Abstract
Background
Treatment of patients with type 2 diabetes mellitus (T2DM) and a history of cardiovascular (CV) disease or CV risk factors may present clinical challenges due to the presence of comorbid conditions and the use of concomitant medications. The sodium glucose co-transporter 2 inhibitor, canagliflozin, has been shown to improve glycaemic control and reduce body weight and blood pressure (BP) with a favourable tolerability profile in a broad range of patients with T2DM. This post hoc analysis assessed the efficacy and safety of canagliflozin in patients with T2DM based on CV disease history or CV risk factors.
Methods
Analyses were based on pooled data from four 26-week, placebo-controlled, Phase 3 studies that evaluated canagliflozin 100 and 300 mg in patients with T2DM (N = 2313; mean HbA1c, 8.0%; body weight, 89 kg; systolic BP, 128 mmHg). Changes from baseline to week 26 in HbA1c, body weight, and systolic BP were assessed based on history of CV disease, history of hypertension, baseline statin use, and number of CV risk factors. Safety was assessed based on adverse event (AE) reports.
Results
At week 26, both canagliflozin doses lowered HbA1c, body weight, and systolic BP compared with placebo in patients with and without CV disease history or risk factors. Placebo-subtracted HbA1c reductions with canagliflozin 100 and 300 mg were similar in patients with a history of CV disease (−0.95 and −1.07%) versus no history of CV disease (−0.71 and −0.90%), history of hypertension (−0.72 and −0.89%) versus no history of hypertension (−0.73 and −0.95%), baseline statin use (−0.77 and −0.99%) versus no statin use (−0.69 and −0.85%), and 0–1 CV risk factor (−0.72 and −0.87%) versus ≥2 CV risk factors (−0.74 and −1.02%). Similar body weight and systolic BP reductions were seen with canagliflozin versus placebo across subgroups. The incidence of AEs, AEs leading to discontinuation, and serious AEs was similar across subgroups.
Conclusions
The efficacy and safety of canagliflozin were generally consistent across subgroups of patients with T2DM and varying degrees of CV disease history or risk factors.
Trial registration numbers and dates ClinicalTrials.gov: NCT01081834, 4 March 2010; NCT01106625, 1 April 2010; NCT01106677, 1 April 2010; NCT01106690, 1 April 2010
Electronic supplementary material
The online version of this article (doi:10.1186/s12933-017-0517-7) contains supplementary material, which is available to authorized users.
Keywords: Canagliflozin, Type 2 diabetes mellitus, Cardiovascular disease, SGLT2 inhibitor, Risk factors
Background
Type 2 diabetes mellitus (T2DM) affects roughly 400 million adults across the globe and contributes to 5 million deaths annually [1]. The majority of these deaths are a result of cardiovascular (CV) complications, which are very common in patients with T2DM due to poorly controlled chronic hyperglycaemia and reduced insulin sensitivity [2–4]. Other major contributors to increased CV risk in patients with T2DM include comorbid conditions such as hypertension and dyslipidaemia [5, 6]. The presence of these comorbid conditions and the associated requirements for concomitant medication use can present significant clinical challenges in the treatment of patients with T2DM and a history of CV disease or CV risk factors [7, 8].
Some pharmacologic agents may not be suitable for patients with T2DM and existing CV disease or CV risk factors. For instance, the prescribing information for the sulphonylureas glipizide [9] and glyburide [10] include a warning for increased risk of CV death, and the prescribing information for pioglitazone includes a warning for congestive heart failure [11]. Additionally, guidelines from the American Diabetes Association recommend against the use of agents associated with hypoglycaemia in patients with T2DM and coronary artery disease [7]. Overall, there remains a need for antihyperglycaemic agents (AHAs) that are efficacious and well tolerated in people with T2DM and CV disease history/risk factors; ideally, such medications would provide not only improved glycaemic control but also favourable effects on CV risk factors such as body weight, hypertension, and dyslipidaemia.
Canagliflozin is a sodium glucose co-transporter 2 (SGLT2) inhibitor approved for the treatment of adults with T2DM. Canagliflozin lowers the renal threshold for glucose, thereby promoting urinary glucose excretion (UGE) and resulting in a mild osmotic diuresis and a net caloric loss [12, 13]. The mechanism of action for canagliflozin is independent of insulin and is complementary to other AHAs, with a low inherent risk for hypoglycaemia. Across Phase 3 clinical trials, canagliflozin has been shown to provide improvements in glycaemic control as well as reductions in body weight and blood pressure (BP) as monotherapy and in combination with other AHAs in a broad range of patients with T2DM [14].
In this analysis, the efficacy and safety of canagliflozin was assessed among patients with T2DM in subgroups based on CV disease history and CV risk factors using pooled data from four 26-week, placebo-controlled, Phase 3 studies [15–18].
Methods
Patients and study design
These post hoc analyses were based on pooled data from four 26-week, randomised, placebo-controlled, Phase 3 studies of canagliflozin 100 and 300 mg in patients with T2DM. These studies included evaluation of canagliflozin compared with placebo as monotherapy in patients with T2DM inadequately controlled with diet and exercise (ClinicalTrials.gov Identifier: NCT01081834) [15], and as combination therapy in patients on background metformin (NCT01106677) [16], metformin plus sulphonylurea (NCT01106625) [17], and metformin plus pioglitazone (NCT01106690) [18]. In all studies, patients were randomised to receive canagliflozin 100 or 300 mg or placebo once daily during a 26-week, double-blind, core treatment period, followed by a 26-week extension period. Data from the 26-week core treatment periods of each study were included in this pooled analysis. The high glycaemic subset (HbA1c >10 and ≤12.0%) of the monotherapy study [15] was not placebo controlled, and the sitagliptin arm of the add-on to metformin study [16] was not prespecified for efficacy comparisons versus canagliflozin at week 26; therefore, these populations were excluded from the analysis.
Key inclusion criteria for these studies are summarised in Table 1. Key exclusion criteria common to all studies included repeated fasting plasma glucose (FPG) generally ≥15.0 mmol/L during the pretreatment phase; history of diabetic ketoacidosis or type 1 diabetes; history of myocardial infarction, unstable angina, revascularisation procedure, or cerebrovascular accident within 3 months of screening; uncontrolled hypertension; and alanine aminotransferase level >2 times the upper limit or normal or total bilirubin >1.5 times the upper limit of normal at screening [19].
Table 1.
Study design and patient population
| Study | Inclusion criteria | Patients contributing data to pooled analysis, n | |||||
|---|---|---|---|---|---|---|---|
| Age, years | HbA1c, % | eGFR, mL/min/1.73 m2 | PBO | CANA 100 mg |
CANA 300 mg |
Total | |
| Monotherapy | 18–80 | 7.0–10.0 | ≥50 | 192 | 195 | 197 | 584 |
| Add-on to MET | 18–80 | 7.0–10.5 | ≥55 | 183 | 368 | 367 | 918 |
| Add-on to MET + SU | 18–80 | 7.0–10.5 | ≥55 | 156 | 157 | 156 | 469 |
| Add-on to MET + PIO | 18–80 | 7.0–10.5 | ≥55 | 115 | 113 | 114 | 342 |
| Overall total, N | 646 | 833 | 834 | 2313 | |||
Details of the individual study designs have been previously reported [15–18]. Briefly, in each study, eligible patients who were on protocol-specified background AHA treatment entered into a 2-week, placebo run-in period. Patients who were not on protocol-specified background AHA treatment (n = 821; 35.5%) entered an 8- to 12-week adjustment/dose stabilisation period prior to the placebo run-in period. After the placebo run-in period, patients were randomised (1:1:1) to canagliflozin 100 or 300 mg or placebo. Glycaemic rescue therapy using an AHA that was complementary to the protocol-specified background therapy was initiated using protocol-specified FPG criteria.
All studies were conducted in accordance with ethical principles originating in the Declaration of Helsinki and were consistent with Good Clinical Practices and applicable regulatory requirements. Approval was obtained from institutional review boards and independent ethics committees for participating centres, and written informed consent was provided by all patients prior to participation.
Study endpoints and assessments
For this post hoc analysis, data from patients who received canagliflozin 100 or 300 mg or placebo in these four clinical trials were pooled and analysed in four different subgroups: (1) history of CV disease (yes/no); (2) history of hypertension (yes/no); (3) statin use at baseline (yes/no); and (4) number of CV risk factors at baseline (0–1 or ≥2), defined as current cigarette smoker, T2DM history of ≥10 years, baseline high-density lipoprotein cholesterol (HDL-C) of <39 mg/dL, micro- or macro-albuminuria (i.e., baseline albumin to creatinine ratio of ≥30 mg/g), and screening systolic BP >140 mmHg. The terms used to define history of CV disease or history of hypertension and statin use at baseline are provided in Additional file 1. Efficacy endpoints assessed at week 26 for each subgroup included changes from baseline in HbA1c, body weight, and systolic BP. Safety assessments across subgroups included overall incidence of adverse events (AEs), AEs leading to discontinuation, AEs related to study drug, serious AEs, and deaths.
Statistical analyses
All analyses used data from the modified intent-to-treat (mITT) population from each study, which consisted of all randomised patients who received ≥1 dose of double-blind study drug. Missing data at week 26 were imputed using the last observation carried forward (LOCF). For patients who received glycaemic rescue therapy, the last post-baseline value prior to the initiation of rescue therapy was used for efficacy analyses. Efficacy endpoints were assessed using an analysis of covariance (ANCOVA) model, with treatment and study factors and the representative baseline value as a covariate. Least squares (LS) means and 95% confidence intervals (CIs) were estimated for comparisons of each canagliflozin dose with placebo. Statistical testing of canagliflozin versus placebo was not prespecified for analyses of efficacy parameters in these post hoc analyses. Therefore, no P values are reported; however, 95% CIs are provided for descriptive purposes.
Results
Patients
A total of 2313 patients were included in the mITT population; of these, 155 patients (6.7%) had a history of CV disease, 1433 (62.0%) had a history of hypertension, and 945 (40.9%) were using statins at baseline; 1727 patients (74.7%) had 0 or 1 CV risk factor and 586 (25.3%) had ≥2 CV risk factors. In the overall population, patient demographic and disease characteristics were generally balanced across treatment groups (Table 2). Baseline HbA1c, body weight, and systolic BP values in subgroups by CV disease history or CV risk factors are shown in Figs. 1, 2, 3. Generally, patients in the higher CV risk subgroups had higher baseline body weight and systolic BP values.
Table 2.
Baseline demographic and disease characteristics (overall population)
| Characteristica | PBO (n = 646) |
CANA 100 mg (n = 833) |
CANA 300 mg (n = 834) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 334 (52) | 408 (49) | 404 (48) |
| Female | 312 (48) | 425 (51) | 430 (52) |
| Age, years | 56.3 (9.8) | 55.9 (10.1) | 55.7 (9.5) |
| Race, n (%) | |||
| White | 470 (73) | 591 (71) | 610 (73) |
| Black or African American | 28 (4) | 43 (5) | 48 (6) |
| Asian | 82 (13) | 103 (12) | 100 (12) |
| Otherb | 66 (10) | 96 (12) | 76 (9) |
| HbA1c, % | 8.0 (0.9) | 8.0 (0.9) | 8.0 (1.0) |
| Body weight, kg | 89.3 (21.7) | 89.8 (22.3) | 88.5 (22.0) |
| Systolic BP, mmHg | 128.2 (13.3) | 128.0 (12.8) | 128.8 (12.8) |
| eGFR, mL/min/1.73 m2 | 87.0 (19.8) | 88.3 (19.0) | 88.8 (18.9) |
| Duration of T2DM, years | 7.5 (6.2) | 7.2 (5.8) | 7.4 (6.2) |
Data have been previously reported [19, 22, 24]
PBO placebo, CANA canagliflozin, BP blood pressure, eGFR estimated glomerular filtration rate, T2DM type 2 diabetes mellitus, SD standard deviation
aData are mean (SD) unless otherwise indicated
bIncludes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiple, other, unknown, and not reported
Fig. 1.
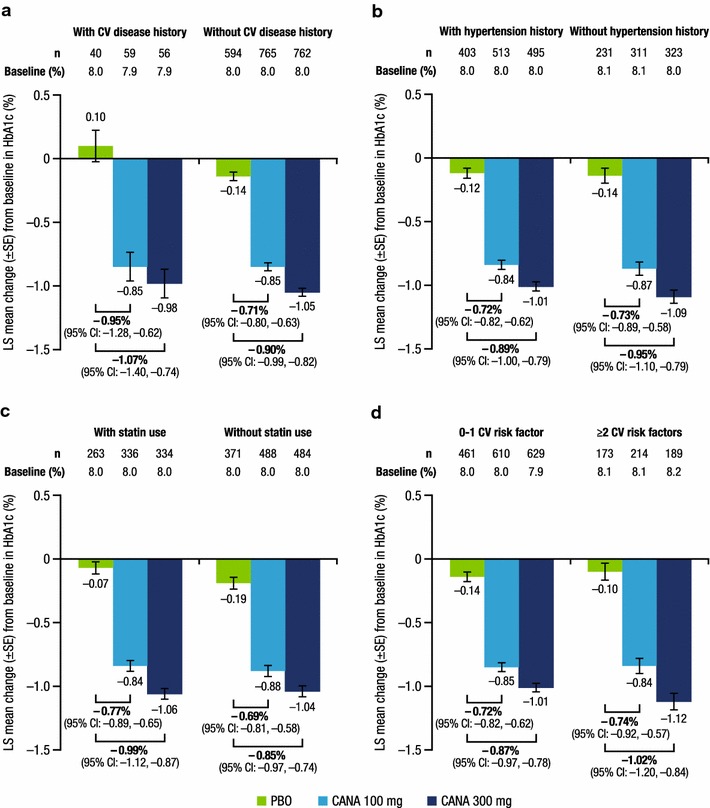
Change from baseline in HbA1c at week 26. a History of CV disease, b history of hypertension, c baseline statin use, d number of CV risk factors. CV cardiovascular, LS least squares, SE standard error, CI confidence interval, PBO placebo, CANA canagliflozin
Fig. 2.
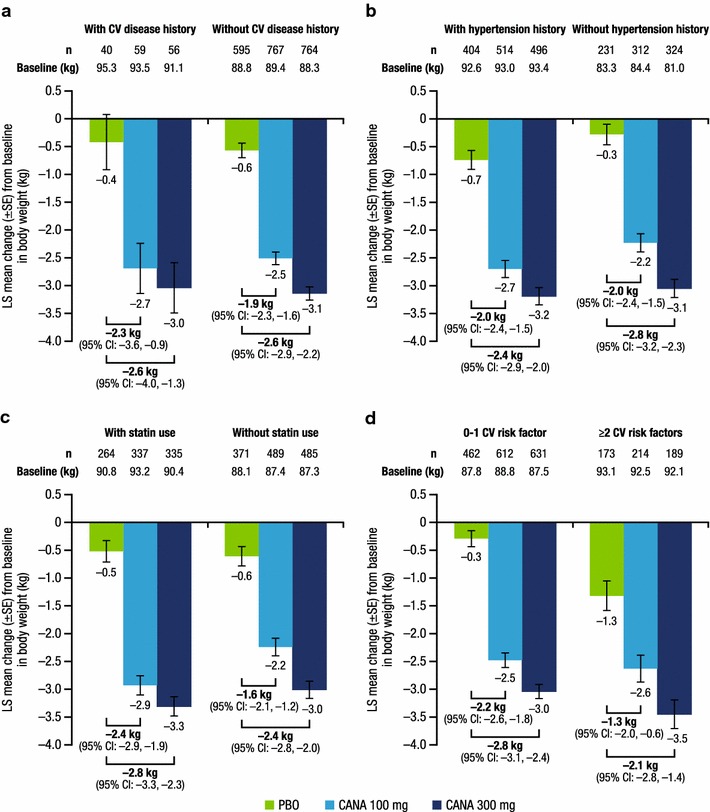
Change from baseline in body weight at week 26. a History of CV disease, b history of hypertension, c baseline statin use, d number of CV risk factors. CV cardiovascular, LS least squares, SE standard error, CI confidence interval, PBO placebo, CANA canagliflozin
Fig. 3.
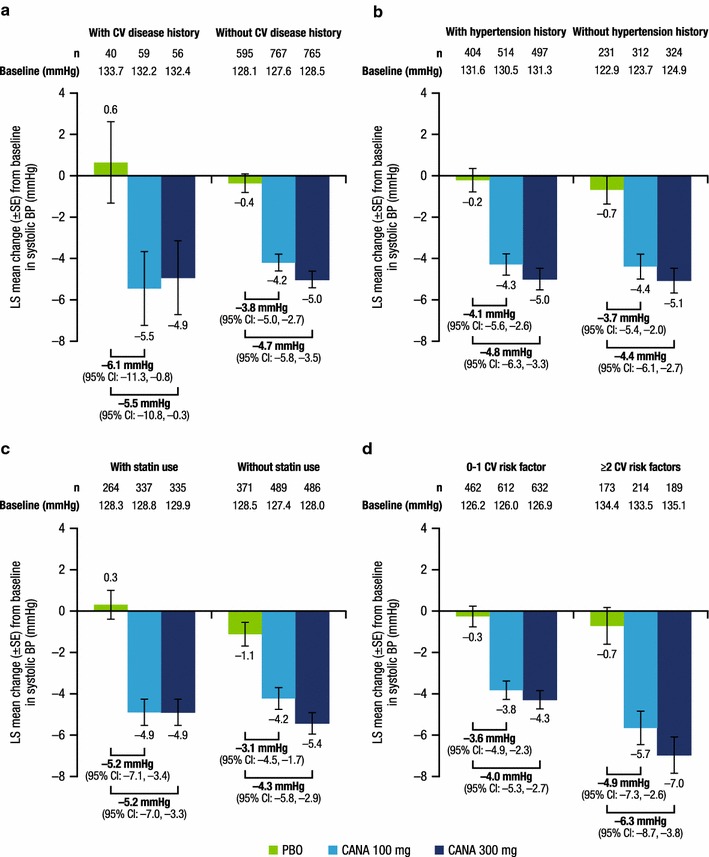
Change from baseline in systolic BP at week 26. a History of CV disease, b history of hypertension, c baseline statin use, d number of CV risk factors. BP blood pressure, CV cardiovascular, LS least squares, SE standard error, CI confidence interval, PBO placebo, CANA canagliflozin
Efficacy
As shown in Fig. 1, LS mean changes in HbA1c from baseline to week 26 were greater with canagliflozin 100 and 300 mg than with placebo in all subgroups. Placebo-subtracted reductions in HbA1c were similar across subgroups, regardless of the presence or absence of history of CV disease or history of hypertension, baseline statin use, or 0–1 versus ≥2 CV risk factors. LS mean changes in body weight (Fig. 2) and systolic BP (Fig. 3) from baseline to week 26 were also greater with canagliflozin 100 and 300 mg than with placebo in all subgroups. Placebo-subtracted reductions in body weight were similar across subgroups, regardless of the presence or absence of history of CV disease or history of hypertension, baseline statin use, or 0–1 versus ≥2 CV risk factors.
Safety
Canagliflozin 100 and 300 mg were generally well tolerated across subgroups by CV disease history or CV risk factors. The incidence of overall AEs, AEs leading to discontinuation, and serious AEs was similar with canagliflozin 100 and 300 mg and placebo across subgroups (Table 3).
Table 3.
Overall safety summary
| Patients, n (%) | History of CV disease | History of hypertension | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||||||||
| PBO (n = 40) |
CANA 100 mg (n = 59) |
CANA 300 mg (n = 56) |
PBO (n = 606) |
CANA 100 mg (n = 774) |
CANA 300 mg (n = 778) |
PBO (n = 409) |
CANA 100 mg (n = 518) |
CANA 300 mg (n = 506) |
PBO (n = 237) |
CANA 100 mg (n = 315) |
CANA 300 mg (n = 328) |
|
| Any AE | 27 (67.5) | 32 (54.2) | 42 (75.0) | 357 (58.9) | 469 (60.6) | 452 (58.1) | 253 (61.9) | 300 (57.9) | 298 (58.9) | 131 (55.3) | 201 (63.8) | 196 (59.8) |
| AEs leading to discontinuation | 0 | 4 (6.8) | 3 (5.4) | 20 (3.3) | 32 (4.1) | 27 (3.5) | 13 (3.2) | 25 (4.8) | 23 (4.5) | 7 (3.0) | 11 (3.5) | 7 (2.1) |
| AEs related to study druga | 5 (12.5) | 8 (13.6) | 18 (32.1) | 80 (13.2) | 163 (21.1) | 173 (22.2) | 62 (15.2) | 101 (19.5) | 111 (21.9) | 23 (9.7) | 70 (22.2) | 80 (24.4) |
| Serious AEs | 2 (5.0) | 2 (3.4) | 4 (7.1) | 20 (3.3) | 26 (3.4) | 18 (2.3) | 19 (4.6) | 17 (3.3) | 11 (2.2) | 3 (1.3) | 11 (3.5) | 11 (3.4) |
| Deaths | 0 | 0 | 1 (1.8) | 2 (0.3) | 1 (0.1) | 0 | 2 (0.5) | 1 (0.2) | 1 (0.2) | 0 | 0 | 0 |
| Patients, n (%) | Baseline statin use | CV risk factors | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | 0–1 | ≥2 | |||||||||
| PBO (n = 266) |
CANA 100 mg (n = 339) |
CANA 300 mg (n = 340) |
PBO (n = 380) |
CANA 100 mg (n = 494) |
CANA 300 mg (n = 494) |
PBO (n = 468) |
CANA 100 mg (n = 616) |
CANA 300 mg (n = 643) |
PBO (n = 178) |
CANA 100 mg (n = 217) |
CANA 300 mg (n = 191) |
|
| Any AE | 168 (63.2) | 212 (62.5) | 220 (64.7) | 216 (56.8) | 289 (58.5) | 274 (55.5) | 275 (58.8) | 380 (61.7) | 381 (59.3) | 109 (61.2) | 121 (55.8) | 113 (59.2) |
| AEs leading to discontinuation | 8 (3.0) | 17 (5.0) | 17 (5.0) | 12 (3.2) | 19 (3.8) | 13 (2.6) | 14 (3.0) | 23 (3.7) | 24 (3.7) | 6 (3.4) | 13 (6.0) | 6 (3.1) |
| AEs related to study druga | 42 (15.8) | 75 (22.1) | 95 (27.9) | 43 (11.3) | 96 (19.4) | 96 (19.4) | 61 (13.0) | 130 (21.1) | 148 (23.0) | 24 (13.5) | 41 (18.9) | 43 (22.5) |
| Serious AEs | 12 (4.5) | 13 (3.8) | 12 (3.5) | 10 (2.6) | 15 (3.0) | 10 (2.0) | 13 (2.8) | 18 (2.9) | 17 (2.6) | 9 (5.1) | 10 (4.6) | 5 (2.6) |
| Deaths | 1 (0.4) | 0 | 0 | 1 (0.3) | 1 (0.2) | 1 (0.2) | 2 (0.4) | 0 | 1 (0.2) | 0 | 1 (0.5) | 0 |
CV cardiovascular, PBO placebo, CANA canagliflozin, AE adverse event
aPossibly, probably, or very likely related to study drug, as assessed by investigators
Discussion
Patients with T2DM are at increased risk for CV disease, in part because of increased prevalence of comorbidities such as hypertension and dyslipidaemia [20, 21]. The presence of CV disease and CV risk factors can complicate T2DM management [8]. This post hoc analysis evaluated the efficacy and safety of canagliflozin 100 and 300 mg in patients with T2DM based on history of CV disease, history of hypertension, baseline use of statins, and the number of CV risk factors. To have a sufficient sample size for analysis that was representative of a diverse population of patients with T2DM, data were pooled from four similarly designed 26-week, placebo-controlled studies of canagliflozin. Results from these analyses showed that treatment with canagliflozin provided meaningful reductions in HbA1c, body weight, and systolic BP that were similar regardless of the presence or absence of history of CV disease, history of hypertension, baseline statin use, or number of CV risk factors. Across subgroups by CV disease history or risk factors, both doses of canagliflozin were generally well tolerated, with a safety profile consistent with that reported across clinical trials of canagliflozin [14, 22, 23]. In the overall population, the incidence of AEs was similar with canagliflozin and placebo; not surprisingly, an increased incidence of AEs related to the mechanism of SGLT2 inhibition (e.g., genital mycotic infections, osmotic diuresis–related AEs) was seen with canagliflozin [19, 22, 24].
Given the high burden of CV disease among patients with T2DM, some guidelines recommend a multifactorial approach to managing T2DM [7, 25]. SGLT2 inhibitors, such as canagliflozin, have been shown to provide significant improvements in HbA1c, body weight, and BP in a broad range of patients with T2DM [23], including older patient populations (≥65 and ≥75 years of age) who may be at elevated risk of CV disease [26, 27]. To improve dyslipidaemia, the European Society of Cardiology recommends statin therapy for all patients with T2DM over 40 years of age and for selected younger patients with elevated CV disease risk [28]. Despite this recommendation, less than 50% of patients in this population were using statins at baseline. Statin therapy has been shown to be associated with increased HbA1c levels in patients with T2DM [29–32]. In the current analysis, canagliflozin treatment provided clinically meaningful improvements in HbA1c in patients with T2DM, regardless of baseline statin use.
Choice of AHAs may be limited for patients with T2DM and CV disease history and CV risk factors based on data suggesting an increased risk for negative CV outcomes with some agents [9–11]. There is a growing body of evidence that SGLT2 inhibitors can provide cardiometabolic benefits beyond glycaemic control. In the EMPA-REG OUTCOME trial, empagliflozin showed a significant reduction in the risk of CV death and heart failure hospitalisation compared with placebo [33]. Based on these results, the US Food and Drug Administration recently approved a new indication for empagliflozin to reduce the risk of CV death in adult patients with T2DM and established CV disease [34]. It is hypothesised that the mechanism for the cardioprotective effect seen with empagliflozin is likely relevant for the entire SGLT2 inhibitor class due to improvements in glycaemic control, as well as BP and body weight reduction via induction of a mild osmotic diuresis, increased natriuresis, and net caloric loss [35–38]. In particular, it has been postulated that the increased osmotic diuresis associated with SGLT2 inhibition may help to reduce cardiac workload via reductions in BP and intravascular volume [37, 38]. Results from the ongoing CANVAS Program [39], including CANagliflozin cardioVascular Assessment Study (CANVAS; ClinicalTrials.gov Identifier: NCT01032629) and CANVAS-R (renal endpoints; NCT01989754) [40, 41], will provide evidence on the CV safety and efficacy of canagliflozin in more than 10,000 patients with CV disease history or CV risk factors upon completion in 2017 and will confirm whether the CV benefits observed with empagliflozin support a class effect. In addition, a separate study is underway to evaluate the effects of canagliflozin versus glimepiride in Japanese patients with T2DM and chronic heart failure [42].
Due to the post hoc nature of these analyses, the current study was limited by a lack of prespecified statistical testing across subgroups. However, calculated 95% CIs allowed for descriptive comparisons for treatment with both canagliflozin doses and placebo. Comparisons were also limited by the small number of patients with CV disease history at baseline, which was not surprising for this general population of patients with T2DM. Nevertheless, the subgroup analysis results were generally consistent with those seen in the overall pooled population [24]. Similar analyses using longer-term efficacy and safety data could provide additional insight into the durability of benefits and risks associated with canagliflozin based on CV disease history and CV risk factors.
Conclusions
Treatment with canagliflozin 100 and 300 mg provided consistent reductions in HbA1c, body weight, and systolic BP, and was generally well tolerated over 26 weeks of treatment in patients with T2DM, regardless of CV disease history or CV risk factors. These findings are noteworthy given the high burden of CV disease among patients with T2DM and the need for diabetes therapies that are safe and efficacious in patients with T2DM and CV disease.
Authors’ contributions
MJD, KM, and RQ contributed to the design and conduct of the study; participated in the acquisition, analysis, and interpretation of data; and drafted, reviewed, and approved the manuscript. UV and JY contributed to the analysis and interpretation of data and drafted, reviewed, and approved the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Medical writing support was provided by Cherie Koch, PhD, of MedErgy, and was funded by Janssen Scientific Affairs, LLC.
Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
Competing interests
MJD and KM are full-time employees of Janssen Scientific Affairs, LLC. UV, JY, and RQ are full-time employees of Janssen Research & Development, LLC.
Availability of data and materials
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All studies included in this post hoc analysis were performed in accordance with the Declaration of Helsinki, and study protocols and amendments were approved by appropriate ethics committees or institutional review boards. Specific details about the ethics approvals for each study are available upon request. Participants in all studies included in this analysis provided written informed consent prior to enrolment.
Funding
This analysis was supported by Janssen Scientific Affairs, LLC. The sponsor was involved in the design of the study; the collection, analysis, and interpretation of data; and writing the manuscript.
Abbreviations
- AE
adverse event
- AHA
antihyperglycaemic agent
- ANCOVA
analysis of covariance
- ATC
Anatomical Therapeutic Chemical
- BMI
body mass index
- BP
blood pressure
- CANA
canagliflozin
- CANVAS
CANagliflozin cardioVascular Assessment Study
- CI
confidence interval
- CV
cardiovascular
- eGFR
estimated glomerular filtration rate
- FPG
fasting plasma glucose
- HDL-C
high-density lipoprotein cholesterol
- LOCF
last observation carried forward
- LS
least squares
- MET
metformin
- mITT
modified intent-to-treat
- PBO
placebo
- PIO
pioglitazone
- SD
standard deviation
- SE
standard error
- SGLT2
sodium glucose co-transporter 2
- SU
sulphonylurea
- T2DM
type 2 diabetes mellitus
- UGE
urinary glucose excretion
Additional file
Additional file 1. Terms used to define history of CV disease, history of hypertension, and statin use at baseline.
Contributor Information
Michael J. Davies, Email: mdavies9@its.jnj.com
Katherine Merton, Email: kmerton@its.jnj.com.
Ujjwala Vijapurkar, Email: UVijapur@its.jnj.com.
Jacqueline Yee, Email: jyee4@its.jnj.com.
Rong Qiu, Email: rqiu17@its.jnj.com.
References
- 1.International Diabetes Federation . IDF diabetes atlas. 7. Brussels: International Diabetes Federation; 2015. [PubMed] [Google Scholar]
- 2.Emerging Risk Factors Collaboration. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48(5):937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 4.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus—mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, Espadero RM, Woerle HJ, Broedl UC, Johansen OE. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diabetes Vasc Dis Res. 2015;12(2):90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen OE. Cardiovascular disease and type 2 diabetes mellitus: a multifaceted symbiosis. Scand J Clin Lab Investig. 2007;67(8):786–800. doi: 10.1080/00365510701408558. [DOI] [PubMed] [Google Scholar]
- 7.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 8.Wami WM, Buntinx F, Bartholomeeusen S, Goderis G, Mathieu C, Aerts M. Influence of chronic comorbidity and medication on the efficacy of treatment in patients with diabetes in general practice. Br J Gen Pract. 2013;63(609):e267–e273. doi: 10.3399/bjgp13X665233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GLUCOTROL® (glipizide) TABLETS [package insert]. New York: Roerig; 2008.
- 10.Glynase® PresTab® (micronized glyburide tablets) [package insert]. New York: Pharmacia & Upjohn Company; 2010.
- 11.ACTOS (pioglitazone) tablets for oral use [package insert]. Deerfield: Takeda Pharmaceuticals America, Inc; 2013.
- 12.Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, Shalayda K, Demarest K, Rothenberg P. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13(7):669–672. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 13.Polidori D, Sha S, Ghosh A, Plum-Mörschel L, Heise T, Rothenberg P. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98(5):E867–E871. doi: 10.1210/jc.2012-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenthal N, Meininger G, Ways K, Polidori D, Desai M, Qiu R, Alba M, Vercruysse F, Balis D, Shaw W, et al. Canagliflozin: a sodium glucose co-transporter 2 inhibitor for the treatment of type 2 diabetes mellitus. Ann N Y Acad Sci. 2015;1358(1):28–43. doi: 10.1111/nyas.12852. [DOI] [PubMed] [Google Scholar]
- 15.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15(4):372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582–2592. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilding JP, Charpentier G, Hollander P, Gonzalez-Galvez G, Mathieu C, Vercruysse F, Usiskin K, Law G, Black S, Canovatchel W, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67(12):1267–1282. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, Stein P. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16(5):467–477. doi: 10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilding JPH, Blonde L, Leiter LA, Cerdas S, Tong C, Yee J, Meininger G. Efficacy and safety of canagliflozin by baseline HbA1c and known duration of type 2 diabetes mellitus. J Diabetes Complications. 2015;29(3):438–444. doi: 10.1016/j.jdiacomp.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Burrows N, Parekh S, Li Y, Geiss L. Prevalence of self-reported cardiovascular disease among persons aged ≥35 years with diabetes—United States, 1997–2005. MMWR Wkly. 2007;56(43):1129–1132. [PubMed] [Google Scholar]
- 21.Jurado J, Ybarra J, Solanas P, Caula J, Gich I, Pou JM, Romeo JH. Prevalence of cardiovascular disease and risk factors in a type 2 diabetic population of the North Catalonia diabetes study. J Am Acad Nurse Pract. 2009;21(3):140–148. doi: 10.1111/j.1745-7599.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- 22.Usiskin K, Kline I, Fung A, Mayer C, Meininger G. Safety and tolerability of canagliflozin in patients with type 2 diabetes: pooled analysis of phase 3 study results. Postgrad Med. 2014;126(3):16–34. doi: 10.3810/pgm.2014.05.2753. [DOI] [PubMed] [Google Scholar]
- 23.Meininger G, Canovatchel W, Polidori D, Rosenthal N. Canagliflozin for the treatment of adults with type 2 diabetes. Diabetes Manag. 2015;5(3):183–201. doi: 10.2217/dmt.15.11. [DOI] [Google Scholar]
- 24.Blonde L, Woo V, Mathieu C, Yee J, Vijapurkar U, Canovatchel W, Meininger G. Achievement of treatment goals with canagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of randomized controlled trials. Curr Med Res Opin. 2015;31(11):1993–2000. doi: 10.1185/03007995.2015.1082991. [DOI] [PubMed] [Google Scholar]
- 25.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Gogo-Jack S, Defronzo RA, Einhorn D, Fonseca VA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocr Pract. 2016;22(1):84–113. doi: 10.4158/EP151126.CS. [DOI] [PubMed] [Google Scholar]
- 26.Sinclair A, Bode B, Harris S, Vijapurkar U, Mayer C, Fung A, Shaw W, Usiskin K, Desai M, Meininger G. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. BMC Endocr Disord. 2014;14(1):37. doi: 10.1186/1472-6823-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinclair AJ, Bode B, Harris S, Vijapurkar U, Shaw W, Desai M, Meininger G. Efficacy and safety of canagliflozin in individuals aged 75 and older with type 2 diabetes mellitus: a pooled analysis. J Am Geriatr Soc. 2016;64(3):543–552. doi: 10.1111/jgs.14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur J Prev Cardiol. 2016;23(11):NP1–NP96. doi: 10.1177/2047487316653709. [DOI] [PubMed] [Google Scholar]
- 29.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 30.Betteridge DJ, Gibson JM. Effects of rosuvastatin on lipids, lipoproteins and apolipoproteins in the dyslipidaemia of diabetes. Diabet Med. 2007;24(5):541–549. doi: 10.1111/j.1464-5491.2007.02095.x. [DOI] [PubMed] [Google Scholar]
- 31.Holman RR, Paul S, Farmer A, Tucker L, Stratton IM, Neil HA. Atorvastatin in factorial with omega-3 EE90 risk reduction in diabetes (AFORRD): a randomised controlled trial. Diabetologia. 2009;52(1):50–59. doi: 10.1007/s00125-008-1179-5. [DOI] [PubMed] [Google Scholar]
- 32.Simsek S, Schalkwijk CG, Wolffenbuttel BH. Effects of rosuvastatin and atorvastatin on glycaemic control in type 2 diabetes-the CORALL study. Diabet Med. 2012;29(5):628–631. doi: 10.1111/j.1464-5491.2011.03553.x. [DOI] [PubMed] [Google Scholar]
- 33.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 34.JARDIANCE® (empagliflozin) tablets, for oral use [package insert]. Ridgefield: Boehringer Ingelheim Pharmaceuticals, Inc; 2016.
- 35.Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115–1122. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 36.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39(7):1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 37.Scheen AJ. Reappraisal of the diuretic effect of empagliflozin in the EMPA-REG OUTCOME trial: comparison with classic diuretics. Diabetes Metab. 2016;42(4):224–233. doi: 10.1016/j.diabet.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes: cardiovascular and kidney effects, potential mechanisms and clinical applications. Circulation. 2016;134(10):752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 39.Neal B, Perkovic V, Mahaffey KW, Fulcher G, Erondu N, Desai M, Shaw W, Law G, Walton MK, Rosenthal N, de Zeeuw D, Matthews DR; CANVAS Program collaborative group. Optimising the analysis strategy for the CANVAS Program - a pre-specified plan for the integrated analyses of the CANVAS and CANVAS-R trials. Diabetes Obes Metab. 2017. doi:10.1111/dom.12924. [DOI] [PMC free article] [PubMed]
- 40.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, Desai M, Shaw W, Jiang J, Vercruysse F, et al. Rationale, design, and baseline characteristics of the canagliflozin cardiovascular assessment study (CANVAS)—a randomized placebo-controlled trial. Am Heart J. 2013;166(2):217–223. doi: 10.1016/j.ahj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Neal B, Perkovic V, Matthews DR, Mahaffey KW, Fulcher G, Meininger G, Erondu N, Desai M, Shaw W, Vercruysse F, et al. Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R): a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19(3):387–393. doi: 10.1111/dom.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka A, Inoue T, Kitakaze M, Oyama J, Sata M, Taguchi I, Shimizu W, Watada H, Tomiyama H, Ako J, et al. Rationale and design of a randomized trial to test the safety and non-inferiority of canagliflozin in patients with diabetes with chronic heart failure: the CANDLE trial. Cardiovasc Diabetol. 2016;15:57. doi: 10.1186/s12933-016-0381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.


