Abstract
Background
Mitochondrial respiration in the dark (R dark) is a critical plant physiological process, and hence a reliable, efficient and high-throughput method of measuring variation in rates of R dark is essential for agronomic and ecological studies. However, currently methods used to measure R dark in plant tissues are typically low throughput. We assessed a high-throughput automated fluorophore system of detecting multiple O2 consumption rates. The fluorophore technique was compared with O2-electrodes, infrared gas analysers (IRGA), and membrane inlet mass spectrometry, to determine accuracy and speed of detecting respiratory fluxes.
Results
The high-throughput fluorophore system provided stable measurements of R dark in detached leaf and root tissues over many hours. High-throughput potential was evident in that the fluorophore system was 10 to 26-fold faster per sample measurement than other conventional methods. The versatility of the technique was evident in its enabling: (1) rapid screening of R dark in 138 genotypes of wheat; and, (2) quantification of rarely-assessed whole-plant R dark through dissection and simultaneous measurements of above- and below-ground organs.
Discussion
Variation in absolute R dark was observed between techniques, likely due to variation in sample conditions (i.e. liquid vs. gas-phase, open vs. closed systems), indicating that comparisons between studies using different measuring apparatus may not be feasible. However, the high-throughput protocol we present provided similar values of R dark to the most commonly used IRGA instrument currently employed by plant scientists. Together with the greater than tenfold increase in sample processing speed, we conclude that the high-throughput protocol enables reliable, stable and reproducible measurements of R dark on multiple samples simultaneously, irrespective of plant or tissue type.
Electronic supplementary material
The online version of this article (doi:10.1186/s13007-017-0169-3) contains supplementary material, which is available to authorized users.
Keywords: Dark respiration, Fluorophore, Gas-exchange, High-throughput, Oxygen consumption, Oxygen electrodes, Respiration, Respiratory flux, Respiratory quotient
Background
Mitochondrial respiration (R) is an essential physiological process in plants required for most energy-dependent metabolic processes. In mature leaves, R takes place in darkness (R dark) and in the light, and is central to processing of carbon assimilates and nitrogen assimilation [1], while also supporting the energy requirements of phloem loading and maintenance processes (e.g. protein turnover and membrane transport) [2–6]. Respiration is also central to the functioning of roots, providing the energy needed for biosynthesis, nutrient uptake and assimilation, as well as maintenance processes [7]. As such, genotypic and/or environmentally-induced variations in leaf and root R play a crucial role in determining growth/survival of individual plants, and productivity/functioning of terrestrial ecosystems [8–10]. Because of this, there is a growing need to describe and predict variability in rates of plant R, which in turn requires provision of large-scale data sets on leaf and root R. Recent studies reporting on expanded global data sets of leaf R dark and its T-dependence [11–13]—compiled over several years using slow, low-throughput gas exchange protocols—are a step forward. However, our understanding of fine-scale temporal, spatial and developmental variation in plant R remains limited, both for natural and managed ecosystems. Addressing the need for new, large-scale datasets on plant R will require development of rapid, high-throughput methods capable of overcoming current bottlenecks in data provision.
One area where there is an urgent need for data on plant R is within the agriculture industry, where more energy-efficient crops are needed to improve global food security. For wheat (Triticum aestivum), only 10–15% of photosynthetic carbon gain contributes to yield [14], demonstrating the untapped potential for improving energy use efficiency. 30–80% of daily carbon gain by photosynthesis is subsequently respired [15–18], with respiratory costs increasing with increasing temperature [19]. Given that the efficiency of ATP synthesis per unit of CO2 or O2 equivalents respired varies (reflecting engagement of phosphorylating and non-phosphorylating pathways of mitochondrial electron transport [20, 21]), there is potential to improve crop yields via selecting for efficient genotypes with reduced rates of R [22, 23]. Indeed, there is growing evidence that physiological screening on a large scale assists crop breeders in identifying beneficial genetic material [24]. However, recombinant inbred line (RIL) populations, diversity panels and/or the structured genetic populations used in genome wide association studies (GWAS) typically include many hundreds of plant variants. Studying these for respiratory traits will require thousands of respiratory measurements to be routinely made on material at the same time of day and developmental stage.
Comprehensive R datasets are also needed to improve modelling of respiratory fluxes in terrestrial ecosystems [9, 25–27]. Using standard leaf gas exchange methods, recent surveys have greatly increased our understanding of biome-to-biome variation in leaf R dark [11–13]; our understanding of how sustained changes in the environment affect respiratory rates is also improving [11, 28–31]. Yet, limitations in available data (e.g. documenting environmental, developmental and/or temporal variations) restrict our ability to fully describe the complexity of plant R that occurs in nature. Similarly, respiratory measurements have been conducted in only a small fraction of extant terrestrial plant species, limiting our ability to explore evolutionary changes in plant energy use efficiency. Addressing these challenges requires development of high-throughput methods for quantifying respiratory fluxes of plants growing in natural ecosystem across the globe.
Protocols using O2-electrodes and infrared gas-analysers have dominated the measuring of plant R dark for several decades (refer to Hunt [32] for a comprehensive review of each techniques application, advantages and disadvantages). The O2-electrode technique was popularised in the form of Clark-type O2-electrodes, being first applied to measure human blood O2 levels [33]. O2-electrodes are often used for measurements of root respiration [34–36] and to assess the impact of exogenous substrates, uncouplers and inhibitors on leaf slices, intact roots and isolated mitochondria [37–39]. While a series of O2 electrodes can be set up in parallel to perform respiratory measurements, in most cases a single electrode is used and each measurement takes an estimated 25–50 min to complete (see Table 1 for a comparison of measurement times associated with this and other methods).
Table 1.
Measurement times required per sample for each of the R dark techniques assessed
| Technique | Step | Description | T (min) |
|---|---|---|---|
| Fluorophore | Calibration | Purge tubes of air using N2 gas or sodium dithionite | 0.02–0.05 |
| Sample preparation | Dissect tissue (e.g. scalpel, scissors or leaf punch) and place in measuring tube | 0.5–1 | |
| Measurements | In general, slopes taken from 1 to 2.5-h. 186 samples per run. Note: more than 186 samples can be simultaneously measured but cycle time between O2 recordings will increase to >6-min, reducing resolution | 0.8 | |
| Total | 1.3–1.9 | ||
| O2-electrode | Calibration | Prepare and assemble electrodes, including application of membrane and electrode solution. Aerate calibration solutions and obtain zero and saturated O2 values after stabilisation of current | 4–9 |
| Sample preparation | Dissect tissue and place inside cuvette and adjust plunger being careful not to introduce air pockets | 1–2 | |
| Measurements | Slopes taken after stabilisation of signal and before depletion of O2, usually within 10–40 min but dependent on sample | 20–40 | |
| Total | 25–51 | ||
| IRGA | Calibration | Change consumables (e.g. soda lime, desiccant, CO2 canister) and zero IRGA chambers | 1–2 |
| Sample preparation | Select and clip measuring chamber onto leaf | 0.5–1 | |
| Measurements | Allow steady-state gas-exchange to be reached | 10–15 | |
| Total | 11.5–18 | ||
| MIMS | Calibration | Apply membrane and test membrane stability. Purge tube and inject known volumes of O2 and CO2. Record background consumption | 5–10 |
| Sample preparation | Dissect tissue and place inside cuvette and air-seal cuvette | 1–3 | |
| Measurements | Allow signal to stabilise (usually 5 min) and record slope between 5 and 20 min | 20 | |
| Total | 26–33 |
T (min) represents the estimated time it takes to measure a single sample in minutes. For example, if 20 samples can be measured without recalibration and it takes 20-min to calibrate, then the calibration T is 1-min
Infrared gas-analysers (IRGA) are also commonly used to measure rates of plant R (as respiratory CO2 efflux), exploiting the infrared absorption properties of CO2. The major benefit of the IRGA systems is that they can be portable and operate as a gas-phase/open system. Such systems have been extensively used in recent times for quantifying plant R dark [12, 40–42], including specialised chambers for whole-plant R dark [16, 19, 43]. While a few research teams have developed multiplex systems for single IRGA measurement of four to 12 samples [e.g. 44], most IRGA measurements are made individually, each requiring 10–20 min per sample (Table 1). Consequently, existing IRGA methods are unlikely to provide the high throughput capacity needed to screen for genetic variations in energy use efficiency and/or improved modelling of ecosystem gas exchange.
Less employed spectroscopy technology for detecting respiratory O2 and/or CO2 exchange include tuneable diode laser (TDL) spectroscopy [45] and cavity ring-down (CRDS) spectroscopy [46]. Mass spectrometry can also be used, with one example of a mass spectrometry technique being membrane inlet mass spectrometry (MIMS), a gas phase method that is used to discriminate between O2 and CO2 isotopes, enabling deeper insight into the photosynthesis/respiratory process [44, 47]. Although MIMS is beneficial in that it can discern gas isotopes, neither it nor the above spectroscopic approaches are high-throughput (Table 1). Similarly, calorimetry measurements of metabolic heat rate and respiratory fluxes [48, 49] while providing an opportunity to explore relationships between respiration and growth—are also not high throughput.
Using O2-sensitive fluorophores in combination with fibre-optic fluorescent detection mechanisms for measuring the O2 evolution of photosynthesis of illuminated leaf disks was occurring by the late 1990s [50]. The technique works by exciting a fluorophore, in most cases a metal porphyrin, whose fluorescence is sensitive to O2 quenching. The measured decay rate of the fluorescent emission is thus proportional to the partial pressure of O2 present [51, 52]. This technology is becoming a more common technique for detecting respiratory O2 consumption of biological samples ranging from bacterial plankton to benthic meiofauna [53, 54]. The power of this technology is that many tissue types of varying abundance can be simultaneously and accurately measured. For example, fluorophore technology has enabled multiple simultaneous measurements of leaf, root and seed respiratory rates [55]. The authors highlight the high-throughput and small tissue size capabilities of the technique, not achievable using conventional Clark-type electrodes, infrared gas-analyser, spectroscopy or calorimetry methods. Yet, take-up of fluorophore technology to facilitate high-throughput measures of plant R remains limited, reflecting the need for more straightforward sample preparation than was possible using the liquid-phase approach of Sew et al. [55]. By contrast, using fluorophore technology in a gas-phase medium is likely to lead to faster processing times and avoid technical issues, such as floating tissues and air-pockets. To date, automated gas-phase measurements of O2 consumption using fluorophore techniques for plants have primarily focused on large-scale analysis of seed germination [56, 57], with automated, high-throughput assessments of non-seed plant R yet to be attempted using gas-phase fluorophore approaches.
To address the urgent need for high-throughput measurements of plant R dark, we have trialled an approach for measuring respiratory O2 uptake which re-purposes equipment designed for seed germination assays and combines the advantages of: (1) fluorophore technology that can accurately measure changes in O2 partial pressure in small measuring volumes that are easily calibrated; (2) closed, gas-phase measurements, which require minimal preparation time; and, (3) an automated sampling mechanism, relying on robotics to take measurements of multiple samples within a short period of time. As part of our study, we compare multiple O2 consumption detection methodologies to ascertain the reliability and compatibility of the different approaches. Further, to illustrate the potential of the high-throughput fluorophore technology to accelerate our understanding of plant R dark, we report on: (1) a screen of R dark in 138 genotypes of wheat (using >550 plants) that was conducted over a few days; and, (2) rapid assessments of respiration in leaf, stem and root tissues that enable whole-plant respiratory fluxes to be estimated by simultaneous analysis of individually dissected plants.
Methods
Plant material
The species used in this study were a grass (wheat—Triticum aestivum), a herb (thale cress—Arabidopsis thaliana) and an evergreen broadleaved tree (red river gum—Eucalyptus camaldulensis), enabling the method to be tested on a range of plant functional types. Considering its agricultural significance, T. aestivum was selected as the primary species of interest, and all experiments, including the high throughput practical applications, were undertaken on T. aestivum, with a sub-set of other experiments conducted using other tissues. All experiments took place at the Research School of Biology at the ANU, Canberra, Australia plants grown in organic potting mix, enriched with Osmocote® OSEX34 EXACT slow-release fertiliser, following manufacturer’s instructions (Scotts Australia, Bella Vista, NSW) with an N/P/K ratio of 16:3.9:10. Plants were watered daily to field capacity. For experiments where roots were analysed, wheat plants were grown hydroponically in a nutrient solution consisting of 1.4 mM NH4NO3, 0.6 mM NaH2PO4·2H2O, 0.5 mM K2SO4, 0.2 mM CaCl2·2H2O, 0.8 mM MgSO4·7H2O, 0.07 mM Fe-EDTA, 0.037 mM H3BO3, 0.009 mM MnCl2·4H2O, 0.00075 mM ZnCl2·7H2O, 0.0003 mM CuSO4·5H2O, 0.0001 mM (NH4)6Mo7O24·4H2O, 0.000138 mM NH4VO3, and 0.0012963 mM Na2SiO3. A pH ranging from 5 to 6 was maintained by adding concentrated sulphuric acid or sodium hydroxide, and monitoring of pH using a portable pH meter (Rowe Scientific Pty. Ltd., NSW, Australia). The hydroponic solution was aerated continuously using Infinity AP-950 aquatic air pumps (Kong’s Pty Ltd, Ingleburn, Australia). Plants were grown at temperatures of 25/20 °C for T. aestivum and E. camaldulensis, in temperature controlled greenhouses with natural photosynthetically active radiation (PAR) of between 400 and 1200 μmol m−2 s−1. A. thaliana was grown at 22/15 °C in temperature-controlled growth chambers (Thermoline, Wetherhill Park, Australia) with a PAR of 200 ± 30 μmol m−2 s−1 and a 12:12 h light/dark photoperiod. For leaf dissection samples, broad-leaved A. thaliana and E. camaldulensis leaf tissue was extracted using brass coring tools of known diameter and for T. aestivum a set distance of leaf blade was dissected with a scalpel. Where sectioned, root segments were dissected transversely from base to tip.
High throughput fluorophore measurements
A Q2 O2-sensor (Astec Global, Maarssen, The Netherlands) designed and marketed for seed germination assays was used to obtain automated, high-throughput fluorophore measurements of dark respiration from plant material. A custom-built frame covered in black cloth was used to maintain darkness during sample measurements. Plant material were freshly dissected and placed in empty tubes (1, 2 or 4 ml in volume) and hermetically sealed with specialised caps (Astec Global). The top surface of caps contained a fluorescent metal organic dye, sensitive to O2 quenching. A blue-spectrum LED excitation pulse (approximately 480 nm) onto the surface of caps, followed by emission detection in the red spectrum (approximately 580 nm), enables the O2 dependent decay in fluorescence signal to be quantified. The fibre optic fluorescence detection unit is attached to a robotic arm which sequentially measures vials placed in racks of 48 tubes each (or 24, 4 ml tubes). The machine can accommodate 16 racks allowing 768 samples (1 or 2 ml tubes) to be measured in a single run. The frequency of measurements was in most cases set to 4 min, enough time to measure approximately 180 samples (a minimum measurement frequency of 1-min is required). The Q2 O2-sensor is calibrated before each set of measurements by measuring a designated tube containing ambient air (designated 100% O2), and a tube purged of all O2 using a sodium dithionite solution, or alternatively purging the tube of air using N2 gas (designated 0% O2). Output is given as an O2 percentage, relative to the calibration readings.
Based on the ideal gas law, raw output as the % O2 relative to the air calibration tube was converted to absolute values of dark respiration rates (R dark) in moles of O2 s−1 using Eq. 1.
| 1 |
P o equals 20.95, the partial pressure of ambient O2 in kPa (i.e. 20.95% of atmospheric pressure), and V equals the volume of the sample tube (1, 2 or 4 ± 0.2 ml tubes were used in this study). S refers to the slope of sample tubes O2 consumption, (as a % of air and subtracting the air calibration tube slope), from 1 to 2.5 h after the beginning of sample measurements, expressed as the % of O2 per second. R is the gas constant (8314 cm3 kPa K−1 mol−1) and T is the temperature in Kelvin (K). The final calculation of O2 consumption rates in moles s−1 were expressed on a leaf area (cm2) basis, calculated from the diameter of the leaf corer (for leaf disks) or ruler measurements for grass leaf sections. Alternatively, for whole-plant developmental partitioning measurements respiration was expressed on a fresh mass basis. To test technical reproducibility of the instrument, a chemical oxidation assay consisting of 100 mM of cysteine in 600 μL of buffered solution (50 mM Hepes, 10 mM MES pH 6.5, 200 μM CaCl2) was used, and the stabilised O2 consumption rate over a 2-h run was measured repeatedly. To test fluorophore sensitivity to O2 depletion, known volumes of pure CO2 gas were injected into tubes through a pin-hole created on the side of tubes and sealed with blu-tack (Bostik, Paris, France) immediately after the gas-injection. All measurements were made at a room temperature of 21.5 ± 1.0 °C.
O2-electrode measurements
Respiratory consumption of O2 by leaves (3–42 mg fresh mass) or roots (56–214 mg fresh mass) were measured in the liquid-phase using Oxytherm Clark-type O2-electrode (Hansatech Instruments, Pentney, UK) in a 2 ml measuring volume. Electrodes were calibrated by bubbling water with compressed air for approximately 2-h to reach saturation followed by adding sodium dithionite to record O2 depleted signals. Leaf and root respiration was measured in a solution containing 20 mM Hepes (pH 7.2), 10 mM MES and 2 mM CaCl2, at 21.5 ± 1 °C. All measurements were made by dark adapting tissue for >30 min, submerging tissue in the Clark-type electrode cuvettes below measuring solution, with no obvious air pockets and continually stirring, and recording O2 consumption using Oxygraph Plus v1.02 software (Hansatech Instruments). The linear part of O2 consumption (approximately 10–30 min into each run) was used to calculate respiration rates.
IRGA measurements
Infrared gas-analysis of CO2 efflux by respiring leaves was measured using a Licor 6400XT with a 3 × 2 cm chamber head ((LI-COR, Lincoln, Nebraska, USA) on >30 min dark-adapted leaves. Attached whole leaves were placed across the measuring chamber and chamber gaskets and measurements recorded after CO2 readings stabilised (~10–15 min). The flow rate was set to 300 µmol s−1, the block temperature set to ambient air temperature of 22 °C and the CO2 reference sample was set to 400 µmol mol−1, to match ambient air. The light source was turned off.
Membrane inlet mass spectrometry
Dark-adapted wheat leaf disks (3 × 0.5 cm2 or 6 × 0.5 cm2) were placed in a 1 mL O-ring sealed cuvette containing only air and a polyethylene membrane sealed outlet attached to a mass spectrometer (MM6: VG, Winsford, UK). O2 (m/z = 32) and CO2 (m/z = 44) detection over a 20-min period was recorded. Prior to leaf disk samples being placed in the cuvette, N2 gas purging of the cuvette and injections of known volumes of O2 and CO2 allowed for conversion of mass detection signal to a gas concentration and the background consumption rate of O2 and CO2 by the mass spectrometer to be accounted for when determining leaf derived O2 consumption and CO2 evolution rates.
Replication and statistical analysis
For all experiments four to six biological replicates, with a biological replicate considered as plant material from individual plants grown in separate pots, or containers (when grown hydroponically) were measured. For the comparison of respiratory techniques, two or more samples from each biological replicate were analysed by each technique and sampling was standardised by selecting a 2 cm long mid-section of young, healthy, fully expanded leaves, or in relation to root samples, a longitudinal section from base to tip of the longest root segment. A one-way ANOVA was used to determine significance between leaf O2 consumption techniques and Two-Sample t-tests for differences between leaf CO2 evolution techniques and root O2 uptake techniques.
Results
Technical and biological reliability and accuracy
The stability of the fluorescent oxygen concentration measurements performed using the Q2 is evident because control tubes containing either ambient air or no O2, gave long-term stable readings at 100 ± 5 or 0 ± 5%, respectively (Fig. 1a). The stability of O2 in the purged tubes demonstrates that the sample tubes were hermetically sealed, providing a closed system, necessary for accurately measuring O2 uptake. Nevertheless, we suggest periodically testing the accuracy of the calibration tubes, in case of drift over time, by placing 100% air and 0% O2 tubes amongst samples during a run. Measuring the spontaneous chemical oxidation of a cysteine solution in replicate vials assessed the technical reproducibility of the O2 consumption measurements. Analysis of 30 tubes in three separate experiments gave an average coefficient of variation of 8.1% (Additional file 1: Table S1).
Fig. 1.
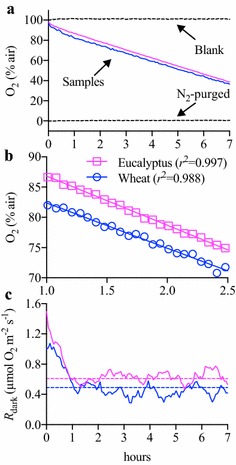
O2 consumption rates measured using the fluorophore technique. a O2 is given as a percentage of O2 in ambient air. A tube containing no sample (labelled as Blank) provided the baseline for no O2 consumption, while a tube devoid of all air (labelled as N2-purged) provided the baseline for total O2 consumption, and samples (magenta for Eucalyptus; blue line for wheat) depleted O2 within this range. b A higher resolution plot of individual data points over a 90-min period, from 1 to 2.5-h and linear regression analysis. c Respiration rates calculated from linear regression of O2 consumption using Eq. 1. Presented are R dark calculated from a 1-h moving slope (solid lines), and R dark calculated from the 1–2.5 h slope as shown in Panel b (dashed horizontal lines). Values are the means of four biological replicates for each species with the % of O2 measured every 4 min
When cut leaf material was placed inside the sample tubes, the fluorophore system was able to measure a consistent decline in O2 over a greater than 7-h period following an initial 1 h period of stabilization (Fig. 1a). The decline was linear in all species and tissues tested. The 90-min O2 consumption slope between 1 and 2.5-h had a mean r 2 of 0.99 across both species (Fig. 1b). Typically, the initial 0–30 min period of each run was associated with sharp declines in the O2 consumption slope. R dark calculated from a 1-h moving average of slopes over 7-h was similar to the slope of O2 consumption over a set 90 min period between 1 and 2.5-h (presented as dashed horizontal lines in Fig. 1c). The O2 consumption slope between 1 and 2.5-h can therefore be used as a standard period for calculating R dark across experiments.
Respiratory rates per unit leaf area were independent of the amount of leaf material placed within a given tube volume, apart from exceedingly small tissue abundance of below 0.1 cm2 mL−1 (Fig. 2a). To test whether the signal was independent of CO2 concentration and linearly related to O2 concentration between 0 and 100% of atmospheric O2 known volumes of pure CO2 gas were injected and sealed in measuring tubes. The measured percentage of O2 in the tube declined linearly in close proximity to the expected values for the amount of air displaced by CO2 (Additional file 1: Fig. S1), validating that for the fluorophore in question, the O2 dependent fluorescence quenching is linear and independent of CO2 concentration. An increase in CO2 concentration was not inhibitory to R dark, evident in maintained R dark when O2 was depleted to less than 40% of ambient levels, equivalent to the gas volume being >8% CO2, assuming a respiratory quotient of one. We provided further support of a lack of CO2 inhibition of R dark by purging tubes containing wheat leaf samples with various concentrations of pure CO2 gas (Fig. 2b). Interestingly, replacing the volume of gas surrounding leaf material with as much as 90% CO2 did not lead to a substantial decline in R dark. When 100% of the air within a tube was replaced with CO2, R dark did essentially stop, understandable considering no O2 would be available for respiration.
Fig. 2.
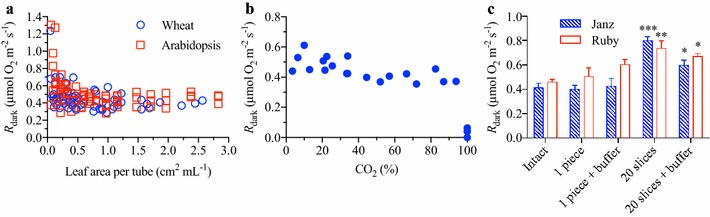
The influence of leaf tissue amount, CO2 concentrations, and mechanical wounding on the dark respiration rate (R dark) of wheat and Arabidopsis leaf tissue. a Differing amounts of leaf area in measuring tube volumes (cm2 mL−1) plotted against corresponding R dark for wheat (open blue circles) and Arabidopsis (open red squares). b R dark of wheat leaves sealed in measuring tubes with varying CO2 as a % of air. c The influence of mechanical wounding during sampling of leaf sections on the dark respiration rate (R dark) of two wheat cultivars, Janz and Ruby. The R dark of a 2 × 0.5 cm transverse section of leaf and a same sized leaf sliced a further 20 times was compared to an intact leaf, which was not mechanically damaged. Alternatively, leaves that were cut were washed with a wounding buffer prior to R dark measurements. The values are mean ± SE of four biological replicates. *significance at P < 0.05, ** at P < 0.01 and *** at P < 0.001, for a one-way ANOVA and a Dunnett’s multiple comparison test with the intact leaf set as the control
Although increased CO2 concentration was not inhibitory to R dark, heavy mechanical wounding of tissue resulted in higher R dark (Fig. 2c). Intact wheat leaves versus a 2 × 0.5 cm transverse section from the middle of leaves (a ratio of 1:1, wounded boundary length to leaf area) did not exhibit significant differences in R dark on an area basis (Fig. 2c). However, if the transverse section was further sliced into 20 smaller pieces (a 20-fold increase in the cut surface length to leaf area ratio), R dark increased by as much as two-fold (Fig. 2c). Applying a buffered saline solution to the heavily wounded leaf partly mitigated the enhancement of R dark by wounding. Thus it is important to reduce the amount of tissue exposed to mechanical damage when processing samples, to avoid the risk of artificially enhancing respiration rates.
Comparisons between leaf gas-exchange methods
Considering the many methods currently in use for determining plant respiratory gas-exchange, and the need to ensure that the fluorophore system was giving comparable rates, we compared R dark values generated using the fluorophore technology, the more conventional Clark-type O2-electrodes, Licor 6400 IRGA gas-exchange system, and membrane inlet mass spectrometry (MIMS). All of these techniques have varying degrees of difference in sample preparation and technical methodology that may influence the final respiratory rate recorded. For example, while we measured O2 consumption in the gas-phase using the fluorophore technique, O2-electrode measurements were made in aqueous-phase. Despite the IRGA measurements being made in gas-phase, measurements were of CO2 rather than O2 flux, and in an open gas-exchange system rather than the closed fluorophore system. Furthermore, IRGA measurements are made on intact not detached leaves. MIMS would be closest in methodology to the fluorophore technique in that both were measuring in the gas phase, in an essentially closed system. However, the MIMS system is not a completely closed system as the gradual leak of gasses through the semi-permeable membrane to the mass spectrometer would lead to changes in partial pressure and water vapour at the site of the leaf.
Understandably, due to the aforementioned differences in methodology, calculations of R dark using matching leaf or root material were significantly different between methods (Fig. 3). On an O2 basis, the conventional O2-electrode technique gave lower values, MIMS gave higher values, and the fluorophore values were intermediate. On a CO2 basis, MIMS measurements were significantly higher than IRGA measurements. MIMS, the only technique that can measure both O2 and CO2 concentrations, gave almost matching R dark measurements on an O2 and CO2 basis, indicating a respiratory quotient near unity for darkened wheat leaf tissue. Root R dark measurements in the gas-phase on the fluorophore system were significantly higher than in the liquid phase measured with O2-electrodes. Thus, while the fluorophore and IRGA approaches provide similar estimates of leaf R dark, both methods yield relatively lower estimated respiratory fluxes compared to MIMS; by contrast, the fluorophore approach yields relatively high values compared to liquid-phase Clark-type O2 electrode measurements.
Fig. 3.
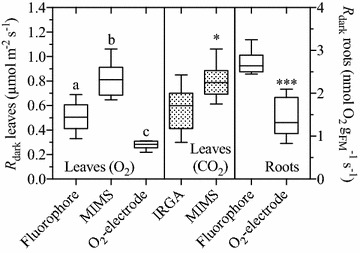
Comparisons of wheat dark respiration (R dark) measurements made using different experimental techniques. Leaf and root R dark was calculated from O2 consumption (open boxes) and CO2 evolution rates (hatched boxes), measured using fluorophore quenching, membrane inlet mass spectrometry (MIMS), Clark-type O2-electrodes, and infrared gas analysis (IRGA). Whiskers of box-plots represent the 5–95 percentile. For leaf O2 analysis, letters indicate significant differences between techniques at P < 0.05, derived from a one-way ANOVA with a Tukey’s multiple comparison test. For leaf CO2 analysis and roots an unpaired t-test was performed and * indicates significance at P < 0.01, while ***significance at P < 0.001. All measurements were made at 21 ± 0.5 °C. The values are based on six biological replicates and greater than 12 technical replicates
High-throughput analysis of respiration
Two studies were undertaken to verify the capabilities and versatility of automated O2 fluorophore technology for measuring high-throughput plant respiration in leaves and other plant tissues.
For the first study we undertook a fully replicated experiment of leaf respiration in 138 wheat cultivars (Fig. 4). There were clear differences in R dark among many genotypes, with a two-fold variation between the lowest and highest respiring cultivars (Fig. 4a). The wheat dataset was used to calculate the average standard deviation among biological replicates. As a proportion, the standard deviation was close to 20% of the overall mean R dark. This coefficient of variation was used to estimate the statistical power for future t test comparisons of R dark between wheat lines as a function of replicate number and difference in means, using a false discovery rate of 5% (α = 0.05; not including corrections for multiple testing). Given the four biological replicates per genotype used in this 138-genotype study, there is sufficient statistical power [(1 − β) > 0.8] to consistently detect only large differences in R dark between two lines equal to 50% of the mean (Fig. 4b). In a further example, to detect a 20% difference in mean respiration rates between any two wheat lines with the conventional statistical power target of (1 − β) = 0.8, 17 replicates would be appropriate (Fig. 4c). Of course, significant differences can still be detected with less replicates and less power, but given the high-throughput capacity of the fluorophore technique, appraisal of statistical power and appropriate biological replication can now be achieved, where previously, such high levels of replication were a barrier to experiments.
Fig. 4.
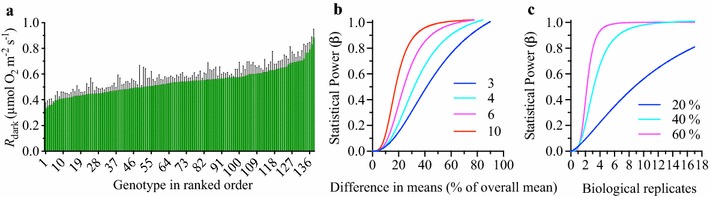
A high-throughput genotype and statistical power analysis of wheat dark respiration (R dark) of the youngest fully expanded leaf. a Leaf respiration in the dark (R dark) across 138 wheat cultivars grown under common conditions in a controlled environment growth room and measured at 21.5 ± 1 °C. The experiment was replicated on four occasions and the means (green columns) ± SE (black bars) of four biological replicates are presented. b Statistical power analysis of the wheat dataset with lines representing different numbers of biological replicates (3, 4, 6, or 10) plotted as a function of statistical power versus difference in genotype mean values. c Statistical power analysis of the wheat dataset with lines representing differences in mean values between genotypes equal to 20, 40 or 60% of the overall mean (equal to effect sizes of 1, 2 and 3 times the standard deviation) plotted as a function of statistical power versus biological replicates
With the potential to run a single sample using the fluorophore system in less than 2 min (Table 1), a single replicate of all 138 genotypes could be processed in less than 4 h, and potentially, a fully replicated 138 genotype study could be achieved in a single day. The number of samples per day is limited by the capacity of the robotic system, and by the time taken to prepare samples. By comparison, the other techniques have significantly longer calibration, sampling and measurement times required to acquire a single measurement (Table 1). Hence, what can be undertaken in 8-h using the high-throughput fluorophore technique, would require a minimum of 83 equivalent hours, or as much as 200-h for other commonly used procedures to measure R.
The second study looked at whole-plant developmental partitioning of R dark between leaves, stems and roots of 46-day-old wheat plants, which had reached the tillering stage of development (Additional file 1: Fig. S2). This type of experiment enables the quantitative attribution of total plant R dark to different parts of the plant at a specific stage of development. The simultaneous measurement of a whole dissected plant saves on the need to combine rates over time from measurements made on different plants. Plants were dissected and the individual leaves (including both leaf blade and sheath), the stem and roots were separated. The R dark of all separated tissues was measured for six entire plants simultaneously. Relative to healthy fully expanded leaves of a tiller; R dark was slightly higher in the oldest and much higher in the youngest leaves, on a fresh mass (i.e. nmol O2 g−1 s−1) basis (Fig. 5a, b). Leaves of intermediate ages exhibited similar rates of mass-based R dark. The total R dark for an entire leaf increased with age, presumably due to the increase in leaf size with stem and tiller developmental maturity. However, the total flux of O2 for the youngest leaf of the main stem or tiller was low (Fig. 5b), due to the smaller leaf size (Fig. 5a). When considered together, the total respiratory output of wheat foliage is dominated by healthy, relatively young, fully-expanded leaves (Fig. 5b) despite the oldest and youngest leaf of a stem or tiller having greater rates of R dark on a mass basis. When considering the partitioning of R dark between all tissues of the entire plant, leaves accounted for 51% (Fig. 5c), roots 37% and stems 12% of the total respiratory flux. Although the stem accounted for 12% of total R flux, it was only 4% of the entire fresh mass of the plant; however, stems had the highest mass based fluxes, likely due to energy expensive processes of cell division and elongation at the site of the apical meristem.
Fig. 5.
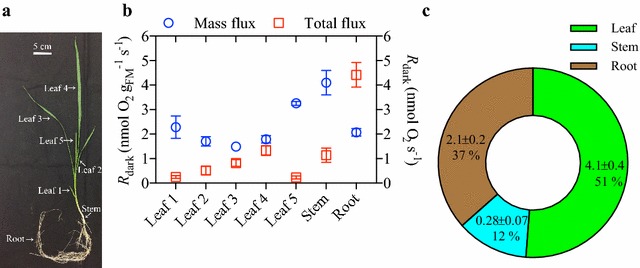
Wheat plants were harvested and separated into individual leaves, stem and roots, enabling an analysis of whole-plant dark respiration (R dark). a A representative image of a tiller with the roots, stem and leaf positions labelled. Leaf position 1 refers to the oldest leaf and position 5 refers to the newest emerging leaf. b Individual leaf, stem and root R dark on a mass basis (Mass flux; plotted on the left-axis) and total flux basis (Total flux; plotted on the right-axis). c A pie diagram illustrating the partitioning of the total flux of R dark between leaves as a whole, the stem and roots. The total plant leaf, stem and root fresh masses (g) are provided, as well as the % flux of total R dark. Values are based on the mean ± SE of six biological and pot replicates
Discussion
We demonstrate that using robotic fluorophore-based gas-phase measurements of O2 consumption in sealed tubes provides a simple yet reliable and reproducible means of measuring R dark for a diverse range of plant tissue types and species. The technique differentiates itself from other conventional methods in that it significantly reduces the time required for sample preparation and has substantial simultaneous measuring capabilities, making the technique a truly high-throughput means for measuring respiration. We demonstrate the potential capabilities of the method by measuring R dark of 138 wheat genotypes, and by measuring R dark of all tissues of six mid-vegetative stage plants simultaneously. A comparison of R dark in absolute terms, generated by different methodologies suggests variation in respiratory rates depending on technique employed, which should be considered when making direct comparisons between methods.
Strengths and weaknesses of high-throughput fluorophore methods
There was an initial spike and rapid decline in respiratory activity within the first 30-min of measurements (Fig. 1b). We dark-adapted leaves for a minimum or 30-min prior to fluorophore analysis, so although it is common to find a spike in respiration of leaves following exposure to light within the initial 30-min post-illumination period [58], post-illumination bursts in respiration do not explain the findings. Furthermore, while the O2-electrode and MIMS measurements continuously recorded in a similar manner to the fluorophore system, neither approach showed the initial spike, followed by rapid decline in R dark that was exhibited by the fluorophore approach (Fig. S3). Consequently, the first 60-min of each run were not used to calculate rates of R dark in the genotypic and developmental studies; the initial stabilisation period, however, can be used as a dark-adaptation period if tissue is not dark-adapted prior to fluorophore experimentation.
CO2 has previously been postulated to inhibit cytochrome c oxidase (COX) activity [59]. Reports initially suggested that a doubling of current atmospheric CO2 (i.e. from 0.04% of atmospheric gas to 0.08%) reduced R dark by 15–30% [60–62]. However, it was later discovered that CO2 inhibition of R dark was mostly likely an artefact of the measuring techniques used to quantify respiratory CO2 release [63–65]. Our results show that CO2 accumulation does not inhibit R dark. In fact, even with CO2 concentrations surrounding the sampled tissue reaching more than 90% of the gas volume (a 450-fold increase in concentration relative to previously reported measurements), no substantial inhibition in respiration occurred (Fig. 2b). We therefore conclude that leaf R dark is highly insensitive to CO2 accumulation over a course of several hours.
One factor that does seem to influence R dark is mechanical wounding (Fig. 2c). Leaf wounding was thought to affect leaf respiration as far back as 1950 [66]. Increased R dark with mechanical wounding is attributed to stimulation of the ATP/ADP ratio and activation of pyruvate kinase due to ion changes associated with wounding [67]. Pre-treatment by washing leaf samples with a buffered saline solution, the same as the measuring solution in liquid phase measurements, reduces any wounding effects on leaf R [38, 68]. We observed an increase in R dark when a large proportion of the sample had a wounded edge, and a reduction in R dark by applying wounding buffer, although not enough of a reduction to eliminate the wounded effect (Fig. 2c). However, minimal wounding did not significantly change R dark. Considering the time required to wash the sample tissue with a wounding solution, we suggest minimising as much as possible the mechanical wounding of tissue, rather than applying a wounding solution, if high-throughput sampling is desired. However, minimising mechanical wounding may require using larger volume tubes (e.g. moving from 1 to 4 mL tubes) to adequately fit sample tissue. By running a preliminary experiment, one could initially check for wounding effects and use the appropriate tissue size thereon after.
The limited effect of leaf wounding and lack of any inhibition to R dark from CO2 accumulation resulted in respiration measurements being stable over a period of many hours (Fig. 1). The stability of R dark for small leaf sections means that although the fluorophore technique we present is a closed-system that destroys the sampled tissue, a small sample of leaf collected in the field can be transported to the lab (making sure to keep detached leaves from desiccating), accurately representing in situ R dark. Thus, the fluorophore method can be considered as a pseudo non-destructive technique for high-throughput analysis for field experiments, as demonstrated below in the 138 wheat genotypes study we present.
Comparisons between respiratory methods
Although Hunt [32] comprehensively compared the strengths and weaknesses of multiple photosynthesis and respiration measurement techniques, no study to our knowledge has directly compared the absolute values of R obtained from the same biological material but measured across multiple techniques. Determining if the fluorophore technique presented in our study is comparable with previously well-established methods is important. Firstly, if results are to be examined among studies that utilised different techniques, it must be established if the analysis is viable, or whether differences among studies are an artefact of measuring technique. Secondly, although in many cases only the relative differences in R between samples may be of interest (for example, the genotypic study we present here), in many circumstances, absolute R will be desired, such as for determining absolute photosynthesis, or modelling the impact of R on terrestrial carbon budgets. Hence, we directly compared fluorophore, O2-electrode, IRGA, and MIMS output (Fig. 3). We found differences did exist between the techniques, suggesting that comparing results between studies utilising different R measuring apparatus may not be appropriate, or at least with the caveat that comparisons may require cross-calibration of method. Differences in measurements based on either O2 consumption or CO2 evolution may be expected considering the respiratory quotient (RQ) will not necessarily be equal to 1 (i.e. respiratory CO2 release being equal to O2 uptake) if pure carbohydrates were not the only source of respiratory substrate, or the oxidation state of respiratory products differed, although a RQ of 1 is usually assumed for higher plants under non-stressed conditions [69]. Indeed, the simultaneous measurement of R dark derived from O2 and CO2 exchange by MIMS gave close to matching values, supporting a RQ of 1, in contrast to a study of wheat leaves measured in the dark, 6-h into the light period (similar conditions to this study), which gave a RQ value of 1.8 ± 0.21 [70]. However, the study by Azcón-Bieto, Lambers and Day [70] used values of R determined separately using O2-electrode and IRGA systems, and since we found lower O2 based O2-electrode values relative to CO2 IRGA values, we emphasise that caution must be taken when comparing R calculated from different methodologies. Of note, the widely used IRGA gas-exchange system on intact leaves gave similar rates to the fluorophore results, suggesting the two techniques may be complementary. We did not undertake subsequent experiments to determine the specific reasons for variations in R dark between the techniques compared, and it will be of interest to further explore the reasons for why the techniques vary in future studies.
Genotypic and whole-plant analysis
Both a comprehensive genotype comparison and whole-plant respiratory balances were successfully obtained by use of the gas-phase automated fluorophore technique. Interestingly, a more than two-fold variation in R was observed between the 138 wheat genotypes (Fig. 4a). This demonstrates the inherent intra-specific divergence of R in Triticum aestivum, and a potential target for future yield improvements, if R not contributing to growth or yield can be minimised. Inherent differences in R dark between species populations have previously been noted, such as in the ryegrass species Lolium perenne, attributed to adenylate limitations on glycolysis and varying ATP turnover rates between populations [71]. R was also highly variable among genotypes. This may not be considered surprising as leaf functional traits vary considerably among populations/genotypes within a given species. For example, a study of 13 common alpine species found that 30% of observable variance in measured traits, such as specific leaf area and leaf nitrogen content, was among populations/genotypes of a given species [72]. Similar results were found for species growing in a dry tropical forest [73]. Considering R is highly variable among genotypes within species, to gain sufficient statistical power a high level of replication is required (Fig. 4b, c), further supporting the benefit of the high-throughput fluorophore technique we present.
Our whole-plant respiratory analysis demonstrated the important effects of plant development on leaf R and partitioning of R between tissue types, as previously demonstrated in Arabidopsis by Sew et al. [55], which could be detrimentally ignored if the power of high-throughput respiratory analysis was not readily available. The results highlight the fact that, when measuring leaf, stem and root O2 uptake in the gas phase, leaf R dark accounted for 51% of the entire R budget. In other words, close to half of all vegetative-stage wheat R occurs in non-leaf tissue, a finding reported for previous studies that quantified whole-plant CO2 fluxes [15–19]. Yet, we tentatively suggest that the majority of plant R reports would focus entirely, or predominantly on leaf R. Furthermore, the oldest and newest emerging leaves had considerably higher mass-based rates of R dark than intermediate aged leaves. In regards to the latter, this is presumably due to the added cost of growth R as well as maintenance R for newly emerging leaves [6]. The spike in R for the oldest leaves may reflect the costs associated with senescence, such as an energy expensive remobilisation of nutrients from the senescing leaf to other parts of the plant. For example, in oats (Avena sativa), promotion of senescence of leaves by withholding light leads to a greater than two-fold increase in O2 consumption, attributed to decoupling of R dark from oxidative phosphorylation, and amino-acid and soluble sugar liberation during senescence [74].
Conclusions
The high-throughput and tissue size versatility of the experiments we conducted highlight the comparative advantages of an automated gas-phase system, over other systems based on the same technology but reliant on aqueous-phase and limited sample tubes and volumes. Although aqueous-phase fluorophore systems may be relatively high-throughput when compared to the older technology of Clark-type O2 electrodes, liquid-phase measurements still require extensive time in preparation of solutions, dispensing of solutions, and delicate sample positioning or sufficient stirring to facilitate O2 movement to the sensor [e.g. 75]. We processed 138 samples, from tissue harvesting to initial O2 uptake measurements, in a period of less than 2-h, which was possible due to the simple procedure of placing tissue in tubes, tightening the caps and placing tubes in the designated instrument position. Such a fast turnaround for sample processing would not be possible in a non-fluorophore and/or aqueous-phase procedure. The speed at which samples can be processed and the versatility in sample size and tissue type enables respiratory analysis that simply would not be feasible using other established approaches. The simultaneous measurement of many genotypes and the construction of multiple whole-plant respiratory budgets emphasise the potential of this method and its wider application.
Authors’ contributions
APS, ACAN, BO, AHM and OKA conceived the idea for the study. APS, ACAN, BO, FAAF, LH, YZ, VC and MRB conducted the experiments. APS wrote the first draft; all authors contributed significantly to subsequent versions. All authors read and approved the final manuscript.
Acknowledgements
The support of the Australian Research Council (CE140100008) to OKA and AHM is acknowledged.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
Australian Research Council (CE140100008).
Additional file
Additional file 1: Table S1. Technical reproducibility of the fluorophore instrument in measuring the chemical oxidation of cysteine. Figure S1. The measured and expected depletion of O2 though replacement of air with known volumes of pure CO2 gas. Figure S2. A representative 46-day-old wheat plant harvested for whole-plant respiration analysis. Figure S3. Derived respiration rates in the dark (Rdark) calculated from measurements between 5 and 20 min from experiment initiation using the various techniques.
Contributor Information
Andrew P. Scafaro, Email: andrew.scafaro@anu.edu.au
A. Clarissa A. Negrini, Email: ana.alves@anu.edu.au
Brendan O’Leary, Email: brendan.oleary@uwa.edu.au.
F. Azzahra Ahmad Rashid, Email: fatimah.azzahra@anu.edu.au.
Lucy Hayes, Email: lucy.hayes@anu.edu.au.
Yuzhen Fan, Email: yuzhen.fan@anu.edu.au.
You Zhang, Email: u4975900@anu.edu.au.
Vincent Chochois, Email: vincent.chochois@anu.edu.au.
Murray R. Badger, Email: murray.badger@anu.edu.au
A. Harvey Millar, Email: harvey.millar@uwa.edu.au.
Owen K. Atkin, Phone: +61 (0)2 6125 5046, Email: Owen.Atkin@anu.edu.au
References
- 1.Tcherkez G, Boex-Fontvieille E, Mahe A, Hodges M. Respiratory carbon fluxes in leaves. Curr Opin Plant Biol. 2012;15:308–314. doi: 10.1016/j.pbi.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Bouma TJ, Devisser R, Janssen JHJA, Dekock MJ, Vanleeuwen PH, Lambers H. Respiratory energy requirements and rate of protein turnover in vivo determined by the use of an inhibitor of protein synthesis and a probe to assess its effect. Physiol Plant. 1994;92:585–594. doi: 10.1111/j.1399-3054.1994.tb03027.x. [DOI] [Google Scholar]
- 3.Bouma TJ, De VR, Van LPH, De KMJ, Lambers H. The respiratory energy requirements involved in nocturnal carbohydrate export from starch-storing mature source leaves and their contribution to leaf dark respiration. J Exp Bot. 1995;46:1185–1194. doi: 10.1093/jxb/46.9.1185. [DOI] [Google Scholar]
- 4.Noguchi K, Yoshida K. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion. 2008;8:87–99. doi: 10.1016/j.mito.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Lambers H. Respiration in intact plants and tissues: its regulation and dependence on environmental factors, metabolism and invaded organisms. In: Douce R, Day DA, editors. Encyclopedia of plant physiology. New York: Springer; 1985. pp. 417–473. [Google Scholar]
- 6.Amthor JS. The McCree–de Wit–Penning de Vries–Thornley respiration paradigms: 30 years later. Ann Bot. 2000;86:1–20. doi: 10.1006/anbo.2000.1175. [DOI] [Google Scholar]
- 7.Lambers H, Atkin OK, Scheurwater I. Respiration patterns in roots in relation to their functioning. In: Waisel Y, Eshel A, Kafkaki U, editors. Plant roots the hidden half. New York: Marcel Dekker; 1996. pp. 323–362. [Google Scholar]
- 8.Gifford RM. Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol. 2003;30:171–186. doi: 10.1071/FP02083. [DOI] [PubMed] [Google Scholar]
- 9.Huntingford C, Zelazowski P, Galbraith D, Mercado LM, Sitch S, Fisher R, Lomas M, Walker AP, Jones CD, Booth BBB, et al. Simulated resilience of tropical rainforests to CO2-induced climate change. Nat Geosci. 2013;6:268–273. doi: 10.1038/ngeo1741. [DOI] [Google Scholar]
- 10.Amthor JS, Wilkinson RE: Plant respiratory responses to the environment and their effects on the carbon balance. In: Plant-environment interactions. volume 1. New York: Marcel Dekker; 1994. p. 501–54.
- 11.Atkin OK, Bloomfield KJ, Reich PB, Tjoelker MG, Asner GP, Bonal D, Bönisch G, Bradford MG, Cernusak LA, Cosio EG, et al. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 2015;206:614–636. doi: 10.1111/nph.13253. [DOI] [PubMed] [Google Scholar]
- 12.Heskel MA, O’Sullivan OS, Reich PB, Tjoelker MG, Weerasinghe KWLK, Penillard A, Xiang J, Egerton JJG, Creek D, Bloomfield KJ, et al. Convergence in the temperature response of leaf respiration across biomes and plant functional types. Proc Natl Acad Sci USA. 2016;113:3832–3837. doi: 10.1073/pnas.1520282113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Sullivan OS, Heskel MA, Reich PB, Tjoelker MG, Weerasinghe KWLK, Penillard A, Zhu L, Egerton JJG, Bloomfield KJ, Creek D, et al. Thermal limits of leaf metabolism across biomes. Glob Change Biol. 2016;23(1):209–223. doi: 10.1111/gcb.13477. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds M, Foulkes J, Furbank R, Griffiths S, King J, Murchie E, Parry M, Slafer G. Achieving yield gains in wheat. Plant Cell Environ. 2012;35:1799–1823. doi: 10.1111/j.1365-3040.2012.02588.x. [DOI] [PubMed] [Google Scholar]
- 15.Poorter H, Remkes C, Lambers H. Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol. 1990;94:621–627. doi: 10.1104/pp.94.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loveys BR, Scheurwater I, Pons TL, Fitter AH, Atkin OK. Growth temperature influences the underlying components of relative growth rate: an investigation using inherently fast- and slow-growing plant species. Plant Cell Environ. 2002;25:975–987. doi: 10.1046/j.1365-3040.2002.00879.x. [DOI] [Google Scholar]
- 17.Atkin OK, Botman B, Lambers H. The causes of inherently slow growth in alpine plants: an analysis based on the underlying carbon economies of alpine and lowland poa species. Funct Ecol. 1996;10:698–707. doi: 10.2307/2390504. [DOI] [Google Scholar]
- 18.Gifford RM. Whole plant respiration and photosynthesis of wheat under increased CO2 concentration and temperature: long-term vs short-term distinctions for modelling. Glob Change Biol. 1995;1:385–396. doi: 10.1111/j.1365-2486.1995.tb00037.x. [DOI] [Google Scholar]
- 19.Atkin OK, Scheurwater I, Pons TL. Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. New Phytol. 2007;174:367–380. doi: 10.1111/j.1469-8137.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 20.Millar AH, Whelan J, Soole KL, Day DA. Organization and regulation of mitochondrial respiration in plants. Annu Rev Plant Biol. 2011;62:79–104. doi: 10.1146/annurev-arplant-042110-103857. [DOI] [PubMed] [Google Scholar]
- 21.Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- 22.Hauben M, Haesendonckx B, Standaert E, Van Der Kelen K, Azmi A, Akpo H, Van Breusegem F, Guisez Y, Bots M, Lambert B, et al. Energy use efficiency is characterized by an epigenetic component that can be directed through artificial selection to increase yield. Proc Natl Acad Sci USA. 2009;106:20109–20114. doi: 10.1073/pnas.0908755106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson D, Jones JG. Effect of selection for dark respiration rate of mature leaves on crop yields of Lolium perenne cv. S23. Ann Bot. 1982;49:313–320. doi: 10.1093/oxfordjournals.aob.a086255. [DOI] [Google Scholar]
- 24.Reynolds M, Langridge P. Physiological breeding. Curr Opin Plant Biol. 2016;31:162–171. doi: 10.1016/j.pbi.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 25.King AW, Gunderson CA, Post WM, Weston DJ, Wullschleger SD. Plant respiration in a warmer world. Science. 2006;312:536–537. doi: 10.1126/science.1114166. [DOI] [PubMed] [Google Scholar]
- 26.Wythers KR, Reich PB, Bradford JB. Incorporating temperature-sensitive Q10 and foliar respiration acclimation algorithms modifies modeled ecosystem responses to global change. J Geophys Res: Biogeosci. 2013;118:77–90. doi: 10.1029/2011JG001897. [DOI] [Google Scholar]
- 27.Atkin OK, Millar AH, Turnbull MH. Plant respiration in a changing world. New Phytol. 2010;187:268–272. doi: 10.1111/j.1469-8137.2010.03343.x. [DOI] [PubMed] [Google Scholar]
- 28.Reich PB, Sendall KM, Stefanski A, Wei X, Rich RL, Montgomery RA. Boreal and temperate trees show strong acclimation of respiration to warming. Nature. 2016;531:633–636. doi: 10.1038/nature17142. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin BC, Xu CY, Rastetter EB, Griffin KL. Predicting ecosystem carbon balance in a warming Arctic: the importance of long-term thermal acclimation potential and inhibitory effects of light on respiration. Glob Change Biol. 2014;20:1901–1912. doi: 10.1111/gcb.12549. [DOI] [PubMed] [Google Scholar]
- 30.Slot M, Rey-Sánchez C, Gerber S, Lichstein JW, Winter K, Kitajima K. Thermal acclimation of leaf respiration of tropical trees and lianas: response to experimental canopy warming, and consequences for tropical forest carbon balance. Glob Change Biol. 2014;20:2915–2926. doi: 10.1111/gcb.12563. [DOI] [PubMed] [Google Scholar]
- 31.Atkin OK, Bruhn D, Hurry VM, Tjoelker MG. The hot and the cold: unraveling the variable response of plant respiration to temperature. Funct Plant Biol. 2005;32:87–105. doi: 10.1071/FP03176. [DOI] [PubMed] [Google Scholar]
- 32.Hunt S. Measurements of photosynthesis and respiration in plants. Physiol Plant. 2003;117:314–325. doi: 10.1034/j.1399-3054.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 33.Clark LC, Wolf R, Granger D, Taylor Z. Continuous recording of blood oxygen tensions by polarography. J Appl Physiol. 1953;6:189–193. doi: 10.1152/jappl.1953.6.3.189. [DOI] [PubMed] [Google Scholar]
- 34.Poorter H, Van Der Werf A, Atkin OK, Lambers H. Respiratory energy requirements of roots vary with the potential growth rate of a species. Physiol Plant. 1991;83:469–475. doi: 10.1111/j.1399-3054.1991.tb00122.x. [DOI] [Google Scholar]
- 35.Loveys BR, Atkinson LJ, Sherlock DJ, Roberts RL, Fitter AH, Atkin OK. Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast- and slow-growing plant species. Glob Change Biol. 2003;9:895–910. doi: 10.1046/j.1365-2486.2003.00611.x. [DOI] [Google Scholar]
- 36.Kurimoto K, Day DA, Lambers H, Noguchi K. Effect of respiratory homeostasis on plant growth in cultivars of wheat and rice. Plant Cell Environ. 2004;27:853–862. doi: 10.1111/j.1365-3040.2004.01191.x. [DOI] [PubMed] [Google Scholar]
- 37.Lambers H, Day DA, Azc¢n-Bieto J. Cyanide-resistant respiration in roots and leaves: measurements with intact tissues and isolated mitochondria. Physiol Plant. 1983;58:148–154. doi: 10.1111/j.1399-3054.1983.tb04159.x. [DOI] [Google Scholar]
- 38.Azcón-Bieto J, Lambers H, Day DA. Respiratory properties of developing bean and pea leaves. Aust J Plant Physiol. 1983;10:237–45.
- 39.Jacoby RP, Millar AH, Taylor NL. Assessment of respiration in isolated plant mitochondria using Clark-type electrodes. In: Whelan J, Murcha WM, editors. Plant mitochondria: methods and protocols. New York: Springer; 2015. pp. 165–185. [DOI] [PubMed] [Google Scholar]
- 40.Vasseur F, Pantin F, Vile D. Changes in light intensity reveal a major role for carbon balance in Arabidopsis responses to high temperature. Plant Cell Environ. 2011;34:1563–1576. doi: 10.1111/j.1365-3040.2011.02353.x. [DOI] [PubMed] [Google Scholar]
- 41.Atkin OK, Scheurwater I, Pons TL. High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Glob Change Biol. 2006;12:500–515. doi: 10.1111/j.1365-2486.2006.01114.x. [DOI] [Google Scholar]
- 42.Whitehead D, Griffin KL, Turnbull MH, Tissue DT, Engel VC, Brown KJ, Schuster WSF, Walcroft AS. Response of total night-time respiration to differences in total daily photosynthesis for leaves in a Quercus rubra L. canopy: implications for modelling canopy CO2 exchange. Glob Change Biol. 2004;10:925–938. doi: 10.1111/j.1529-8817.2003.00739.x. [DOI] [Google Scholar]
- 43.den Hertog J, Stulen I, Lambers H. Assimilation, respiration and allocation of carbon in Plantago major as affected by atmospheric CO2 levels: a case-study. Vegetatio. 1993;104:369–378. doi: 10.1007/BF00048166. [DOI] [Google Scholar]
- 44.Kolling K, George GM, Kunzli R, Flutsch P, Zeeman SC. A whole-plant chamber system for parallel gas exchange measurements of Arabidopsis and other herbaceous species. Plant Methods. 2015;11:48. doi: 10.1186/s13007-015-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbour MM, McDowell NG, Tcherkez GUIL, Bickford CP, Hanson DT. A new measurement technique reveals rapid post-illumination changes in the carbon isotope composition of leaf-respired CO2. Plant Cell Environ. 2007;30:469–482. doi: 10.1111/j.1365-3040.2007.01634.x. [DOI] [PubMed] [Google Scholar]
- 46.Nakaema WM, Hao Z-Q, Rohwetter P, Wöste L, Stelmaszczyk K. PCF-based cavity enhanced spectroscopic sensors for simultaneous multicomponent trace gas analysis. Sensors. 2011;11:1620. doi: 10.3390/s110201620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beckmann K, Messinger J, Badger MR, Wydrzynski T, Hillier W. On-line mass spectrometry: membrane inlet sampling. Photosynth Res. 2009;102:511–522. doi: 10.1007/s11120-009-9474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen LD, Smith BN, Criddle RS, Breidenbach RW. Calorimetry of plant respiration. J Therm Anal Calorim. 1998;51:757–763. doi: 10.1007/BF03341452. [DOI] [Google Scholar]
- 49.Macfarlane C, Adams MA, Hansen LD. Application of an enthalpy balance model of the relation between growth and respiration to temperature acclimation of Eucalyptus globulus seedlings. Proc R Soc B: Biol Sci. 2002;269:1499–1507. doi: 10.1098/rspb.2002.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tyystjärvi E, Karunen J, Lemmetyinen H. Measurement of photosynthetic oxygen evolution with a new type of oxygen sensor. Photosynth Res. 1998;56:223–227. doi: 10.1023/A:1005994311121. [DOI] [Google Scholar]
- 51.Ast C, Schmälzlin E, Löhmannsröben H-G, van Dongen JT. Optical oxygen micro- and nanosensors for plant applications. Sensors. 2012;12:7015. doi: 10.3390/s120607015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J, Wang Z, Li Y, Zhuang Q, Gu J. Real-time monitoring of dissolved oxygen with inherent oxygen-sensitive centers in metal–organic frameworks. Chem Mater. 2016;28:2652–2658. doi: 10.1021/acs.chemmater.6b00016. [DOI] [Google Scholar]
- 53.Warkentin M, Freese HM, Karsten U, Schumann R. New and fast method to quantify respiration rates of bacterial and plankton communities in freshwater ecosystems by using optical oxygen sensor spots. Appl Environ Microbiol. 2007;73:6722–6729. doi: 10.1128/AEM.00405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moodley L, Steyaert M, Epping E, Middelburg JJ, Vincx M, van Avesaath P, Moens T, Soetaert K. Biomass-specific respiration rates of benthic meiofauna: demonstrating a novel oxygen micro-respiration system. J Exp Mar Biol Ecol. 2008;357:41–47. doi: 10.1016/j.jembe.2007.12.025. [DOI] [Google Scholar]
- 55.Sew YS, Ströher E, Holzmann C, Huang S, Taylor NL, Jordana X, Millar AH. Multiplex micro-respiratory measurements of Arabidopsis tissues. New Phytol. 2013;200:922–932. doi: 10.1111/nph.12394. [DOI] [PubMed] [Google Scholar]
- 56.Zhao G-W, Zhong T-L. Improving the assessment method of seed vigor in Cunninghamia lanceolata and Pinus massoniana based on oxygen sensing technology. J For Res. 2012;23:95–101. doi: 10.1007/s11676-012-0238-4. [DOI] [Google Scholar]
- 57.Guangwu Z, Xuwen J. Roles of gibberellin and auxin in promoting seed germination and seedling vigor in Pinus massoniana. For Sci. 2014;60:367–373. [Google Scholar]
- 58.Atkin OK, Evans JR, Siebke K. Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Aust J Plant Physiol. 1998;25:437–443. doi: 10.1071/PP97159. [DOI] [Google Scholar]
- 59.Gonzalez-Meler MA, Ribas-Carbo M, Siedow JN, Drake BG. Direct inhibition of plant mitochondrial respiration by elevated CO2. Plant Physiol. 1996;112:1349–1355. doi: 10.1104/pp.112.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amthor JS, Koch GW, Bloom AJ. CO2 inhibits respiration in leaves of Rumex crispus L. Plant Physiol. 1992;98:757–760. doi: 10.1104/pp.98.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drake BG, Azcon-Bieto J, Berry J, Bunce J, Dijkstra P, Farrar J, Gifford RM, Gonzalez-Meler MA, Koch G, Lambers H, et al. Does elevated atmospheric CO2 concentration inhibit mitochondrial respiration in green plants? Plant Cell Environ. 1999;22:649–657. doi: 10.1046/j.1365-3040.1999.00438.x. [DOI] [Google Scholar]
- 62.Curtis PS, Wang XZ. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia. 1998;113:299–313. doi: 10.1007/s004420050381. [DOI] [PubMed] [Google Scholar]
- 63.Jahnke S. Atmospheric CO2 concentration does not directly affect leaf respiration in bean or poplar. Plant Cell Environ. 2001;24:1139–1151. doi: 10.1046/j.0016-8025.2001.00776.x. [DOI] [Google Scholar]
- 64.Bruhn D, Mikkelsen TN, Atkin OK. Does the direct effect of atmospheric CO2 concentration on leaf respiration vary with temperature? Responses in two species of Plantago that differ in relative growth rate. Physiol Plant. 2002;114:57–64. doi: 10.1034/j.1399-3054.2001.1140109.x. [DOI] [PubMed] [Google Scholar]
- 65.Tjoelker MG, Oleksyn J, Lee TD, Reich PB. Direct inhibition of leaf dark respiration by elevated CO2 is minor in 12 grassland species. New Phytol. 2001;150:419–424. doi: 10.1046/j.1469-8137.2001.00117.x. [DOI] [Google Scholar]
- 66.Klinker JE. A modification of the warburg respirometer to measure the respiration rate of tomato leaf discs. Plant Physiol. 1950;25:354–355. doi: 10.1104/pp.25.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Macnicol PK. Rapid metabolic changes in the wounding response of leaf discs following excision. Plant Physiol. 1976;57:80–84. doi: 10.1104/pp.57.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azcón-Bieto J, Day DA, Lambers H. The regulation of respiration in the dark in wheat (Triticum aestivum cultivar Gabo) leaf slices. Plant Sci Lett. 1983;32:313–320. doi: 10.1016/0304-4211(83)90037-8. [DOI] [Google Scholar]
- 69.Reich PB, Tjoelker MG, Pregitzer KS, Wright IJ, Oleksyn J, Machado JL. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol Lett. 2008;11:793–801. doi: 10.1111/j.1461-0248.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 70.Azcón-Bieto J, Lambers H, Day DA. Effect of photosynthesis and carbohydrate status on respiratory rates and the involvement of the alternative pathway in leaf respiration. Plant Physiol. 1983;72:598–603. doi: 10.1104/pp.72.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Day DA, de Vos OC, Wilson D, Lambers H. Regulation of respiration in the leaves and roots of two Lolium perenne populations with contrasting mature leaf respiration rates and crop yields. Plant Physiol. 1985;78:678–683. doi: 10.1104/pp.78.4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Albert CH, Thuiller W, Yoccoz NG, Douzet R, Aubert S, Lavorel S. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Funct Ecol. 2010;24:1192–1201. doi: 10.1111/j.1365-2435.2010.01727.x. [DOI] [Google Scholar]
- 73.Hulshof CM, Swenson NG. Variation in leaf functional trait values within and across individuals and species: an example from a Costa Rican dry forest. Funct Ecol. 2010;24:217–223. doi: 10.1111/j.1365-2435.2009.01614.x. [DOI] [Google Scholar]
- 74.Tetley RM, Thimann KV. The metabolism of oat leaves during senescence: I. Respiration, carbohydrate metabolism, and the action of cytokinins. Plant Physiol. 1974;54:294–303. doi: 10.1104/pp.54.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sew YS, Millar AH, Stroeher E. Micro-respiratory measurements in plants. In: Whelan J, Murcha WM, editors. Plant mitochondria: methods and protocols. New York: Springer; 2015. pp. 187–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


