Abstract
The objective of this study was to determine whether atopy and other clinical and environmental variables predict the risk of childhood habitual snoring (HS) in a birth cohort born to atopic parents. Participants completed clinical evaluations and questionnaires at ages 1–4 and age 7. HS was defined as snoring ≥3 nights/week. Traffic-related air pollution (TRAP) exposure was estimated using land-use regression. The association between early (≤age 4) and current (age 7) allergic disease, environmental exposures, and snoring at age 7 was examined using adjusted logistic regression. Of the 609 children analyzed the prevalence of HS at age 7 was 21%. Early tobacco smoke exposure [environmental tobacco smoke (ETS)] [odds ratio (OR) 1.79, 95% CI (confidence interval) 1.12–2.84], rhinitis (OR 1.74, 95% CI 1.06–2.92), wheezing (OR 1.63, 95% CI 1.05–2.53), maternal HS (OR 2.08, 95% CI 1.36–3.18), and paternal HS (OR 1.83, 95% CI 1.14–3.00) were significantly associated with HS at age 7. Current TRAP (OR 1.93, 95% CI 1.13–3.26), respiratory infections (OR 1.16, 95% 1.03–1.35), maternal HS (OR 2.86, 95% CI 1.69–4.84), and paternal HS (OR 3.01, 95% CI 1.82–5.09) were significantly associated with HS at age 7. To our knowledge, this is the largest birth cohort examining longitudinal predictors of snoring in children born to atopic parents. Parental HS was the only variable consistently associated with childhood HS from ages 1 to 7. Early rhinitis, early ETS exposure, and concurrent traffic pollution exposure increased the risk of HS at age 7, while aeroallergen sensitization did not. Children with these characteristics should be considered for screening of sleep disorders.
Keywords: : snoring, birth cohort, atopy, parental snoring, environmental pollution
Introduction
Sleep disordered breathing (SDB) includes a range of diseases from primary snoring to obstructive sleep apnea (OSA) and has a variable prevalence reported between 4.0% and 34.5%.1,2 Children with SDB have numerous complications described including delayed growth, impaired cognitive functioning, lipid abnormalities, abnormal pulmonary artery pressures, hypertension, body weight abnormalities, hyperactivity, irritability, and poorer school performance.3–5
Gender, race, breastfeeding, family history, allergic rhinitis, asthma, eczema, respiratory infections, tobacco smoke, traffic pollution, and obesity have also been associated.6–12 Polysomnography (PSG) is the gold standard for diagnosis; however, it is expensive and not always available.13,14 Therefore, identifying clinical and environmental factors could help categorize children in need of screening and provide modifiable characteristics that could prevent complications. Further, studies indicate that adenotonsillectomy (AT) can be curative. Thus, identifying predictors could help stratify those in need of surgical intervention.15,16
A paucity of prospective birth cohort studies has examined predictors of snoring longitudinally,15,17–21 and only a few have been conducted on children residing in the United States.22–25 The Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS), a prospective birth cohort with longitudinal follow-up, has characterized snoring throughout childhood.26 Sensitization at age 1 was identified as a risk factor for habitual snoring (HS) in this birth cohort born to atopic parents.23 Thus, the study objectives were to determine whether atopy, along with other early clinical and environmental risk factors, predicts the risk of HS at age 7. In addition, cross-sectional factors associated with HS were evaluated at age 7.
Materials and Methods
Study population
The design, recruitment, environmental exposure assessment, and health evaluations of the CCAAPS cohort have been previously reported.26,27 Briefly, CCAAPS children were born in the Cincinnati, Ohio area between 2001 and 2003.27 Study eligibility required having a parent with at least 1 allergy or asthma symptom along with confirmed aeroallergen sensitization, and residing either near (<400 m) or far (>1,500 m) from the nearest major roadway (defined as >1,000 trucks/day).27 The University of Cincinnati Institutional Review Board approved the study protocol before subject recruitment and written informed consent was obtained from the parents before participation.
Clinical evaluations
Children enrolled completed demographic and clinical evaluations annually from ages 1 to 4 and at age 7. At each visit children underwent skin prick testing (SPT) to milk and egg, and a panel of 15 aeroallergens: pollen (meadow fescue, timothy, white oak, maple mix, American elm, red cedar, short ragweed), mold (Alternaria, Aspergillus fumigatus, Penicillium mix, Cladosporium), pet (cat, dog), and dust [German cockroach (Blattella germanica), dust mite mix (Dermatophagoides farinae and Dermatophagoides pteronyssinus)].26 SPT was defined as positive when the wheal was ≥3 mm larger than the saline control after 15 min.26 At each clinical examination, parents completed questionnaires regarding their child's respiratory and allergic health history, and reported environmental exposures.26 Parents were also queried regarding the frequency of their child's snoring: never, rarely (<1 night/week), sometimes (1–2 nights/week), frequently (3–4 nights/week), and almost always (5–7 nights/week).23 The same questions were also asked of the mother and father's snoring.
Children's height and weight were measured at each visit and body mass index (BMI) was calculated as weight in kilograms/height in meters.2 Early and current BMI was defined at ages 4 and 7, respectively. BMI change was defined as the difference from ages 4 to 7.
Allergic and respiratory disease definitions
Variables were defined as “early” if reported between the ages of 1 and 4 and “current” if reported at age 7. Early aeroallergen sensitization was defined as having at least 1 positive SPT to the panel of aeroallergens on 2 clinical visits between ages 1 and 4. Current aeroallergen sensitization was defined as at least 1 positive SPT at age 7. Early and current food sensitization were defined as positive SPT to either milk and/or egg on at least 1 visit during ages 1–4 and at age 7, respectively. Rhinitis was defined by parental report of symptoms of sneezing, runny, or blocked nose in the preceding 12 months outside of a cold or flu. Upper respiratory infections (URIs) included colds, ear infections, sinus infections, strep throat, tonsillitis, respiratory flu, and colored drainage as defined by parental report in the past 12 months. Wheezing was described by parental report 2 or more times in the previous 12 months. Eczema was defined as parental report of frequent skin scratching, skin redness, red spots, or rough and dry skin ≥6 months.
During the age 7 visit, all children underwent pulmonary function testing according to American Thoracic Society (ATS) guidelines.28 Based on spirometry testing or methacholine challenge test (MCCT), children were diagnosed with asthma if they had (1) parental report of asthma symptoms and (2) demonstrable airway reversibility (≥12% increase in FEV1 after bronchodilator) or evidence of bronchial hyperresponsiveness with a positive MCCT result (PC20 ≤ 4 mg/mL).29
Environmental exposure assessment
A previously validated land-use regression (LUR) model was used to estimate traffic-related air pollution (TRAP) exposure for all CCAAPS participants.30 Briefly, ambient air sampling was conducted on a rotating basis in the Cincinnati area from 2001 through 2006, and the average daily concentration of elemental carbon attributable to traffic (ECAT) was determined for each monitoring station.31 A LUR model was developed, validated, and applied to all addresses where children were reported to have lived and spent time to provide an estimate of ECAT,32 which is a surrogate marker of the diesel exhaust component of the TRAP mixture.33 Early and current environmental tobacco smoke (ETS) exposure was defined by parental report of 1 or more cigarettes smoked by residents of the child's household at ages 1 and 7, respectively. Early and current dog and cat ownership were defined as having 1 or more dog or cat in the child's household at ages 1 and 7, respectively.
Statistical analysis
For this analysis, the 5 categories of reported snoring were combined into 2 as reported in previous studies.23,34 HS included those who snored ≥3 nights/week and answered “frequently” and “almost always.”35,36 Non-habitual snoring (non-HS) included the responses of “never,” “rarely,” and “sometimes.” All characteristics in Tables 1, 2, and 4 were compared across levels of snoring at age 7 by either analysis of variance or a chi-squared test, as appropriate. Two adjusted logistic regression models estimated the association between the risk factors and HS: 1 model for early characteristics (Table 3 and Fig. 1) and another for current, cross-sectional characteristics (Table 5 and Fig. 2). Characteristics were included in each model if these were univariately associated with the ordered level of snoring at age 7 (p < 0.1).
Table 1.
Demographic Variables and Snoring Frequency of the Cohort (n = 609)
| Snoring frequency | ||||
|---|---|---|---|---|
| Variable | n (%) | Non-habitual (n = 480), n (%) | Habitual (n = 129), n (%) | p |
| African Americana | 131 (22) | 90 (19) | 41 (32) | 0.002 |
| Male | 336 (55) | 268 (56) | 68 (53) | 0.549 |
| Income ≥$40,000 | 339 (56) | 289 (60) | 50 (39) | <0.001 |
| Breastfeeding ≥4 months | 322 (53)b | 271 (56) | 51 (40) | 0.001 |
Remainder of the cohort represented by 475 Caucasians and 3 children of other races.
Unavailable data for 1 patient.
Table 2.
Early Variables at Age 4 and Their Association with Snoring Frequency at Age 7 (n = 609)
| Snoring frequency | ||||
|---|---|---|---|---|
| Variable | n (%) | Non-habitual (n = 480), n (%) | Habitual (n = 129), n (%) | p |
| BMIa | 16.07b | 16.04 | 16.20 | 0.387 |
| ETSc | 154 (25) | 102 (21) | 52 (40) | <0.001 |
| Dog ownershipd | 217 (36) | 168 (35) | 49 (38) | 0.535 |
| Cat ownershipe | 138 (23) | 113 (24) | 25 (19) | 0.345 |
| TRAPf | 138 (23) | 94 (20) | 44 (34) | 0.001 |
| Aeroallergen sensitizationg | 275 (45) | 208 (43) | 67 (52) | 0.09 |
| Food sensitizationh | 44 (7) | 37 (8) | 7 (5) | 0.448 |
| Rhinitisi | 404 (66) | 302 (63) | 102 (79) | 0.001 |
| Wheezingj | 206 (34) | 146 (30) | 60 (47) | 0.001 |
| URIk | 3.22l | 3.10 | 3.64 | 0.39 |
| Eczemam | 191 (31) | 148 (31) | 43 (33) | 0.594 |
| Maternal HS | 215 (35) | 146 (30) | 69 (53) | <0.001 |
| Paternal HS | 387 (64) | 287 (60) | 100 (78) | <0.001 |
Defined as the numerical value at age 4.
Represents mean numerical value for BMI. Unavailable data for 37 children.
ETS exposure defined by parental report of 1 or more cigarettes smoked by residents of the child's household through age 1.
One or more dog in the child's household at age 1.
One or more cat in the child's household at age 1.
TRAP exposure as defined by a land-use regression model.
Defined as at least 1 positive (≥3 mm greater than negative control) to a panel of 15 aeroallergens.
Defined as at least 1 positive SPT to milk and/or egg.
Defined by parental report of symptoms of sneezing, runny, or blocked nose in the preceding 12 months outside of a cold or flu.
Defined by parental report of wheezing 2 or more times in the previous 12 months.
Upper respiratory tract conditions (cold, ear infection, sinus infection, strep throat, tonsillitis, respiratory flu, colored drainage) were defined as the mean number as described by parental report in the past 12 months.
Represents mean number of URIs reported by parents in preceding 12 months.
Parental report of frequent skin scratching, skin redness, red spots, or rough and dry skin for ≥6 months.
BMI, body mass index; ETS, environmental tobacco smoke; HS, habitual snoring; SPT, skin prick testing; TRAP, traffic-related air pollution; URI, upper respiratory infection.
Table 4.
Current Variables at Age 7 and Their Association with Snoring Frequency at Age 7 (n = 609)
| Snoring frequency | ||||
|---|---|---|---|---|
| Variable | N (%) | Non-habitual (n = 480) | Habitual (n = 129) | p |
| BMIa | 16.48b | 16.33 | 17.01 | 0.012 |
| BMI change | 0.38c | 0.26 | 0.82 | 0.023 |
| ETSd | 118 (19)e | 81 (17) | 37 (29) | 0.003 |
| Dog ownershipf | 269 (44) | 208 (43) | 61 (47) | 0.425 |
| Cat ownershipg | 134 (22) | 109 (23) | 25 (19) | 0.472 |
| TRAPh | 148 (24) | 104 (22) | 44 (34) | 0.004 |
| Aeroallergen sensitizationi | 254 (42) | 196 (41) | 58 (45) | 0.422 |
| Food sensitizationj | 12 (2) | 11 (2) | 1 (1) | 0.324 |
| Rhinitisk | 271 (45)l | 200 (42) | 71 (55) | 0.009 |
| Asthmam | 95 (16) | 58 (12) | 37 (29) | <0.001 |
| URIn | 0.87o | 0.76 | 1.26 | 0.002 |
| Eczemap | 82 (13) | 55 (11) | 27 (21) | 0.007 |
| Maternal HS | 130 (22)q | 78 (17) | 52 (41) | <0.001 |
| Paternal HS | 310 (53)q | 222 (48) | 88 (73) | <0.001 |
Defined as the mean value as defined at age 7.
Represents mean numerical BMI at age 7. Unavailable data for 20 children.
Represents mean change in numerical BMI from ages 4 to 7. Unavailable data for 53 children.
ETS exposure as defined by parental report of more than 1 cigarette smoked by residents of the child's household through age 7.
Unavailable data for 53 children.
One or more dog in the child's household at age 7.
One or more cat in the child's household at age 7.
TRAP exposure as defined by a land-use regression model.
Defined as atleast 1 positive (≥ 3 mm greater than negative control) to a panel of 15 aeroallergens.
Positive SPT to milk and/or egg.
Defined by parental report of symptoms of sneezing, runny, or blocked nose in the preceding 12 months outside of a cold or flu.
Unavailable data for 3 children.
As defined by American Thoracic Society guidelines by either spirometry or methacholine challenge test.
Upper respiratory tract conditions (cold, ear infection, sinus infection, strep throat, tonsillitis, respiratory flu, colored drainage) were defined as the mean number as described by parental report in the past 12 months.
Represents mean number of URIs reported by parents in preceding 12 months.
Parental report of frequent skin scratching, skin redness, red spots, or rough and dry skin ≥6 months.
Missing data for 25 parents.
Table 3.
Early Model of Variables at Age 4 Predicting Snoring at Age 7
| Variable | Odds ratio | Lower CI | Upper CI |
|---|---|---|---|
| African American | 1.27 | 0.74 | 2.14 |
| Income ≥$40,000 | 0.75 | 0.46 | 1.23 |
| Breastfeeding ≥4 months | 0.71 | 0.45 | 1.11 |
| ETSa | 1.79 | 1.12 | 2.84 |
| TRAPb | 1.41 | 0.87 | 2.26 |
| Aeroallergen sensitizationc | 1.39 | 0.91 | 2.13 |
| Rhinitisd | 1.74 | 1.06 | 2.92 |
| Wheezinge | 1.63 | 1.05 | 2.53 |
| Maternal HS | 2.08 | 1.36 | 3.18 |
| Paternal HS | 1.83 | 1.14 | 3.00 |
ETS Exposure as defined by parental report of more than 1 cigarette smoked by residents of the child's household through age 1.
TRAP exposure as defined by a land-use regression model.
Defined as at least 1 positive (≥3 mm greater than negative control) to a panel of 15 aeroallergens.
Defined by parental report of symptoms of sneezing, runny, or blocked nose in the preceding 12 months outside of a cold or flu.
Defined by parental report of wheezing 2 or more times in the previous 12 months.
CI, confidence interval.
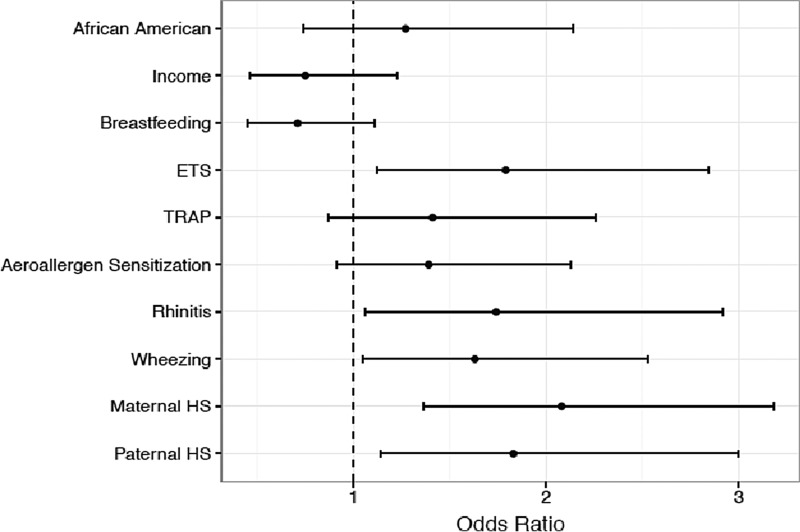
FIG. 1.
Early model of variables at age 4 predicting snoring at age 7.
Table 5.
Current Model of Variables at Age 7 Predicting Snoring at Age 7
| Variable | Odds ratio | Lower CI | Upper CI |
|---|---|---|---|
| African American | 1.53 | 0.84 | 2.77 |
| Income ≥$40,000 | 0.62 | 0.36 | 1.08 |
| BMIa | 1.01 | 0.88 | 1.15 |
| BMI changeb | 1.03 | 0.90 | 1.21 |
| ETSc | 1.29 | 0.71 | 2.31 |
| TRAPd | 1.93 | 1.13 | 3.26 |
| Rhinitise | 1.42 | 0.88 | 2.30 |
| Asthmaf | 1.68 | 0.89 | 3.14 |
| URIg | 1.16 | 1.03 | 1.35 |
| Eczemah | 1.57 | 0.82 | 2.91 |
| Maternal HS | 2.86 | 1.69 | 4.84 |
| Paternal HS | 3.01 | 1.82 | 5.09 |
Defined as the mean numerical BMI at age 7.
Change in BMI as defined from numerical values between ages 4 and 7.
ETS exposure as defined by parental report of more than 1 cigarette smoked by residents of the child's household through age 1.
TRAP exposure as defined by a land-use regression model.
Defined by parental report of symptoms of sneezing, runny, or blocked nose in the preceding 12 months outside of a cold or flu.
As defined by American Thoracic Society guidelines by either spirometry or methacholine challenge test.
Upper respiratory tract conditions (cold, ear infection, sinus infection, strep throat, tonsillitis, respiratory flu, colored drainage) were defined by parental report as the mean number in past 12 months.
Parental report of frequent skin scratching, skin redness, red spots, or rough and dry skin ≥6 months.
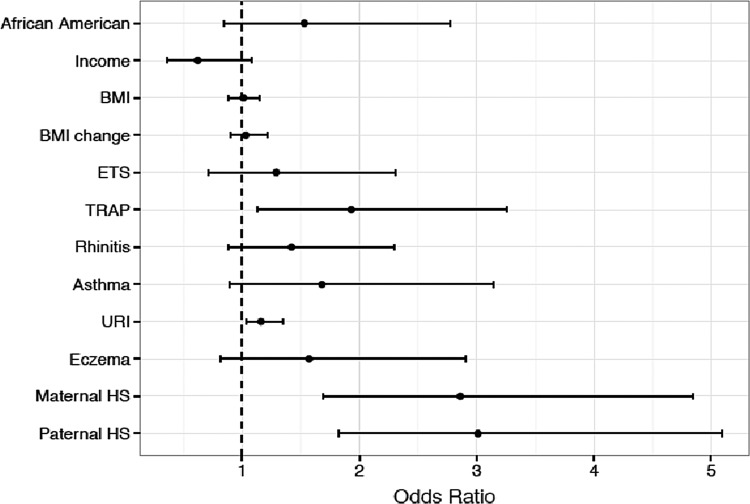
FIG. 2.
Current model of variables at age 7 predicting snoring at age 7.
Results
Of 762 children, 609 had available information regarding snoring and were included in the analysis. The prevalence of HS was 22% and 21% at ages 4 and 7, respectively. Sixty-three children with HS at age 4 (50%) continued to snore at age 7. Sixty-four children with HS at age 4 (50%) no longer snored at age 7. Fifty-five children without HS at age 4 (12%) developed HS by age 7. Therefore, half of the children in this study continued to snore at ages 4 and 7. A small percentage of children developed snoring at age 7, while half the children with HS at age 4 no longer had HS at age 7.
Demographic variables along with snoring frequency are listed in Table 1. When comparing non-HS versus HS, there was a significant difference in snoring frequency among African Americans (p = 0.002), and they represented 32% of HS. Children with a household income ≥$40,000 (p < 0.001), and breastfeeding for ≥4 months (p = 0.001) had significant differences in snoring and were more likely to be non-HS.
Early variables
Early variables from ages 1 to 4 and prevalence of HS at age 7 are shown in Table 2. Significant differences in non-HS and HS were observed in those exposed to ETS (p < 0.001), with a higher proportion of HS having ETS exposure (40%). Children exposed to TRAP also had significant differences (p = 0.001), with a higher proportion of HS reporting higher TRAP exposure. Children sensitized to aeroallergens also differed significantly (p = 0.09), with higher proportion of HS being sensitized. A higher proportion of HS reported rhinitis (p = 0.001). Wheezing was also significant (p = 0.001), with a higher proportion of HS reporting wheezing. Maternal (p < 0.001) and paternal (p < 0.001) snoring also had significant differences.
The early variables that were significantly associated with differences in snoring frequency (race, income, breastfeeding, ETS, TRAP, aeroallergen sensitization, rhinitis, wheezing, and parental HS) were combined into an adjusted model (Table 3 and Fig. 1) to predict the factors associated HS at age 7. ETS exposure [odds ratio (OR) 1.79, 95% confidence interval (CI) 1.12–2.84], rhinitis (OR 1.74, 95% CI 1.06–2.92), wheezing (OR 1.63, 95% CI 1.05–2.53), maternal HS (OR 2.08, 95% CI 1.36–3.18), and paternal HS (OR 1.83, 95% CI 1.14–3.00) were significantly associated with HS at age 7.
Current variables
Cross-sectional current variables at age 7 and snoring frequency are shown in Table 4. Significant differences were associated with BMI (p = 0.012) and change in BMI from ages 4 to 7 (p = 0.023), with HS having higher BMIs and larger changes in BMI. ETS exposure was significant (p = 0.003), with a higher proportion of HS having exposure. TRAP exposure (p = 0.004) was also significant with the higher proportion of HS being exposed. Rhinitis (p = 0.009), asthma (p < 0.001), and eczema (p = 0.007) significantly differed with higher proportions of HS having these diseases. HS reported more URIs, and had significant differences in non-HS versus HS (p = 0.002). Maternal (p < 0.001) and paternal (p < 0.001) HS also had significant differences.
The current variables that were significantly associated with differences in snoring frequency (race, income, BMI, change in BMI, ETS, TRAP, rhinitis, asthma, URIs, eczema, parental HS) were combined into an adjusted model (Table 5 and Fig. 2) to determine which factors were associated with snoring at age 7. TRAP (OR 1.93, 95% CI 1.13–3.26), URIs (OR 1.16, 95% 1.03–1.35), maternal HS (OR 2.86, 95% CI 1.69–4.84), and paternal HS (OR 3.01, 95% CI 1.82–5.09) were significantly associated with HS at age 7.
Discussion
Birth cohorts examining snoring in children with a personal and family history of atopy have been studied in other countries,34,36,37 but to our knowledge, this is the largest birth cohort study examining longitudinal predictors of snoring in children born to atopic parents and the first in the United States. In multivariate analysis, early ETS exposure, rhinitis, wheezing, and parental HS were found to significantly increase the risk of HS at age 7. In cross-sectional analysis, traffic pollution, URIs, and parental HS were significantly associated with childhood HS at age 7.
The objective of this study was to expand upon the findings of this cohort at age 1, and determine whether sensitization, along with other clinical and environmental variables increased the risk of HS. Compared to the 15% prevalence of HS at age 1,23 the prevalence increased to 21% at age 7. Although this prevalence is higher than reported in some studies,6,18 these results could possibly reflect this population's predisposition for atopy, given its high reported prevalence of rhinitis.8 Skin prick positivity was used to define atopy at age 1; however, aeroallergen sensitization was not found to be a predictor of HS at age 7. This discrepancy might be explained by the lack of reproducibility of positive aeroallergen skin tests at age 1 when compared with subsequent years, as previously demonstrated in this cohort.38
Parental HS was the only variable to consistently predict childhood HS in this birth cohort. Family studies have revealed an inherited predisposition to SDB,39,40 and cross-sectional studies have found associations with a family history of snoring.41,42 To our knowledge, this is the first birth cohort study showing parental HS as a risk factor for childhood HS in a population enriched for atopy. It is hypothesized to result from a possible interaction between genetic and environmental determinants,7 which further supports our finding of parental HS predicting childhood HS from ages 1 to 4 and its association at age 7. Hereditable factors could determine recognized patient characteristics associated with snoring including craniofacial structure, body composition, and neuromuscular control of the upper airway.43,44 Interestingly, parental history of AT has been linked to a child's risk of snoring and OSA, hypothesized to be related to adenotonsillar tissue growth rate.42,45 In addition, parents in this study were atopic, therefore, another plausible hypothesis is that parents are snoring due to their allergic rhinitis.
It is interesting that early, but not current, rhinitis was found to be a significant predictor of HS. This finding could be attributed to the increased prevalence of rhinitis at age 4 (66%) compared to age 7 (45%). These results support previous studies reporting rhinitis to be associated with snoring.7,8 Allergen-triggered mucus and edema can lead to airflow resistance and nasal cavity obstruction, which can lead to HS.46–48 Therapies for allergic rhinitis, including intranasal fluticasone, azelastine, and beclomethasone have been shown to decrease symptoms of nasal airway obstruction, adenoidal size, and apnea.49–51 It is possible that the decrease in prevalence of rhinitis at age 7 can be explained by concomitant use of allergic medications, which are more likely to be prescribed at this age due to dosing indications.52 Unfortunately, this study did not have enough information to analyze medication use. In addition, since rhinitis was based on parental report, other subtypes of rhinitis (infectious, nonallergic, idiopathic) could not be further discriminated. Therefore, it could be plausible these etiologies are playing a role.
Wheezing was found to be an early predictor of HS, which correlates with data showing children with a history of wheezing having more frequent tonsillar enlargement that may explain its association with SDB.53–55 Wheezing can be interpreted as a surrogate for asthma,56 however, asthma at age 7 was not found to be a predictor of HS. It could also be interpreted as a sign of URIs,57 however, early URIs were not significant for HS. Given the nonspecific nature of wheezing, it is possible parents confused it with other disorders, including snoring.58 Since wheezing was not confirmed with objective measures by a practitioner, it is difficult to ascertain whether parental report of wheezing truly represented the disorder. In addition, tonsillar tissue size and history of surgical intervention were not collected in this study, making it difficult to ascertain its effects on wheezing.
Early ETS exposure was also found to be a predictor of HS in this cohort, which may explain both children and parental snoring. Previous studies have demonstrated passive cigarette exposure is associated with HS,46,59 possibly due to chemical-induced pharyngeal inflammation and edema leading to obstruction.11,46,60. Effects of second hand smoke exposure on HS have been shown to be dose dependent,11 however, ETS exposure was not found as a current predictor. This may be explained by the decreased prevalence of HS exposed to ETS at ages 4 (40%) and 7 (29%).
In cross-sectional analysis, URIs were found to be associated with HS. Childhood respiratory tract infections have been associated with snoring,61,62 and Respiratory Syncytial Virus has been suggested to induce proliferative changes from leukotriene and neutrophil production in tonsillar tissue, which could lead to snoring.63,64 Why URIs were found as a significant predictor of HS at age 7, but not age 4, is unclear, especially since URIs early in life are common.65 Perhaps, those with persistent URIs at 7 represent a more severe phenotype. Another hypothesis could be that mouth breathing during HS could lead to oral flora exposure leading to URIs.66,67 Otitis media has been linked to mouth breathing,68 and given otitis media's association with URIs, it could be hypothesized that they are linked.69
Traffic pollution exposure was found to be cross-sectionally associated with snoring at age 7, which supports a study showing that HS was associated with road traffic.18 Another study found children aged 6–12 who resided in neighborhoods with greater traffic pollution had higher rates of HS, hypothesized to be related to pollutants' promotion of inflammatory changes in lymph-adenoid tissue.12 The fact that traffic pollution was not found as an early predictor may suggest that a certain duration of exposure is needed before developing snoring. Similar to the development of incident asthma in school-aged children exposed to traffic pollution,70 it could be speculated that a similar process is playing a role in childhood HS. TRAP exposure could also be hypothesized to be contributing to parental snoring.
Although not found to be a significant predictor of HS in this study, breastfeeding has been found to be protective against HS in previous studies.15,35 One study of children born to families with a history of asthma found that children breastfed for more than 1 month had a reduced risk of HS and witnessed apneas.36 Proposed mechanisms include the physical effect of the breast in oropharyngeal development and passive immunoglobulin transfer that protects against respiratory viruses that can lead to SDB.36
Strengths of this study include its prospective design and focus on high-risk children born to atopic families, recognizing that atopy has been associated with HS.8 Our study design allowed for examining multiple predictors of snoring at different points in a child's life. The major limitation was parental report of snoring that was not confirmed with objective measures. While parental report is often subject to recall bias and confusion with other disorders, it has been shown to accurately correlate with PSG results.71 In addition, other symptoms reported by parents, such as wheezing, were not confirmed by practitioners. Although parental aeroallergen sensitization was verified by SPT, objective evidence of rhinitis and asthma were not collected in the parents, making it impossible to determine whether all parents were truly atopic in this study. Additionally, adenoidal size and the effects of AT were not examined. This study did not have enough information to study the effects of medication use, such as inhaled or nasal corticosteroids to assess whether these had any impact on snoring frequencies. It is also recognized that the results of this study may not apply to children born to nonatopic parents.
Future areas of study include continued reassessment of this cohort into adulthood to determine the persistence of snoring and development of OSA. Confirmation of allergic symptoms (rhinitis, wheezing, URIs) and obstructive breathing with PSG would be paramount. In addition, examining the effect of interventions such as AT and nasal steroids on snoring should be considered.
Conclusions
Children born to atopic families with a family history of snoring, early exposure to ETS, and early diagnoses of rhinitis and wheezing are at increased risk for HS later in childhood. In addition, concurrent parental snoring, TRAP exposure, and URIs at age 7 was associated with HS at age 7. Children with these characteristics should be considered for screening of sleep disorders, which may prevent adverse health outcomes. Furthermore, these results suggest children with a family history of snoring and atopy would benefit the greatest from screening. Further research is needed to determine whether interventions can prevent these children from developing persistent snoring.
Acknowledgment
This study was supported by the National Institute of Environmental Health Sciences (NIEHS) R01ES11170, R01ES019890.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Li AM, Zhu Y, Au CT, Lee DL, Ho C, Wing YK. Natural history of primary snoring in school-aged children: a 4-year follow-up study. Chest 2013; 143:729–735 [DOI] [PubMed] [Google Scholar]
- 2.Li AM, Au CT, So HK, Lau J, Ng PC, Wing YK. Prevalence and risk factors of habitual snoring in primary school children. Chest 2010; 138:519–527 [DOI] [PubMed] [Google Scholar]
- 3.Guilleminault C, Eldridge FL, Simmons FB, Dement WC. Sleep apnea in eight children. Pediatrics 1976; 58:23–30 [PubMed] [Google Scholar]
- 4.Cai XH, Li XC, Hu QQ, Yu CY, Zhou YH, Su MS, et al. Multiple system morbidities associated with children with snore symptom. Pediatr Pulmonol 2013; 48:381–389 [DOI] [PubMed] [Google Scholar]
- 5.Gill AI, Schaughency E, Galland BC. Prevalence and factors associated with snoring in 3-year olds: early links with behavioral adjustment. Sleep Med 2012; 13:1191–1197 [DOI] [PubMed] [Google Scholar]
- 6.Ng DK, Chan CH, Ng EP. Natural history of snoring in Hong Kong adolescents. J Paediatr Child Health 2014; 50:596–604 [DOI] [PubMed] [Google Scholar]
- 7.Li S, Jin X, Yan C, Wu S, Jiang F, Shen X. Habitual snoring in school-aged children: environmental and biological predictors. Respir Res 2010; 11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chng SY, Goh DY, Wang XS, Tan TN, Ong NB. Snoring and atopic disease: a strong association. Pediatr Pulmonol 2004; 38:210–216 [DOI] [PubMed] [Google Scholar]
- 9.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med 1999; 159(5 Pt 1):1527–1532 [DOI] [PubMed] [Google Scholar]
- 10.Kheirandish-Gozal L, Dayyat EA, Eid NS, Morton RL, Gozal D. Obstructive sleep apnea in poorly controlled asthmatic children: effect of adenotonsillectomy. Pediatr Pulmonol 2011; 46:913–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbo GM, Fuciarelli F, Foresi A, De Benedetto F. Snoring in children: association with respiratory symptoms and passive smoking. BMJ 1989; 299:1491–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kheirandish-Gozal L, Ghalebandi M, Salehi M, Salarifar MH, Gozal D. Neighbourhood air quality and snoring in school-aged children. Eur Respir J 2014; 43:824–832 [DOI] [PubMed] [Google Scholar]
- 13.Beck SE, Marcus CL. Pediatric Polysomnography. Sleep medicine clinics 2009; 4:393–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaditis A, Kheirandish-Gozal L, Gozal D. Pediatric OSAS: oximetry can provide answers when polysomnography is not available. Sleep Med Rev 2015; 27:96–105 [DOI] [PubMed] [Google Scholar]
- 15.Bonuck KA, Chervin RD, Cole TJ, Emond A, Henderson J, Xu L, et al. Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study. Sleep 2011; 34:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suen JS, Arnold JE, Brooks LJ. Adenotonsillectomy for treatment of obstructive sleep apnea in children. Arch Otolaryngol Head Neck Surg 1995; 121:525–530 [DOI] [PubMed] [Google Scholar]
- 17.Freeman K, Bonuck K. Snoring, mouth-breathing, and apnea trajectories in a population-based cohort followed from infancy to 81 months: a cluster analysis. Int J Pediatr Otorhinolaryngol 2012; 76:122–130 [DOI] [PubMed] [Google Scholar]
- 18.Kuehni CE, Strippoli MP, Chauliac ES, Silverman M. Snoring in preschool children: prevalence, severity and risk factors. Eur Respir J 2008; 31:326–333 [DOI] [PubMed] [Google Scholar]
- 19.Ali NJ, Pitson D, Stradling JR. Natural history of snoring and related behaviour problems between the ages of 4 and 7 years. Arch Dis Child 1994; 71:74–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anuntaseree W, Sangsupawanich P, Mo-suwan L, Ruangnapa K, Pruphetkaew N. Prospective cohort study on change in weight status and occurrence of habitual snoring in children. Clin Otolaryngol 2014; 39:164–168 [DOI] [PubMed] [Google Scholar]
- 21.Luo R, Schaughency E, Gill AI, Dawes PJ, Galland BC. Natural history of snoring and other sleep-disordered breathing (SDB) symptoms in 7-year-old New Zealand children: a follow-up from age 3. Sleep Breath 2015; 19:977–985 [DOI] [PubMed] [Google Scholar]
- 22.Beebe DW, Rausch J, Byars KC, Lanphear B, Yolton K. Persistent snoring in preschool children: predictors and behavioral and developmental correlates. Pediatrics 2012; 130:382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalra M, Lemasters G, Bernstein D, Wilson K, Levin L, Cohen A, et al. Atopy as a risk factor for habitual snoring at age 1 year. Chest 2006; 129:942–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottlieb DJ, Chase C, Vezina RM, Heeren TC, Corwin MJ, Auerbach SH, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr 2004; 145:458–464 [DOI] [PubMed] [Google Scholar]
- 25.Rosen CL, Storfer-Isser A, Taylor HG, Kirchner HL, Emancipator JL, Redline S. Increased behavioral morbidity in school-aged children with sleep-disordered breathing. Pediatrics 2004; 114:1640–1648 [DOI] [PubMed] [Google Scholar]
- 26.LeMasters GK, Wilson K, Levin L, Biagini J, Ryan P, Lockey JE, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr 2006; 149:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol 2005; 116:279–284 [DOI] [PubMed] [Google Scholar]
- 28.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43:343–373 [DOI] [PubMed] [Google Scholar]
- 29.Reponen T, Lockey J, Bernstein DI, Vesper SJ, Levin L, Khurana Hershey GK, et al. Infant origins of childhood asthma associated with specific molds. J Allergy Clin Immunol 2012; 130:639–644.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan PH, Lemasters GK, Biswas P, Levin L, Hu S, Lindsey M, et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect 2007; 115:278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman NC, Ryan P, Lemasters G, Levin L, Bernstein D, Hershey GK, et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ Health Perspect 2013; 121:731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman NC, Ryan PH, Huang B, Beck AF, Sauers HS, Kahn RS. Traffic-related air pollution and asthma hospital readmission in children: a longitudinal cohort study. J Pediatr 2014; 164:1396–1402.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahu M, Hu S, Ryan PH, Le Masters G, Grinshpun SA, Chow JC, et al. Chemical compositions and source identification of PM(2).(5) aerosols for estimation of a diesel source surrogate. Sci Total Environ 2011; 409:2642–2651 [DOI] [PubMed] [Google Scholar]
- 34.Zicari AM, Occasi F, Cesoni Marcelli A, Lollobrigida V, Celani C, Indinnimeo L, et al. Habitual snoring in children with previous allergic sensitization. Int J Immunopathol Pharmacol 2013; 26:565–570 [DOI] [PubMed] [Google Scholar]
- 35.Li AM, Sadeh A, Au CT, Goh DY, Mindell JA. Prevalence of habitual snoring and its correlates in young children across the Asia Pacific. J Paediatr Child Health 2013; 49:E153–E159 [DOI] [PubMed] [Google Scholar]
- 36.Brew BK, Marks GB, Almqvist C, Cistulli PA, Webb K, Marshall NS. Breastfeeding and snoring: a birth cohort study. PloS One 2014; 9:e84956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall NS, Almqvist C, Grunstein RR, Marks GB, Childhood Asthma Prevention S. Predictors for snoring in children with rhinitis at age 5. Pediatr Pulmonol 2007; 42:584–591 [DOI] [PubMed] [Google Scholar]
- 38.Codispoti CD, Bernstein DI, Levin L, Reponen T, Ryan PH, Biagini Myers JM, et al. Early-life mold and tree sensitivity is associated with allergic eosinophilic rhinitis at 4 years of age. Ann Allergy Asthma Immunol 2015; 114:193–198.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Redline S, Tishler PV, Tosteson TD, Williamson J, Kump K, Browner I, et al. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med 1995; 151(3 Pt 1):682–687 [DOI] [PubMed] [Google Scholar]
- 40.Mathur R, Douglas NJ. Family studies in patients with the sleep apnea-hypopnea syndrome. Ann Intern Med 1995; 122:174–178 [DOI] [PubMed] [Google Scholar]
- 41.Ng DK, Kwok KL, Cheung JM, Leung SY, Chow PY, Wong WH, et al. Prevalence of sleep problems in Hong Kong primary school children: a community-based telephone survey. Chest 2005; 128:1315–1323 [DOI] [PubMed] [Google Scholar]
- 42.Kaditis AG, Finder J, Alexopoulos EI, Starantzis K, Tanou K, Gampeta S, et al. Sleep-disordered breathing in 3,680 Greek children. Pediatr Pulmonol 2004; 37:499–509 [DOI] [PubMed] [Google Scholar]
- 43.Redline S, Tishler PV. The genetics of sleep apnea. Sleep Med Rev 2000; 4:583–602 [DOI] [PubMed] [Google Scholar]
- 44.Guilleminault C, Partinen M, Hollman K, Powell N, Stoohs R. Familial aggregates in obstructive sleep apnea syndrome. Chest 1995; 107:1545–1551 [DOI] [PubMed] [Google Scholar]
- 45.Alexopoulos EI, Charitos G, Malakasioti G, Varlami V, Gourgoulianis K, Zintzaras E, et al. Parental history of adenotonsillectomy is associated with obstructive sleep apnea severity in children with snoring. J Pediatr 2014; 164:1352–1357 [DOI] [PubMed] [Google Scholar]
- 46.Corbo GM, Forastiere F, Agabiti N, Pistelli R, Dell'Orco V, Perucci CA, et al. Snoring in 9- to 15-year-old children: risk factors and clinical relevance. Pediatrics 2001; 108:1149–1154 [DOI] [PubMed] [Google Scholar]
- 47.Zicari AM, Marzo G, Rugiano A, Celani C, Carbone MP, Tecco S, et al. Habitual snoring and atopic state: correlations with respiratory function and teeth occlusion. BMC Pediatr 2012; 12:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anuntaseree W, Rookkapan K, Kuasirikul S, Thongsuksai P. Snoring and obstructive sleep apnea in Thai school-age children: prevalence and predisposing factors. Pediatr Pulmonol 2001; 32:222–227 [DOI] [PubMed] [Google Scholar]
- 49.Brouillette RT, Manoukian JJ, Ducharme FM, Oudjhane K, Earle LG, Ladan S, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr 2001; 138:838–844 [DOI] [PubMed] [Google Scholar]
- 50.Demain JG, Goetz DW. Pediatric adenoidal hypertrophy and nasal airway obstruction: reduction with aqueous nasal beclomethasone. Pediatrics 1995; 95:355–364 [PubMed] [Google Scholar]
- 51.Berkiten G, Kumral TL, Cakir O, Yildirim G, Salturk Z, Uyar Y, et al. Effectiveness of azelastine nasal spray in the treatment of adenoidal hyper-trophy in children. Hippokratia 2014; 18:340–345 [PMC free article] [PubMed] [Google Scholar]
- 52.Fan Y, Ji P, Leonard-Segal A, Sahajwalla CG. An overview of the pediatric medications for the symptomatic treatment of allergic rhinitis, cough, and cold. J Pharm Sci 2013; 102:4213–4229 [DOI] [PubMed] [Google Scholar]
- 53.Kaditis AG, Kalampouka E, Hatzinikolaou S, Lianou L, Papaefthimiou M, Gartagani-Panagiotopoulou P, et al. Associations of tonsillar hypertrophy and snoring with history of wheezing in childhood. Pediatr Pulmonol 2010; 45:275–280 [DOI] [PubMed] [Google Scholar]
- 54.Desager KN, Nelen V, Weyler JJ, De Backer WA. Sleep disturbance and daytime symptoms in wheezing school-aged children. J Sleep Res 2005; 14:77–82 [DOI] [PubMed] [Google Scholar]
- 55.Verhulst SL, Vekemans K, Ho E, Aerts L, Jacobs S, De Backer LA, et al. Is wheezing associated with decreased sleep quality in Sri Lankan children? A questionnaire study. Pediatr Pulmonol 2007; 42:579–583 [DOI] [PubMed] [Google Scholar]
- 56.National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007; 120(5 Suppl):S94–S138 [DOI] [PubMed] [Google Scholar]
- 57.Brand PL, Baraldi E, Bisgaard H, Boner AL, Castro-Rodriguez JA, Custovic A, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J 2008; 32:1096–1110 [DOI] [PubMed] [Google Scholar]
- 58.Cane RS, McKenzie SA. Parents' interpretations of children's respiratory symptoms on video. Arch Dis Child 2001; 84:31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang G, Spickett J, Rumchev K, Lee AH, Stick S. Snoring in primary school children and domestic environment: a Perth school based study. Respir Res 2004; 5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bloom JW, Kaltenborn WT, Quan SF. Risk factors in a general population for snoring. Importance of cigarette smoking and obesity. Chest 1988; 93:678–683 [DOI] [PubMed] [Google Scholar]
- 61.Mitchell EA, Thompson JM. Snoring in the first year of life. Acta Paediatr 2003; 92:425–429 [DOI] [PubMed] [Google Scholar]
- 62.Urschitz MS, Guenther A, Eitner S, Urschitz-Duprat PM, Schlaud M, Ipsiroglu OS, et al. Risk factors and natural history of habitual snoring. Chest 2004; 126:790–800 [DOI] [PubMed] [Google Scholar]
- 63.Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med 2005; 172:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldstein NA, Aronin C, Kantrowitz B, Hershcopf R, Fishkin S, Lee H, et al. The prevalence of sleep-disordered breathing in children with asthma and its behavioral effects. Pediatr Pulmonol 2015; 50:1128–1136 [DOI] [PubMed] [Google Scholar]
- 65.Fahey T, Stocks N, Thomas T. Systematic review of the treatment of upper respiratory tract infection. Arch Dis Child 1998; 79:225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol 1999; 70:793–802 [DOI] [PubMed] [Google Scholar]
- 67.Juliano ML, Machado MA, de Carvalho LB, Zancanella E, Santos GM, do Prado LB, et al. Polysomnographic findings are associated with cephalometric measurements in mouth-breathing children. J Clin Sleep Med 2009; 5:554–561 [PMC free article] [PubMed] [Google Scholar]
- 68.van Bon MJ, Zielhuis GA, Rach GH, van den Broek P. Otitis media with effusion and habitual mouth breathing in Dutch preschool children. Int J Pediatr Otorhinolaryngol 1989; 17:119–125 [DOI] [PubMed] [Google Scholar]
- 69.Chonmaitree T, Revai K, Grady JJ, Clos A, Patel JA, Nair S, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis 2008; 46:815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect 2010; 118:1021–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montgomery-Downs HE, O'Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep 2004; 27:87–94 [DOI] [PubMed] [Google Scholar]