Abstract
Background
Brain oscillatory responses can be used for non-invasive analyses of cortico-cortical connectivity, local neuronal synchronization, and coherence of oscillations in many neuropsychiatric conditions including Alzheimer's disease (AD). In the present paper, we examine sensory-evoked and event-related gamma coherences elicited by visual stimuli in three sub-gamma bands in two sub-groups of patients with AD (i.e., acetylcholinesterase-inhibitor treated and untreated) and healthy controls.
Methods
We studied a total of 39 patients with probable mild AD (according to NINCDS-ADRDA criteria) who had been sub-divided into untreated (n = 21) and treated (n = 18) (patients either on cholinergic monotherapy or combined therapy with memantine) AD groups, and 21 age-, gender-, and education-matched healthy elderly controls. A simple flash visual paradigm was applied for the acquisition of sensory-evoked coherences. Event-related coherences were elicited using a classical visual oddball paradigm. Both sensory-evoked and event-related gamma coherences were calculated for long-distance intrahemispheric pairs for three frequency ranges: 25–30 Hz, 30–35 Hz, and 40–48 Hz in post-stimulus 0–800 ms duration. The long-distance intrahemispheric pairs from both sides were fronto-parietal, fronto-temporal, fronto-temporoparietal, fronto-occipital, centro-occipital and parieto-occipital.
Results
The sensory-evoked or event-related gamma coherences revealed that both treated and untreated AD patients had significantly increased values compared to healthy controls in all three sub-gamma bands. Moreover, the treated AD patients demonstrated significantly higher fronto-parietal gamma coherences during both sensory stimulation and oddball paradigm and lower occipito-parietal coherences during oddball paradigm in comparison to untreated AD patients.
Conclusion
The present study demonstrated that an increase of gamma coherences was present in response to both visual sensory and cognitive stimulation in AD patients in all gamma sub-bands. Therefore, gamma oscillatory activity seems to be fundamental in brain functions at both the sensory and cognitive levels. The increase of gamma coherence values was not due to cholinergic treatment to any significant extent, as both treated and untreated AD patients had increased gamma coherence values compared to healthy controls. The use of coherence values reflecting brain connectivity holds potential for neuroimaging of AD and understanding brain dynamics related to the effects of medication.
Keywords: Event-related, Sensory-evoked, Coherence, Gamma, Alzheimer's disease, EEG, Connectivity
Highlights
-
•
Treated and untreated Alzheimer's disease (AD) patients and controls were examined.
-
•
Visual sensory-evoked and event-related gamma coherences were investigated.
-
•
Long-distance intrahemispheric pairs for three sub-gamma bands were calculated.
-
•
Gamma coherences were significantly higher in AD patients than healthy controls.
1. Introduction
Alzheimer's disease (AD) is one of the most devastating illnesses that threatens public health.
It is characterized by progressive synaptic failure and brain atrophy related to neurodegeneration (Jack et al., 2010). The investigation of candidate biomarkers for the early detection of mild cognitive impairment (MCI) and/or AD by using several neuroimaging techniques has recently been a hot topic (Jack et al., 2010, Yener and Başar, 2013, Başar, 2013, Babiloni et al., 2016, Rossini et al., 2006, Frisoni et al., 2011).
Brain oscillatory responses can be used for non-invasive analyses of cortico-cortical connectivity, local neuronal synchronization, and coherence of oscillations (Rossini et al., 2007). Event-related oscillations (EROs), used as a powerful technique with high temporal resolution, can be elicited upon application of cognitive stimuli. It is a useful tool for detecting subtle abnormalities in cognitive processes (Başar, 1980, Başar, 2004).
Our research group has published reports on the analyses of EROs and electrophysiological connectivity measurements in AD/MCI over the last decade. In addition to EROs, we have explored sensory-evoked oscillations (SEOs), and the evoked- or event-related coherences of AD/MCI patients using visual and/or auditory sensory and cognitive stimulation (Yener et al., 2008, Yener et al., 2009, Yener et al., 2012, Güntekin et al., 2008, Başar et al., 2010, Yener and Başar, 2010). The term “event-related” is used for a potential that is elicited after a cognitive task, while the term “sensory-evoked” is used for a potential that is elicited after a simple sensory stimulus of auditory or visual modality (Başar et al., 1997).
In our previous studies, frontal delta EROs were found to be associated with frontal volume in patients with MCI and healthy elderly controls (Yener et al., 2016), indicating that EROs can be used as a biomarker candidate in diagnosis. The use of electrophysiological measures in diagnosis and monitoring of treatment responses is important. In the present paper, we aim to explore evoked- and event-related gamma coherences in AD.
Although the history of gamma activity began in the 1940s (Adrian, 1942), it was discovered in later years by Freeman (1975) and Başar et al., 1975a, Başar et al., 1975b, Başar et al., 1975c that gamma oscillatory activity reflects a wide variety of cognitive functions. In 1973, the terminology “gamma response” was introduced by Başar and Ungan (1973) to describe hippocampal gamma band activity elicited by stimuli in cats. Galambos (1981) later indicated that there are sensory and cognitive correlates of gamma responses in human participants. Gamma oscillatory responses do not appear to have a specific function in the nervous system, even though they are selectively distributed in widespread brain regions including the cortex, hippocampus, thalamus, and reticular formations in both animal and human brains (Başar, 2013). Thus, it can be speculated that gamma synchronization is a fundamental process for all brain functions (Başar et al., 1999, Başar et al., 2013, Başar-Eroglu et al., 1996a). Furthermore, gamma oscillatory activity is related to proper functioning of inhibitory interneurons which mostly consists of GABAergic neurons (Palop and Mucke, 2016).
As gamma oscillatory responses have a fundamental role in many cognitive and sensory processes, they are reported to play a role in attention, perception, object recognition, memory processes, face recognition, and emotional paradigms (Güntekin and Başar, 2014, Keil et al., 1999, Busch et al., 2004, Busch et al., 2006, Tallon-Baudry et al., 1998, Gruber et al., 2004, Herrmann et al., 2004a, Müller and Keil, 2004, Senkowski and Herrmann, 2002). (For further information on gamma responses, please see reviews from Başar, 2013, Başar-Eroglu et al., 1996b, Herrmann et al., 2004b, Singer, 1999, Tallon-Baudry and Bertrand, 1999.)
The limited literature that exists on gamma responses in AD is highly controversial. Ribary et al. (1991) showed by magnetic field tomography that the cortical component of the thalamocortical coherence of 40 Hz oscillations was reduced in AD patients. Van Deursen et al. (2008) reported significantly higher gamma band power in AD patients as compared with healthy controls in all resting state, music listening, story listening and visual stimulation conditions. The authors stated that both groups showed increased gamma band power during tasks in comparison to resting state (van Deursen et al., 2008). Moreover, both magnetoencephalography (MEG) and EEG studies demonstrated increased 40 Hz steady-state responses in AD patients compared to healthy controls (Osipova et al., 2006, van Deursen et al., 2011). Koenig et al. (2005) reported decreased gamma global field synchronization (GSF) values in AD patients compared to controls and stated that the inter-individual variance of gamma GSF values was much larger than the other frequency bands. Stam et al. (2002) showed decreased gamma band synchronization in AD patients compared to controls but no differences on gamma coherence were found between groups. However, Stam et al. (2006) later reported increased functional connectivity in the occipito-parietal regions (measured by resting state coherence) in the gamma band in AD patients. Rossini et al. (2006) demonstrated that higher resting state gamma coherence (fronto-parietal regions) is associated with faster conversion of MCI to AD. The discrepancies between these studies likely involve methodological differences regarding the recording condition (i.e., task versus resting state), gamma band frequency range, measurement techniques (i.e., power, coherence, synchronization, etc.) and the medication status of the AD patients. To the best of our knowledge, there is no study investigating gamma coherence in treated versus untreated AD patients using an event-related design. Moreover, previous studies included either only psychoactive drug naïve AD patients (Osipova et al., 2006, van Deursen et al., 2008, van Deursen et al., 2011, Koenig et al., 2005, Rossini et al., 2006) or mixed groups involving both treated and untreated AD patients (Stam et al., 2002, Stam et al., 2006).
Güntekin et al. (2008) reported a study on electrophysiological connectivity measurement, i.e., coherence, upon oddball paradigm in AD. A later comprehensive article by Başar et al. (2010) covered coherence measurements of low frequency ranges and the gamma window in the same group of participants. However, these reports did not thoroughly analyze the gamma coherences by separating the sub-gamma windows. Başar et al. (2015) showed that a more comprehensive gamma response analysis includes division of the gamma window into three sub-gamma bands (25–30, 30–35 and 40–48 Hz). A detailed analysis of gamma sub-bands in many time-windows indicated that the AD group showed a delayed gamma response, most likely due to a delay in reverberating memory circuits. The present study now introduces the analysis of connectivity by means of coherence measurement in the three sub-gamma groups.
In the literature, it is standard that analyses of gamma responses include a single frequency and time window. Our previous study in healthy control participants emphasized the importance of analyzing the gamma responses in multiple frequencies and time windows (Başar et al., 2015). Başar et al.'s (2015) study demonstrated that during a cognitive paradigm (e.g., oddball), at least 3–4 phase/time-locked gamma responses between 25 and 45 Hz occur in multiple time-windows (between 0 and 800 ms). In our recent study on AD, we investigated sensory and cognitive gamma responses in three frequency ranges (25–30, 30–35, 40–48 Hz) over four time windows (0–200, 200–400, 400–600, 600–800 ms) and found that AD patients show decreased early sensory gamma responses and delayed cognitive gamma responses compared to healthy controls (Başar et al., 2016a). Overall, the cognitive gamma responses were delayed about 100 ms in AD patients, and this delay was probably related to delays in propagation, reverberation of signals, or recurrent excitation.
The present study aims to investigate the sensory-evoked and event-related gamma coherences in both treated and untreated AD patients in comparison to healthy controls using visual sensory stimulation and a visual oddball paradigm. To the best of our knowledge, this is the first study that explores evoked and/or event-related gamma coherence in AD patients as well as drug effects. AD animal models suggest disrupted inhibitory interneuron activity causes gamma abnormalities (Verret et al., 2012, Palop and Mucke, 2016). We expect abnormalities in the gamma networks of AD patients. We hypothesized that the evoked and event-related gamma networks and the evoked/event-related gamma coherence would be abnormal in AD patients as AD patients had increased late gamma responses in our previous study (Başar et al., 2016a). These compensatory late gamma responses could also be represented in the long-distance gamma networks.
2. Materials and methods
2.1. Participants
A total of 39 patients with probable mild AD who were diagnosed according to DSM-IV and NINCDS-ADRDA criteria and 21 age-, gender-, and education-matched healthy elderly controls took part in the study. AD patients were divided into two groups—treated and untreated AD; the treated AD group consisted of 18 patients who were either taking cholinergic monotherapy (n = 12) or combined cholinergic treatment with memantine (n = 6), and the untreated AD group consisted of 21 patients. All AD patients were within the first year of their diagnosis.
The mean age was 69.00 years (SD 7.11) for the healthy controls, 75.09 years (SD 8.23) for the untreated AD patients, and 72.72 years (SD 6.38) for the treated AD patients. The mean number of educational years was 7.61 years (SD 4.30) for the healthy controls, 5.58 years (SD 2.64) for the untreated AD patients, and 9.11 years (SD 4.31) for the treated AD patients. There were 13 women in the control group, 11 women in the untreated AD group, and 7 women in the treated AD group. The mean of mini-mental state examination (MMSE) scores was 28.85 (SD 1.03) for the control group, 22.55 (SD 3.88) for the untreated AD patients, and 23.52 (SD 3.89) for the treated AD patients, out of a possible 30 points. The general demographic and clinical characteristics of the groups are shown in Table 1. The local ethical committee approved the study, and all participants and/or their relatives provided informed consent.
Table 1.
General demographic and clinical features of participants.
| Healthy controls (n = 21) | Untreated AD patients (n = 21) | Treated AD patients (n = 18) | p | |
|---|---|---|---|---|
| Age (SD) | 69.00 ± 7.11 | 76.19 ± 7.64 | 72.72 ± 6.39 | 0.007a |
| Education (SD) | 10.48 ± 4.95 | 7.76 ± 4.27 | 9.11 ± 4.43 | 0.165a |
| Gender (M/F) | 8/13 | 10/11 | 11/7 | 0.357b |
| MMSE (SD) | 28.81 ± 1.03 | 22.85 ± 3.83 | 23.53 ± 3.89 | 0.000a |
SD: standard deviation, M: male, F: female, AD: Alzheimer's disease, MMSE: Mini-Mental State Examination.
One-way ANOVA.
Chi-square.
2.2. Paradigms
2.2.1. Visual sensory stimulation
A visual sensory paradigm was applied to all participants for the acquisition of sensory-evoked coherences. A white screen with 40 cd/cm2 luminance was used for the stimulation, and the duration of stimuli was 1000 ms. Sixty stimulation signals were applied, and the inter-stimulus interval varied randomly between 3 and 7 s.
2.2.2. Visual event-related stimulation
Event-related coherences were elicited using a classical visual oddball paradigm. A total of 120 stimulation signals were used, and there were 40 target and 80 standard stimuli. A white screen with a 10 cd/cm2 luminance was used for the standard stimuli, and 40 cd/cm2 was used for the target stimuli. The duration of stimuli was 1000 ms. The target stimuli were embedded randomly within a series of standard stimuli, and the inter-stimulus interval varied randomly between 3 and 7 s. The participants were required to count the target stimuli in the oddball paradigm, and only the individuals who demonstrated sufficient accuracy in their mental count of target stimuli were included in the study. There was no significant difference between groups in terms of counting the target stimuli (p = 0.162).
2.3. Electrophysiological recording
EEGs were recorded from 30 Ag-AgCl electrodes mounted in an elastic cap (Easy-cap), according to the International 10–20 system. Two additional linked Ag-AgCl earlobe electrodes (A1 + A2) were used as references. The electrooculogram (EOG) was registered from both the medial upper and the lateral orbital rim of the right eye. All electrode impedances were less than 10 kΩ. The EEG was amplified with a BrainAmp 32-channel DC system with band limits of 0.01–250 Hz, and a sampling rate of 500 Hz was used.
Artifact-containing epochs were manually rejected prior to averaging the data (i.e., single sweep EOG recordings were visually studied, and trials with eye movement or blink artifacts were rejected). Sweep numbers were randomly equalized between the event-related target and simple visual stimulation conditions. A notch filter of 50 Hz was applied to the EEG data.
2.4. Data analysis
BrainVision Analyzer Software was used for signal analysis, evaluation of oscillatory dynamics, and sensory-evoked and event-related coherence analyses. First, the Fast Fourier Transform (FFT) of each epoch (0–800 ms duration) was performed, and then sensory-evoked and event-related gamma coherences were calculated for long-distance intrahemispheric pairs for three frequency ranges: 25–30 Hz, 30–35 Hz, 40–48 Hz. The long distance intrahemispheric pairs were F3–T7, F3–TP7, F3–P3, F3–O1, C3–O1 and P3-O1 on the left side and F4–T8, F4–TP8, F4–P4, F4–O2, C4–O2 and P4-O2 on the right side. The method used was cross-spectrum/autospectrum, and the mathematical relations are described in the following:
in conjunction with
Fisher's Z transformation was then used to normalize the distribution of average coherence values. The peak with the maximum coherence Z score within this frequency range for each person was included for the statistical analysis.
2.5. Statistical analysis
Statistical analyses were performed with Statistica Software. Repeated measures ANOVA was used for statistical analysis. Age was taken as a covariate as it was significantly different between groups (p = 0.007). Repeated measures ANOVAs were run separately for sensory-evoked and event-related coherences for three different gamma frequency ranges (25–30 Hz, 30–35 Hz, 40–48 Hz). The repeated measures ANOVA included Group (three levels: untreated AD patients, treated AD patients, and healthy controls) as the between-participant factor, and Region (six levels: fronto-parietal, fronto-temporal, fronto-temporoparietal, fronto-occipital, centro-occipital, parieto-occipital) and Hemisphere (two levels: left, right) as within-participant factors. Greenhouse–Geisser corrected p-values are reported. Post hoc analyses were performed using the Bonferroni test. Significance level was p < 0.05 for all comparisons.
3. Results
3.1. Visual sensory-evoked gamma coherences at three frequency ranges
Fig. 1 illustrates the grand averages of coherence analysis in the 25–48 Hz frequency band upon application of visual sensory stimulation over F3-P3 and F4-P4 electrode pairs. Green line represents the healthy controls, red line represents the untreated AD patients, and blue line represents the treated AD patients. As can be seen in the figure, average gamma coherence values upon presentation of visual sensory stimulation are higher in AD patients in comparison to healthy controls. Treated AD patients had the highest coherence value.
Fig. 1.
Grand averages of visual sensory-evoked gamma (25–48 Hz) coherences for a) F3-P3 and b) F4-P4 electrode pairs. Green line refers healthy controls, red line refers untreated AD patients, and blue line refers treated AD patients.
3.1.1. Visual sensory-evoked gamma coherences in the 25–30 Hz frequency range
There was no main GROUP effect on visual sensory-evoked gamma coherences in the 25–30 Hz frequency range. However, there was an interaction effect for REGION × GROUP [F10.280 = 5.284; p < 0.001], indicating the visual sensory-evoked coherence values in the 25–30 Hz frequency range were significantly different in the fronto-parietal (p < 0.001), fronto-temporoparietal (p = 0.002), and fronto-occipital (p = 0.002) regions between groups. Post hoc analysis revealed that both treated and untreated AD patients had significantly increased sensory-evoked coherences compared with healthy controls in the fronto-parietal, fronto-temporoparietal, and fronto-occipital regions (all, p < 0.03). Moreover, treated AD patients had significantly higher coherences than untreated AD patients in the fronto-parietal region (p = 0.021), (Fig. 2, Fig. 3).
Fig. 2.
Mean Z values with standard deviation of healthy controls, treated AD patients, and untreated AD patients for visual sensory-evoked coherences in the 25–30 Hz frequency range upon simple light stimulation (“*” represents p < 0.05).
Fig. 3.
Visual sensory-evoked coherences in the 25–30 Hz frequency range were significantly different between groups in the fronto-parietal, fronto-temporoparietal, and fronto-occipital regions. “*” indicates that untreated AD patients had significantly higher sensory-evoked coherences than healthy controls; “†” indicates that treated AD patients had significantly higher sensory-evoked coherences than healthy controls; “‡” indicates that treated AD patients had significantly higher sensory-evoked coherences than untreated AD patients; “§” indicates untreated AD patients had significantly higher sensory-evoked coherences than treated AD patients. Error bars indicate standard errors of the mean.
3.1.2. Visual sensory-evoked gamma coherences in the 30–35 Hz frequency range
Repeated measures ANOVA yielded a main GROUP effect on visual sensory-evoked gamma coherence values in the 30–35 Hz frequency range [F2.56 = 3.646; p = 0.032]. Post hoc comparisons showed that the treated AD patients had higher sensory-evoked coherences than healthy controls in the F3-P3 (p < 0.001), F4-P4 (p = 0.002), F3-O1 (p = 0.001), and F4-O2 (p = 0.021) electrode pairs; the untreated AD patients had higher sensory-evoked coherences than healthy controls in the F4-P4 (p = 0.049) electrode pair, and the treated AD patients had higher sensory-evoked coherences than the untreated AD patients in the F3-P3 (p = 0.021) electrode pair (Fig. 4).
Fig. 4.
Mean Z values with standard deviation of healthy controls, treated AD patients, and untreated AD patients for visual sensory-evoked coherences in the 30–35 Hz frequency range upon simple light stimulation (“*” represents p < 0.05).
There was also an interaction effect for REGION × GROUP [F10.280 = 5.260; p = 0.001], indicating the visual sensory-evoked coherence values in the 30–35 Hz frequency range were significantly different in the fronto-parietal (p < 0.001), fronto-temporoparietal (p = 0.008), fronto-occipital (p < 0.001), and centro-occipital (p = 0.045) regions between groups. Post hoc analysis revealed that both treated and untreated AD patients had significantly higher sensory-evoked coherences than healthy controls in the fronto-parietal, fronto-temporoparietal, and fronto-occipital regions (all, p < 0.03). Moreover, treated AD patients had significantly higher coherences than untreated AD patients in the fronto-parietal region (p = 0.021) (Fig. 5).
Fig. 5.
Visual sensory-evoked coherences in the 30–35 Hz frequency range were significantly different between groups in the fronto-parietal, fronto-temporoparietal, fronto-occipital, and centro-occipital regions. “*” indicates that untreated AD patients had significantly higher sensory-evoked coherences than healthy controls; “†” indicates that treated AD patients had significantly higher sensory-evoked coherences than healthy controls; “‡” indicates that treated AD patients had significantly higher sensory-evoked coherences than untreated AD patients; “§” indicates untreated AD patients had significantly higher sensory-evoked coherences than treated AD patients. Error bars indicate standard errors of the mean.
3.1.3. Visual sensory-evoked gamma coherences in the 40–48 Hz frequency range
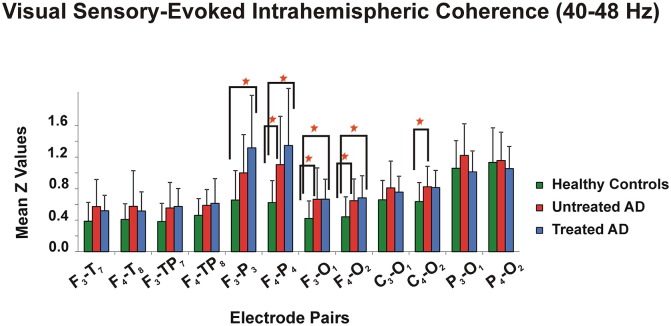
Repeated measures ANOVA showed a main GROUP effect on visual sensory-evoked gamma coherence values in the 40–48 Hz frequency range [F2.56 = 4.142; p = 0.021]. Post hoc comparisons revealed that the treated AD patients had higher sensory-evoked coherences than healthy controls in the F3-P3 (p = 0.001), F4-P4 (p = 0.001), F3-O1 (p = 0.045) and F4-O2 (p = 0.02) electrode pairs, and the untreated AD patients had higher coherences than healthy controls in the F4-P4 (p = 0.023), F3-O1 (p = 0.036), and C4-O2 (p = 0.046) electrode pairs (Fig. 6).
Fig. 6.
Mean Z values with standard deviation of healthy controls, treated AD patients, and untreated AD patients for visual sensory-evoked coherences in the 40–48 Hz frequency range upon simple light stimulation (“*” represents p < 0.05).
A significant interaction effect for REGION × GROUP [F10.280 = 5.488; p = 0.001] was also observed, indicating the visual sensory-evoked coherences in the 40–48 Hz frequency range were significantly different in the fronto-parietal (p < 0.001), fronto-temporal (p = 0.021), fronto-temporoparietal (p = 0.005), fronto-occipital (p < 0.001), and centro-occipital (p = 0.007) regions between groups. Post hoc analysis showed that both treated and untreated AD patients had significantly higher sensory-evoked coherences than healthy controls in the fronto-parietal, fronto-temporoparietal, and fronto-occipital regions (all, p < 0.03). Moreover, untreated AD patients had significantly higher coherences than healthy controls in the fronto-temporal and centro-occipital locations (all, p < 0.02) (Fig. 7).
Fig. 7.
Visual sensory-evoked coherences in the 40–48 Hz frequency range were significantly different between groups in the fronto-parietal, fronto-temporal, fronto-temporoparietal, fronto-occipital, and centro-occipital regions. “*” indicates that untreated AD patients had significantly higher sensory-evoked coherences than healthy controls; “†” indicates that treated AD patients had significantly higher sensory-evoked coherences than healthy controls; “‡” indicates that treated AD patients had significantly higher sensory-evoked coherences than untreated AD patients; “§” indicates untreated AD patients had significantly higher sensory-evoked coherences than treated AD patients. Error bars indicate standard errors of the mean.
3.2. Visual event-related gamma coherences in three frequency ranges
Fig. 8 illustrates the grand averages of coherence analysis in the 25–48 Hz frequency band upon application of target stimulation (oddball paradigm) over F3-P3 and F4-P4 electrode pairs. Green line represents the healthy controls, red line represents the untreated AD patients, and blue line represents the treated AD patients. As can be seen in the figure, average gamma coherence values upon presentation of target stimulation are higher in AD patients in comparison to healthy controls. Treated AD patients had the highest coherence values.
Fig. 8.
Grand averages of visual event-related gamma (25–48 Hz) coherences for a) F3-P3 and b) F4-P4 electrode pairs. Green line refers healthy controls, red line refers untreated AD patients, and blue line refers treated AD patients.
3.2.1. Visual event-related gamma coherences in the 25–30 Hz frequency range
Repeated measures ANOVA revealed a main GROUP effect on visual event-related gamma coherence values in the 25–30 Hz frequency range [F2.56 = 3.491; p = 0.037]. Post hoc comparisons showed that the treated AD patients had higher event-related coherences than healthy controls in the F3-P3 (p = 0.002), F4-P4 (p = 0.001), F3-TP7 (p = 0.035), F4-TP8 (p = 0.024) and F4-O2 (p = 0.012) electrode pairs; the untreated AD patients had higher coherences than healthy controls in the F4-P4 (p = 0.011), F4-TP8 (p = 0.017), and F4-O2 (p = 0.021) electrode pairs, and the treated AD patients had higher coherences than the untreated AD patients in the F3-P3 (p = 0.03) electrode pair (Fig. 9).
Fig. 9.
Mean Z values with standard deviation of healthy controls, treated AD patients, and untreated AD patients for visual event-related coherences in the 25–30 Hz frequency range upon application of target stimuli (“*” represents p < 0.05).
There was also an interaction effect for REGION × GROUP [F10.280 = 4.837; p = 0.001], indicating the visual event-related coherence values in the 25–30 Hz frequency range were significantly different in the fronto-parietal (p < 0.001), fronto-temporal (p = 0.04), fronto-temporoparietal (p < 0.001), fronto-occipital (p = 0.001), centro-occipital (p = 0.013), and parieto-occipital (p = 0.02) regions between groups. Post hoc comparisons indicated that both treated and untreated AD patients had significantly higher event-related coherences than healthy controls in the fronto-parietal, fronto-temporoparietal and fronto-occipital regions (all, p < 0.02). Moreover, untreated AD patients had higher coherences than healthy controls in the centro-occipital region (p = 0.015), and untreated AD patients showed significantly higher coherences than treated AD patients in the parieto-occipital region (p = 0.019) (Fig. 10).
Fig. 10.
Visual event-related coherences in the 25–30 Hz frequency range were significantly different between groups in the fronto-parietal, fronto-temporal, fronto-temporoparietal, fronto-occipital, centro-occipital, and parieto-occipital regions. “*” indicates that untreated AD patients had significantly higher event-related coherences than healthy controls; “†” indicates that treated AD patients had significantly higher event-related coherences than healthy controls; “‡” indicates that treated AD patients had significantly higher event-related coherences than untreated AD patients; “§” indicates untreated AD patients had significantly higher event-related coherences than treated AD patients. Error bars indicate standard errors of the mean.
3.2.2. Visual event-related gamma coherences in the 30–35 Hz frequency range
There was a main GROUP effect on visual event-related gamma coherence values in the 30–35 Hz frequency range [F2.56 = 4.419; p = 0.017]. Post hoc analysis revealed that the treated AD patients had higher event-related coherences than healthy controls in the F3-P3 (p < 0.001), F4-P4 (p < 0.001), F3-TP7 (p = 0.035), F4-TP8 (p = 0.031), F3-O1 (p = 0.004), and F4-O2 (p = 0.006) electrode pairs; the untreated AD patients had higher coherences than healthy controls in the F4-P4 (p = 0.004) and F4-O2 (p = 0.017) electrode pairs, and the treated AD patients had higher coherences than the untreated AD patients in the F3-P3 (p = 0.005) and F4-O2 (p = 0.023) electrode pairs (Fig. 11).
Fig. 11.
Mean Z values with standard deviation of healthy controls, treated AD patients, and untreated AD patients for visual event-related coherences in the 30–35 Hz frequency range upon application of target stimuli (“*” represents p < 0.05).
A significant interaction effect for REGION x GROUP [F10.280 = 7.593; p < 0.001] was also found, indicating the visual event-related coherence values in the 30–35 Hz frequency range were significantly different in the fronto-parietal (p < 0.001), fronto-temporoparietal (p = 0.001), fronto-occipital (p < 0.001), centro-occipital (p = 0.013), and parieto-occipital (p = 0.003) regions between groups. Post hoc analysis showed that both treated and untreated AD patients had significantly higher event-related coherences than healthy controls in the fronto-parietal, fronto-temporoparietal, and fronto-occipital regions (all, p < 0.02). Moreover, untreated AD patients had significantly higher coherences than healthy controls in the centro-occipital region (p = 0.015). Furthermore, treated AD patients demonstrated higher coherences in the fronto-parietal (p = 0.015) region and lower coherences in the parieto-occipital (p = 0.002) region than untreated AD patients (Fig. 12).
Fig. 12.
Visual event-related coherences in the 30–35 Hz frequency range were significantly different between groups in the fronto-parietal, fronto-temporoparietal, fronto-occipital, centro-occipital, and parieto-occipital regions. “*” indicates that untreated AD patients had significantly higher event-related coherences than healthy controls; “†” indicates that treated AD patients had significantly higher event-related coherences than healthy controls; “‡” indicates that treated AD patients had significantly higher event-related coherences than untreated AD patients; “§” indicates untreated AD patients had significantly higher event-related coherences than treated AD patients. Error bars indicate standard errors of the mean.
3.2.3. Visual event-related gamma coherences in the 40–48 Hz frequency range
There was a main GROUP effect on visual event-related gamma coherence values in the 40–48 Hz frequency range [F2.56 = 9.489; p < 0.001]. Post hoc analysis yielded that the treated AD patients had higher event-related coherences than healthy controls in the F3-P3 (p < 0.001), F4-P4 (p < 0.001), F3-T7 (p = 0.027), F3-TP7 (p = 0.013), F4-TP8 (p = 0.005), F3-O1 (p = 0.005), and F4-O2 (p < 0.001) electrode pairs; the untreated AD patients had higher coherences than healthy controls in the F4-P4 (p = 0.002), F4-T8 (p = 0.004), F4-TP8 (0.005), F3-O1 (p = 0.002), F4-O2 (p < 0.001), C3-O1 (p = 0.006), C4-O2 (p = 0.006), and P3-O1 (p = 0.043) electrode pairs, and the treated AD patients had higher coherences than the untreated AD patients in the F3-P3 (p = 0.01) electrode pair and lower coherences in the P3-O1 (p = 0.048) electrode pair (Fig. 13).
Fig. 13.
Mean Z values with standard deviation of healthy controls, treated AD patients, and untreated AD patients for visual event-related coherences in the 40–48 Hz frequency range upon application of target stimuli (“*” represents p < 0.05).
There was also an interaction effect for REGION x GROUP [F10.280 = 7.211; p < 0.001], indicating the visual event-related coherence values in the 40–48 Hz frequency range were significantly different in the fronto-parietal (p < 0.001), fronto-temporal (p = 0.002), fronto-temporoparietal (p < 0.001), fronto-occipital (p < 0.001), centro-occipital (p < 0.001), and parieto-occipital (p = 0.003) regions between groups. Post hoc comparisons showed that both treated and untreated AD patients had significantly higher coherences than healthy controls in the fronto-parietal, fronto-temporal, fronto-temporoparietal, fronto-occipital, and centro-occipital regions (all, p < 0.008). Moreover, untreated AD patients had higher coherences than healthy controls in the parieto-occipital region (p = 0.025). Furthermore, treated AD patients demonstrated higher coherences in the fronto-parietal (p = 0.016) region and lower coherences in the parieto-occipital (p = 0.005) region compared to untreated AD patients (Fig. 14).
Fig. 14.
Visual event-related coherences in the 40–48 Hz frequency range were significantly different between groups in the fronto-parietal, fronto-temporal, fronto-temporoparietal, fronto-occipital and centro-occipital regions. “*” indicates that untreated AD patients had significantly higher event-related coherences than healthy controls; “†” indicates that treated AD patients had significantly higher event-related coherences than healthy controls; “‡” indicates that treated AD patients had significantly higher event-related coherences than untreated AD patients; “§” indicates untreated AD patients had significantly higher event-related coherences than treated AD patients. Error bars indicate standard errors of the mean.
4. Discussion
4.1. Increase of gamma coherence: unusual or strong finding?
The results of the present study showed that AD patients had increased sensory-evoked and event-related gamma coherence values compared to healthy controls. Both treated and untreated AD patients had higher coherence values than healthy controls in all three gamma frequency sub-bands (25–30 Hz, 30–35 Hz, 40–48 Hz). In our previous study, we presented our preliminary findings on event-related gamma coherences in twelve untreated AD patients and twelve healthy controls (Başar et al., 2016b). An increase of gamma coherence values in three different frequency ranges was reported during a visual oddball paradigm. The present study, with the addition of a treated AD group of participants, further showed that the increase in gamma coherence values was not affected by drug therapy. Both treated and untreated AD patients had increased gamma coherence values compared to healthy controls. Furthermore, the present study demonstrated that this abnormal increase of gamma coherences in AD existed both during sensory visual stimulation as well as during target stimulation of the visual oddball paradigm. In the current study, the treated AD patients demonstrated significantly higher fronto-parietal gamma coherences during both sensory stimulation and oddball paradigm and lower occipito-parietal coherences compared to untreated AD patients in response to the target stimulation of the oddball paradigm.
Previous studies on gamma oscillations in AD presented mixed results. Some authors reported decreased gamma band synchronization in AD patients (Koenig et al., 2005, Stam et al., 2002), while others indicated increased gamma band power (van Deursen et al., 2008) and/or steady-state gamma responses in AD patients (Osipova et al., 2006, van Deursen et al., 2011). Likewise, gamma coherence was found to be similar to healthy controls (Stam et al., 2002), higher in AD patients in the occipito-parietal regions (Stam et al., 2006), and higher in MCI patients with faster conversion rates to AD in the fronto-parietal regions (Rossini et al., 2006). The finding of higher gamma coherence in the occipito-parietal regions was explained by impaired long distance cortico-cortical connections, which in turn may cause compensatory increases in local connections in AD (Stam et al., 2006, van Deursen et al., 2008). In a MEG study, Bajo et al. (2010) demonstrated that during a memory task MCI patients had higher posterior inter-hemispheric synchronization in the gamma band; on the other hand, healthy controls showed higher gamma synchronization than MCI patients between central-posterior and frontal-posterior channels. According to these results, the authors concluded that increased inter-hemispheric connectivity could be due to a compensatory mechanism for the lack of efficiency of the memory networks in MCI patients (Bajo et al., 2010). Moreover, compared to healthy controls MCI patients showed a hypersynchronization in the gamma band during an internal mental calculation task, which was related to a poorer cognitive performance (López et al., 2014).
In other neuropsychiatric conditions, our group has reported that bipolar disorder patients had decreased gamma coherence values both in manic (Özerdem et al., 2010) and euthymic stages (Özerdem et al., 2011). Özerdem et al. (2010) analyzed the event-related coherence of drug-free manic patients and healthy controls upon application of a visual oddball paradigm. Drug-free manic patients had lower gamma coherence values than healthy controls in the first recordings; after six weeks of valproate monotherapy there were no significant differences between groups. Moreover, Özerdem et al. (2011) showed that euthymic bipolar disorder patients had reduced event-related gamma coherence values compared to healthy controls both in target and non-target stimulation. However, there were no significant differences between patients and healthy controls during simple sensory visual stimulation. Özerdem et al., 2010, Özerdem et al., 2011 discussed that the reason for reduced gamma coherence in bipolar disorder patients could be related to application of cognitive stimulation but not sensory stimulation. The authors concluded that there was an inadequate connectivity in the brain of bipolar disorder patients during cognitive load but not during sensory stimulation. These findings were explained in conjunction with the results of cognitive dysfunction in bipolar disorder patients (Martínez-Arán et al., 2004).
4.2. Possible mechanisms for increased sensory-evoked and event-related gamma coherences in AD
There are many possible mechanisms related to the increase of sensory-evoked and event-related gamma oscillations and coherence in AD. These include, (1) the different dynamics of resting state EEG and evoked and/or event-related oscillations upon application of a sensory or cognitive task (i.e., oddball paradigm) (Başar, 1980, Başar et al., 2016b); (2) inhibitory interneuron impairment in AD; (3) disrupted Ca2 + signaling; (4) drug effects (i.e., cholinergic and glutamatergic modulation).
Previous studies using animal models reported abnormal gamma oscillations; the authors mostly demonstrated reduced gamma oscillations in AD models (Verret et al., 2012). However, these results are mostly performed under resting conditions; during exploratory behaviors, gamma oscillations were increased both in healthy mice and AD models (Verret et al., 2012). The studies of gamma oscillations in human participants have contradictory results. Some authors reported decreased gamma band synchronization in AD (Koenig et al., 2005, Stam et al., 2002, Herrmann and Demiralp, 2005), while others presented increased gamma activity (Osipova et al., 2006, Stam et al., 2006, van Deursen et al., 2008, van Deursen et al., 2011). In our previous study, we have found that healthy controls had higher gamma responses than AD subjects in early time windows during cognitive paradigms (Başar et al., 2016a). Furthermore, we have indicated that AD subjects had higher gamma responses in late time windows than healthy controls.
The controversies between animal studies and human studies could be due to several factors: (1) The animal studies were mostly conducted during resting conditions; the dynamics of spontaneous gamma activity could be different than the evoked/event-related gamma responses elicited during cognitive paradigms. The animal studies also showed that the resting animal and behaving animal have different gamma activity (Verret et al., 2012). (2) The analysis of local dynamics and long-range dynamics could be different. Analysis of evoked power with single electrodes could present different results than analysis of evoked coherence between different electrode pairs. (3) The network properties of animals could be different than humans. The AD models mostly performed with mice or rats, the human brain network properties could be more complicated than the network properties of mice or rats. (4) Although gamma oscillations were defined mostly between 25 and 100 Hz, there could be more than one gamma band. Most likely in the near future, researchers will define gamma oscillations with multiple sub-bands. A recent study by Başar et al. (2015) showed that during cognitive load, three different gamma bands were differentiated in four different time windows.
There is an important relationship between gamma oscillations and inhibitory GABAergic interneurons (Gray and McCormick, 1996). In earlier publications, the neurotransmitters that affect the gamma frequency band have been identified as GABA, GABA/glutamate and dopamine respectively (Whittington et al., 1995, Muthukumaraswamy et al., 2009, Gray and McCormick, 1996, Kömek et al., 2012). Combinations of many transmitters play a role in even the simplest cognitive responses. As reported earlier, the synchrony of gamma oscillations is essential for integration of distributed sets of neurons and cortico-cortical interaction (Rodriguez et al., 1999).
The abnormalities of gamma coherence in AD patients during sensory and cognitive stimulation could be due to interneuron impairment in AD patients. Inhibitory interneurons produce gamma activity; impairment in these inhibitory interneurons should cause a decrease of gamma activity (Verret et al., 2012, Palop and Mucke, 2016). Decreased GABAergic inhibition was demonstrated in a mice model of AD and was associated with hyperactivity of cortical neurons (Busche et al., 2008). Decreased activity of high-frequency oscillations in AD patients may be associated with this decreased inhibition (Nimmrich et al., 2015). However, these results mostly come from spontaneous EEG studies as reviewed by Herrmann and Demiralp (2005). More recent studies presented increased gamma responses in AD patients (Stam et al., 2006, Osipova et al., 2006, van Deursen et al., 2008, van Deursen et al., 2011, Başar et al., 2016a). In the present study, we report increased gamma coherence in AD after both sensory and cognitive stimulation. It is not possible elucidate the exact reason for the increase of evoked gamma coherence with the current studies in the literature. There is no previous research in AD models in which gamma responses were investigated with coherence analysis (the connectivity in multiple brain areas) during cognitive load. Further research is needed to understand how the gamma networks change during cognitive load in animal models. Application of similar methodologies to both animal models and AD patients could clarify the results on gamma activity, evoked gamma responses and evoked gamma coherence.
Moreover, gamma band activity was found to be related with Ca2 + channels (Luster et al., 2015); modulation of gamma oscillations in the pedunculopontine nucleus by neural calcium sensor protein was reported by D'onofrio et al. (2015). Berridge (2013) reported recently that there was an increase of Ca2 + signaling in AD. The author further stated that “Ca2 + has two diametrically opposed actions: it can both form and erase memories, and alternation in Ca2 + signaling will contribute to apoptosis resulting in the neurodegeneration”. In bipolar disorder, Ca2 + signaling is also increased, which causes membrane excitability rather than apoptosis and memory loss (Berridge, 2013). According to the present manuscript and according to the results of Özerdem et al., 2010, Özerdem et al., 2011, there were inverse results between bipolar disorder and AD. The complex signaling patterns of Ca2 + and the complex relationship between different neurotransmitters could result in this differentiation. Both bipolar disorder patients and AD patients had abnormalities in Ca2 + signaling; Ca2 + signaling was found to be related to gamma oscillations. The abnormal gamma coherence values in both diseases could be related to Ca2 + signaling abnormalities.
Cholinergic modulation causes augmentation of long-distance thalamo-cortical tracts, while diminishing short cortico-cortical connections in the neocortex (Hasselmo and Sarter, 2011). This in turn results in attentional increase in experimental animals.
For an investigation of drug effects, we compared treated and untreated AD patients in the present study. We observed increased evoked and event-related long distance gamma connectivity and decreased short distance event-related coherence in treated AD patients compared to untreated AD patients. These findings may share similar mechanisms with the observed increase in long-distance connectivity in addition to the decrease of short-distance connectivity. Moreover, GABAergic interneurons coexpress alpha7 nicotinic receptors (Voytenko et al., 2015). Therefore, cholinergic treatment can increase GABA activity, resulting in increased gamma responses.
Memantine, a drug used widely in AD, is a glutamatergic NMDA receptor antagonist that modulates calcium influx. It helps in restoration of the signal-to-noise ratio in excited neurons (Chen et al., 1992). As some of our treated AD patients had taken memantine in addition to cholinergic treatment, the effect of memantine on event-related gamma coherence increase should be investigated in future studies. However, the increase of event-related gamma coherence in AD cannot be solely explained by drug effects, as untreated AD patients also showed increased event-related gamma coherences in comparison to healthy controls.
4.3. Synthesis of all results on event-related α, β, γ, θ, δ oscillations
For the first time in the literature, the present manuscript demonstrated that AD patients had increased abnormal gamma coherences during both simple sensory and cognitive stimulation. Previously, our group and other groups in the literature reported event-related EEG biomarkers for AD in different frequency bands with different methodologies. A decrease of the delta response in AD patients in comparison to healthy controls during both visual and auditory cognitive paradigms was reported (Caravaglios et al., 2008, Yener et al., 2008, Yener et al., 2012). Decrease of delta responses upon application of cognitive paradigms was also found for patients with MCI (Yener et al., 2013, Kurt et al., 2014). Furthermore, the decrease of delta responses in frontal areas was correlated with the decrease of frontal volume in MCI patients (Yener et al., 2016). Frontal theta phase-locking was also impaired in AD patients. AD patients without any medication had decreased frontal theta phase-locking in comparison to healthy controls. On the other hand, cholinesterase inhibitors had positive effects on theta phase-locking; increased frontal theta phase-locking was reported for AD patients treated with cholinesterase inhibitors (Yener et al., 2007). Caravaglios et al. (2010) reported decreased poststimulus theta power in AD patients in comparison to healthy controls during auditory oddball paradigm. Increased gamma activity during steady-state stimulation was reported by Osipova et al. (2006) and by van Deursen et al., 2008, van Deursen et al., 2011. (Please see Table 2 for a short review of results; please see review article by Yener and Başar, 2013 for detailed information.)
Table 2.
Event-related oscillatory responses.
| ● | AD | References |
|---|---|---|
| Delta | ▼ Decreased in cognitive paradigms ▲ Increased in occipital areas during visual sensory stimulation |
Yener et al., 2008, Yener et al., 2009, Yener et al., 2012, Yener et al., 2013 Kurt et al. (2014) |
| Theta | ▼Decreased frontal phase-locking in untreated AD patients ▲Cholinesterase inhibitors had positive effects on frontal theta phase-locking |
Yener et al. (2007) Cummins et al. (2008) Caravaglios et al. (2010) |
| Alpha | ▼Decreased event-related desynchronization (ERD) in AD ▼Decreased alpha activity in AD |
Karrasch et al. (2006) Zervakis et al. (2011) |
| Beta | ▼ Decreased in cognitive paradigms |
Güntekin et al. (2013) Missonnier et al. (2007) |
| Gamma | ▼Decreased in the first 200 ms during cognitive load ▲Delay of gamma responses, increased in the late time windows during cognitive load ▲ Increased during auditory steady-state stimulation |
Başar et al. (2016a) Osipova et al. (2006) van Deursen et al., 2008, van Deursen et al., 2011 Haupt et al. (2008) |
Event-related coherence analysis in AD patients was performed by Başar et al. (2010) and by Güntekin et al. (2008). Decreases of delta, theta, and alpha coherence values were reported upon application of cognitive paradigms (Başar et al., 2010, Güntekin et al., 2008). There were no differences in the coherence values between healthy controls and AD patients upon application of simple visual paradigm (Başar et al., 2010). Başar et al. (2010) also performed event-related coherence analysis in the gamma frequency band and reported that there was an increase in gamma coherence values in AD patients in comparison to healthy controls but in very few electrode pairs. Başar et al. (2010) analyzed the event-related coherence values in the 28–48 Hz frequency range. Başar et al. (2015) showed that analyzing gamma frequency in different sub-bands was necessary and brought out important results. The present manuscript analyzed event-related gamma coherences in three different frequency sub-bands (25–30 Hz, 30–35 Hz, 40–48 Hz). Analysis of event-related gamma coherences in three sub-bands indicated more significant results in comparison to analysis of gamma coherence in one broad range (28–48 Hz, as performed in Başar et al., 2010). Gamma coherence values were overall higher in AD patients in comparison to healthy controls, but most significantly for the 40–48 Hz frequency band (Fig. 14). (Please see Table 3 for a short review of results; please see review article by Yener and Başar, 2013 for detailed information).
Table 3.
Event-related coherence analysis.
| ●▬● | AD | References |
|---|---|---|
| Delta | ▼ Decreased in cognitive paradigms ►Does not change in sensory paradigms |
Başar et al. (2010) Güntekin et al. (2008) |
| Theta | ▼ Decreased in cognitive paradigms ► Does not change in sensory paradigms |
Başar et al. (2010) Güntekin et al. (2008) |
| Alpha | ▼ Decreased in cognitive paradigms ► Does not change in sensory paradigms ▼ Decreased in visual photic stimulation |
Başar et al. (2010) Hogan et al. (2003), Güntekin et al. (2008) Kikuchi et al. (2002) Jiang (2005) |
| Beta | ▲ Trend towards increase for both sensory and cognitive paradigms | Başar et al. (2010) |
| Gamma | ▲ Increased in both sensory and cognitive paradigms | Present manuscript |
5. Conclusions
The present study showed that the increase of gamma coherence values was not due to AD treatment to any great extent, as both treated and untreated AD patients had increased gamma coherence values compared to healthy controls. Furthermore, the present study showed that this abnormal increase of gamma coherences was present in response to both visual sensory and cognitive stimulation. The treated AD patients had significantly higher fronto-parietal gamma coherences during both sensory stimulation and oddball paradigm; and lower occipito-parietal coherences during the oddball paradigm compared to untreated AD patients. Whether this is a general result of the impairment of the inhibitory interneurons in AD or a compensatory effect related to the cholinergic modulation is unclear. Future studies on animal models and human participants are needed to clarify this issue.
There are several limitations of the present study. We did not compare the prestimulus and/or spontaneous EEG coherence with evoked and/or event-related coherence. To the best of our knowledge, there are only two studies in the literature investigating spontaneous EEG gamma coherence in MCI or AD. Rossini et al. (2006) reported that increased gamma coherence values were associated with higher rates of conversion from MCI to AD. Stam et al. (2006) found that AD patients had increased functional connectivity in the gamma band in the occipito-parietal regions as compared with healthy controls. Despite this, we interpreted the increased gamma coherences in AD as related to dysfunctional sensory and/or cognitive processing; it could be argued that the observed effect might be related to more basic neuro-compensation processes also present during resting or non-sensory or cognitive processing. Further studies should compare the spontaneous, evoked and event-related gamma coherences.
Other well-studied biomarkers of AD or other patient groups were not included in the present study. Previous studies demonstrated correlations of EEG findings with well-defined AD biomarkers such as CSF (Papaliagkas et al., 2010), PET (Dierks et al., 2000) and MRI (Babiloni et al., 2015, Moretti, 2015, Vecchio et al., 2016, Yener et al., 2016) in MCI/AD. Future studies using multimodal biomarkers including MRI, PET and CSF and comparing AD patients with different patient groups would potentiate the use of electrophysiological techniques as biomarkers and strengthen differential diagnosis. The relationship of well-defined AD biomarkers to oscillatory responses in various frequency bands awaits exploration in future studies. Using some of the most discriminating frequency bands over certain brain regions may give rise to a model of oscillatory responses (Başar et al., 2015) in diagnostics of neuropsychiatric conditions in future studies.
Acknowledgments
This work (grant number 112S459) was supported by the Turkish National Science and Research Council (TUBITAK). The funding agency had no involvement in the conduct of the research or preparation of the article.
References
- Adrian E.D. Olfactory reactions in the brain of the hedgehog. J. Physiol. 1942;31:459–473. doi: 10.1113/jphysiol.1942.sp003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C., Del Percio C., Boccardi M., Lizio R., Lopez S., Carducci F., Marzano N., Soricelli A., Ferri R., Triggiani A.I., Prestia A., Salinari S., Rasser P.E., Basar E., Famà F., Nobili F., Yener G., Emek-Savaş D.D., Gesualdo L., Mundi C., Thompson P.M., Rossini P.M., Frisoni G.B. Occipital sources of resting-state alpha rhythms are related to local gray matter density in subjects with amnesic mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2015;36(2):556–570. doi: 10.1016/j.neurobiolaging.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C., Triggiani A.I., Lizio R., Cordone S., Tattoli G., Bevilacqua V., Soricelli A., Ferri R., Nobili F., Gesualdo L., Millán-Calenti J.C., Buján A., Tortelli R., Cardinali V., Barulli M.R., Giannini A., Spagnolo P., Armenise S., Buenza G., Scianatico G., Logroscino G., Frisoni G.B., Del Percio C. Classification of single normal and Alzheimer's disease individuals from cortical sources of resting state EEG rhythms. Front. Neurosci. 2016;10:47. doi: 10.3389/fnins.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo R., Maestú F., Nevado A., Sancho M., Gutiérrez R., Campo P., Castellanos N.P., Gil P., Moratti S., Pereda E., Del-Pozo F. Functional connectivity in mild cognitive impairment during a memory task: implications for the disconnection hypothesis. J. Alzheimers Dis. 2010;22(1):183–193. doi: 10.3233/JAD-2010-100177. [DOI] [PubMed] [Google Scholar]
- Başar E. Elsevier; Amsterdam: 1980. EEG–Brain Dynamics. Relation Between EEG and Brain Evoked Potentials; pp. 1–411. [Google Scholar]
- Başar E. CRC Press; Florida: 2004. Memory and Brain Dynamics. Oscillations Integrating Attention, Perception, Learning, and Memory; pp. 1–261. [Google Scholar]
- Başar E. A review of gamma oscillations in healthy subjects and in cognitive impairment. Int. J. Psychophysiol. 2013;90:99–117. doi: 10.1016/j.ijpsycho.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Başar E., Ungan P. A component analysis and principles derived for the understanding of evoked potentials of the brain: studies in the hippocampus. Kybernetik. 1973;12:133–140. doi: 10.1007/BF00289165. [DOI] [PubMed] [Google Scholar]
- Başar E., Gönder A., Ozesmi C., Ungan P. Dynamics of brain rhythmic and evoked potentials. III. Studies in the auditory pathway, reticular formation, and hippocampus during sleep. Biol. Cybern. 1975;20:161–169. doi: 10.1007/BF00342636. [DOI] [PubMed] [Google Scholar]
- Başar E., Gönder A., Ozesmi C., Ungan P. Dynamics of brain rhythmic and evoked potentials. II. Studies in the auditory pathway, reticular formation, and hippocampus during the waking stage. Biol. Cybern. 1975;20:145–160. doi: 10.1007/BF00342635. [DOI] [PubMed] [Google Scholar]
- Başar E., Gönder A., Ozesmi C., Ungan P. Dynamics of brain rhythmic and evoked potentials. I. Some computational methods for the analysis of electrical signals from the brain. Biol. Cybern. 1975;20:137–143. doi: 10.1007/BF00342634. [DOI] [PubMed] [Google Scholar]
- Başar E., Schürmann M., Başar-Eroglu C., Karakas S. Alpha oscillations in brain functioning: an integrative theory. Int. J. Psychophysiol. 1997;26:5–29. doi: 10.1016/s0167-8760(97)00753-8. [DOI] [PubMed] [Google Scholar]
- Başar E., Demiralp T., Schürmann M., Başar-Eroğlu C., Ademoğlu A. Oscillatory brain dynamics, wavelet analysis and cognition. Brain Lang. 1999;66:146–183. doi: 10.1006/brln.1998.2029. [DOI] [PubMed] [Google Scholar]
- Başar E., Güntekin B., Tülay E., Yener G.G. Evoked and event related coherence of Alzheimer patients manifest differentiation of sensory–cognitive networks. Brain Res. 2010;1357:79–90. doi: 10.1016/j.brainres.2010.08.054. [DOI] [PubMed] [Google Scholar]
- Başar E., Başar-Eroğlu C., Güntekin B., Yener G.G. Brain's alpha, beta, gamma, delta, and theta oscillations in neuropsychiatric diseases: proposal for biomarker strategies. Suppl. Clin. Neurophysiol. 2013;62:19–54. doi: 10.1016/b978-0-7020-5307-8.00002-8. [DOI] [PubMed] [Google Scholar]
- Başar E., Tülay E., Güntekin B. Multiple gamma oscillations in the brain: a new strategy to differentiate functional correlates and P300 dynamics. Int. J. Psychophysiol. 2015;95(3):406–420. doi: 10.1016/j.ijpsycho.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Başar E., Emek-Savaş D.D., Güntekin B., Yener G.G. Delay of cognitive gamma responses in Alzheimer's disease. Neuroimage Clin. 2016;11:106–115. doi: 10.1016/j.nicl.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başar E., Schmiedt-Fehr C., Mathes B., Femir B., Emek-Savaş D.D., Tülay E., Tan D., Düzgün A., Güntekin B., Özerdem A., Yener G., Başar-Eroğlu C. What does the broken brain say to the neuroscientist? Oscillations and connectivity in schizophrenia, Alzheimer's disease, and bipolar disorder. Int. J. Psychophysiol. 2016;103:135–148. doi: 10.1016/j.ijpsycho.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Başar-Eroglu C., Strüber D., Schürmann M., Stadler M., Başar E. Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. Int. J. Psychophysiol. 1996;24:101–112. doi: 10.1016/s0167-8760(96)00051-7. [DOI] [PubMed] [Google Scholar]
- Başar-Eroglu C., Strüber D., Kruse P., Başar E., Stadler M. Frontal gamma band enhancement during multistable visual perception. Int. J. Psychol. Physiol. 1996;24:113–125. doi: 10.1016/s0167-8760(96)00055-4. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Calcium regulation of neural rhythms, memory and Alzheimer's disease. J. Physiol. 2013;592(2):281–293. doi: 10.1113/jphysiol.2013.257527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch N.A., Debener S., Kranczioch C., Engel A.K., Herrmann C.S. Size matters: effects of stimulus size, duration and eccentricity on the visual gamma-band response. Clin. Neurophysiol. 2004;115:1810–1820. doi: 10.1016/j.clinph.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Busch N.A., Herrmann C.S., Müller M.M., Lenz D., Gruber T. A cross-laboratory study of event-related gamma activity in a standard object recognition paradigm. NeuroImage. 2006;33:1169–1177. doi: 10.1016/j.neuroimage.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Busche M.A., Eichhoff G., Adelsberger H., Abramowski D., Wiederhold K.H., Haass C., Staufenbiel M., Konnerth A., Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321(5896):1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Caravaglios G., Costanzo E., Palermo F., Muscoso E.G. Decreased amplitude of auditory event-related delta responses in Alzheimer's disease. Int. J. Psychophysiol. 2008;70:23–32. doi: 10.1016/j.ijpsycho.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Caravaglios G., Castro G., Costanzo E., Maria di G., Mancuso D., Muscoso E.G. Theta power responses in mild Alzheimer's disease during an auditory oddball paradigm: lack of theta enhancement during stimulus processing. J. Neural Transm. 2010;117(10):1195–1208. doi: 10.1007/s00702-010-0488-2. [DOI] [PubMed] [Google Scholar]
- Chen H.S., Pellegrini J.W., Aggarwal S.K., Lei S.Z., Warach S., Jensen F.E., Liptopn S.A. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J. Neurosci. 1992;12(11):4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins T.D., Broughton M., Finnigan S. Theta oscillations are affected by amnestic mild cognitive impairment and cognitive load. Int. J. Psychophysiol. 2008;70:75–81. doi: 10.1016/j.ijpsycho.2008.06.002. [DOI] [PubMed] [Google Scholar]
- van Deursen J.A., Vuurman E.F., Verhey F.R., van Kranen-Mastenbroek V.H., Riedel W.J. Increased EEG gamma band activity in Alzheimer's disease and mild cognitive impairment. J. Neural Transm. 2008;115(9):1301–1311. doi: 10.1007/s00702-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen J.A., Vuurman E.F., van Kranen-Mastenbroek V.H., Verhey F.R., Riedel W.J. 40-Hz steady state response in Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging. 2011;32:24–30. doi: 10.1016/j.neurobiolaging.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Dierks T., Jelic V., Pascual-Marqui R.D., Wahlund L., Julin P., Linden D.E., Maurer K., Winblad B., Nordberg A. Spatial pattern of cerebral glucose metabolism (PET) correlates with localization of intracerebral EEG-generators in Alzheimer's disease. Clin. Neurophysiol. 2000;111(10):1817–1824. doi: 10.1016/s1388-2457(00)00427-2. [DOI] [PubMed] [Google Scholar]
- D'Onofrio S., Kezunovic N., Hyde J.R., Luster B., Messias E., Urbano F.J. Modulation of gamma oscillations in the pedunculopontine nucleus by neuronal calcium sensor protein-1: relevance to schizophrenia and bipolar disorder. J. Neurophysiol. 2015;113:709–719. doi: 10.1152/jn.00828.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman W.J. Academic Press; New York: 1975. Mass Action in the Nervous System. [Google Scholar]
- Frisoni G.B., Fox N.C., Jack C.R., Jr., Scheltens P., Thompson P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2011;6(2):67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos L. A prospectus of knowledge. Science. 1981;212:775–776. doi: 10.1126/science.212.4496.775. [DOI] [PubMed] [Google Scholar]
- Gray C.M., McCormick D.A. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274:109–113. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- Gruber T., Tsivilis D., Montaldi D., Müller M.M. Induced gamma band responses: an early marker of memory encoding and retrieval. Neuroreport. 2004;15:1837–1841. doi: 10.1097/01.wnr.0000137077.26010.12. [DOI] [PubMed] [Google Scholar]
- Güntekin B., Başar E. A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia. 2014;58:33–51. doi: 10.1016/j.neuropsychologia.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Güntekin B., Saatçi E., Yener G. Decrease of evoked delta, theta and alpha coherences in Alzheimer patients during a visual oddball paradigm. Brain Res. 2008;1235:109–116. doi: 10.1016/j.brainres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Güntekin B., Emek-Savaş D.D., Kurt P., Görsev G.G., Başar E. Beta oscillatory responses in healthy subjects and subjects with mild cognitive impairment. Neuroimage Clin. 2013;3:39–46. doi: 10.1016/j.nicl.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo M.E., Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36(1):52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt M., González-Hernández J.A., Scherbaum W.A. Regions with different evoked frequency band responses during early-stage visual processing distinguish mild Alzheimer dementia from mild cognitive impairment and normal aging. Neurosci. Lett. 2008;442(3):273–278. doi: 10.1016/j.neulet.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Herrmann C.S., Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin. Neurophysiol. 2005;116(12):2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Herrmann C.S., Lenz D., Junge S., Busch N.A., Maess B. Memory-matches evoke human gamma-responses. BMC Neurosci. 2004;5(13) doi: 10.1186/1471-2202-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann C.S., Munk M.H., Engel A.K. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn. Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hogan M.J., Swanwick G.R.J., Kaiser J., Rowman M., Lawlor B. Memory-related EEG power and coherence reduction in mild Alzheimer's disease. Int. J. Psychophysiol. 2003;49(2):147–163. doi: 10.1016/s0167-8760(03)00118-1. [DOI] [PubMed] [Google Scholar]
- Jack C.R., Jr., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W., Petersen R.C., Trojanowski J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z.Y. Abnormal cortical functional connections in Alzheimer's disease: analysis of inter- and intra-hemispheric EEG coherence. J. Zhejiang Univ. (Sci.) 2005;6B(4):259–264. doi: 10.1631/jzus.2005.B0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrasch M., Laine M., Rinne J.O., Rapinoja P., Sinerva E., Krause C.M. Brain oscillatory responses to an auditory–verbal working memory task in mild cognitive impairment and Alzheimer's disease. Int. J. Psychophysiol. 2006;59(2):168–178. doi: 10.1016/j.ijpsycho.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Keil A., Müller M.M., William J.R., Gruber T., Elbert T. Human gamma band activity and perception of a gestalt. J. Neurosci. 1999;19:7152–7161. doi: 10.1523/JNEUROSCI.19-16-07152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M., Wada Y., Takeda T., Oe H., Hashimoto T., Koshino Y. EEG harmonic responses to photic stimulation in normal aging and Alzheimer's disease: differences in interhemispheric coherence. Clin. Neurophysiol. 2002;113(7):1045–1051. doi: 10.1016/s1388-2457(02)00129-3. [DOI] [PubMed] [Google Scholar]
- Koenig T., Prichep L., Dierks T., Hubl D., Wahlund L.O., John E.R., Jelic V. Decreased EEG synchronization in Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging. 2005;26(2):165–171. doi: 10.1016/j.neurobiolaging.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kömek K., Ermentrout G.B., Walker C.P., Cho R.Y. Dopamine and gamma band synchrony in schizophrenia–insights from computational and empirical studies. Eur. J. Neurosci. 2012;36:2146–2155. doi: 10.1111/j.1460-9568.2012.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt P., Emek-Savaş D.D., Batum K., Turp B., Güntekin B., Karşıdağ S., Yener G.G. Patients with mild cognitive impairment display reduced auditory event-related delta oscillatory responses. Behav. Neurol. 2014;2014:268967. doi: 10.1155/2014/268967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López M.E., Garcés P., Cuesta P., Castellanos N.P., Aurtenetxe S., Bajo R., Marcos A., Montenegro M., Yubero R., del Pozo F., Sancho M., Maestú F. Synchronization during an internally directed cognitive state in healthy aging and mild cognitive impairment: a MEG study. Age (Dordr.) 2014;36(3):9643. doi: 10.1007/s11357-014-9643-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster B., D'Onofrio S., Urbano F., Garcia-Rill E. High-threshold Ca2 + channels behind gamma band activity in the pedunculopontine nucleus (PPN) Physiol. Rep. 2015;3:1–11. doi: 10.14814/phy2.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Arán A., Vieta E., Colom F., Torrent C., Sánchez-Moreno J., Reinares M. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6(3):224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- Missonnier P., Deiber M.P., Gold G., Herrmann F.R., Millet P., Michon A., Fazio-Costa L., Ibanez V., Giannakopoulos P. Working memory load-related electroencephalographic parameters can differentiate progressive from stable mild cognitive impairment. Neuroscience. 2007;150:346–356. doi: 10.1016/j.neuroscience.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Moretti D.V. Conversion of mild cognitive impairment patients in Alzheimer's disease: prognostic value of Alpha3/Alpha2 electroencephalographic rhythms power ratio. Alzheimers Res. Ther. 2015;7:80. doi: 10.1186/s13195-015-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M.M., Keil A. Neuronal synchronization and selective color processing in the human brain. J. Cogn. Neurosci. 2004;16:503–522. doi: 10.1162/089892904322926827. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Edden R.A., Jones D.K., Swettenham J.B., Singh K.D. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. U. S. A. 2009;106(20):8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmrich V., Draguhn A., Axmacher N. Neuronal network oscillations in neurodegenerative diseases. Neruomol. Med. 2015;17(3):270–284. doi: 10.1007/s12017-015-8355-9. [DOI] [PubMed] [Google Scholar]
- Osipova D., Pekkonen E., Ahveninen J. Enhanced magnetic auditory steady-state response in early Alzheimer's disease. Clin. Neurophysiol. 2006;117:1990–1995. doi: 10.1016/j.clinph.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Özerdem A., Güntekin B., Saatçi E., Tunca Z., Başar E. Disturbance in long distance gamma coherence in bipolar disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34(6):861–865. doi: 10.1016/j.pnpbp.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Özerdem A., Güntekin B., Atagün I., Turp B., Başar E. Reduced long distance gamma (28–48 Hz) coherence in euthymic patients with bipolar disorder. J. Affect. Disord. 2011;132(3):325–332. doi: 10.1016/j.jad.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Palop J.J., Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016;17(12):777–792. doi: 10.1038/nrn.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaliagkas V.T., Anogianakis G., Tsolaki M.N., Koliakos G., Kimiskidis V.K. Combination of P300 and CSF β-amyloid(1-42) assays may provide a potential tool in the early diagnosis of Alzheimer's disease. Curr. Alzheimer Res. 2010;7(4):295–299. doi: 10.2174/156720510791162421. [DOI] [PubMed] [Google Scholar]
- Ribary U., Ioannides A.A., Singh K.D., Hasson R., Bolton J.P., Lado F., Mogilner A., Llinás R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc. Natl. Acad. Sci. U. S. A. 1991;88(24):11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E., George N., Lachaux J.P., Martinerie J., Renault B., Varela F.J. Perception's shadow: long-distance synchronization of human brain activity. Nature. 1999;397(6718):430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Rossini P.M., Del Percio C., Pasqualetti P., Cassetta E., Binetti G., Dal Forno G., Ferreri F., Frisoni G., Chiovenda P., Miniussi C., Parisi L., Tombini M., Vecchio F., Babiloni C. Conversion from mild cognitive impairment to Alzheimer's disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience. 2006;143:793–803. doi: 10.1016/j.neuroscience.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Rossini P.M., Rossi S., Babiloni C., Polich J. Clinical neurophysiology of aging brain, from normal aging to neurodegeneration. Prog. Neurobiol. 2007;83:375–400. doi: 10.1016/j.pneurobio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Senkowski D., Herrmann C.S. Effects of task difficulty on evoked gamma activity and ERPs in a visual discrimination task. Clin. Neurophysiol. 2002;113:1742–1753. doi: 10.1016/s1388-2457(02)00266-3. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Stam C.J., van Cappellen van Walsum A.M., Pijnenburg Y.A., Berendse H.W., de Munck J.C., Scheltens P., van Dijk B.W. Generalized synchronization of MEG recordings in Alzheimer's Disease: evidence for involvement of the gamma band. J. Clin. Neurophysiol. 2002;19(6):562–574. doi: 10.1097/00004691-200212000-00010. [DOI] [PubMed] [Google Scholar]
- Stam C.J., Jones B.F., Manshanden I., van Cappellen van Walsum A.M., Montez T., Verbunt J.P., de Munck J.C., van Dijk B.W., Berendse H.W., Scheltens P. Magnetoencephalographic evaluation of resting-state functional connectivity in Alzheimer's disease. NeuroImage. 2006;32(3):1335–1344. doi: 10.1016/j.neuroimage.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C., Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C., Bertrand O., Peronnet F., Pernier J. Induced gamma band activity during the delay of a visual short-term memory task in humans. J. Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio F., Miraglia F., Piludu F., Granata G., Romanello R., Caulo M., Onofrj V., Bramanti P., Colosimo C., Rossini P.M. “Small World” architecture in brain connectivity and hippocampal volume in Alzheimer's disease: a study via graph theory from EEG data. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9528-3. 10.1007/s11682-016-9528-3 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Verret L., Mann E.O., Hang G.B., Barth A.M., Cobos I., Ho K., Devidze N., Masliah E., Kreitzer A.C., Mody I., Mucke L., Palop J.J. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149(3):708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytenko L.P., Lushnikova I.V., Sayotchenko A.V., Isaeva E.V., Skok E.V., Lykhmus O.Y., Patseva M.A., Skibo G.G. Hippocampal GABAergic interneurons coexpressing alpha7-nicotinic receptors and connexin-36 are able to improve neuronal viability under oxygen-glucose deprivation. Brain Res. 2015;1616:134–145. doi: 10.1016/j.brainres.2015.04.061. [DOI] [PubMed] [Google Scholar]
- Whittington M.A., Traub R.D., Jefferys J.G. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373(6515):612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Yener G.G., Başar E. Sensory evoked and event related oscillations in Alzheimer's disease: a short review. Cogn. Neurodyn. 2010;4:263–274. doi: 10.1007/s11571-010-9138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yener G.G., Başar E. Biomarkers in Alzheimer's disease with a special emphasis on event-related oscillatory responses. Suppl Clin Neurophysiol. 2013;62:237–273. doi: 10.1016/b978-0-7020-5307-8.00020-x. [DOI] [PubMed] [Google Scholar]
- Yener G., Guntekin B., Öniz A., Başar E. Increased frontal phase-locking of event related theta oscillations in Alzheimer patients treated with cholinesterase inhibitors. Int. J. Psychophysiol. 2007;64(1):46–52. doi: 10.1016/j.ijpsycho.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Yener G., Guntekin B., Basar E. Event-related delta oscillatory responses of Alzheimer patients. Eur. J. Neurol. 2008;15:540–547. doi: 10.1111/j.1468-1331.2008.02100.x. [DOI] [PubMed] [Google Scholar]
- Yener G.G., Güntekin B., Tülay E., Başar E. A comparative analysis of sensory visual evoked oscillations with visual cognitive event related oscillations in Alzheimer's disease. Neurosci. Lett. 2009;462:193–197. doi: 10.1016/j.neulet.2009.07.036. [DOI] [PubMed] [Google Scholar]
- Yener G.G., Güntekin B., Örken D.N., Tülay E., Forta H., Başar E. Auditory delta event-related oscillatory responses are decreased in Alzheimer's disease. Behav. Neurol. 2012;25:3–11. doi: 10.3233/BEN-2012-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yener G.G., Kurt P., Emek-Savaş D.D., Güntekin B., Başar E. Reduced visual event-related delta oscillatory responses in amnestic mild cognitive impairment. J. Alzheimers Dis. 2013;37(4):759–767. doi: 10.3233/JAD-130569. [DOI] [PubMed] [Google Scholar]
- Yener G.G., Emek-Savaş D.D., Lizio R., Çavuşoğlu B., Carducci P., Ada E., Güntekin B., Babiloni C., Başar E. Frontal delta event-related oscillations relate to frontal volume in mild cognitive impairment and healthy controls. Int. J. Psychophysiol. 2016;103:110–117. doi: 10.1016/j.ijpsycho.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Zervakis M., Michalopoulos K., Iordanidou V., Sakkalis V. Intertrial coherence and causal interaction among independent EEG components. J. Neurosci. Methods. 2011;197(2):302–314. doi: 10.1016/j.jneumeth.2011.02.001. [DOI] [PubMed] [Google Scholar]