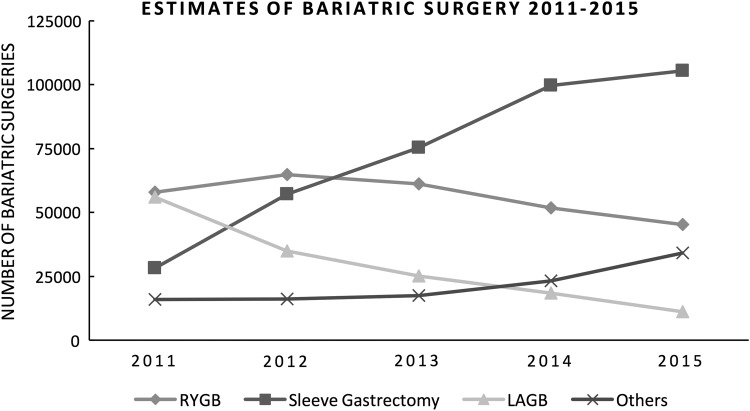
In 2013 (Fig. 1), sleeve gastrectomy (SG) surpassed Roux-en-Y gastric bypass (RYGB) surgery as the most common bariatric procedure in the United States.8 RYGB, however, still comprises a significant portion of bariatric surgeries performed in the United States and is reported to have better resolution of obesity-related comorbidities than SG.9 From 1998 to 2008, almost 750,000 RYGB were performed in the United States, accounting for ∼80% of all bariatric surgeries during this 10-year period and reaching over 1 million RYGB surgeries up to 2015.10 In addition to a number of gastrointestinal complications, RYGB, and its associated fat malabsorption, have the potential to cause a number of other long-term complications, including metabolic derangements, nutritional deficiencies, and kidney stones.11
FIG. 1.
Estimates of the trends in bariatric surgery from 2011 to 2015, modified from the American Society for Metabolic and Bariatric Surgery website, published in July 2016. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers
With such high prevalence of RYGB over the last 10 years, physicians should monitor this group of patients more closely due to their risk of renal stones. Prevalence of nephrolithiasis—10.6% in men and 7.1% in women—has been noted to be higher in obese individuals (11.2%) compared with normal-weight individuals (6.1%) in the United States, p < 0.001.12 RYGB patients with previous stone history were found to have kidney stone recurrence rate as high as 18.6% just 2 years after RYGB surgery. Alterations in urine chemistry profiles and metabolic derangements have been the current focus of study to reveal associations of kidney stone formation after bariatric surgery, especially RYGB, to better assist patients and manage their risk factors for nephrolithiasis after successful weight loss surgery.11 In addition to metabolic abnormalities, RYGB patients demonstrate higher supersaturation of calcium oxalate, higher urinary oxalate levels, lower urine volumes, and hypocitraturia in 24-h urine analysis, placing them at increased stone risk. Supersaturation of the urine with stone-forming salts is a critical factor in crystallization and increasing water intake can significantly decrease lithogenesis. Efforts are currently underway to further stratify risk factors in this population and provide tactics in lowering stone risk. In this review of the current literature, we tabulate and summarize the existing data and provide patient strategies to prevent stone formation and its limitations.
Methods
The most recent studies present in the literature were reviewed using MEDLINE. Key words included all forms and abbreviations of nephrolithiasis, kidney stone formation, calcium oxalate supersaturation, and hyperoxaluria with regard to restrictive bariatric procedures, laparoscopic adjustable gastric banding (LAGB), and SG, and malabsorptive bariatric procedures, biliopancreatic diversion with duodenal switch (BPD), and RYGB surgery.
Results and Discussion
Changes in urinary chemistry profiles following bariatric surgery
Among previous reports, RYGB has been the principal bariatric surgery associated with significantly higher 24-h urine oxalate levels and urinary calcium oxalate supersaturation (CaOx SS), both of which contribute to developing calcium oxalate stones. In a 2005 study of a 23 patient cohort with confirmed oxalate nephropathy (n = 2) and calcium oxalate nephrolithiasis (n = 21) after RYGB surgery, Nelson et al. first described RYGB-associated enteric hyperoxaluria and high rates of calcium oxalate nephrolithiasis.13 They reported means of 2.38 μmL/L (normal range <1.77) in CaOx SS in eight standard RYGB and 2.69 μmL/L in six malabsorptive/distal RYGB patients. Thereafter, number of groups have reported high incidence of calcium oxalate stones, elevated levels of oxalate, CaOx SS, hypocitraturia, and low urine volumes in 24-h urine profiles in similar retrospective and prospective studies. A summary of mean 24-h urine data from retrospective RYGB, SG, or gastric banding surgeries are stratified by stone history in Table 1.13–28
Table 1.
Summary of Mean 24-h Urine Data from Roux-en-Y Gastric Bypass or Restrictive Procedures Stratified by Stone History
| Patient number | Oxalate (mg/day) | Citrate (mg/day) | Volume (L/day) | |
|---|---|---|---|---|
| RYGB and 24-h urine (∼12 months F/U) | ||||
| Adult NSF, prospective14–19 | 277 | 44 | 442 | 1.1 |
| Adult NSF, retrospective13,20–24 | 177 | 54 | 312 | 1.1 |
| Adolescents NSF, retrospective25 | 10 | 41.8 | 646 | 1.49 |
| Primarily stone formers, any type26–28 | 166 | 71 | 415 | 1.4 |
| LAGB or SG 24-h urine (∼12 months F/U) | ||||
| Adult NSF, retrospective21,22 | 30 | 36 | NR | 1.3 |
| Adolescents NSF, retrospective25 | 7 | 26.4 | 687 | 0.95 |
Prospectively collected 24-h urine chemistry profiles from primarily nonstone formers before and after either RYGB (n = 275) or BPD (n = 2) procedure are summarized in Table 1.14–19 In the six prospective studies, 24-h urine chemistry profiles collected from 277 patients in a mean of 11 months after RYGB or BPD, revealed a 36.4% increase (28 to 44 mg/day) in urine oxalate levels after their bariatric surgery. Park et al. reported data from 45 RYGB patients in a prospective, longitudinal study, before and after their procedure.14 Their group noted a statistically significant increase in CaOx SS and urine oxalate levels, along with a decrease in urinary total volume in L/daily (p = 0.002) and a decrease in median urinary citrate (p = 0.0006). Urine citrate levels should be of high relevance due to their known endogenous inhibition effect on calcium oxalate crystallization by forming soluble complexes and decreasing stones.29
In RYGB patients, citrate levels have been shown to decrease over time. Duffey et al. demonstrated a 38% increase in the number of patients with hypocitraturia in a cohort of 21 nonstone formers over a 2-year period after RYGB.15 Their group also reported a twofold increase in urine oxalate excretion from 33 to 29 mg/day (p ≤ 0.001), although CaOx SS and urine volume did not significantly change. Similarly, Kumar et al. analyzed urine chemistry profiles for 9 RYGB and 2 BPD patients (mean pre-BMI 45.7 kg/m2 and post-BMI 28.4 kg/m2) before and after their surgeries.16 Although urine oxalate only increased 23% (26.4 to 32.6 mg/day) 12 months after surgery and citrate levels did not show significant changes, CaOx SS doubled (2.3) in 6 months (p = 0.003), and urine volume was significantly lower (p = 0.018) at 6 months postsurgery. Of note, a 1.5-fold increase in oxalate absorption was observed after oral oxalate load 12 months after surgery, which correlated with a similar increase in plasma oxalate levels at 12 months (p = 0.018), suggesting a correlation between dietary oxalate absorption and lithogenesis risk in this population.
Analogous findings were found by Valezi et al. who studied a large prospective cohort of 151 pre and post-RYGB subjects (median BMI changed from 44.1 to 27.0 kg/m2; p < 0.001).17 Twelve months after RYGB, both mean urinary citrate levels (268 to 170 mg/day) and urine volumes (1.31 to 0.93 L/day) significantly (p < 0.001) decreased by 36% and 29%, respectively. Of note, all 151 patients were found to be hypocitraturic at 12 months post-RYGB. Overall, urinary oxalate also increased significantly from 24 mg/day preop to 41 mg/day postop, p < 0.001. Notably, urinary oxalate level was found to be a significant predictor for de novo stone formers postop (n = 11, p = 0.015). When compared postoperatively, urine oxalate levels were lower in nonstone formers (n = 135) versus de novo stone formers, 40 mg/day versus 47 mg/day, respectively. Moreover, Wu et al. followed 38 obese patients for 6 months after their RYGB surgery and noted significant increase in urinary CaOx SS and oxalate levels in their 24-h urine parameters compared with prebariatric surgery baseline levels.18 Equivalent findings were reported by Agrawal et al. in a prospective cohort of 13 morbidly obese patients. Their group collected baseline 24-h urines 4 weeks before and 1, 2, 4, and 6 months after RYGB, noting a significant twofold increase in urinary oxalate (12.6 to 28.4 mg/day, p = 0.005), an increase (p = 0.001) in CaOx supersaturation, and a decrease (p = 0.006) in urine volume at 6 months from baseline. Additionally, urinary citrate levels continuously decreased reaching a 6-month low of 305 mg/day compared with 540 mg/day at baseline.19
Recently, Lieske et al. analyzed 24-h urinary chemistry profiles in 55 bariatric surgical patients with stone formation and 248 bariatric surgical patients without stones after either standard RYGB, BDP, very long limb RYGB, and SG; as well as 20 obese stone former controls without previous history of bariatric surgery.30 A significant difference (p < 0.001) was seen in urine oxalate excretion (0.70 mmol/day = 63.0 mg/day) in 42 of the 55 stone formers at >8 months after their respective bariatric surgeries compared with 112 nonstone formers at >8 months (42.3 mg/day). When mean urine oxalate levels in controls (35.1 mg/day) were compared only to RYGB stone formers, a significant (p < 0.05) increase was also found. Lieske et al. also reported significant (p < 0.005) increase in mean CaOx SS, delta Gibbs between RYGB stone formers and obese controls (1.69). Overall, urine citrate levels were significantly lower >8 months postbariatric surgery (mean of 448 mg/day) versus nonstone formers >8 months postsurgery (610 mg/day); p < 0.05. As expected, CaOx SS was significantly (p < 0.001) higher throughout postbariatric surgery stone formers (2.12) versus nonstone formers (1.50) after surgery. Interestingly, CaOx SS remained above the mean (1.77) in both obese controls with stones and in the nonstone postbariatric surgery group at all time periods, but was highest in postbariatric surgery stone formers.
Similarly, obesity in children and adolescents is a public health issue of alarming concern. Prevalence of obesity (BMI >95 percentile for the BMI-for-age growth charts) has been estimated at 16.9% from ages of 2 to 19.31 Because of this, bariatric surgery is more commonly utilized in this population. However, urinary metabolic indices after bariatric surgery in severely obese adolescents have not previously been reported. DeFoor et al. are the first to report a comparative analysis of urinary parameters in 17 obese adolescents and 14 obese controls (mean ∼18 years of age) ∼12 months after bariatric surgery.25 In their population, adolescents who underwent RYGB had higher urine oxalate excretion compared with SG (p = 0.04) and obese nonoperative controls (p < 0.01), with hyperoxaluria (>40 mg/day) being identified in half (50%) of the RYGB group and in a third (30%) of the control group. Urinary citrate levels and CaOx SS were similar in all groups.
Kidney stone incidence in postbariatric surgery patients
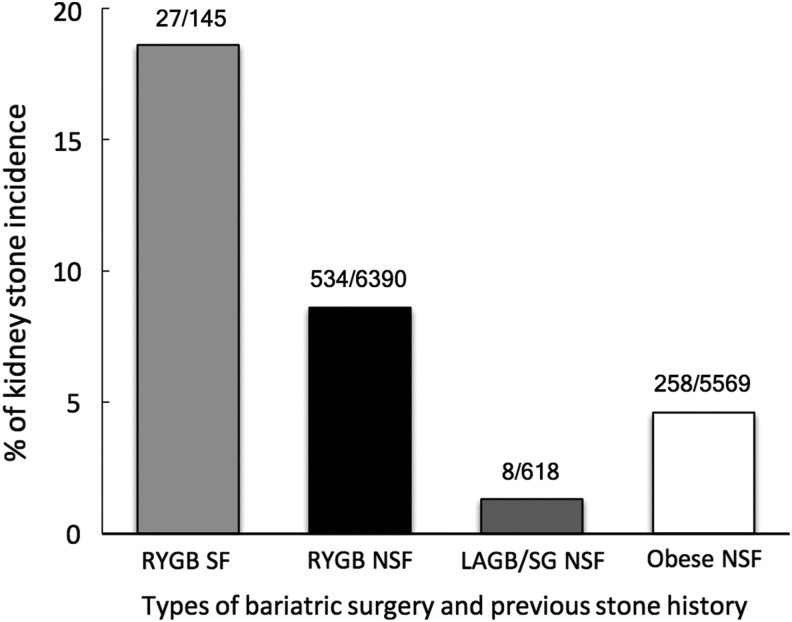
Kidney stone rates after bariatric surgery has reportedly increased up to four times in patients with a previous history of nephrolithiasis.11 The seven studies that describe kidney stone incidence after bariatric surgery or in obese controls are summarized in Figure 2.17,32–37 RYGB individuals with previous stone history were found to have kidney stone recurrence rate as high as 18.6% (27/145 patients) within ∼2 years of RYGB compared with 8.6% (534/6390) of patients with no previous kidney stone history. The results are limited, however, as only 4 small series have reported rates of recurrent stone disease following RYGB versus 6390 subjects without previous stone history. Restrictive procedures (LAGB and SG) had a much lower stone incidence rate of 1.3% (8/618) compared with obese controls 4.6% (258/5569) during a similar 2-year time frame.35,36
FIG. 2.
Kidney stone incidence with at least 2 years follow-up in obese patients or following bariatric surgery.17,32–37
The literature on the incidence of nephrolithiasis after RYGB consists of only a small sample of studies and more efforts are needed to delineate direct causation. Matlaga et al. presented the first large-scale claims data report in a case–control study of 4639 patients in which they found a 7.65% incidence of urolithiasis in post-RYGB patients versus 4.63% in obese patients in the control group (p < 0.0001).32 The mean time to develop a stone was 1.5 years after bariatric surgery. Their claim data analysis indicates a significant 1.71-fold increased risk of stone formation within ∼4 years of RYGB surgery. The first report of increased stone prevalence in a cohort of 972 RYGB patients (8.8%) compared with rates from a control (5.2%) population in the United States dates back to 2006 when Durrani et al. reported a prevalence of 3.6% de novo stones in post-RYGB patients (mean time to stone formation of 2.8 years).33 Although the incidence of de novo stone formation postsurgery was lower (3.2%), it still implies a significant risk. Valezi et al. examined 24-h urine levels 1 year post-RYGB in 135 nonstone formers and 11 de novo (8% incidence) stone formers and found that urine oxalate was a significant predictor for de novo stone formers using multivariable analysis (OR 1.41, 95% CI 1.101–1.803; p = 0.006). In addition, their group also found hyperuricosuria to be a significant predictor of developing nephrolithiasis 1 year after RYGB (OR 1.09, 95% CI 1.002–1.016; p = 0.013).17
In 2015 Lieske et al. reported an 11.1% incidence in bariatric patients over a mean follow-up of 6 years compared with a 4.3% in obese controls.30 Most of the bariatric procedures performed (n = 591, 78%) were standard RYGB surgeries. At baseline bariatric surgical patients and controls had a similar rate of stone formation, 4.0% and 4.2% respectively. Their group established that kidney stone events increased as early as the first 2 years after surgery, doubling after 10 years. Using multivariable analysis, Lieske et al. reported standard RYGB surgery as a statistically significant risk factor (OR 2.13, 95% CI 1.30–3.49; p = 0.003) for the development of kidney stones. Not surprisingly, patients who had undergone malabsorptive procedures had the highest risk of forming stones, while standard RYGB showed an intermediate stone risk and the risk was lowest in those who underwent restrictive procedures.
Most recently, Haddad et al. conducted a standardized telephone questionnaire with ∼50% response rate in 478 patients who had undergone RYGB.34 The median BMI before surgery was 51 kg/m2 and the mean follow-up after RYGB was 7 years. The rate of overall post-RYGB symptomatic urolithiasis was 7.3% (35 patients out of 478) with a median stone incident time of 3.1 years following RYGB. The incidence of de novo symptomatic stone incident was 5.7% (25 patients out of 435), whereas that of recurrence was 23% (10 patients out of 43) in previous stone formers, with a median time from RYGB surgery to stone incident of 3.3 and 2 years, respectively.
In contrast to the RYGB procedure, the risk of kidney stones does not appear to increase after restrictive types of bariatric surgeries, such as LAGB or SG. In a retrospective study, Chen et al. found a 1.2% risk of stones in 85 SG and 332 gastric banding surgical patients over a 4.5-year period. In another study, Semins et al. identified a 1.5% rate of stone formation over 2.5 years in 201 gastric banding patients versus 6% in matched obese controls (n = 201). Although taken from a small sample, the combined risk of 1.3% (n = 618) appears to be lower than the risk present in obese control individuals of 4.6% (n = 5569).35,36
Strategies, limitations, and solutions to reduce calcium oxalate stone risk after RYGB
Largely, the key strategies to prevent kidney stones after bariatric surgery are similar to those recommended to all stone formers and a summary is provided in Table 2. As discussed above, low urine volume status in bariatric surgical patients can significantly increase the risk of nephrolithiasis due to supersaturation of stone-forming solute particles. Recommending all patients to increase their intake of water to 2 L/day is a widely used prevention strategy by all physicians. Although a seemingly simple task, compliance with these recommendations can be difficult, as RYGB patients have small gastric pouches which can limit fluid intake, decrease urine volume, and increase urinary crystal supersaturation. This setback is not only limited to RYGB patients, but is also present in patients with restrictive-only procedures, for which prevalence has been steadily increasing. Two groups of researchers highlighted the importance of appropriate hydration in these subjects with restrictive-only procedures when they found that postoperative elevations in CaOx SS do occur due to decreased urinary volumes, even though urinary oxalate levels were not significantly higher.21,22 To address this problem, kidney stone formers may consider using smartphone application reminders to remind them to drink fluid regularly. In addition, combining supportive therapies, such as drinking fluids containing high citrate levels (e.g., lemonade) can double the positive effect.
Table 2.
Reducing Calcium Oxalate Stone Risk After Roux-en-Y Gastric Bypass
| Risk factors | Recommendations | Restrictions in RYGB patients | Solutions |
|---|---|---|---|
| Hyperoxaluria | Low-oxalate diet (<80–100 mg/day) | Component in vegetables and “healthy” foods (peanuts, bran, soy), bioavailability variable. | Patient education,a downloadable phone applications, “balance” versus avoidance |
| Low-fat diet (<25% daily calories) | High prevalence of fatty foods in diets | Early satiety after surgery, patient education | |
| Probiotics | No commercially available Oxalobacter, unknown efficacy of Lactobacillus sp. | Most yogurts contain protein, calcium, and forms of probiotics | |
| Vitamin B6 (pyridoxine) | Well studied in primary hyperoxaluria; potential for neurotoxicity at high doses | Consider supplementing 50 mg/day (low dose) × 6 months then discontinue | |
| Calcium citrate and dietary calcium to bind enteric oxalate | Tolerability, absorption efficacy, compliance, expense | Patient education, low-dose chewable Citracal (250 mg) taken 5–6 × daily with small meals | |
| Hypocitraturia | Potassium citrate | Tolerability, absorption efficacy, expense | Dispense as liquid or crystal/powder forms |
| Low urine volume | Urine output >2 L/day | Compliance, small stomach pouch | Push fluids high in citrate (i.e., lemonade), downloadable phone application reminders |
| High-sodium and protein diet | Low-salt (<2300 mg/day) and animal protein (0.8–1.0 g/kg/day) intake | Both ubiquitous, particularly in American diet | Patient education, follow Dietary Approaches to Stop Hypertension-style diet |
High oxalate food contents can be found at: https://regepi.bwh.harvard.edu/health/Oxalate/files
Modified from Canales and Hatch.11
Citrate
Hypocitraturia is defined as a urinary excretion of citrate <320 mg/day. Decreased urinary citrate levels in RYGB surgical patients has been linked to increased stone formation, as citrate is a known critical inhibitor of crystallization.29In the renal tubules, citrate has the capability to bind calcium molecules forming soluble complexes. However, when citrate is at low levels, calcium is free to bind molecules, such as oxalate, forming insoluble complexes and promoting calcium oxalate agglomeration and stone formation. Several groups have found experimental RYGB surgery to be associated with a development of a metabolic acidosis in rodents.38,39 Likewise, the literature reports that citrate salts, such as potassium citrate, and alkali are able to play a role correcting hypocitraturia and metabolic acidosis.15,28,32,40 Randomized controlled trials have demonstrated that potassium citrate is associated with decreased calcium stone risks in patients with low oxalate urine excretion levels.41 Recently, Sakhaee et al. assessed 24 and 15 patients, at a mean of 4.7 and 4.2 years after RYGB, respectively, in two-phase, randomized placebo crossover studies comparing the effect of a potassium citrate combined formula, potassium–calcium citrate (PCC), on calcium oxalate crystallization.42 Their group reported that PCC treatment (40 mEq potassium, 800 mg calcium, 100 mEq citrate/d) led to a raise in urine pH and citrate levels, as well as a decrease in calcium oxalate agglomeration, leading to inhibition of calcium oxalate crystallization. Moreover, due to its liquid formulation and rapidly dissolving properties, PCC appears to be more effective in raising acute serum calcium levels and have better bioavailability than calcium citrate.43 Although PCC is not commercially available, it has the potential to correct key metabolic abnormalities in RYGB patients and decrease calcium oxalate stone risk factors.
Oxalate and oral calcium
Oxalate is a common constituent in the Western diet, ranging from 100 to 200 mg intake daily.44 Efforts need to be taken to instruct bariatric patients to maintain a low oxalate intake ranging from 50 to 80 mg/day, since an oxalate-free diet is practically impossible. In patients with enteric hyperoxaluria after bariatric surgery, dietary oxalate loads lead to an increase in urinary oxalate levels. Evidence provided by Froeder et al. showed a twofold mean increase in oxaluria after an oxalate load test in a cohort of RYGB patients compared with controls, suggested that RYGB patients absorb more oxalate from their diet.20 A valuable list of oxalate-containing food products and alternatives is available at https://regepi.bwh.harvard.edu/health/Oxalate/files. Phone applications and brochure strategies can also be employed by clinicians to further educate and help patients. Finally, calcium supplementation, which is encouraged in bariatric patients to maintain bone health, is also felt to be an important method to limit oxalate absorption. Calcium is a key player for the inhibition of oxalate reabsorption in the gut. A cohort observational study revealed a significant inverse relationship between oxalate excretion indices and dietary calcium intake.45 Enteric binding of oxalate by low-dose dietary calcium is an effective, and routinely recommended, clinical strategy to combat hyperoxaluria. Penniston and Nakada showed significantly decreased calcium oxalate supersaturation indices in hyperoxaluric renal stone formers supplemented with either calcium citrate (300–500 mg) or a targeted nutritional therapy of calcium-containing foods (≥300 mg) during meals.46 This retrospective study of 22 patients demonstrated significantly decreased urinary oxalate excretion in both cohorts without changes in urinary calcium excretion. However, the addition of calcium citrate to a low-oxalate diet in this group did not result in a greater decrease of urinary oxalate excretion than the low-oxalate calcium nutritional therapy group alone. Nevertheless, their data support the importance of calcium nutritional therapy with meals to effectively manage hyperoxaluria. Supporting evidence for the benefits of calcium intake with meals as a successful medical therapy for urinary oxalate excretion has been scarce, but abundant calcium should bind oxalate loads during meals and prevent the complex absorption from the gastrointestinal tract. Some studies have reported that calcium supplementation with meals protects against the risk of calcium oxalate nephrolithiasis in stone and nonstone formers, reinforcing the needed balance of calcium and oxalate intake to avoid increasing the risk of calcium oxalate lithogenesis.47,48 Efforts should be made by clinicians to recommend low-dose dietary calcium intake routinely to bariatric patients to counterbalance the metabolic alterations of bariatric surgery.
Dietary fat and vitamin B6
Considering the amount of malabsorptive bariatric procedures done in past years, a low-fat diet (<25% daily calories) is another crucial recommendation for bariatric patients. Fatty acids not absorbed in the proximal small intestine, due to the surgical redirecting of food contents in bariatric patients, reach the distal bowel and combine with calcium molecules, which were destined to prevent high oxalate reabsorption. In turn, the elevated levels of fatty acids in the distal intestines chelate calcium and oxalate is free to be reabsorbed and excreted into the urine, leading to high levels of oxaluria. Other factors that can play a role in the urinary level of oxalate are probiotics use and Vitamin B6 (pyridoxine) levels. Vitamin B6 helps to decrease hepatic oxalogenesis in primary hyperoxaluria. Although poorly studied in RYGB patients, a recent study reported that 20% of RYGB patients are deficient in serum vitamin B6 levels 12 to 24 months after their bariatric procedure.49 More studies are needed in this area to further clarify if B6 supplementation can decrease 24-h urinary oxalate levels.
Conclusion
Based on the literature, metabolic derangements found in bariatric surgery patients lead to an elevated risk of nephrolithiasis. The strongest associations have been demonstrated to be decreased 24-h urine volume, increased urinary oxalate levels, and hypocitraturia. Patients at risk of kidney stones and its complications deserve special consideration before and after RYGB surgery is performed. RYGB can increase stone risk in patients with or without previous kidney stone history. Restrictive-only bariatric surgeries, such as SG, do not appear to increase stone risk, but can lower 24-h urine volume, a potential stone risk factor. Preoperative and dietary counseling is warranted in this population and physicians should be actively vigilant of their bariatric surgery patients, and encourage them to follow the above recommendations to decrease some of the risk factors for nephrolithiasis after successful bariatric surgeries.
Acknowledgment
B.K.C. was funded by NIH Grant K08-89000.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ogden CL, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295:1549–1555 [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, et al. The spread of the obesity epidemic in the United States, 1991–1998. JAMA 1999;282:1519–1522 [DOI] [PubMed] [Google Scholar]
- 3.Weiner JP, et al. Impact of bariatric surgery on health care costs of obese persons: a 6-year follow-up of surgical and comparison cohorts using health plan data. JAMA Surg 2013;148:555–562 [DOI] [PubMed] [Google Scholar]
- 4.Picot J, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009;13:1–190, 215–357, iii–iv [DOI] [PubMed] [Google Scholar]
- 5.Sjostrom L, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 6.Buchwald H, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen NT, et al. Trends in use of bariatric surgery, 2003–2008. J Am Coll Surg 2011;213:261–266 [DOI] [PubMed] [Google Scholar]
- 8.American Society for Metabolic and Bariatric Surgery. Estimate of Bariatric Surgery Numbers, 2011–2015. American Society for Metabolic and Bariatric Surgery. 2016. Available at https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers (accessed October19, 2016)
- 9.Li JF, et al. Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis of randomized and nonrandomized trials. Surg Laparosc Endosc Percutan Tech 2014;24:1–11 [DOI] [PubMed] [Google Scholar]
- 10.Pope GD, Birkmeyer JD, Finlayson SR. National trends in utilization and in-hospital outcomes of bariatric surgery. J Gastrointest Surg 2002;6:855–860; discussion 861 [DOI] [PubMed] [Google Scholar]
- 11.Canales BK, Hatch M. Kidney stone incidence and metabolic urinary changes after modern bariatric surgery: review of clinical studies, experimental models, and prevention strategies. Surg Obes Relat Dis 2014;10:734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scales CD Jr., et al. Prevalence of kidney stones in the United States. Eur Urol 2012;62:160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson WK, et al. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis 2005;1:481–485 [DOI] [PubMed] [Google Scholar]
- 14.Park AM, et al. A prospective study of risk factors for nephrolithiasis after Roux-en-Y gastric bypass surgery. J Urol 2009;182:2334–2339 [DOI] [PubMed] [Google Scholar]
- 15.Duffey BG, et al. , Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: a 2-year prospective longitudinal study. J Am Coll Surg 2010;211:8–15 [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, et al. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery 2011;149:654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valezi AC, et al. Urinary evaluation after RYGBP: a lithogenic profile with early postoperative increase in the incidence of urolithiasis. Obes Surg 2013;23:1575–1580 [DOI] [PubMed] [Google Scholar]
- 18.Wu JN, et al. Urolithiasis risk factors in the bariatric population undergoing gastric bypass surgery. Surg Obes Relat Dis 2013;9:83–87 [DOI] [PubMed] [Google Scholar]
- 19.Agrawal V, et al. Calcium oxalate supersaturation increases early after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2014;10:88–94 [DOI] [PubMed] [Google Scholar]
- 20.Froeder L, et al. Response to dietary oxalate after bariatric surgery. Clin J Am Soc Nephrol 2012;7:2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penniston KL, et al. Gastric band placement for obesity is not associated with increased urinary risk of urolithiasis compared to bypass. J Urol 2009;182:2340–2346 [DOI] [PubMed] [Google Scholar]
- 22.Semins MJ, et al. The effect of restrictive bariatric surgery on urinary stone risk factors. Urology 2010;76:826–829 [DOI] [PubMed] [Google Scholar]
- 23.Patel BN, et al. Prevalence of hyperoxaluria after bariatric surgery. J Urol 2009;181:161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maalouf NM, et al. Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol 2010;183:1026–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeFoor WR, et al. Prospective evaluation of urinary metabolic indices in severely obese adolescents after weight loss surgery. Surg Obes Relat Dis 2016;12:363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pang R, et al. Controlled metabolic diet reduces calcium oxalate supersaturation but not oxalate excretion after bariatric surgery. Urology 2012;80:250–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asplin JR, Coe FL. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol 2007;177:565–569 [DOI] [PubMed] [Google Scholar]
- 28.Sinha MK, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int 2007;72:100–107 [DOI] [PubMed] [Google Scholar]
- 29.Kok DJ, Papapoulos SE, Bijvoet OL. Excessive crystal agglomeration with low citrate excretion in recurrent stone-formers. Lancet 1986;1:1056–1058 [DOI] [PubMed] [Google Scholar]
- 30.Lieske JC, et al. Kidney stones are common after bariatric surgery. Kidney Int 2015;87:839–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogden CL, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matlaga BR, et al. Effect of gastric bypass surgery on kidney stone disease. J Urol 2009;181:2573–2577 [DOI] [PubMed] [Google Scholar]
- 33.Durrani O, et al. Analysis of stone disease in morbidly obese patients undergoing gastric bypass surgery. J Endourol 2006;20:749–752 [DOI] [PubMed] [Google Scholar]
- 34.Haddad N, et al. Long-term incidence of symptomatic urolithiasis post-bariatric surgery. Can Urol Assoc J 2014;8:E688–E694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semins MJ, et al. The effect of gastric banding on kidney stone disease. Urology 2009;74:746–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen T, et al. The effect of restrictive bariatric surgery on urolithiasis. J Endourol 2013;27:242–244 [DOI] [PubMed] [Google Scholar]
- 37.Costa-Matos A, et al. Is there an association between urolithiasis and Roux-en-y gastric bypass surgery? Int Braz J Urol 2009;35:432–435 [DOI] [PubMed] [Google Scholar]
- 38.Canales BK, et al. Steatorrhea and hyperoxaluria occur after gastric bypass surgery in obese rats regardless of dietary fat or oxalate. J Urol 2013;190:1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abegg K, et al. Roux-en-Y gastric bypass surgery reduces bone mineral density and induces metabolic acidosis in rats. Am J Physiol Regul Integr Comp Physiol 2013;305:R999–R1009 [DOI] [PubMed] [Google Scholar]
- 40.Shimizu H, et al. Evaluation of postoperative nephrolithiasis and renal dysfunction in gastric cancer patients. Gastric Cancer 2013;16:338–344 [DOI] [PubMed] [Google Scholar]
- 41.Pearle MS, et al. Medical management of kidney stones: AUA guideline. J Urol 2014;192:316–324 [DOI] [PubMed] [Google Scholar]
- 42.Sakhaee K, Griffith C, Pak CY. Biochemical control of bone loss and stone-forming propensity by potassium-calcium citrate after bariatric surgery. Surg Obes Relat Dis 2012;8:67–72 [DOI] [PubMed] [Google Scholar]
- 43.Sakhaee K, Pak C. Superior calcium bioavailability of effervescent potassium calcium citrate over tablet formulation of calcium citrate after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2013;9:743–748 [DOI] [PubMed] [Google Scholar]
- 44.Asplin JR. The management of patients with enteric hyperoxaluria. Urolithiasis 2016;44:33–43 [DOI] [PubMed] [Google Scholar]
- 45.Trinchieri A, et al. Hyperoxaluria in patients with idiopathic calcium nephrolithiasis. J Nephrol 1998;11(Suppl. 1):70–72 [PubMed] [Google Scholar]
- 46.Penniston KL, Nakada SY. Effect of dietary changes on urinary oxalate excretion and calcium oxalate supersaturation in patients with hyperoxaluric stone formation. Urology 2009;73:484–489 [DOI] [PubMed] [Google Scholar]
- 47.de OGMC., et al. Effects of an oxalate load on urinary oxalate excretion in calcium stone formers. J Ren Nutr 2003;13:39–46 [DOI] [PubMed] [Google Scholar]
- 48.Domrongkitchaiporn S, et al. Schedule of taking calcium supplement and the risk of nephrolithiasis. Kidney Int 2004;65:1835–1841 [DOI] [PubMed] [Google Scholar]
- 49.Ortiz-Alvarado O, et al. Pyridoxine and dietary counseling for the management of idiopathic hyperoxaluria in stone-forming patients. Urology 2011;77:1054–1058 [DOI] [PubMed] [Google Scholar]