Abstract
The kidney’s maintenance of the metabolic component of acid-base homeostasis is critical for normal health. The study by Esche and colleagues in this issue of Kidney International shows that normal children with higher levels of renal net acid excretion and of dietary acid loads have stimulation of glucocorticoid hormone metabolism. Thus, normal variations in dietary acid intake and renal net acid excretion have important biological correlates.
Keywords: acid-base, ammonia, glucocorticoid hormones, net acid excretion
Maintenance of acid-base homeostasis is critical for normal health. At its simplest level, acid-base homeostasis involves CO2 elimination by the lungs in combination with net acid excretion (NAE) by the kidneys. The kidney’s response to acid and alkali loads involves interactions with other organs, most prominently skeletal muscle, and with the endocrine system. The adrenal gland is a critical endocrine organ in this response, and glucocorticoid hormones are a necessary component of the adrenal response.1,2 However, both the deficiency of and excess amounts of glucocorticoid hormones are associated with development of a number of pathophysiologic processes. The manuscript by Esche et al.3 (2016) in the current issue of Kidney International is an important contribution to our understanding of this complex process.
Acid-base homeostasis is essential for normal health. Chronic acid-base disturbances lead to skeletal bone defects, growth defects in children, recurrent nephrolithiasis, insulin resistance, thyroid hormone resistance, and, in individuals with chronic kidney disease, accelerated progression of their chronic kidney disease. Kidneys facilitate prevention of these conditions through their contribution to acid-base homeostasis through the processes of filtered bicarbonate reabsorption and new bicarbonate generation, also known as net NAE. The major component of new bicarbonate generation is renal ammonia generation and excretion. Kidneys generate ammonia from amino acid, predominantly glutamine, and metabolism, and the ammonia generated then undergoes regulated renal epithelial cell transport.
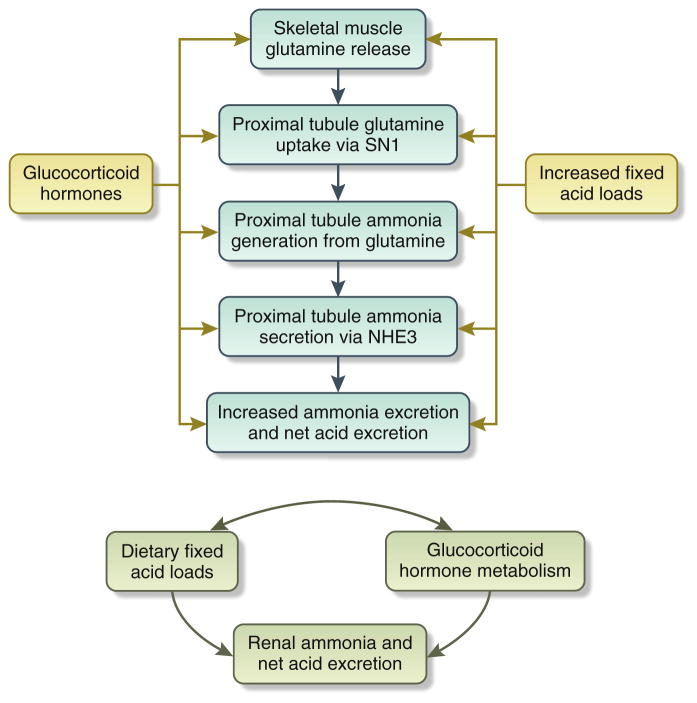
Glucocorticoid hormones have a critical role in the renal maintenance of acid-base homeostasis (Figure 1).1,2 During metabolic acidosis, glucocorticoid hormones are critical for induction of skeletal muscle catabolism and release of glutamine, which is then used by the kidneys for ammonia generation. In the kidneys, glucocorticoids are critical for the renal cortical expression of SN1, the primary renal transporter activated to increase proximal tubule cell glutamine uptake, the primary substrate for renal ammonia generation. Additional studies have shown that glucocorticoids are critical for the increase in proximal tubule ammonia generation and proximal tubule ammonia secretion via NHE3. The role of glucocorticoids in other aspects of renal ammonia metabolism, that is, loop of Henle ammonia reabsorption, interstitial sulfa-tide ammonia binding, or in collection of duct ammonia secretion, has not been studied carefully.
Figure 1. Interrelationship of dietary acid loads, renal net acid secretion, and glucocorticoid hormone metabolism.
The top panel shows that both glucocorticoid hormones and increased dietary acid load stimulate multiple components of renal ammonia metabolism, leading to increased net acid excretion. The bottom panel shows the complex interrelationship between dietary acid load, net acid excretion, and glucocorticoid hormone metabolism.
However, increased glucocorticoid hormone metabolism in response to metabolic acidosis, although necessary for the renal adaptive response of increased NAE, also has the potential of contributing to adverse effects. Glucocorticoid hormones are catabolic, contribute to skeletal muscle breakdown, impair glucose tolerance, alter appetite, and can lead to impaired bone characteristics.
The study by Esche et al. in this volume of Kidney International is an important addition to our understanding of the interaction of glucocorticoid hormones, diet, and renal acid excretion.3 This is a carefully performed study that examined normal community-living children and sought to correlate differences in dietary acid loads and renal NAE with glucocorticoid hormone metabolism. It used children 6 to 10 years of age enrolled in the prospective, observational Dortmund Nutritional and Anthropometric Longitudinally Designed Study that began in 1985. The authors identified 401 children with 24-hour urine samples that met criteria for adequacy of collection and for whom NAE could be determined. They then subdivided these into the 50 male and 50 female subjects with highest NAE and the 50 male and female subjects with the lowest NAE. Glucocorticoid hormone metabolism was assessed through extensive analysis of urinary steroid profiles. Because NAE is determined by a variety of factors in addition to dietary acid loads,4 they also calculated a urinary estimate of dietary acid loads as potential renal acid loads (PRAL) using well-established formulae. Extensive statistical analysis was performed by investigators expert in these types of analyses.
This study has several important strengths. These include the following: (i) its examination of normal children; (ii) it being a component of the large observational study performed over several decades; (iii) its design that specifically compared children with evidence of the lowest rate of NAE with those with the highest rate; (iv) extensive availability of glucocorticoid metabolite analyses; and (v) careful statistical analyses to adjust for numerous interactive factors. When multiple 24-hour collections were available, only the first was used in the analyses. Results of the studies show a clear correlation between renal net acid secretion, dietary PRAL, and glucocorticoid metabolism. Thus, normal children with normal variations in NAE and dietary PRAL show significant variations in glucocorticoid hormone metabolism.
However, the observational nature of the study mandates several considerations. One important issue is the causal relationship of NAE and glucocorticoid metabolism. Exogenous acid loads, typically of greater magnitude than the variations observed in the article by Esche et al.,3 increase NAE excretion and stimulate glucocorticoid metabolism. On the other hand, primary increases in glucocorticoid metabolism, as shown in studies using exogenous glucocorticoid administration, increase renal NAE.1 Thus, which “comes first,” increases in the need for net acid secretion, resulting from increased dietary acid loads, or an increase in glucocorticoid metabolism, cannot be determined definitively. This article partially addresses this issue by quantifying PRAL. However, glucocorticoid administration can also increase protein intake,5 and dietary protein intake is a major component of PRAL. Thus, the possibility remains that a primary increase in glucocorticoid metabolism may cause spontaneous dietary changes that increase PRAL and stimulate renal NAE.
Another important consideration is the clinical implication of changes in glucocorticoid hormone metabolism observed in the study by Esche et al.3 A previous report from the Dortmund Nutritional and Anthropometric Longitudinally Designed Study showed significant correlation between higher glucocorticoid hormone levels and impaired bone characteristics in children.6 Thus, one possible extrapolation is that higher dietary acid loads lead to impaired bone formation in children. However, higher dietary protein intake, a major component of PRAL, actually correlates with greater bone mineral content and bone strength.7 It was only after controlling for protein intake, that is, for the major component of PRAL, that higher glucocorticoid hormone levels correlated with impaired bone characteristics in children.6 Thus, increased dietary protein intake appears to correlate with improved bone characteristics, but “excessive” glucocorticoid hormone response may blunt these benefits. Also, increases in NAE are important in the response to higher PRAL. Specifically, a dissociation between acid loads and NAE, as occurs during glucocorticoid deficiency, leads to metabolic acidosis, which has adverse effects, independent of glucocorticoid hormones, that impair bone modeling and remodeling.8 Perhaps increased glucocorticoid levels are necessary, but excessive increases can be counterproductive.
In summary, the study by Esche et al.3 adds important information to our knowledge of the normal biology of children. This study provides strong evidence of a correlation between PRAL, NAE, and glucocorticoid metabolism in normal children with a normal expected range of diet. Previous studies using exogenous acid or glucocorticoid loads showed similar findings, but it was important to determine whether adaptive responses associated with the normal range of physiologic variations are similar to those observed with non-physiologic, experimental stimuli. Future studies hopefully will address the question of the interaction between dietary acid loads, renal acid excretion, and glucocorticoid metabolism, determine the primary/initiating process(es), and determine the specific characteristics of the optimal glucocorticoid hormone response.
Acknowledgments
Preparation of this commentary was supported by funds from the National Institutes of Health (R01-DK045788 and R01-DK107798). Support from the Research Service of the Gainesville VA Medical Center is gratefully acknowledged. This publication does not reflect official policy of the Department of Veterans Affairs.
Footnotes
DISCLOSURE
The author declared no competing interests.
References
- 1.Hamm LL, Ambuhl PM, Alpern RJ. Role of glucocorticoids in acidosis. Am J Kidney Dis. 1999;34:960–965. doi: 10.1016/s0272-6386(99)70059-4. [DOI] [PubMed] [Google Scholar]
- 2.Weiner ID, Mitch WE, Sands JM. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol. 2014;10:1444–1458. doi: 10.2215/CJN.10311013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esche J, Shi L, Sánchez-Guijo A, et al. Higher diet-dependent renal acid load associates with higher glucocorticoid secretion and potentially bioactive free glucocorticoids in healthy children. Kidney Int. 2016;90:325–333. doi: 10.1016/j.kint.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 4.Weiner ID, Verlander JW. Renal ammonia metabolism and transport. Compr Physiol. 2012;3:201–220. doi: 10.1002/cphy.c120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grigson PS, Johnson DF, Collier GH, et al. The effect of dexamethasone-21-acetate on meal size, meal frequency and macronutrient self-selection in rats. Physiol Behav. 1989;46:211–216. doi: 10.1016/0031-9384(89)90258-8. [DOI] [PubMed] [Google Scholar]
- 6.Shi L, Sanchez-Guijo A. Higher glucocorticoid secretion in the physiological range is associated with lower bone strength at the proximal radius in healthy children: importance of protein intake adjustment. J Bone Min Res. 2015;30:240–248. doi: 10.1002/jbmr.2347. [DOI] [PubMed] [Google Scholar]
- 7.Alexy U, Remer T, Manz F, et al. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr. 2005;82:1107–1114. doi: 10.1093/ajcn/82.5.1107. [DOI] [PubMed] [Google Scholar]
- 8.Kraut JA, Coburn JW. Bone, acid, and osteoporosis. N Engl J Med. 1995;330:1821–1822. doi: 10.1056/NEJM199406233302510. [DOI] [PubMed] [Google Scholar]